Self-Assembly Behavior, Aggregation Structure, and the Charge Carrier Transport Properties of S-Heterocyclic Annulated Perylene Diimide Derivatives
Abstract
1. Introduction
2. Results
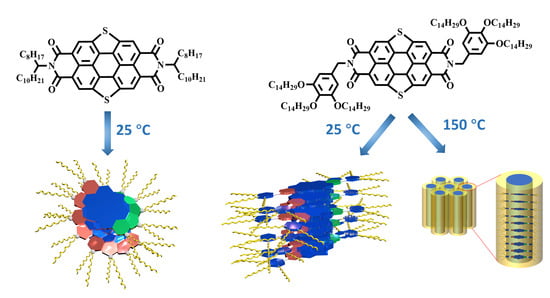
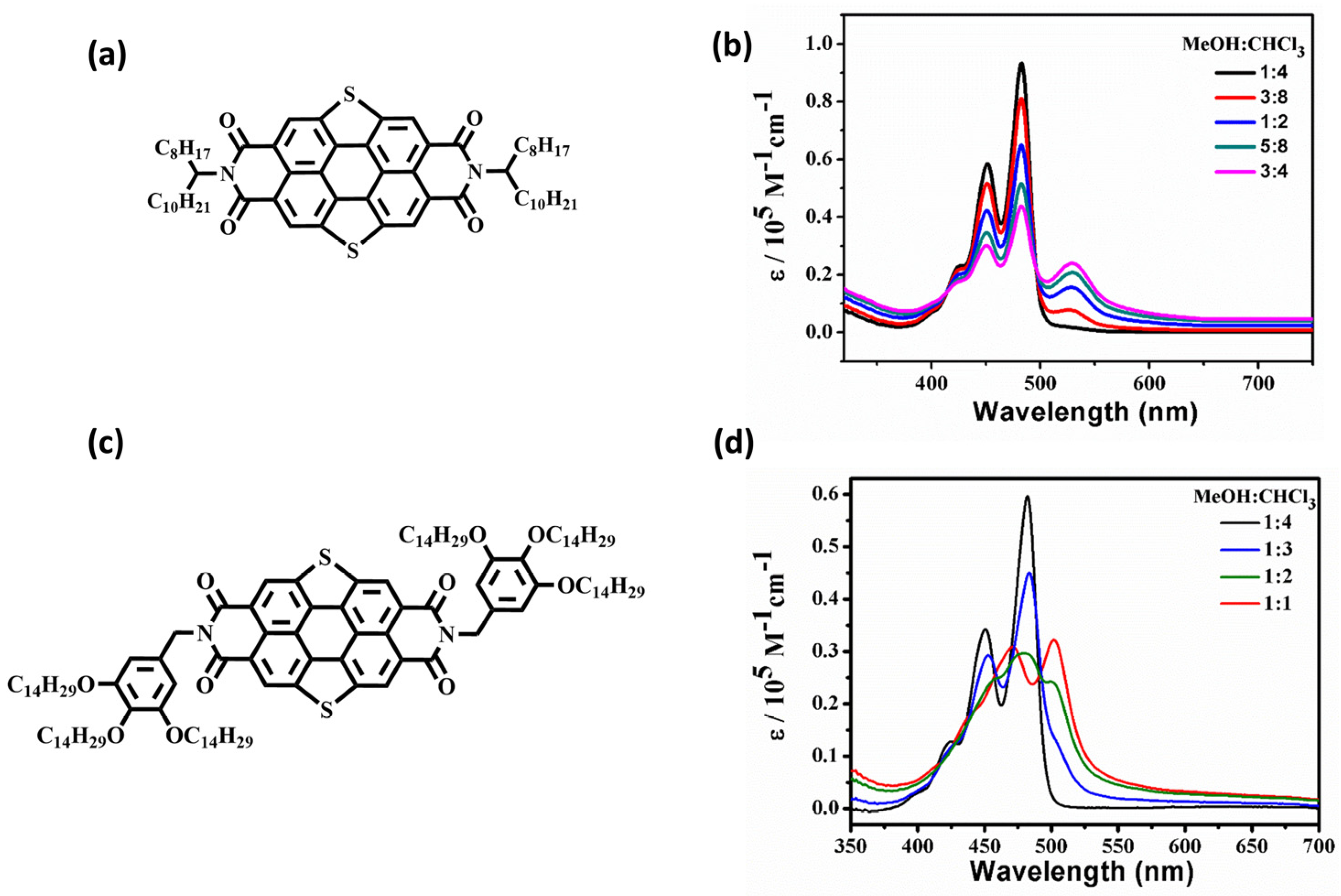
2.1. The Self-Assembly Behavior of SPDIs in Solution
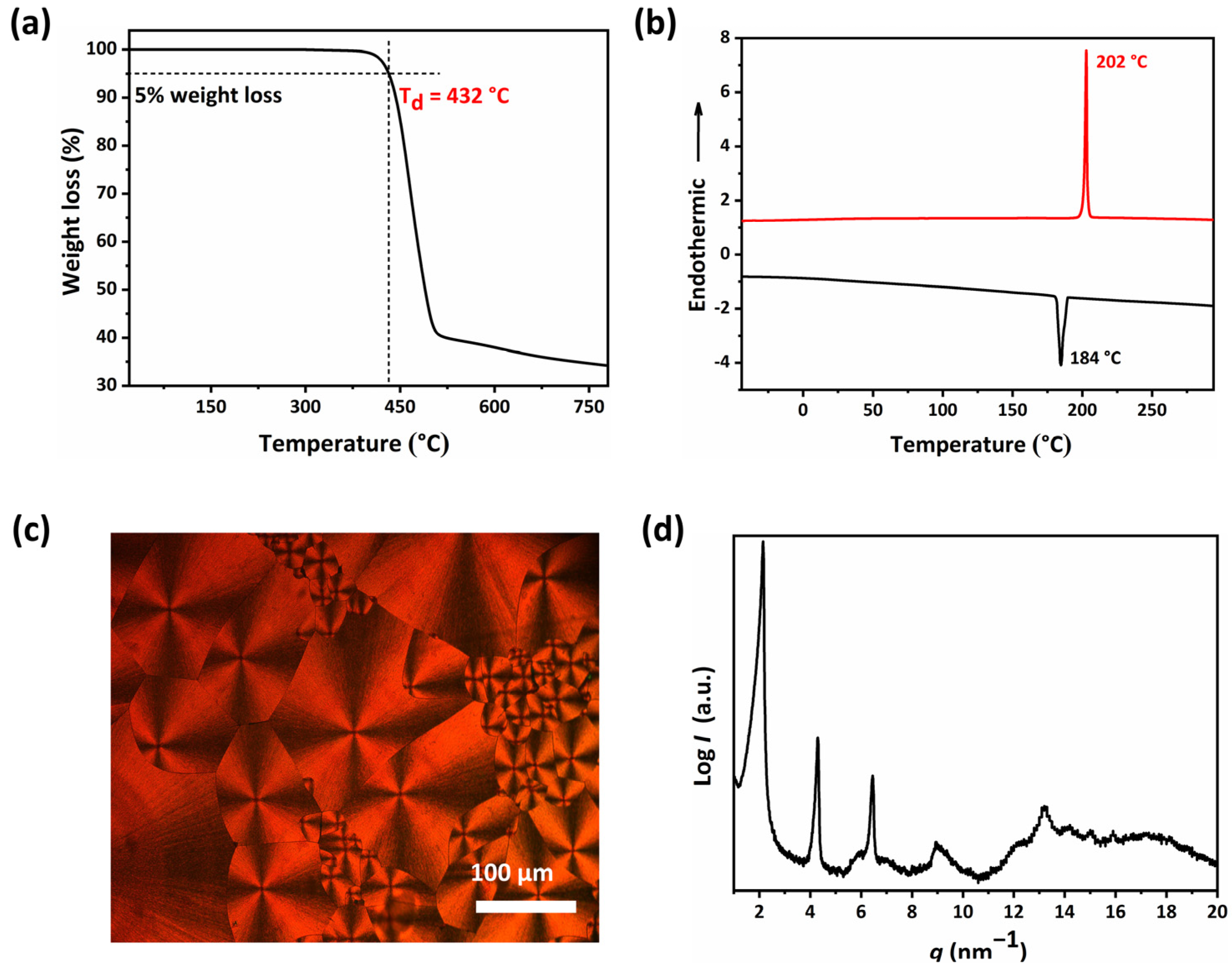
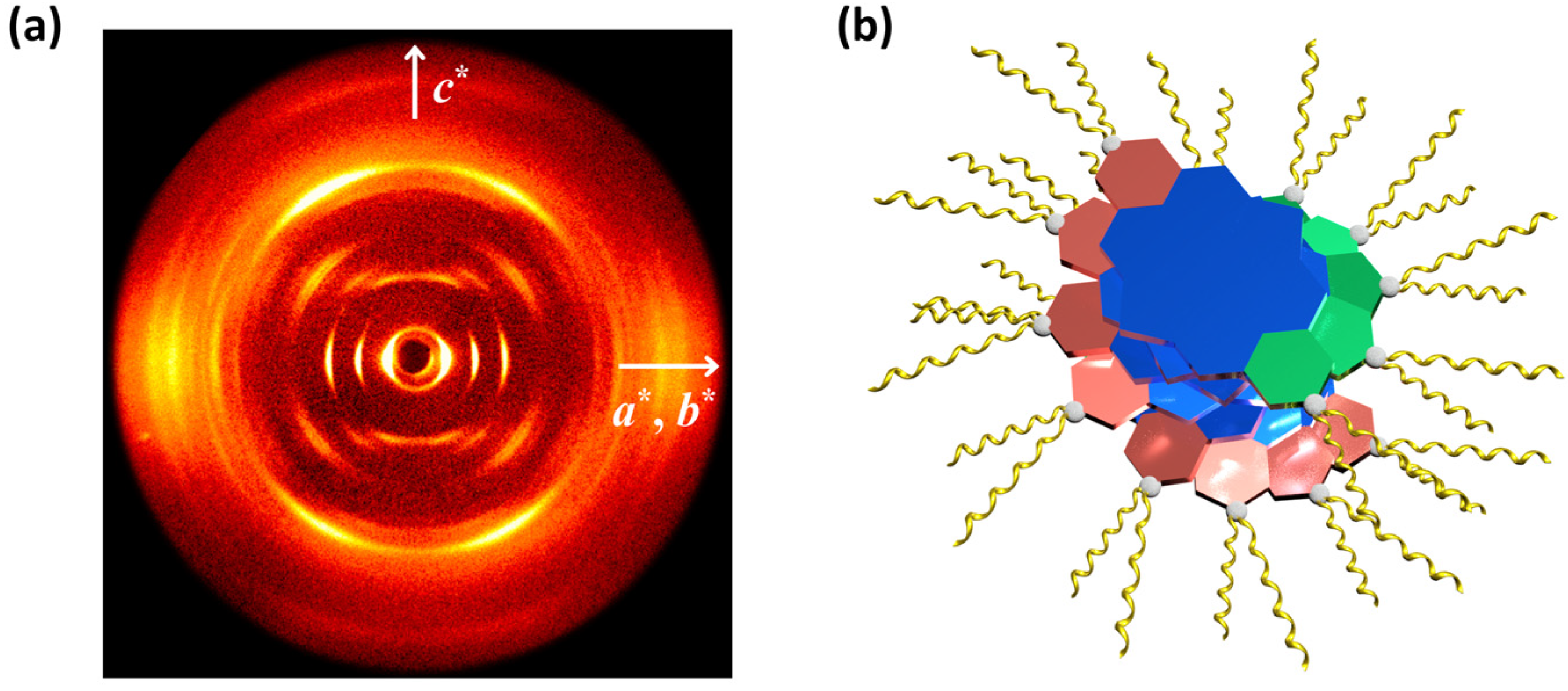
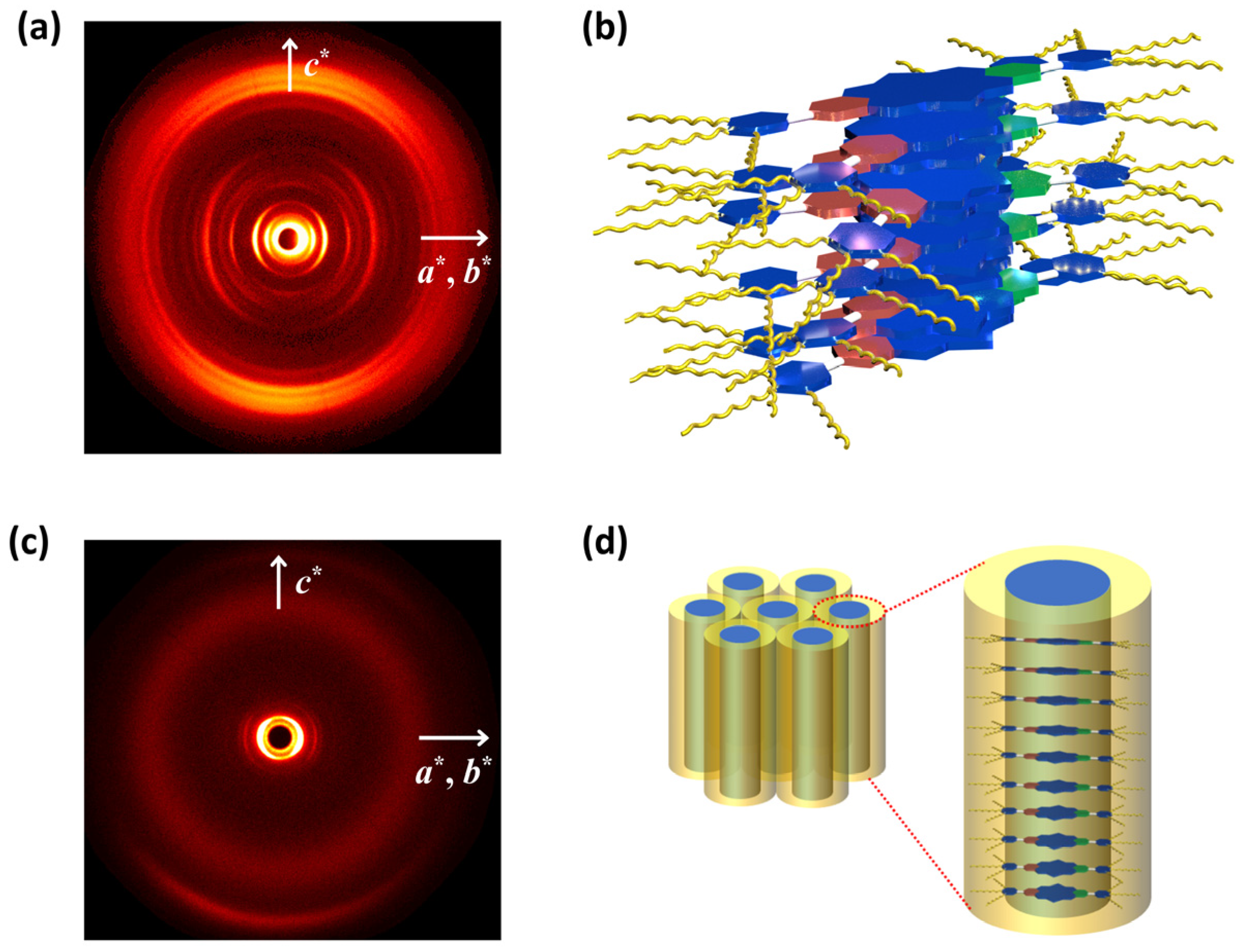
2.2. The Thermal Properties and Self-Assembly Behavior of SPDIs in Solid
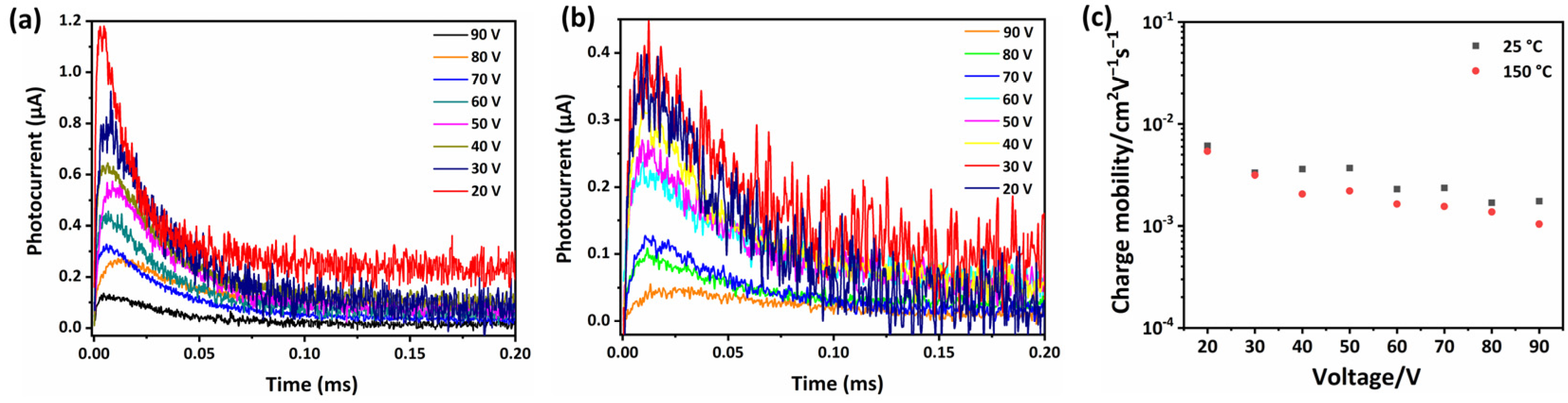
2.3. The Charge Carrier Transport Properties of SPDIs
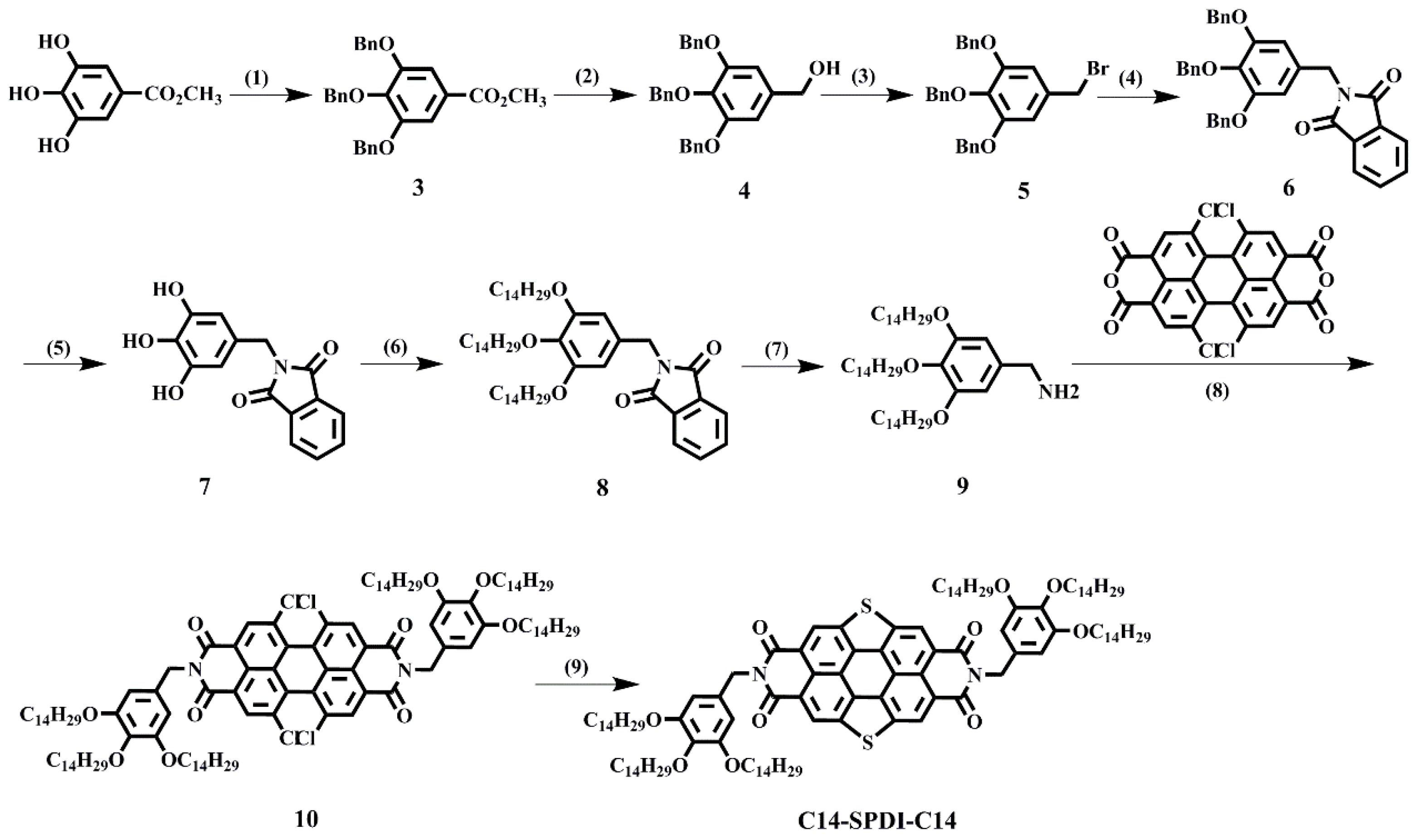
3. Materials and Methods
3.1. Materials
3.2. Equipment and Characterization
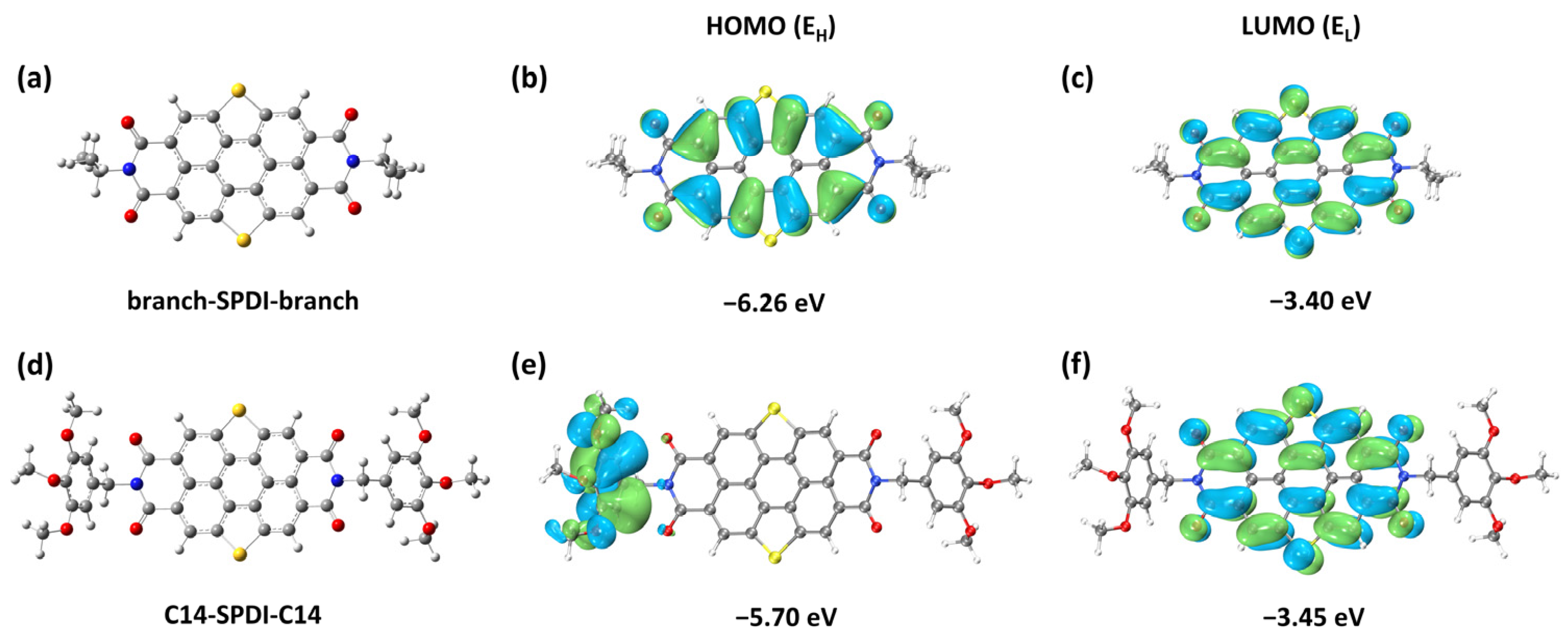
3.3. Theoretical Calculation Methods
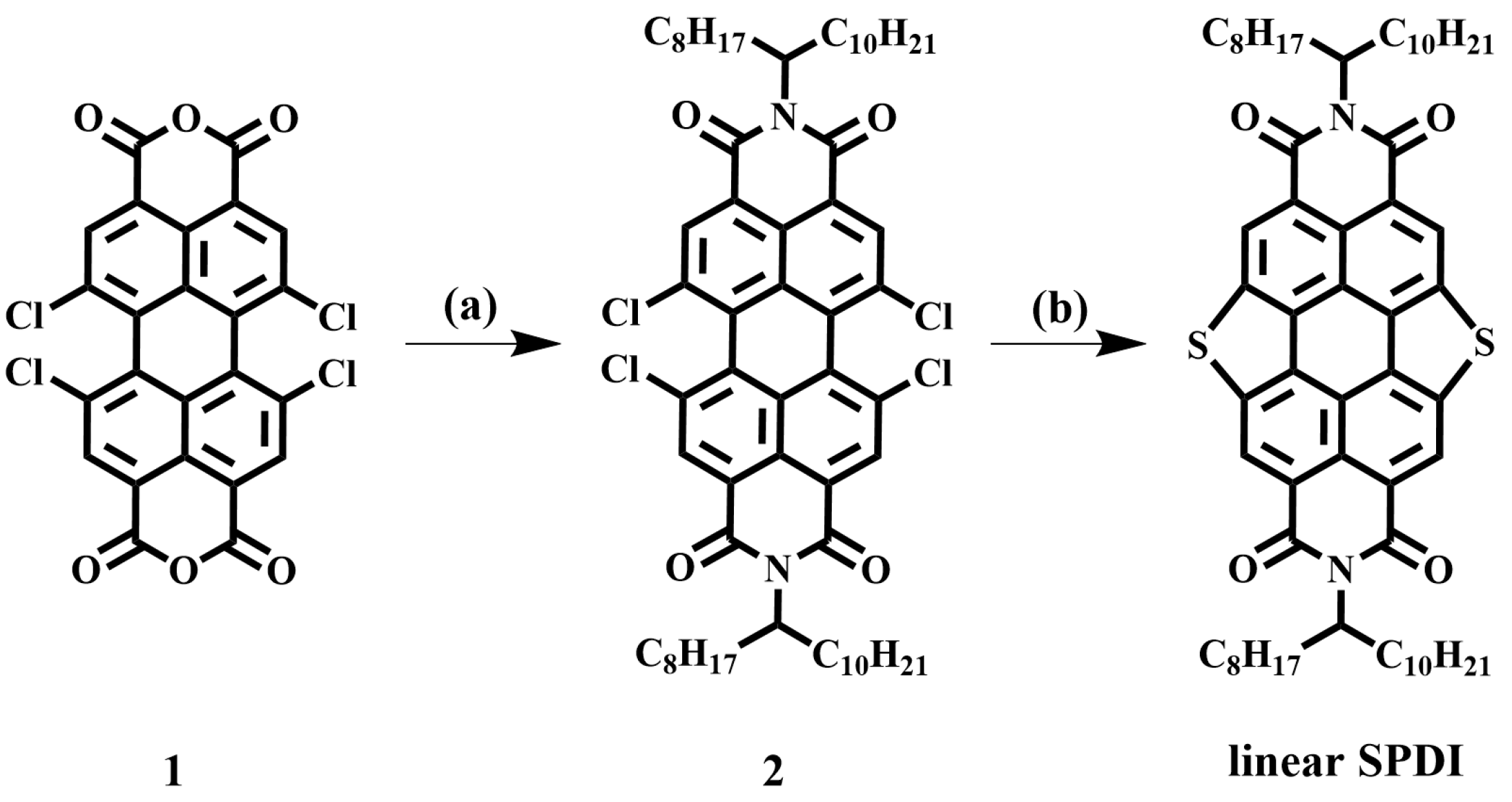
3.4. The Synthesis of Linear SPDI
3.5. The Synthesis of Dendronized SPDI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawatzki, M.; Wang, S.; Kleemann, H.; Leo, K. Highly ordered small molecule organic semiconductor thin-films enabling complex, high-performance multi-junction devices. Chem. Rev. 2023, 123, 8232–8250. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.; Dong, R.; Feng, X.; Kaskel, S.; Matoga, D.; Stavila, V. Electronic devices using open framework materials. Chem. Rev. 2020, 120, 8581–8640. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Cao, L.; Wu, T. Meniscus-guided deposition of organic semiconductor thin films: Materials, mechanism, and application in organic field-effect transistors. Small 2023, 19, 2300151. [Google Scholar] [CrossRef]
- Guan, Y.; Qiao, J.; Liang, Y.; Bisoyi, H.; Wang, C.; Xu, W.; Zhu, D.; Li, Q. A high mobility air-stable n-type organic small molecule semiconductor with high UV–visible-to-NIR photoresponse. Light Sci. Appl. 2022, 11, 236. [Google Scholar] [CrossRef]
- Hota, M.; Chandra, S.; Lei, Y.; Xu, X.; Hedhili, M.; Emwas, A.; Shekhah, O.; Eddaoudi, M.; Alshareef, H. Electrochemical thin-film transistors using covalent organic framework channel. Adv. Funct. Mater. 2022, 32, 2201120. [Google Scholar] [CrossRef]
- Griggs, S.; Marks, A.; Bristow, H.; McCulloch, I. n-Type organic semiconducting polymers: Stability limitations, design considerations and applications. J. Mater. Chem. C 2021, 9, 8099–8128. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Kumagai, S.; Fukuzaki, E.; Ishii, H.; Watanabe, G.; Niitsu, N.; Annaka, T.; Yamagishi, M.; Tani, Y.; Sugiura, H.; et al. Robust, high-performance n-type organic semiconductors. Sci. Adv. 2020, 6, eaaz0632. [Google Scholar] [CrossRef]
- Chen, L.; Qin, Z.; Huang, H.; Zhang, J.; Yin, Z.; Yu, X.; Zhang, X.; Li, C.; Zhang, G.; Huang, M.; et al. High-performance ambipolar and n-type emissive semiconductors based on perfluorophenyl-substituted perylene and anthracene. Adv. Sci. 2023, 10, 2300530. [Google Scholar] [CrossRef]
- Li, D.; An, N.; Tan, K.; Ren, Y.; Wang, H.; Li, S.; Deng, Q.; Song, J.; Bu, L.; Lu, G. Insulating electrets converted from organic semiconductor for high-performance transistors, memories, and artificial synapses. Adv. Funct. Mater. 2023, 33, 2305012. [Google Scholar] [CrossRef]
- Feng, K.; Guo, H.; Sun, H.; Guo, X. n-Type Organic and Polymeric Semiconductors based on bithiophene imide derivatives. Acc. Chem. Res. 2021, 54, 3804–3817. [Google Scholar] [CrossRef]
- Kumagai, S.; Ishii, H.; Watanabe, G.; Yu, C.; Watanabe, S.; Takeya, J.; Okamoto, T. Nitrogen-containing perylene diimides: Molecular design, robust aggregated structures, and advances in n-type organic semiconductors. Acc. Chem. Res. 2022, 55, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Ravindran, E.; Sangeetha, V.; Liu, S.; Jung, J. Perylene-diimide for organic solar cells: Current scenario and prospects in molecular geometric, functionalization, and optoelectronic properties. J. Mater. Chem. A 2023, 11, 26393–26425. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Zhou, Y.; Hao, P.; Shang, Q.; Wang, S.; Zhang, Y.; Li, D.; Yang, S.; Zhang, Q.; et al. Facile syntheses, characterization, and physical properties of sulfur-decorated pyran-annulated perylene diimides. Asian J. Org. Chem. 2018, 7, 702–706. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, Z.; Zhang, A.; Li, J.; Wei, X.; Jiang, T.; Li, Y.; Wang, X. Self-assembly, optical and electrical properties of five membered O- or S-heterocyclic annulated perylene diimides. Dyes Pigment. 2016, 135, 41–48. [Google Scholar] [CrossRef]
- Gsanger, M.; Bialas, D.; Huang, L.; Stolte, M.; Wuerthner, F. Organic semiconductors based on dyes and color pigments. Adv. Mater. 2016, 28, 3615–3645. [Google Scholar] [CrossRef]
- Shinamura, S.; Osaka, I.; Miyazaki, E.; Takimiya, K. Air-stable and high-mobility organic semiconductors based on heteroarenes for field-effect transistors. Heterocycles 2011, 83, 1187–1204. [Google Scholar] [CrossRef]
- Behera, P.; Yadav, K.; Rao, D.; Pandey, U.; Sudhakar, A. Ambipolar columnar self-assembled organic semiconductors based on heteroatom bay-annulated perylene bisimides. Chem Asian J. 2023, 18, e202300086. [Google Scholar] [CrossRef]
- Gupta, R.; Dey, A.; Singh, A.; Lyer, P.; Sudhakar, A. Heteroatom bay-annulated perylene bisimides: New materials for organic field effect transistors. ACS Appl. Electron. Mater. 2019, 1, 1378–1386. [Google Scholar] [CrossRef]
- Hecht, M.; Wuerthner, F. Supramolecularly engineered J-aggregates based on perylene bisimide dyes. Acc. Chem. Res. 2021, 54, 642–653. [Google Scholar] [CrossRef]
- Konidaris, K.; Zambra, M.; Giannici, F.; Guagliardi, A.; Masciocchi, N. Forcing twisted 1,7-dibromoperylene diimides to flatten in the solid state: What a difference an atom makes. Angew. Chem. Int. Ed. 2023, 62, e202310445. [Google Scholar] [CrossRef]
- Ben, H.; Ren, X.; Song, B.; Li, X.; Feng, Y.; Jiang, W.; Chen, E.; Wang, Z.; Jiang, S. Synthesis, crystal structure, enhanced photoluminescence properties and fluoride detection ability of S-heterocyclic annulated perylene diimide-polyhedral oligosilsesquioxane dye. J. Mater. Chem. C 2017, 5, 2566–2576. [Google Scholar] [CrossRef]
- Kozma, E.; Grisci, G.; Mroz, W.; Catellani, M.; Eckstein-Andicsova, A.; Pagano, K.; Galeotti, F. Water-soluble aminoacid functionalized perylene diimides: The effect of aggregation on the optical properties in organic and aqueous media. Dyes Pigment. 2016, 125, 201–209. [Google Scholar] [CrossRef]
- Barra, M.; Chiarella, F.; Chianese, F.; Vaglio, R.; Cassinese, A. Perylene-diimide molecules with cyano functionalization for electron-transporting transistors. Electronics 2019, 8, 249. [Google Scholar] [CrossRef]
- Chen, Z.; Fimmel, B.; Wuerthner, F. Solvent and substituent effects on aggregation constants of perylene bisimide π-stacks-a linear free energy relationship analysis. Org. Biomol. Chem. 2012, 10, 5845–5855. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zhang, J.; Gong, Y.; Tang, S.; Tu, J.; Xie, Y.; Peng, Q.; Yu, G.; Li, Z. Alkyl chain engineering of pyrene-fused perylene diimides: Impact on transport ability and microfiber self-assembly. Mater. Chem. Front. 2017, 1, 2341–2348. [Google Scholar] [CrossRef]
- Dehm, V.; Chen, Z.; Baumeister, U.; Prins, P.; Siebbeles, L.; Wuerthner, F. Helical growth of semiconducting columnar dye assemblies based on chiral perylene bisimides. Org. Lett. 2007, 9, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, W.; Wang, L. Folding versus self-assembling. Chem. Eur. J. 2003, 9, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Dehm, V.; Buechner, M.; Seibt, J.; Engel, V.; Wuerthner, F. Foldamer with a spiral perylene bisimide staircase aggregate structure. Chem. Sci. 2011, 2, 2094–2100. [Google Scholar] [CrossRef]
- Shao, C.; Gruene, M.; Stolte, M.; Wuerthner, F. Perylene bisimide dimer aggregates: Fundamental insights into self-assembly by NMR and UV/Vis spectroscopy. Chem. Eur. J. 2012, 18, 13665–13677. [Google Scholar] [CrossRef]
- Tang, B.; Geng, Y.; Lam, J.; Li, B.; Jing, X.; Wang, X.; Wang, F.; Pakhomov, A.; Zhang, X. Processible nanostructured materials with electrical conductivity and magnetic susceptibility: preparation and properties of maghemite/polyaniline nanocomposite films. Chem. Mater. 1999, 11, 1581–1589. [Google Scholar] [CrossRef]
- Eashoo, M.; Wu, Z.; Zhang, A.; Shen, D.; Tse, C.; Harris, F.; Cheng, S. High performance aromatic polyimide fibers, 3. A polyimide synthesized from 3,3′,4,4′-biphenyltetracarboxylic dianhydride and 2,2′-dimethyl-4,4′-diaminobiphenyl. Macromol. Chem. Phys. 1994, 195, 2207–2225. [Google Scholar] [CrossRef]
- Jing, A.; Taikun, O.; Li, C.; Harris, F.; Cheng, S. Phase identifications and monotropic transition behaviors in a thermotropic main-chain liquid crystalline polyether. Polymer 2002, 43, 3431–3440. [Google Scholar] [CrossRef]
- Jing, H.; Lu, L.; Feng, Y.; Zheng, J.; Deng, L.; Chen, E.; Ren, X. Synthesis, Aggregation-induced emission, and liquid crystalline structure of tetraphenylethylene–surfactant complex via ionic self-assembly. J. Phys. Chem. C 2016, 120, 27577–27586. [Google Scholar] [CrossRef]
- Lu, C.; Luo, Z. Critical evaluation of (110) texture in lithium electrodeposits on isotropic Cu polycrystals. Nat. Commun. 2022, 13, 5673. [Google Scholar] [CrossRef]
- Lopezcarrasquero, F.; Montserrat, S.; Ilarduya, A.; Munozguerra, S. Structure and thermal properties of new comblike polyamides: Helical poly(.beta.-L-aspartate)s containing linear alkyl side chains. Macromolecules 1995, 28, 5535–5546. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, Y.; Dong, X.; Zhou, Y.; Wang, D. Frustrated crystallisation and hierarchical self-assembly behaviour of comb-like polymers. Chem. Soc. Rev. 2013, 42, 2075–2099. [Google Scholar] [CrossRef]
- Lu, L.; Sun, H.; Zeng, Y.; Shao, Y.; Bermeshev, M.; Zhao, Y.; Sun, B.; Chen, Z.; Ren, X.; Zhu, M. Perylene diimide derivative via ionic self-assembly: Helical supramolecular structure and selective detection of ATP. J. Mater. Chem. C 2020, 8, 10422–10430. [Google Scholar] [CrossRef]
- Wang, M.; Fu, S.; Petkov, P.; Fu, Y.; Zhang, Z.; Liu, Y.; Ma, J.; Chen, G.; Gali, S.; Gao, L.; et al. Exceptionally high charge mobility in phthalocyanine-based poly(benzimidazobenzophenanthroline)-ladder-type two-dimensional conjugated polymers. Nat. Mater. 2023, 22, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, J.; Gao, S.; Bermeshev, M.; Chen, Z.; Ren, X. Siloxane tethered perylene diimide: From monotropic phase structures to tunable photoconductivity. J. Mater. Chem. C 2021, 9, 9236–9241. [Google Scholar] [CrossRef]
- Funahashi, M.; Sonoda, A. Liquid–crystalline perylene tetracarboxylic acid bisimide bearing oligosiloxane chains with high electron mobility and solubility. Org. Electron. 2012, 13, 1633–1640. [Google Scholar] [CrossRef]
- Reghu, R.; Bisoyi, H.; Grazulevicius, J.; Anjukandi, P.; Gaidelis, V.; Jankauskas, V. Air stable electron-transporting and ambipolar bay substituted perylene bisimides. J. Mater. Chem. 2011, 21, 7811–7819. [Google Scholar] [CrossRef]
- Muth, M.; Gupta, G.; Wicklein, A.; Carrasco, M.; Thurn, T.; Thelakkat, M. Crystalline vs. Liquid Crystalline Perylene Bisimides: Improved Electron Mobility via Substituent Alteration. J. Phys. Chem. C 2014, 118, 92–102. [Google Scholar] [CrossRef]
- Yang, T.; Wu, Q.; Dai, F.; Huang, K.; Xu, H.; Liu, C.; Chen, C.; Hu, S.; Liang, X.; Liu, X.; et al. Understanding, Optimizing, and Utilizing Nonideal Transistors Based on Organic or Organic Hybrid Semiconductors. Adv. Funct. Mater. 2020, 30, 1903889. [Google Scholar] [CrossRef]
- Becke, A. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of Becke and Lee. Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.; Seeger, R.; Pople, J. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD-Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Peterca, M.; Sienkowska, M.; Ilies, M.; Aqad, E.; Smidrkal, J.; Heiney, P. Synthesis and retrostructural analysis of libraries of AB3 and constitutional isomeric AB2 phenylpropyl ether-based supramolecular dendrimers. J. Am. Chem. Soc. 2006, 128, 3324–3334. [Google Scholar] [CrossRef] [PubMed]
- Eich, E.; Pertz, H.; Kaloga, M.; Schulz, J.; Fesen, M.; Mazumder, A.; Pommier, Y. (−)-Arctigenin as a lead structure for inhibitors of human immunodeficiency virus type-1 integrase. J. Med. Chem. 1996, 1, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Sun, H.; Leowanawat, P.; Peterca, M.; Graf, R.; Spiess, H.; Zeng, X.; Ungar, G.; Heiney, P. Transformation from kinetically into thermodynamically controlled self-organization of complex helical columns with 3D periodicity assembled from dendronized perylene bisimides. J. Am. Chem. Soc. 2013, 135, 4129–4148. [Google Scholar] [CrossRef]
- Percec, V.; Peterca, M.; Tadjiev, T.; Zeng, X.; Ungar, G.; Leowanawat, P.; Aqad, E.; Imam, M.; Rosen, B.; Akbey, U.; et al. Self-assembly of dendronized perylene bisimides into complex helical columns. J. Am. Chem. Soc. 2011, 133, 12197–12219. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben, H.; Yan, G.; Wang, Y.; Zeng, H.; Wu, Y.; Lin, F.; Zhao, J.; Du, W.; Zhang, S.; Zhou, S.; et al. Self-Assembly Behavior, Aggregation Structure, and the Charge Carrier Transport Properties of S-Heterocyclic Annulated Perylene Diimide Derivatives. Molecules 2024, 29, 1964. https://doi.org/10.3390/molecules29091964
Ben H, Yan G, Wang Y, Zeng H, Wu Y, Lin F, Zhao J, Du W, Zhang S, Zhou S, et al. Self-Assembly Behavior, Aggregation Structure, and the Charge Carrier Transport Properties of S-Heterocyclic Annulated Perylene Diimide Derivatives. Molecules. 2024; 29(9):1964. https://doi.org/10.3390/molecules29091964
Chicago/Turabian StyleBen, Haijie, Gaojie Yan, Yulin Wang, Huiming Zeng, Yuechao Wu, Feng Lin, Junhua Zhao, Wanglong Du, Shaojie Zhang, Shijia Zhou, and et al. 2024. "Self-Assembly Behavior, Aggregation Structure, and the Charge Carrier Transport Properties of S-Heterocyclic Annulated Perylene Diimide Derivatives" Molecules 29, no. 9: 1964. https://doi.org/10.3390/molecules29091964
APA StyleBen, H., Yan, G., Wang, Y., Zeng, H., Wu, Y., Lin, F., Zhao, J., Du, W., Zhang, S., Zhou, S., Pu, J., Ye, M., Ji, H., & Lv, L. (2024). Self-Assembly Behavior, Aggregation Structure, and the Charge Carrier Transport Properties of S-Heterocyclic Annulated Perylene Diimide Derivatives. Molecules, 29(9), 1964. https://doi.org/10.3390/molecules29091964