In Vivo, In Vitro and In Silico Anticancer Activity of Ilama Leaves: An Edible and Medicinal Plant in Mexico
Abstract
1. Introduction
2. Results
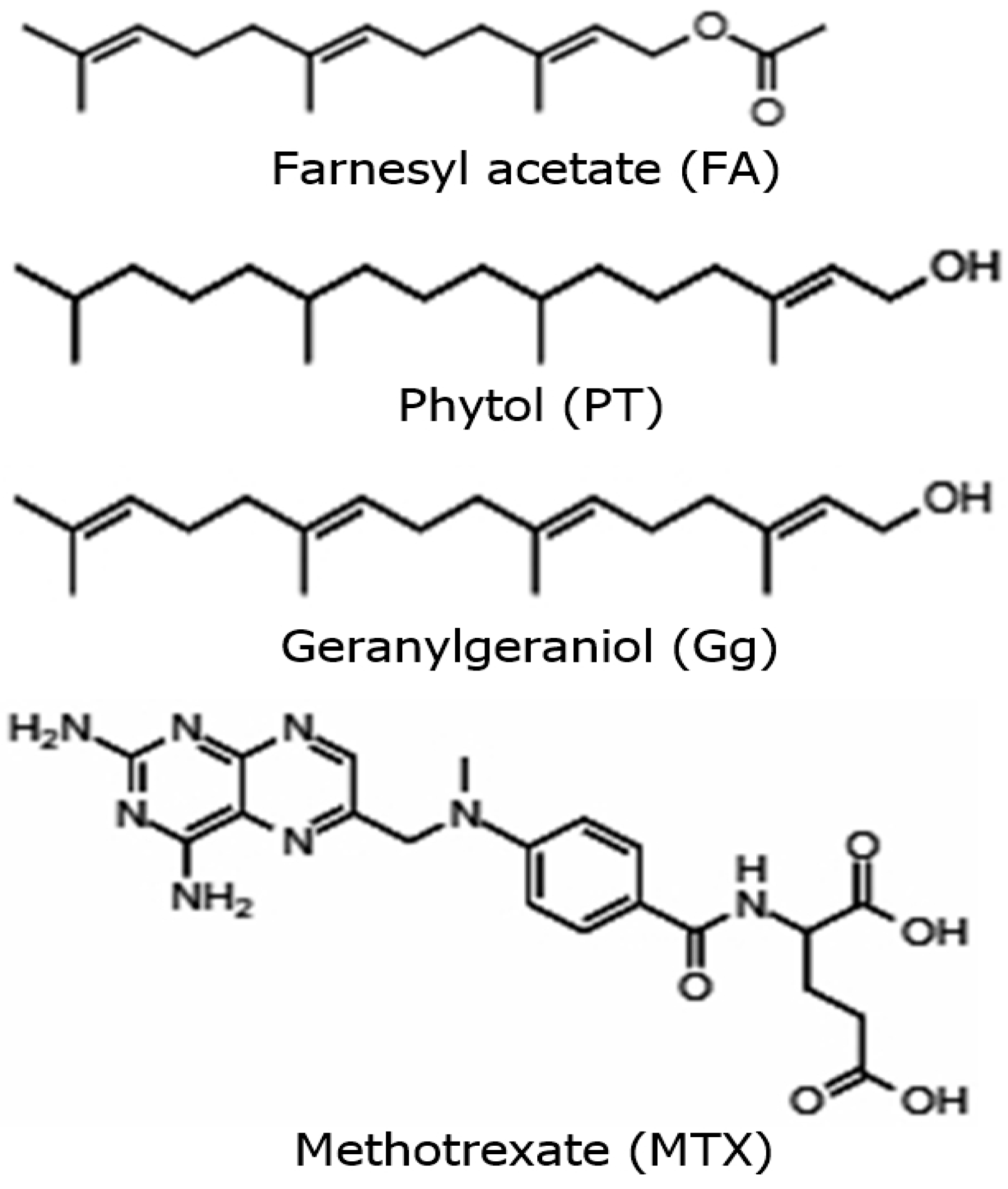
2.1. Antilymphoma Activity of Acyclic Terpenoids Isolated from Ilama Leaves
2.2. Effect of Geranylgeraniol, Phytol and Farnesyl Acetate Isolated from Ilama Leaves on Apoptosis and Necrosis in U-937 Cells
2.3. Effect of Geranylgeraniol, Phytol and Farnesyl Acetate Isolated from Ilama Leaves on Induction of Generation of ROS in U-937 Cells
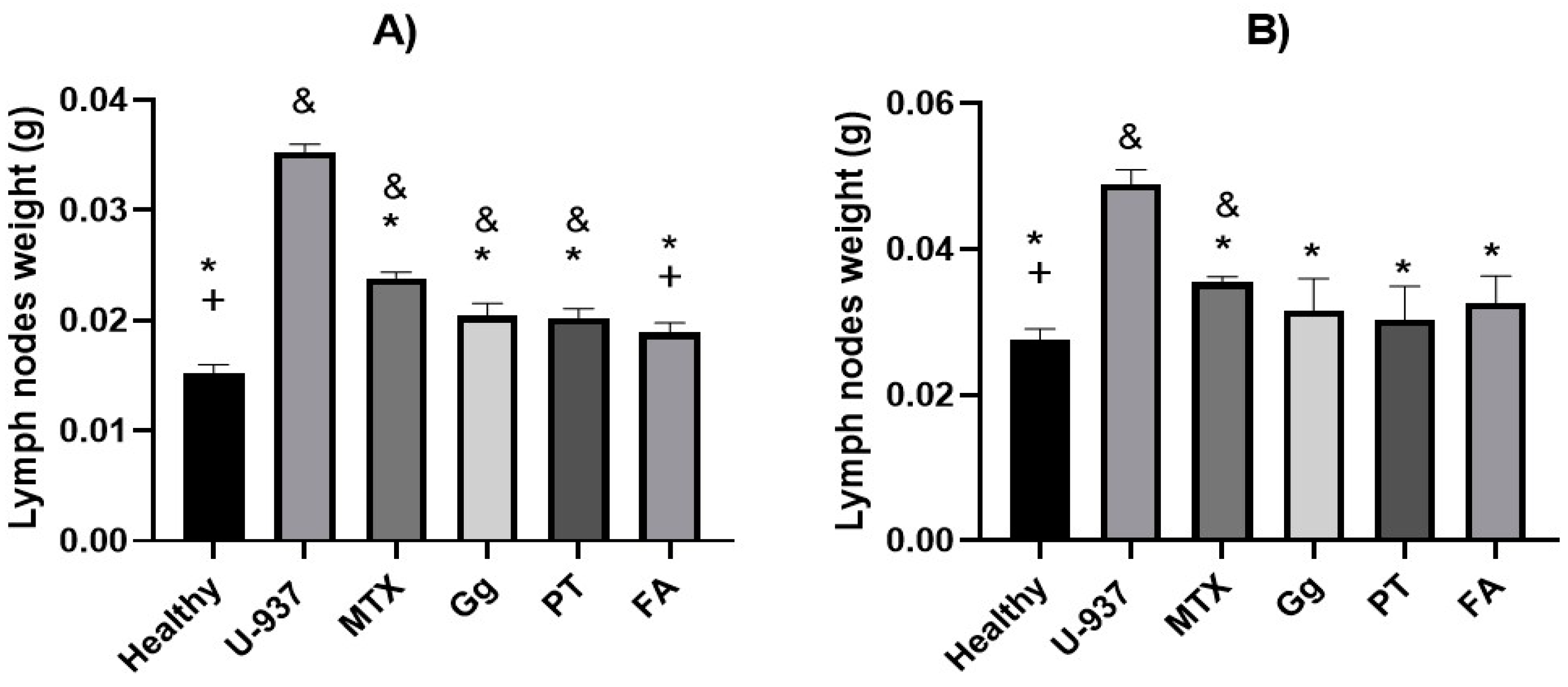
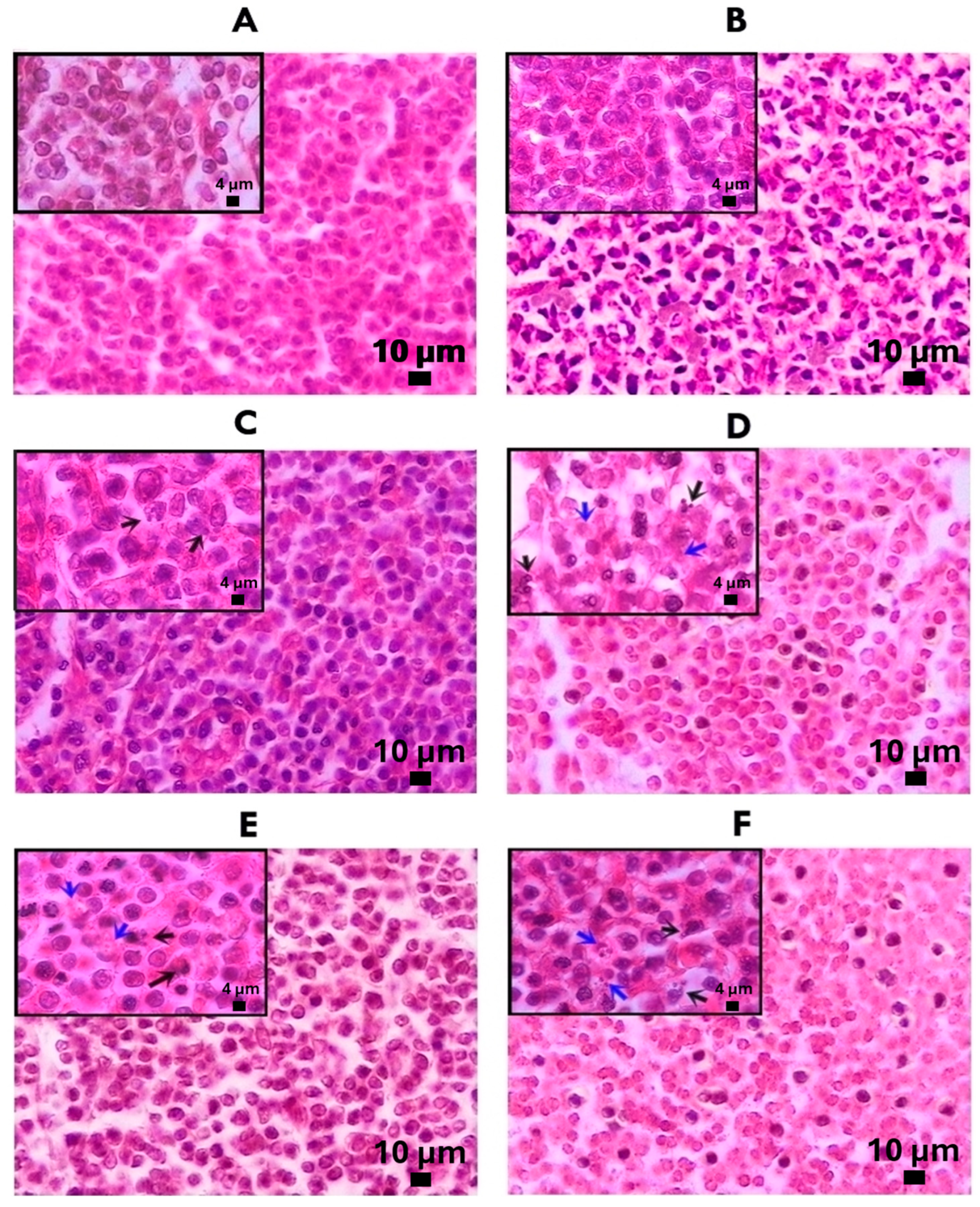
2.4. Morphological Analysis Using Histology of Axillary Lymph Nodes
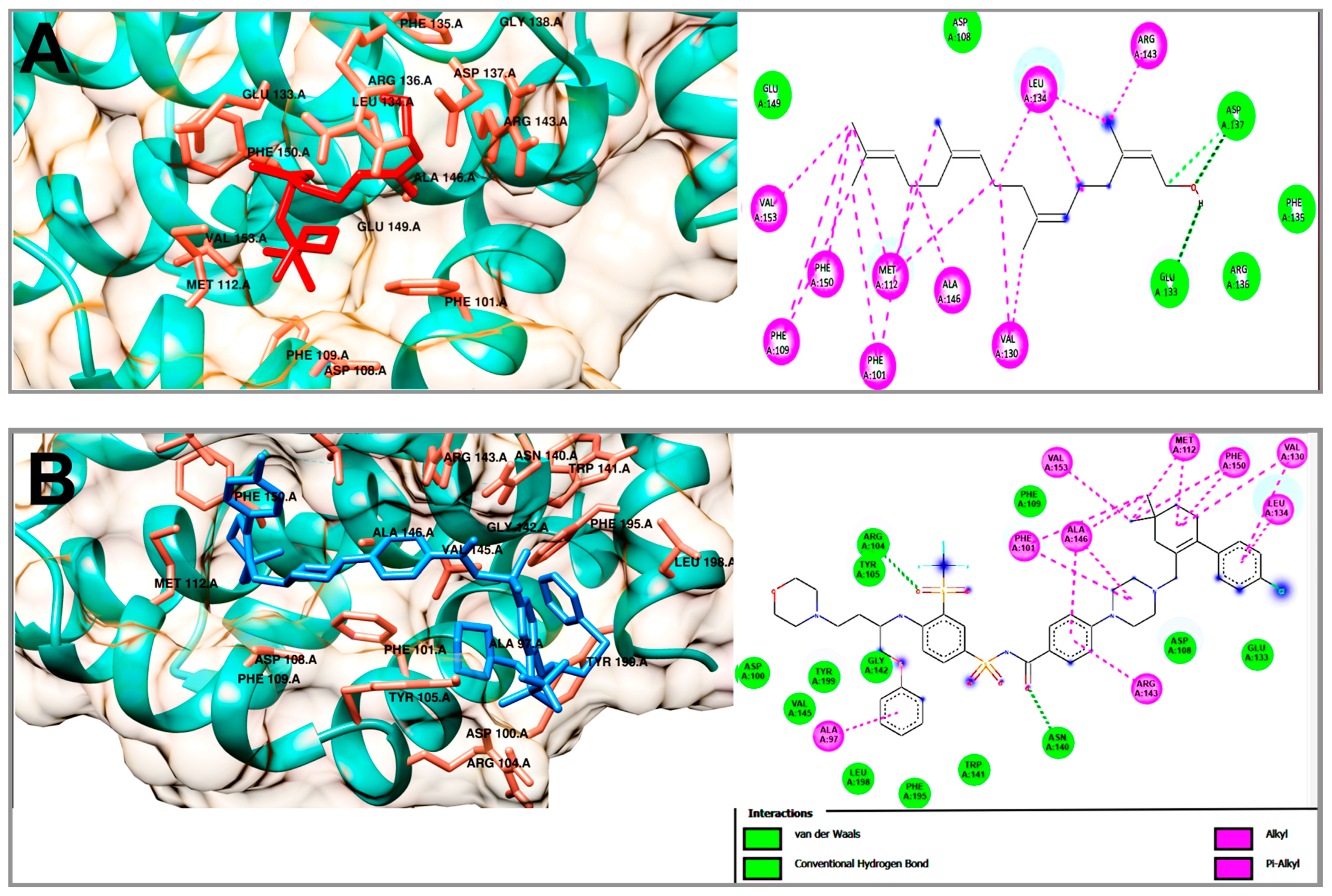
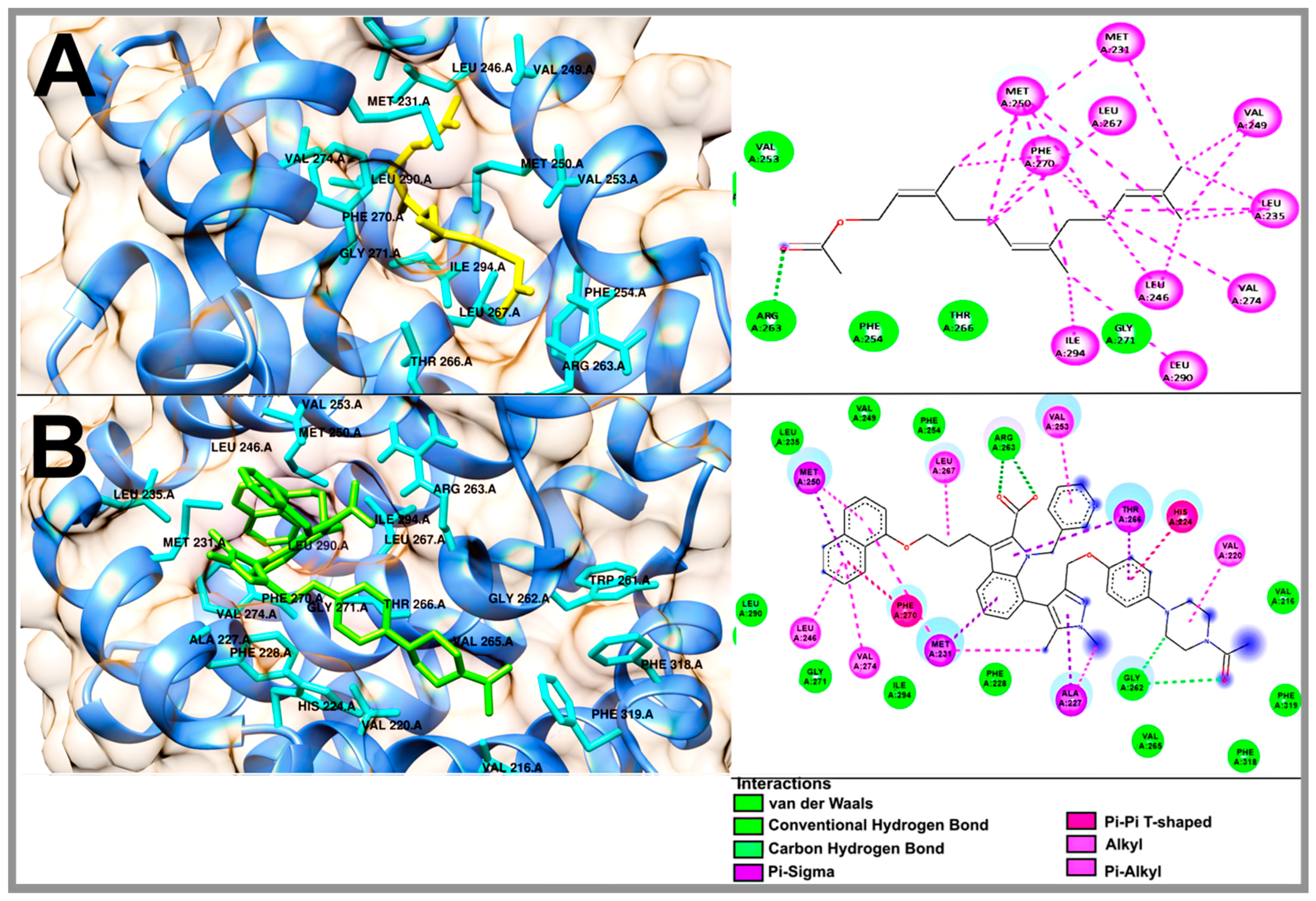
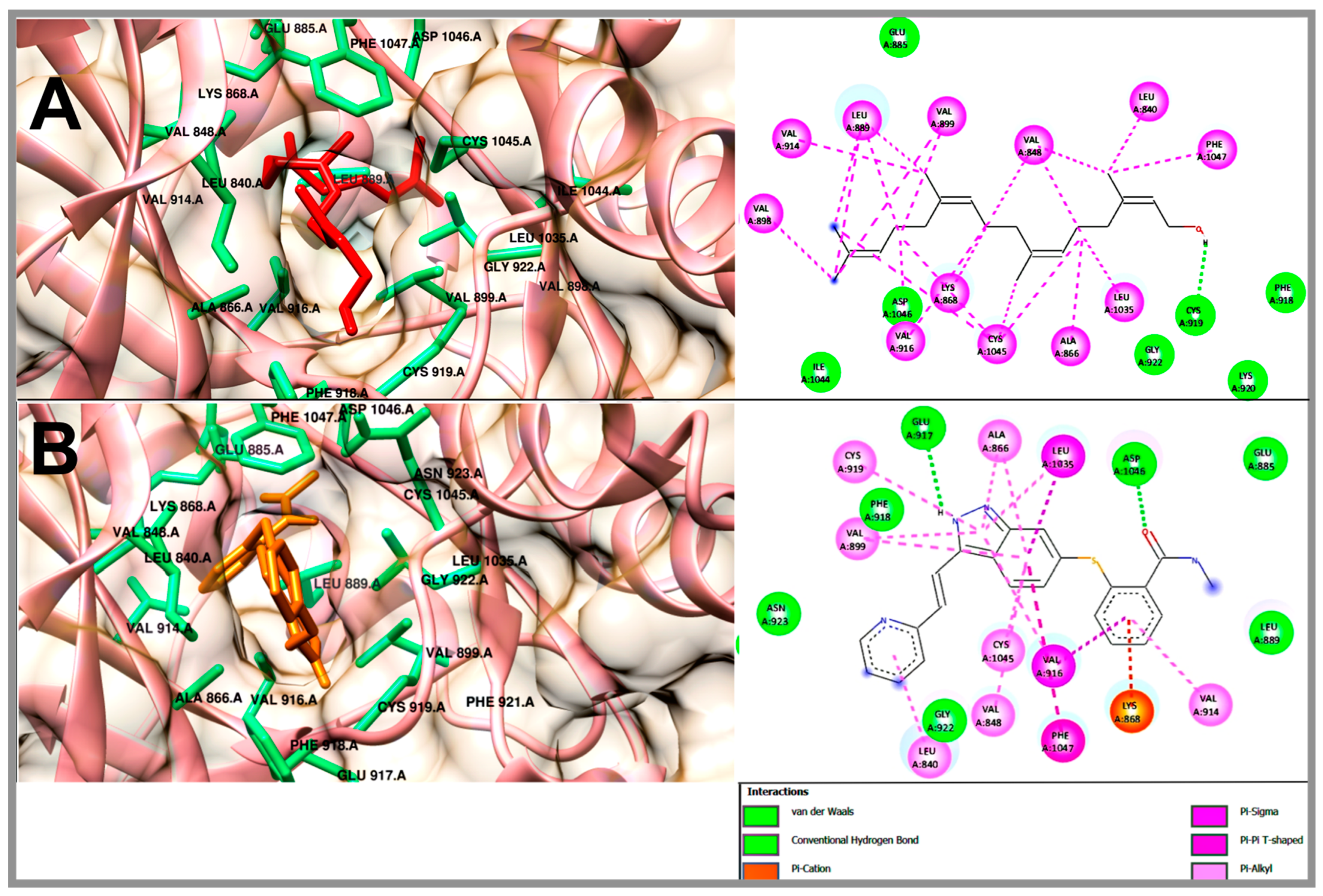
2.5. Molecular Docking Studies of Geranylgeraniol, Farnesyl Acetate and Phytol
3. Discussion
4. Materials and Methods
4.1. Preparation of Petroleum Ether Extract of Leaves from A. macroprophyllata
4.2. Isolation of Geranylgeraniol, Phytol and Farnesyl Acetate
4.3. Animals
Antilymphoma Activity
4.4. Cell-Based Assay
4.4.1. Culture
4.4.2. Annexin V-FITC/IP Staining
4.4.3. Measurement of Intracellular ROS
4.5. Histology of Axillary Lymph Nodes
4.6. Molecular Docking Studies
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Julián-Loaeza, A.P.; Santos-Sánchez, N.F.; Valadez-Blanco, R.; Sánchez-Guzman, B.S.; Salas-Coronado, R. Chemical composition, color, and antioxidant activity of three varieties of Annona diversifolia Safford fruits. Ind. Crops Prod. 2011, 34, 1262–1268. [Google Scholar] [CrossRef]
- Brindis, F.; González-Trujano, M.E.; González-Andrade, M.; Aguirre-Hernández, E.; Villalobos-Molina, R. Aqueous extract of Annona macroprophyllata: A potential a-glucosidase inhibitor. Biomed. Res. Int. 2013, 2013, 591313. [Google Scholar] [CrossRef] [PubMed]
- Otero-Sánchez, M.A.; Becerril-Román, A.E.; Castillo-Morales, A.; Michel-Aceves, A.C.; Ariza-Flores, R.; Barrios-Ayala, A.; Rebolledo-Martínez, A. Producción de ilama (Annona diversifolia Saff.) en el trópico seco de Guerrero, México. Rev. Chapingo Ser. Hortic. 2006, 12, 137–146. [Google Scholar] [CrossRef]
- Lefebvre, C.; Segura, S.; Carmona, A.; Mathuriau, C.; Barrios, S.; Andrés, J.; Medellín-Azuara, J. Linking local Appreciation with conservation of an edible fruit species: The case study of Ilama (Annona diversifolia Saff.) in Tierra Caliente, Mexico. Nat. Resour. 2018, 9, 337–353. [Google Scholar] [CrossRef][Green Version]
- González-Trujano, M.E.; Martínez, A.L.; Reyes-Ramírez, A.; Reyes-Trejo, B.; Navarrete, A. Palmitone isolated from Annona diversifolia induces an anxiolytic-like effect in mice. Plant Med. 2006, 72, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Santos, J.; Calzada, F.; Mendieta-Wejebe, J.E.; Ordoñez-Razo, R.M.; Martinez-Casares, R.M.; Valdes, M. Understanding the Antilymphoma Activity of Annona macroprophyllata Donn and Its Acyclic Terpenoids: In Vivo, In Vitro, and In Silico Studies. Molecules 2022, 27, 7123. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/es/news-room/fact-sheets/detail/cancer (accessed on 22 February 2023).
- World Health Organization. Available online: https://www.iarc.who.int/news-events/latest-global-cancer-data-cancer-burden-rises-to-19-3-million-new-cases-and-10-0-million-cancer-deaths-in-2020/ (accessed on 25 August 2023).
- Rubio-Jurado, B.; Sosa-Quintero, L.; Carrasco-Martinez, I.; Norato-Delgado, A.; Garcia-Luna, E.; Guzmán-Silahua, S.; Nava-Zavala, A.H. New biomarkers in non-Hodgkin lymphoma and acute leukemias. Adv. Clin. Chem. 2020, 96, 19–53. [Google Scholar] [PubMed]
- International Agency for Research on Cancer. GLOBOCAN 2020. Global Cancer Observatory. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/34-Non-hodgkin-lymphoma-fact-sheet.pdf (accessed on 30 August 2023).
- Koff, J.L.; Chihara, D.; Phan, A.; Nastoupil, L.J.; Williams, J.N.; Flowers, C.R. To each its own: Linking the biology and epidemiology of NHL subtypes. Curr. Hematol. Malig. Rep. 2015, 10, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Omari, N.; Bakrim, S.; Bakha, M.; Lorenzo, J.M.; Rebezov, M.; Shariati, M.A.; Bouyahya, A. Natural bioactive compounds targeting epigenetic pathways in cancer: A review on alkaloids, terpenoids, quinones, and isothiocyanates. Nutrients 2021, 13, 3714. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef] [PubMed]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ anti-cancer effects: Focus on autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef]
- Jain, H.; Dhingra, N.; Narsinghani, T.; Sharma, R. Insights into the mechanism of natural terpenoids as NF-κB inhibitors: An overview on their anticancer potential. Exp. Oncol. 2016, 38, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Leslie, L.A.; Skarbnik, A.P.; Bejot, C.; Stives, S.; Feldman, T.A.; Goy, A.H. Targeting indolent non-Hodgkin lymphoma. Expert Rev. Hematol. 2017, 10, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Roberts, A.W.; Spencer, A.; Rosenberg, A.S.; Siegel, D.; Walter, R.B.; Stein, A. Targeting MCL-1 in hematologic malignancies: Rationale and progress. Blood Rev. 2020, 44, 100672. [Google Scholar] [CrossRef] [PubMed]
- Cho-Vega, J.H.; Rassidakis, G.Z.; Admirand, J.H.; Oyarzo, M.; Ramalingam, P.; Paraguya, A.; Medeiros, L.J. MCL-1 expression in B-cell non-Hodgkin’s lymphomas. Hum. Pathol. 2004, 35, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, S.; Zhang, L.; Li, X.; Fu, X.; Wang, X.; Zhang, M. An open label, single-armed, exploratory study of apatinib (a novel VEGFR-2 tyrosine kinase inhibitor) in patients with relapsed or refractory non-Hodgkin lymphoma. Oncotarget 2018, 9, 16213. [Google Scholar] [CrossRef] [PubMed]
- Sangande, F.; Julianti, E.; Tjahjono, D.H. Ligand-based pharmacophore modeling, molecular docking, and molecular dynamic studies of dual tyrosine kinase inhibitor of EGFR and VEGFR2. Int. J. Mol. Sci. 2020, 21, 7779. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Ranieri, G.; Specchia, G.; Vacca, A. The role of angiogenesis in human non-Hodgkin lymphomas. Neoplasia 2013, 15, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Moradipoodeh, B.; Jamalan, M.; Zeinali, M.; Fereidoonnezhad, M.; Mohammadzadeh, G. In vitro and in silico anticancer activity of amygdalin on the SK-BR-3 human breast cancer cell line. Mol. Biol. Rep. 2019, 46, 6361–6370. [Google Scholar] [CrossRef] [PubMed]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Elmore, S.W. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Bruncko, M.; Wang, L.; Sheppard, G.S.; Phillips, D.C.; Tahir, S.K.; Xue, J.; Souers, A.J. Structure-guided design of a series of MCL-1 inhibitors with high affinity and selectivity. J. Med. Chem. 2015, 58, 2180–2194. [Google Scholar] [CrossRef] [PubMed]
- Sanphanya, K.; Wattanapitayakul, S.K.; Phowichit, S.; Fokin, V.V.; Vajragupta, O. Novel VEGFR-2 kinase inhibitors identified by the back-to-front approach. Bioorg. Med. Chem. Lett. 2013, 23, 2962–2967. [Google Scholar] [CrossRef] [PubMed]
- Babaei, G.; Aliarab, A.; Abroon, S.; Rasmi, Y.; Gholizadeh-Ghaleh Aziz, S. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018, 106, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.; Alshawsh, M.A. Therapeutic potential of certain terpenoids as anticancer agents: A scoping review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and medicinal uses of terpenes. Med. Plants 2019, 333–359. [Google Scholar]
- Howard, S.C.; McCormick, J.; Pui, C.H.; Buddington, R.K.; Harvey, R.D. Preventing and managing toxicities of high-dose methotrexate. Oncologist 2016, 21, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Thakor, P.; Mehta, J.B.; Patel, R.R.; Patel, D.D.; Subramanian, R.B.; Thakkar, V.R. Extraction and purification of phytol from Abutilon indicum: Cytotoxic and apoptotic activity. RSC Adv. 2016, 6, 48336–48345. [Google Scholar] [CrossRef]
- Kim, C.W.; Lee, H.J.; Jung, J.H.; Kim, Y.H.; Jung, D.B.; Sohn, E.J.; Kim, S.H. Activation of caspase-9/3 and inhibition of epithelial mesenchymal transition are critically involved in antitumor effect of phytol in hepatocellular carcinoma cells. Phytother. Res. 2015, 29, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.V.; Yeganehjoo, H.; Katuru, R.; DeBose-Boyd, R.; Morris, L.; Michon, R.; Mo, H. Geranylgeraniol suppresses the viability of human DU145 prostate carcinoma cells and the level of HMG CoA reductase. Exp. Biol. Med. 2013, 238, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Yeganehjoo, H.; DeBose-Boyd, R.; McFarlin, B.; Mo, H. Synergistic impact of d-δ-tocotrienol and geranylgeraniol on the growth and HMG CoA reductase of human DU145 prostate carcinoma cells. Nutr. Cancer. 2017, 69, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Yamada, J.; Tsuno, N.; Okaji, Y.; Kawai, K.; Tsuchiya, T.; Takahashi, K. Plaunotol and geranylgeraniol induce caspase-mediated apoptosis in colon cancer. J. Surg. Res. 2009, 153, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Ohizumi, H.; Masuda, Y.; Nakajo, S.; Sakai, I.; Ohsawa, S.; Nakaya, K. Geranylgeraniol is a potent inducer of apoptosis in tumor cells. J. Biochem. 1995, 117, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, R.; Malar, D.S.; Devi, K.P. Phytol shows anti-angiogenic activity and induces apoptosis in A549 cells by depolarizing the mitochondrial membrane potential. Biomed. Pharmacother. 2018, 105, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cho, S.K. Phytol induces apoptosis and ROS-mediated protective autophagy in human gastric adenocarcinoma AGS cells. Biochem. Analytic. Biochem. 2015, 4, 1. [Google Scholar]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Synergistic interaction of β-caryophyllene with aromadendrene oxide 2 and phytol induces apoptosis on skin epidermoid cancer cells. Phytomedicine 2018, 47, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Shibayama-Imazu, T.; Sonoda, I.; Sakairi, S.; Aiuchi, T.; Ann, W.W.; Nakajo, S.; Nakaya, K. Production of superoxide and dissipation of mitochondrial transmembrane potential by vitamin K 2 trigger apoptosis in human ovarian cancer TYK-nu cells. Apoptosis 2006, 11, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signaling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Pileri, S.A.; Grogan, T.M.; Harris, N.L.; Banks, P.; Campo, E.; Chan, J.K.C.; Weiss, L.M. Tumours of histiocytes and accessory dendritic cells: An immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology 2002, 41, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Willard-Mack, C.L. Normal structure, function, and histology of lymph nodes. Toxicol. Pathol. 2006, 34, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yi, Y.; Chen, C.; Wang, J.; Liu, Z. Discordant lymphoma consisting of mediastinal large B-cell lymphoma and nodular sclerosis Hodgkin lymphoma in the right supraclavicular lymph nodes: A case report. Diagn. Pathol. 2015, 10, 215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kroemer, G. Early work on the role of mitochondria in apoptosis, an interview with Guido Kroemer. Cell Death Differ. 2004, 11, S33–S36. [Google Scholar] [CrossRef] [PubMed]
- Choundry, G.S.; Al-Harbi, S.; Mazmumder, S.; Hill, B.T.; Smith, M.R.; Bodo, J.; His, E.D.; Almasan, A. MCL-1 and BCL-xL- dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015, 6, e1593. [Google Scholar]
- Roberts, A.W.; Wei, A.H.; Huang, D.C. BCL2 and MCL1 inhibitors for hematologic malignancies. Blood 2021, 138, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Norma Oficial Mexicana. NOM-062-ZOO-1999: Especificaciones Técnicas Para la Producción, Cuidado y Uso de Los Animales de Laboratorio. 1999. Available online: https://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF (accessed on 9 April 2022).
- Calzada, F.; Solares-Pascasio, J.I.; Valdes, M.; Garcia-Hernandez, N.; Velazquez, C.; Ordoñez-Razo, R.M.; Barbosa, E. Antilymphoma potential of the ethanol extract and rutin obtained of the leaves from Schinus molle linn. Pharmacogn. Res. 2018, 10, 119–123. [Google Scholar] [CrossRef]
- Luna, L.G. Manual de Métodos de Tinción Histológica del Instituto de Patología de las Fuerzas Armadas, 3rd ed.; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Hanwell, M.; Curtis, D.; Lonie, D.; Vandermeersch, T.; Zurek, E.; Hutchison, G. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Morris, G.; Lindstrom, W.; Sanner, M.; Belew, R.; Goodshell, D.; Olson, A. Autodock4 and AutodockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]



| Sample | Mean Fluorescence Displacement (%) |
|---|---|
| U-937 cells | - |
| DMSO | 5.11 |
| H2O2 | 71.30 * |
| Geranylgeraniol (Gg) | 58.7 * |
| Phytol (PT) | 31.5 * |
| Farnesyl acetate (FA) | 5.36 |
| Methotrexate (MTX) | 6.06 |
| Compound | Bcl-2 | |||
|---|---|---|---|---|
| ΔG (kcal/mol) | H-BR | NPI | RMSD | |
| Geranylgeraniol | −7.33 | Asp 108, Glu 133, Phe 135, Arg 136, Asp 137, Glu 149 | Phe 101, Phe 109, Met 112, Val 130, Leu 134, Arg 143, Ala 146, Phe 150, Val 153 | - |
| Phytol | −6.91 | Asp 108, Glu 133, Phe 135, Asp 137, Phe 150 | Phe 101, Phe 109, Met 112, Val 130, Leu 134, Arg 143, Ala 146, Val 153 | - |
| Farnesyl acetate | −6.47 | Asp 108, Phe 135, Arg 136, Asp 137, Arg 143, Phe 147, Glu 149 | Phe 101, Phe 109, Met 112, Val 130, Leu 134, Ala 146, Val 153 | - |
| Navitoclax (ABT-263) | −12.54 | Asp 100, Arg 104, Tyr 105, Asp 108, Phe 109, Glu 133, Asn 140, Trp 141, Gly 142, Val 145, Phe 195, Leu 198, Tyr 199 | Ala 97, Phe 101, Met 112, Val 130, Leu 134, Arg 143, Ala 146, Phe 150 | 1.20 |
| Compound | Mcl1-1 | |||
| ΔG (kcal/mol) | H-BR | NPI | RMSD | |
| Geranylgeraniol | −6.46 | Val 253, Thr 266, Leu 267, Ile 268, Gly 271, Ile 294 | Ala 227, Phe 228, Met 231, Leu 235, Leu 246, Val 249, Met 250, Phe 270, Val 274, Leu 290 | - |
| Phytol | −6.35 | Phe 228, Arg 263, Thr 266, Gly 271, Val 274, Ile 294 | Met 231, Leu 235, Leu 246, Val 249, Met 250, Val 253, Phe 254, Leu 267, Phe 270, Leu 290 | - |
| Farnesyl acetate | −6.69 | Val 253, Phe 254, Arg 263, Thr 266, Gly 271 | Met 231, Leu 235, Leu 246, Val 249, Met 250, Leu 267, Phe 270, Val 274, Leu 290, Ile 294 | - |
| 9EA | −10.77 | Val 216, Phe 228, Leu 235, Val 249, Phe 254, Gly 262, Arg 263, Val 265, Gly 271, Leu 290, Ile 294, Phe 318, Phe 319 | Val 220, His 224, Ala 227, Met 231, Leu 246, Met 250, Val 253, Thr 266, Leu 267, Phe 270, Val 274 | 1.45 |
| Compound | VEGFR-2 | |||
| ΔG (kcal/mol) | H-BR | NPI | RMSD | |
| Geranylgeraniol | −6.3 | Glu 885, Phe 918, Cys 919, Lys 920, Gly 922, Ile 1044, Asp 1046 | Leu 840, Val 848, Ala 866, Lys 868, Leu 889, Val 898, Val 899, Val 914, Val 916, Leu 1035, Cys 1045, Phe 1047 | - |
| Phytol | −5.98 | Lys 868, Val 898, Phe 918, Cys 919, Lys 920, Gly 922, Asn 923, His 1026, Ile 1044, Asp 1046 | Leu 840, Val 848, Ala 866, Leu 889, Val 899, Val 914, Val 916, Leu 1035, Cys 1045, Phe 1047 | - |
| Farnesyl acetate | −5.82 | Leu 840, Ala 866, Phe 918, Cys 919, Gly 922, Ile 1044, Asp 1046 | Val 848, Lys 868, Leu 889, Val 899, Val 916, Leu 1035, Cys 1045, Phe 1047 | - |
| Axitinib (AG-013736) | −8.57 | Glu 885, Leu 889, Glu 917, Phe 918, Cys 919, Gly 922, Asn 923, Asp 1046 | Leu 840, Val 848, Ala 866, Lys 868, Val 899, Val 914, Val 916, Cys 919, Leu 1035, Cys 1045, Phe 1047 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Santos, J.; Calzada, F.; Ordoñez-Razo, R.M.; Mendieta-Wejebe, J.E.; Velázquez-Domínguez, J.A.; Argüello-García, R.; Velázquez, C.; Barbosa, E. In Vivo, In Vitro and In Silico Anticancer Activity of Ilama Leaves: An Edible and Medicinal Plant in Mexico. Molecules 2024, 29, 1956. https://doi.org/10.3390/molecules29091956
Ramírez-Santos J, Calzada F, Ordoñez-Razo RM, Mendieta-Wejebe JE, Velázquez-Domínguez JA, Argüello-García R, Velázquez C, Barbosa E. In Vivo, In Vitro and In Silico Anticancer Activity of Ilama Leaves: An Edible and Medicinal Plant in Mexico. Molecules. 2024; 29(9):1956. https://doi.org/10.3390/molecules29091956
Chicago/Turabian StyleRamírez-Santos, Jesica, Fernando Calzada, Rosa María Ordoñez-Razo, Jessica Elena Mendieta-Wejebe, José Antonio Velázquez-Domínguez, Raúl Argüello-García, Claudia Velázquez, and Elizabeth Barbosa. 2024. "In Vivo, In Vitro and In Silico Anticancer Activity of Ilama Leaves: An Edible and Medicinal Plant in Mexico" Molecules 29, no. 9: 1956. https://doi.org/10.3390/molecules29091956
APA StyleRamírez-Santos, J., Calzada, F., Ordoñez-Razo, R. M., Mendieta-Wejebe, J. E., Velázquez-Domínguez, J. A., Argüello-García, R., Velázquez, C., & Barbosa, E. (2024). In Vivo, In Vitro and In Silico Anticancer Activity of Ilama Leaves: An Edible and Medicinal Plant in Mexico. Molecules, 29(9), 1956. https://doi.org/10.3390/molecules29091956








