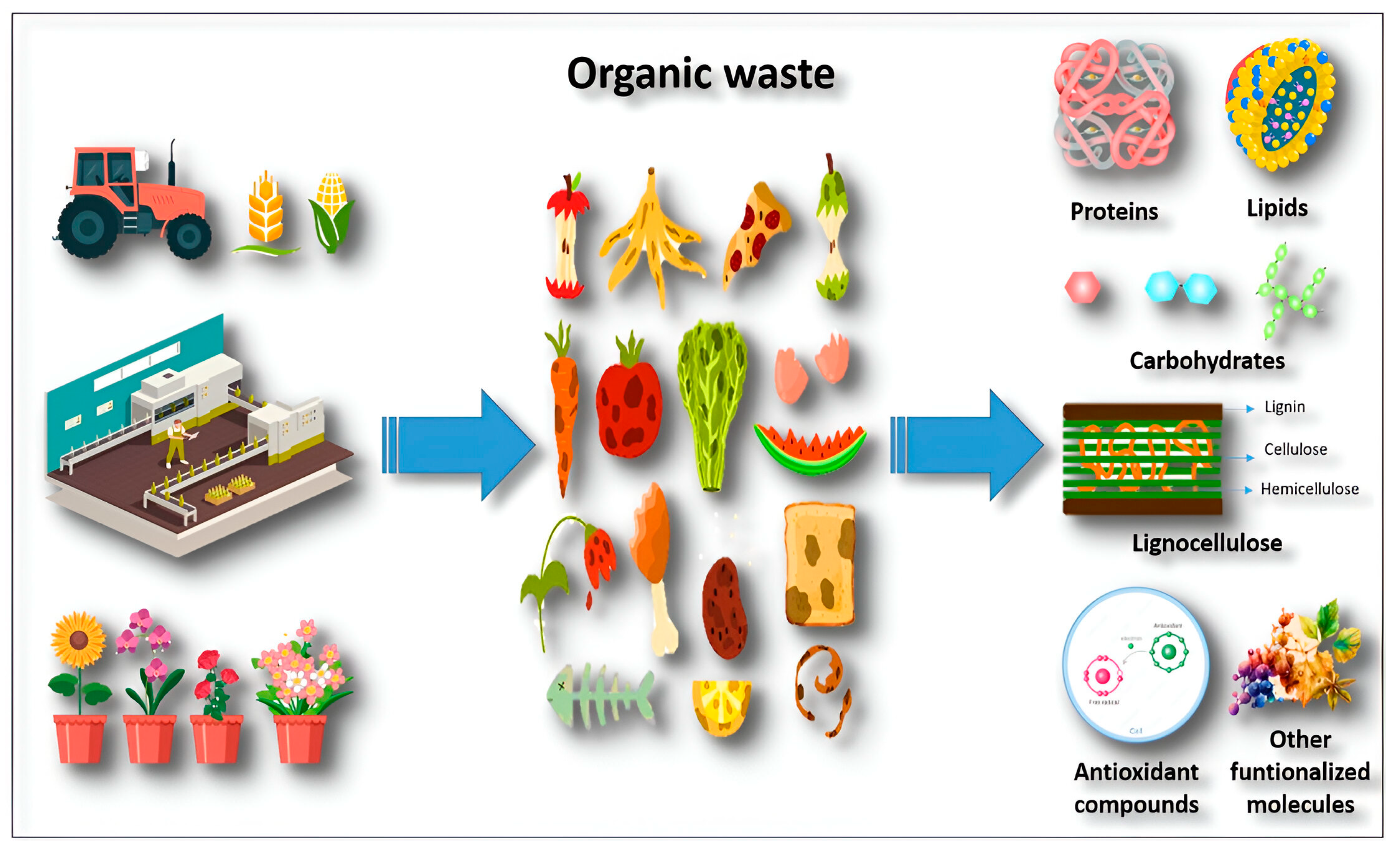
Bioactive Compounds from Organic Waste
Abstract
1. Introduction
2. Methodology
3. Waste Derived from Food Industry
| Method | Solvent | Advantage | Disadvantage | Author | |
|---|---|---|---|---|---|
| Conventional extraction methods | Percolation | Water and Solvents | The equipment is simple and applicable to a wide range of organic matter. | Unstable components due to temperature. High solvent and energy consumption. Long extraction time. | [82] |
| Maceration | Water and solvents | Easy-to-use material and implementation. Minor energy consumption (electricity). | It takes a long time to extract components (days to weeks). Significant consumption of solvent. Non-exhaustive extraction. | [83] | |
| Decoction | Water | Used for phenolic compounds. Easy to use. | Significant energy consumption. Heating takes minutes to hours. Not for thermolabile and volatile compounds. | [84] | |
| Soxhlet extraction | Solvents | Lower amount of solvent. Filtration after extraction is not required. Easy-to-use equipment. | Long time for extraction, and high volume of solvent. | [85] | |
| Hydrodistilation | Water | Volatile compounds extraction. Easy-to-use material and equipment. | Cannot be used for thermolabile compounds. Long extraction time. Possible chemical change. | [86] | |
| Eco-Friendly/Green Extraction Method | Ultrasound-assisted extraction (UAE) | Solvents | Lower amounts of solvent, low energy consumption and extraction time, major extraction efficiency, preservation of bioactive compound stability, and widespread industrial applications. | Not recommended for thermolabile compounds. Heat generated during extraction can modify compound’s structure. | [87] |
| Microwave-assisted extraction (MAE) | Solvents | Low-cost equipment that requires reduced extraction time and solvent quantity. Batch extraction. | More complicated and time-consuming than blending. High pressure. | [88] | |
| Supercritical fluid extraction (SFE) | Solvents, mainly carbon dioxide | High selectivity for non-polar compounds. Recommended for thermolabile compounds. | High cost and complex operation. | [89] | |
| Pressurized liquid extraction (PLE) | Solvents | Lower solvent consumption. Short processing time. Possibility to perform more extraction cycles and samples throughout. | High instrumentation cost and long cell preparation | [90] |
4. Crop Residues
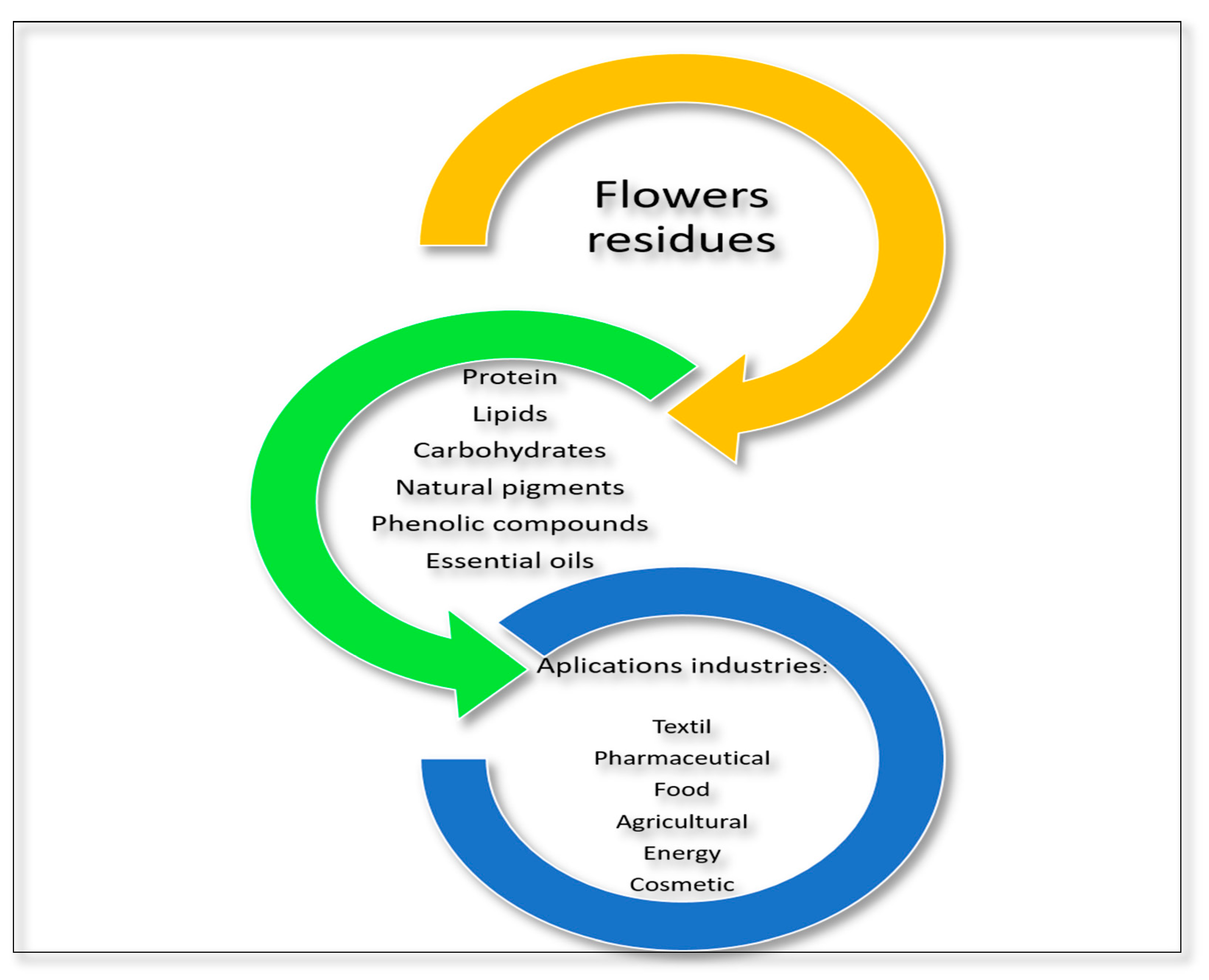
5. Flower Residues
| Biocompounds | Organic Waste | Reference | |
|---|---|---|---|
| Bioactive compounds from food industry and crops. | Lemon seed oil (DM) Yield (%): 26.65–35.85 Total polyphenol: 82.46–165.90 µg GAE/mL Flavonoids: 11.88–21.69 µg QE/mL | Lemon seed | [139] |
| Total polyphenols in peel: 3.51–5.17 mg GAE g−1 (FW) Total phenol content in seed: 4.44 mg GAE g−1 (FW) Condensed tannins in peel: 14.7 mg CE g−1 (FW) and seeds: 15.5 mg CE g−1 (FW) Main phenolic compounds in peel (DM): Delphinidin-3-O-glucoside: 2032–2644 µg L−1 Delphinidin rutinoside: 3255–4407 µg L−1 Quercetin-3-glucoside: 2048–2654 µg L−1 Valoneic acid dilactone: 103–1430 µg L−1 Sinensetin: 1769–4164 µg L−1 Rutin: 3608–4055 µg L−1 Main phenolic compounds in seed (DM): Cyanidin-3-O-glucoside: 174–345 µg L−1 Valoneic acid dilactone: 5947–13,127.81 µg L−1 6-Malonyldaidzin: 67–143 µg L−1 Myricitrin: 25–74 µg L−1 Gallic acid: 28–71 µg L−1 | Peel and seeds of ripe, semi-ripe, and ripe fruits of Citrus reticulata Blanco. | [140] | |
| 64 volatile compounds detected (DM): Tomato branches had β-carotene (37.23 mg/kg−1) and lycopene (3.08 mg/kg−1) concentrations. Phenolic compounds in rotten fruit, tomato branches, and green tomato were present at 27.54, 27.09 and 9.90 mg GAE/g, respectively. | Rotten and green tomato fruit and branches of tomato plant. | [141] | |
| Compounds present (mg/100 g DM): 3-Caffeoylquinic acid: 8.3 - 104 5-Caffeoylquinic acid: 167 - 385 Caffeine: 194–391 Caffeic acid: 3.7 4,5-Dicaffeoylquinic acid: 3.6 - 11.0 1,5-Dicaffeoylquinic acid: 2.3 3,4-Dicaffeoylquinic acid: 0.6 - 7.0 | Coffee grounds | [142] | |
| Compounds (µg/g DM): Gallic acid: 0.72–60.22 Chlorogenic acid: 19.64–337 Caffeic acid: 1.19–6.15 | Coffee husks | [143] | |
| Compounds present in dry matter (%): Crude protein (11.53), crude fiber (1.00), crude fat (15.00), carbohydrate (58.39), pectin (18.2) | Watermelon rind | [144] | |
| Major compounds (mg/100 g DM): Caftaric acid: 22.4 Viferin: 10.4 Procyanidin B2: 24.6 Quercetin-β-D-glucoside: 288.9 | Grape skin | [145] | |
| Total phenols: 309.14–666.41 mg GAE/100 g DM Total flavonoids: 74.75–120.47 mg QE/100 g DM Total anthocyanins: 8.39–8.95 mg CGE/100 g DM Vitamin C: 68.40–108.04 mg/100 g DM | Peach waste | [146] | |
| Concentrations of phenolic compounds (mg/100 g DM): Caffeic acid: 57.88 Caffeic acid derivative: 6.41 Chlorogenic acid derivative: 454.34 p-Coumaric acid: 7.23 Quercetin derivative: 11.32 Kaempferol derivative: 1.88 Fatty acids (%): Palmitic (C16:0): 29.05–35.60 Stearic (C18:0): 6.71–11.96 Oleic (C18:1-9c): 14.59–22.25 Linoleic (C18:2-9,12c; w-6): 35.62–41.33 | Tomato peel | [147] | |
| Compounds in g/kg−1 DM: Chlorophylls: <0.03 Polyphenols: <1.0 Carotenoids: <0.07 | [148] | ||
| Lycopene: 9068–17532 mg/kg DM Lycopene: 272 mg/100 g DM | [149] [150] | ||
| Lycopene: 33.83–135 mg/100 g DM Total carotenoids: 65.09–160.04 mg/100 g DM | Guava powder | [151] | |
| Pectin yield in dry matter (%): Lemon: 10.11 Mandarin: 11.29 Kiwi: 17.30 | Peel of lemon, mandarin, and kiwi. | [152] | |
| Biocompounds presents in flowers and flower waste. | Total carotenoids: 0.129–0.173 mg/g FW Total chlorophyll-a: 1.02 mg/g FW Total chlorophyll-b: 0.315 mg/g FW Phenolic content: 130.10–202.30mg GAE/100g FW | Younger and mature leaf of Hibiscus sabdariffa var. sabdariffa. | [153] |
| Total phenols: 5.65 μg GAE/mL (DM) Total flavonoids: 0.43 μg QE/mL (DM) | Flower of Crotalaria juncea | [154] | |
| Identification of 42 phenolic compounds, some of them were the following (DM): Acid gallic: 13.402–54.318 (mgGAE/gE) HHDP digalloyl hexose: 4.907–11.884 (mgGAE/gE) Flavogallonic acid: 2.810–6.891 (mgGAE/gE) Ellagic acid: 6.591–67.784 (mgGAE/gE) Kaempferol-3-O-β-d-galactopyranoside: 7.124–29.525 (mg HypE/gE) Quercetin: 3.859–16.758 (mg HypE/gE) Kaempferol: 4.751–20.206 (mg HypE/gE) | Rose blossom (flower) | [155] | |
| Major compounds (DM): Total phenols: 167.23 mg/g Total flavonoids: 76.11 mg/g Chlorogenic acid: 3.36 mg/g Catechin: 5.21 mg/g Quercetin: 11.01 mg/g | Flowering shoots of Scrophularia striata Boiss | [156] | |
| Yield (% per gram of DM): Deep pink (Portulaca grandiflora): 0.73–1.65 Red (Rosa ards rovar): 3.59–6.79 Light red (Celosia argentea ver. cristia): 0.67–1.43 Orange (Periskia bleo): 1.19–3.3 Bluish green (Alternanthera ficoidea): 1.1–2.67 | Petals Petals Comb of roster Petals Leaves | [157] |
6. Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Gimeno, A.; Navarro-Pedreño, J.; Almendro-Candel, M.B.; Gómez, I.; Zorpas, A.A. The use of wastes (organic and inorganic) in land restoration in relation to their characteristics and cost. Waste Manag. Res. J. Sustain. Circ. Econ. 2019, 37, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- García-Mahecha, M.; Soto-Valdez, H.; Carvajal-Millan, E.; Madera-Santana, T.J.; Lomelí-Ramírez, M.G.; Colín-Chávez, C. Bioactive Compounds in Extracts from the Agro-Industrial Waste of Mango. Molecules 2023, 28, 458. (In English) [Google Scholar] [CrossRef]
- Lopes, F.C.; Ligabue-Braun, R. Agro-Industrial Residues: Eco-Friendly and Inexpensive Substrates for Microbial Pigments Production. Front. Sustain. Food Syst. 2021, 5, 589414. (In English) [Google Scholar] [CrossRef]
- Eades, P.; Kusch-Brandt, S.; Heaven, S.; Banks, C.J. Estimating the Generation of Garden Waste in England and the Differences between Rural and Urban Areas. Resources 2020, 9, 8. [Google Scholar] [CrossRef]
- Regadío, M.; Ruiz, A.I.; Rodríguez-Rastrero, M.; Cuevas, J. Containment and attenuating layers: An affordable strategy that preserves soil and water from landfill pollution. Waste Manag. 2015, 46, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Mourad, M. Recycling, recovering and preventing “food waste”: Competing solutions for food systems sustainability in the United States and France. J. Clean. Prod. 2016, 126, 461–477. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Biriș, S. Sustainable Valorization of Waste and By-Products from Sugarcane Processing. Sustainability 2022, 14, 11089. [Google Scholar] [CrossRef]
- Alves, E.; Simoes, A.; Domingues, M.R. Fruit seeds and their oils as promising sources of value-added lipids from agro-industrial byproducts: Oil content, lipid composition, lipid analysis, biological activity and potential biotechnological applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 1305–1339. [Google Scholar] [CrossRef]
- Segatto, M.L.; Stahl, A.M.; Zanotti, K.; Zuin, V.G. Green and sustainable extraction of proteins from agro-industrial waste: An overview and a closer look to Latin America. Curr. Opin. Green Sustain. Chem. 2022, 37, 100661. [Google Scholar] [CrossRef]
- Peinemann, J.C.; Pleissner, D. Material Utilization of Organic Residues. Appl. Biochem. Biotechnol. 2018, 184, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Osorio, P.D.; Ramírez-Mejía, J.M.; Mejía-Avellaneda, L.F.; Mesa, L.; Bautista, E.J. Agro-industrial residues for microbial bioproducts: A key booster for bioeconomy. Bioresour. Technol. Rep. 2022, 20, 101232. [Google Scholar] [CrossRef]
- Manubothula, S.; Gorre, M. Influence of rice husk ash on compressive strength of an aerated concrete. Mater. Today Proc. 2022, 65, 1982–1986. [Google Scholar] [CrossRef]
- Mattey, P.E.; Robayo, R.A.; Díaz, J.E.; Delvasto, S.; Monzó, J. Aplicación de ceniza de cascarilla de arroz obtenida de un proceso agro-industrial para la fabricación de bloques en concreto no estructurales. Rev. Latinoam. Metal. Mater. 2015, 35, 285–294. [Google Scholar]
- Costa, S.; Summa, D.; Semeraro, B.; Zappaterra, F.; Rugiero, I.; Tamburini, E. Fermentation as a Strategy for Bio-Transforming Waste into Resources: Lactic Acid Production from Agri-Food Residues. Fermentation 2021, 7, 3. [Google Scholar] [CrossRef]
- Zhang, Y.; Vadlani, P.V. Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J. Biosci. Bioeng. 2015, 119, 694–699. (In English) [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Li, C.; Lin, C.S.K. Restoring of Glucose Metabolism of Engineered Yarrowia lipolytica for Succinic Acid Production via a Simple and Efficient Adaptive Evolution Strategy. J. Agric. Food Chem. 2017, 65, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Bakhiet, E.K.; Hussein, H.R.; Ali, S.G. Statistical optimization studies for polyhydroxybutyrate (PHB) production by novel Bacillus subtilis using agricultural and industrial wastes. Int. J. Environ. Sci. Technol. 2019, 16, 3497–3512. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. (In English) [Google Scholar] [CrossRef]
- Choo, W.S.; Saik, A.Y.H. Chapter 4—Valorization of fruit and vegetable waste for bioactive pigments: Extraction and utilization. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 61–81. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Valdespino-León, M.; Calderón-Domínguez, G.; Salgado-Cruz, M.D.L.P.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Morales-Sánchez, E.; Gaona-Sánchez, V.A.; Terrazas-Valencia, F. Biodegradable Electrosprayed Pectin Films: An Alternative to Valorize Coffee Mucilage. Waste Biomass Valorization 2021, 12, 2477–2494. [Google Scholar] [CrossRef]
- Vaez, S.; Karimi, K.; Mirmohamadsadeghi, S.; Jeihanipour, A. An optimal biorefinery development for pectin and biofuels production from orange wastes without enzyme consumption. Process Saf. Environ. Prot. 2021, 152, 513–526. [Google Scholar] [CrossRef]
- Li, H.; Zhou, M.; Mohammed, A.E.A.Y.; Chen, L.; Zhou, C. From fruit and vegetable waste to degradable bioplastic films and advanced materials: A review. Sustain. Chem. Pharm. 2022, 30, 100859. [Google Scholar] [CrossRef]
- Moeinian, K.; Mehdinia, S.M. Removing Methylene Blue from Aqueous Solutions Using Rice Husk Silica Adsorbent. Pol. J. Environ. Stud. 2019, 28, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Onu, C.E.; Ohale, P.E.; Obiora-Okafo, I.A.; Asadu, C.O.; Okoye, C.C.; Ojukwu, E.V.; Ezennajiego, E.E. Application of Rice Husk-Based Biomaterial in Textile Wastewater Treatment. In Textile Wastewater Treatment: Sustainable Bio-Nano Materials and Macromolecules; Muthu, S.S., Khadir, A., Eds.; Springer Nature: Singapore, 2022; Volume 2, pp. 231–250. [Google Scholar] [CrossRef]
- Georganas, A.; Giamouri, E.; Pappas, A.C.; Papadomichelakis, G.; Galliou, F.; Manios, T.; Tsiplakou, E.; Fegeros, K.; Zervas, G. Bioactive Compounds in Food Waste: A Review on the Transformation of Food Waste to Animal Feed. Foods 2020, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals (In English). 2020, 10, 131. [Google Scholar] [CrossRef]
- Rajeh, C.; Saoud, I.P.; Kharroubi, S.; Naalbandian, S.; Abiad, M.G. Food loss and food waste recovery as animal feed: A systematic review. J. Mater. Cycles Waste Manag. 2021, 23, 1–17. [Google Scholar] [CrossRef]
- Machineni, L. Lignocellulosic biofuel production: Review of alternatives. Biomass Convers. Biorefinery 2020, 10, 779–791. [Google Scholar] [CrossRef]
- Malode, S.J.; Prabhu, K.K.; Mascarenhas, R.J.; Shetti, N.P.; Aminabhavi, T.M. Recent advances and viability in biofuel production. Energy Convers. Manag. X 2021, 10, 100070. [Google Scholar] [CrossRef]
- Darras, A. Overview of the Dynamic Role of Specialty Cut Flowers in the International Cut Flower Market. Horticulturae 2021, 7, 51. [Google Scholar] [CrossRef]
- Nation Master. Floriculture. Available online: https://www.nationmaster.com/nmx/ranking/flowers-production (accessed on 4 January 2024).
- Masure, P.; Patil, B. Extraction of Waste Flowers. Int. J. Eng. Res. Technol. 2014, 3, 43–44. [Google Scholar]
- Sharma, D.; Yadav, K.D.; Kumar, S. Biotransformation of flower waste composting: Optimization of waste combinations using response surface methodology. Bioresour. Technol. 2018, 270, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Waghmode, M.S.; Gunjal, A.B.; Nawani, N.N.; Patil, N.N. Management of Floral Waste by Conversion to Value-Added Products and Their Other Applications. Waste Biomass Valorization 2018, 9, 33–43. [Google Scholar] [CrossRef]
- Sharma, D.; Yadav, K.D.; Kumar, S. Role of sawdust and cow dung on compost maturity during rotary drum composting of flower waste. Bioresour. Technol. 2018, 264, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Arici, M.; Karasu, S.; Baslar, M.; Toker, O.S.; Sagdic, O.; Karaagacli, M. Tulip petal as a novel natural food colorant source: Extraction optimization and stability studies. Ind. Crops Prod. 2016, 91, 215–222. [Google Scholar] [CrossRef]
- Dutta, S.; Kumar, M. Potential of value-added chemicals extracted from floral waste: A review. J. Clean. Prod. 2021, 294, 126280. [Google Scholar] [CrossRef]
- Yadav, K.D.; Sharma, D.; Prasad, R. 5—Challenges and opportunities for disposal of floral waste in developing countries by using composting method. In Advanced Organic Waste Management; Hussain, C., Hait, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 55–77. [Google Scholar] [CrossRef]
- Mohan, V.; Priyanka, R.; Ananda, H.; Sarwad, T. Paper Production from Flower: Recycling of Flower Waste. J. Altern. Energy Sources Technol. 2018, 9, 77–80. [Google Scholar]
- Terral, J.-F.; Bonhomme, V.; Pagnoux, C.; Ivorra, S.; Newton, C.; Paradis, L.; Ater, M.; Kassout, J.; Limier, B.; Bouby, L.; et al. The Shape Diversity of Olive Stones Resulting from Domestication and Diversification Unveils Traits of the Oldest Known 6500-Years-Old Table Olives from Hishuley Carmel Site (Israel). Agronomy 2021, 11, 2187. [Google Scholar] [CrossRef]
- Nation Master. Olives Production. 2019. Available online: https://www.nationmaster.com/nmx/ranking/olives-production-fao (accessed on 7 January 2024).
- Dias, M.C.; Araújo, M.; Silva, S.; Santos, C. Sustainable Olive Culture under Climate Change: The Potential of Biostimulants. Horticulturae 2022, 8, 1048. [Google Scholar] [CrossRef]
- Naureen, Z.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Foods of the Mediterranean diet: Citrus, cucumber and grape. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E21–E27. (In English) [Google Scholar] [CrossRef]
- Peralbo-Molina, Á.; de Castro, M.D.L. Potential of residues from the Mediterranean agriculture and agrifood industry. Trends Food Sci. Technol. 2013, 32, 16–24. [Google Scholar] [CrossRef]
- Conde, E.; Cara, C.; Moure, A.; Ruiz, E.; Castro, E.; Domínguez, H. Antioxidant activity of the phenolic compounds released by hydrothermal treatments of olive tree pruning. Food Chem. 2009, 114, 806–812. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. (In English) [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Qu, J.; Xu, Z.; Feng, S.; Chen, T.; Ding, C. Optimizing Total Phenolic and Oleuropein of Chinese Olive (Olea europaea) Leaves for Enhancement of the Phenols Content and Antioxidant Activity. Agronomy 2021, 11, 686. [Google Scholar] [CrossRef]
- Abdelgawad, S.M.; El Hassab, M.A.; Abourehab, M.A.S.; Elkaeed, E.B.; Eldehna, W.M. Olive Leaves as a Potential Phytotherapy in the Treatment of COVID-19 Disease; A Mini-Review. Front. Pharmacol. 2022, 13, 879118. (In English) [Google Scholar] [CrossRef]
- Caballero-Guerrero, B.; Garrido-Fernández, A.; Fermoso, F.G.; Rodríguez-Gutierrez, G.; Fernández-Prior, M.; Reinhard, C.; Nyström, L.; Benítez-Cabello, A.; Arroyo-López, F.N. Antimicrobial effects of treated olive mill waste on foodborne pathogens. LWT 2022, 164, 113628. [Google Scholar] [CrossRef]
- Pontes, P.V.d.A.; Czaikoski, A.; Almeida, N.A.; Fraga, S.; Rocha, L.d.O.; Cunha, R.L.; Maximo, G.J.; Batista, E.A.C. Extraction optimization, biological activities, and application in O/W emulsion of deep eutectic solvents-based phenolic extracts from olive pomace. Food Res. Int. 2022, 161, 111753. [Google Scholar] [CrossRef]
- Criado-Navarro, I.; Ledesma-Escobar, C.A.; Parrado-Martínez, M.J.; Marchal-López, R.M.; Olmo-Peinado, J.M.; Espejo-Calvo, J.A.; Priego-Capote, F. Monitoring the partition of bioactive compounds in the extraction of extra virgin olive oil. LWT 2022, 162, 113433. [Google Scholar] [CrossRef]
- Lazzerini, C.; Domenici, V. Pigments in Extra-Virgin Olive Oils Produced in Tuscany (Italy) in Different Years. Foods 2017, 6, 25. (In English) [Google Scholar] [CrossRef]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.P. Olive by-products: Challenge application in cosmetic industry. Ind. Crops Prod. 2015, 70, 116–124. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Plaza, M.; García, M.C.; Turner, C.; Marina, M.L. A sustainable approach for the extraction of cholesterol-lowering compounds from an olive by-product based on CO2-expanded ethyl acetate. Anal. Bioanal. Chem. 2019, 411, 5885–5896. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Ruggio, D.M.; Toivo, J.I.; Swank, M.A.; Simpkins, A.H. Free and Esterified Sterol Composition of Edible Oils and Fats. J. Food Compos. Anal. 2002, 15, 123–142. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for recycling and valorization of grape marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. (In English) [Google Scholar] [CrossRef]
- Sochorova, L.; Prusova, B.; Cebova, M.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Nedomova, S.; Baron, M.; Sochor, J. Health Effects of Grape Seed and Skin Extracts and Their Influence on Biochemical Markers. Molecules 2020, 25, 5311. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Singhal, S.; Hulle, N.R.S. Citrus pectins: Structural properties, extraction methods, modifications and applications in food systems—A review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef]
- Labanca, F.; Faraone, I.; Nolè, M.R.; Hornedo-Ortega, R.; Russo, D.; García-Parrilla, M.C.; Chiummiento, L.; Bonomo, M.G.; Milella, L. New Insights into the Exploitation of Vitis vinifera L. cv. Aglianico Leaf Extracts for Nutraceutical Purposes. Antioxidants 2020, 9, 708. [Google Scholar] [CrossRef]
- Cantwell, M.; Hong, G.; Albornoz, K.; Berlanga, M. Fresh grapevine (Vitis vinifera L.) leaves: Postharvest biology and handling recommendations. Sci. Hortic. 2022, 292, 110627. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic Profiles of Leaves, Grapes and Wine of Grapevine Variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. (In English) [Google Scholar] [CrossRef] [PubMed]
- International Coffee Organization (ICO). Historical Data on the Global Coffee Trade. 2023. Available online: https://www.ico.org/new_historical.asp (accessed on 10 January 2024).
- Thenepalli, T.; Ramakrishna, C.; Ahn, J.W. Environmental effect of the coffee waste and anti-microbial property of oyster shell waste treatment. J. Energy Eng. 2017, 26, 39–49. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Cho, E.; Trinh, L.T.P.; Jeong, J.-S.; Bae, H.-J. Development of an integrated process to produce d-mannose and bioethanol from coffee residue waste. Bioresour. Technol. 2017, 244, 1039–1048. [Google Scholar] [CrossRef]
- Cerino-Córdova, F.; Davila-Guzman, N.E.; León, A.; Salazar-Rábago, J.J.; Soto-Regalado, E. Revalorization of Coffee Waste. In Coffee—Production and Research; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Lestari, W.; Hasballah, K.; Listiawan, M.Y.; Sofia, S. Coffee by-products as the source of antioxidants: A systematic review. F1000Research 2022, 11, 220. (In English) [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The wastes of coffee bean processing for utilization in food: A review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef]
- International Cocoa Organization (ICCO). Data on Production and Grindings of Cocoa Beans. 2018. Available online: https://www.icco.org/ (accessed on 21 January 2024).
- The Observatory of Economic Complexity (OEC). Cocoa Shells, Husks, Skins and Waste. 2020. Available online: https://oec.world/en/profile/hs/cocoa-shells-husks-skins-and-waste?redirect=true (accessed on 25 January 2024).
- Handojo, L.; Triharyogi, H.; Indarto, A. Cocoa bean shell waste as potential raw material for dietary fiber powder. Int. J. Recycl. Org. Waste Agric. 2019, 8, 485–491. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological approaches for cocoa waste management: A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Żyżelewicz, D. Antioxidants in Cocoa. Antioxidants 2020, 9, 1230. [Google Scholar] [CrossRef]
- Belwal, T.; Cravotto, C.; Ramola, S.; Thakur, M.; Chemat, F.; Cravotto, G. Bioactive Compounds from Cocoa Husk: Extraction, Analysis and Applications in Food Production Chain. Foods 2022, 11, 798. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. (In English) [Google Scholar] [CrossRef]
- Wang, W.-Y.; Qu, H.-B.; Gong, X.-C. Research progress on percolation extraction process of traditional Chinese medicines. Zhongguo Zhong Yao Za Zhi 2020, 45, 1039–1046. (In Chinese) [Google Scholar] [CrossRef]
- Morata, A.; González, C.; Tesfaye, W.; Loira, I.; Suárez-Lepe, J.A. Chapter 3—Maceration and Fermentation: New Technologies to Increase Extraction. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 35–49. [Google Scholar]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Zygler, A.; Słomińska, M.; Namieśnik, J. 2.04—Soxhlet Extraction and New Developments Such as Soxtec. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 65–82. [Google Scholar]
- Khan, S.; Sahar, A.; Tariq, T.; Sameen, A.; Tariq, F. Chapter 1—Essential oils in plants: Plant physiology, the chemical composition of the oil, and natural variation of the oils (chemotaxonomy and environmental effects, etc.). In Essential Oils; Nayik, G.A., Ansari, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–36. [Google Scholar]
- Ponphaiboon, J.; Krongrawa, W.; Aung, W.W.; Chinatangkul, N.; Limmatvapirat, S.; Limmatvapirat, C. Advances in Natural Product Extraction Techniques, Electrospun Fiber Fabrication, and the Integration of Experimental Design: A Comprehensive Review. Molecules 2023, 28, 5163. [Google Scholar] [CrossRef] [PubMed]
- Llompart, M.; Garcia-Jares, C.; Celeiro, M.; Dagnac, T. Extraction | Microwave-Assisted Extraction☆. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 67–77. [Google Scholar]
- Drennan, B.W.; Wicker, A.P.; Berger, B.K.; Schug, K.A. Chapter 4—Measurements of drugs and metabolites in biological matrices using SFC and SFE-SFC-MS. In Separation Science and Technology; Hicks, M., Ferguson, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 14, pp. 73–99. [Google Scholar]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A Powerful Tool to Implement Extraction and Purification of Food Contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Akao, S. Nitrogen, Phosphorus, and Antioxidant Contents in Crop Residues for Potential Cascade Utilization. Waste Biomass Valorization 2018, 9, 1535–1542. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Bergamo, T.F.; Kikas, T. Potential of cereal-based agricultural residues available for bioenergy production. Data Brief 2019, 23, 103829. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Gupta, C.; Jat, S.L.; Parmar, M. Crop residue recycling for economic and environmental sustainability: The case of India. Open Agric. 2017, 2, 486–494. [Google Scholar] [CrossRef]
- Hassan, G.; Shabbir, M.A.; Ahmad, F.; Pasha, I.; Aslam, N.; Ahmad, T.; Rehman, A.; Manzoor, M.F.; Inam-Ur-Raheem, M.; Aadil, R.M. Cereal processing waste, an environmental impact and value addition perspectives: A comprehensive treatise. Food Chem. 2021, 363, 130352. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, M.; Sharma, M.; Sharma, G.D.; Passari, A.K.; Bhasin, S. Valorization of agro-industrial residues for production of commercial biorefinery products. Fuel 2022, 322, 124284. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Júnior, M.R.M. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef]
- Palencia, M.; Lerma, T.A.; Garcés, V.; Mora, M.A.; Martínez, J.M.; Palencia, S.L. Chapter 25—Separation of secondary metabolites and bioactive substances from agricultural residues. In Eco-Friendly Functional Polymers; Palencia, M., Lerma, T.A., Garcés, V., Mora, M.A., Martínez, J.M., Palencia, S.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 381–394. [Google Scholar]
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Kumar, S.S.; Swapna, T.S.; Sabu, A. Coffee Husk: A Potential Agro-Industrial Residue for Bioprocess. In Waste to Wealth; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; pp. 97–109. [Google Scholar]
- Bondam, A.F.; da Silveira, D.D.; dos Santos, J.P.; Hoffmann, J.F. Phenolic compounds from coffee by-products: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 123, 172–186. [Google Scholar] [CrossRef]
- Joglekar, S.N.; Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Process of fruit peel waste biorefinery: A case study of citrus waste biorefinery, its environmental impacts and recommendations. Environ. Sci. Pollut. Res. 2019, 26, 34713–34722. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Ashour, M.; Yehia, H.; Chaidir, C. Utilization of Agro-industrial by-products for production of bioactive natural products from endophytic fungi. J. Nat. Prod. 2011, 4, 108–114. [Google Scholar]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018, 376, 3. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Rezaei, M.; Liu, B. Food Loss and Waste in the Food Supply Chain; International Nut and Dried Fruit Council: Reus, Spain, 2017; pp. 26–27. [Google Scholar]
- Fidelis, M.; De Moura, C.; Junior, T.K.; Pap, N.; Mattila, P.H.; Mäkinen, S.; Putnik, P.; Kovačević, D.B.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef]
- Karnopp, A.R.; Margraf, T.; Maciel, L.; Santos, J.S.; Granato, D. Chemical composition, nutritional and in vitro functional properties of by-products from the Brazilian organic grape juice industry. Int. Food Res. J. 2017, 24, 207–214. [Google Scholar]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT 2019, 105, 242–249. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Warnakulasuriya, S.N.; Rupasinghe, H.V.; Shahidi, F. Antioxidant ability of fractionated apple peel phenolics to inhibit fish oil oxidation. Food Chem. 2013, 140, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Santana, I.; Castelo-Branco, V.N.; Guimarães, B.M.; Silva, L.d.O.; Peixoto, V.O.D.S.; Cabral, L.M.C.; Freitas, S.P.; Torres, A.G. Hass avocado (Persea americana Mill.) oil enriched in phenolic compounds and tocopherols by expeller-pressing the unpeeled microwave dried fruit. Food Chem. 2019, 286, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, D.Y.; Kim, O.Y.; Kang, H.J.; Kim, H.S.; Hur, S.J. Overview of Studies on the Use of Natural Antioxidative Materials in Meat Products. Food Sci. Anim. Resour. 2020, 40, 863–880. (In English) [Google Scholar] [CrossRef]
- Powell, M.J.; Sebranek, J.G.; Prusa, K.J.; Tarté, R. Evaluation of citrus fiber as a natural replacer of sodium phosphate in alternatively-cured all-pork Bologna sausage. Meat Sci. 2019, 157, 107883. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Pradhan, S.R.; Das, A.; Nanda, P.K.; Bandyopadhyay, N.; Das, A.K. Inhibition of lipid and protein oxidation in raw ground pork by Terminalia arjuna fruit extract during refrigerated storage. Asian-Australas. J. Anim. Sci. 2019, 32, 265–273. (In English) [Google Scholar] [CrossRef] [PubMed]
- International Association of Horticultural Producers. Available online: https://aiph.org/ (accessed on 25 January 2024).
- Jadhav, A.R. Flower Waste Degradation Using Microbial Consortium. J. Agric. Vet. Sci. 2013, 3, 1–4. [Google Scholar] [CrossRef]
- Yousuf, B.; Panesar, P.S.; Chopra, H.K.; Gul, K. Characterization of Secondary Metabolites from Various Solvent Extracts of Saffron Floral Waste. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 89–100. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Kumar, A. An endeavor to achieve sustainable development goals through floral waste management: A short review. J. Clean. Prod. 2021, 283, 124669. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, H.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Res. Int. 2019, 126, 108660. [Google Scholar] [CrossRef] [PubMed]
- López-Cruz, R.; Sandoval-Contreras, T.; Iñiguez-Moreno, M. Plant Pigments: Classification, Extraction, and Challenge of Their Application in the Food Industry. Food Bioprocess Technol. 2023, 16, 2725–2741. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Li, H.-B.; Xu, D.-P.; Xu, X.-R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Jiang, J.; Yuan, F.; Gao, Y. Subcritical water extraction and antioxidant activity evaluation with on-line HPLC-ABTS(·+) assay of phenolic compounds from marigold (Tagetes erecta L.) flower residues. J. Food Sci. Technol. 2015, 52, 3803–3811. (In English) [Google Scholar] [CrossRef]
- Mak, Y.W.; Chuah, L.O.; Ahmad, R.; Bhat, R. Antioxidant and antibacterial activities of hibiscus (Hibiscus rosa-sinensis L.) and Cassia (Senna bicapsularis L.) flower extracts. J. King Saud Univ.-Sci. 2013, 25, 275–282. [Google Scholar] [CrossRef]
- Waghmode, M.; Gaikwad, P.; Kabade, S.; Gunjal, A.; Nawani, N.; Kapadnis, B.; Patil, N. Production of Surface active compounds by Microbispora sp. V2 using flower extract of Madhuca latifolia L. Natl. J. Interdiscip. Res. 2015, 1, 130–137. [Google Scholar]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Sinaga, H.Y.; Jaya, M.K.A. The potential of frangipani flower extract (Plumeria alba L.) as an antibacterial: A literature review. J. Pharm. Sci. Appl. 2022, 4, 33–38. [Google Scholar] [CrossRef]
- Leja, K.; Szudera-Kończal, K.; Świtała, E.; Juzwa, W.; Kowalczewski, P.; Czaczyk, K. The Influence of Selected Plant Essential Oils on Morphological and Physiological Characteristics in Pseudomonas Orientalis. Foods 2019, 8, 277. [Google Scholar] [CrossRef]
- Álvarez, C.A.; Barriga, A.; Albericio, F.; Romero, M.S.; Guzmán, F. Identification of Peptides in Flowers of Sambucus nigra with Antimicrobial Activity against Aquaculture Pathogens. Molecules 2018, 23, 1033. (In English) [Google Scholar] [CrossRef]
- Janda, K.; Wojtkowska, K.; Jakubczyk, K.; Antoniewicz, J.; Skonieczna-Żydecka, K. Passiflora incarnata in Neuropsychiatric Disorders—A Systematic Review. Nutrients 2020, 12, 3894. (In English) [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-L.; Li, Y.; Wang, Q.; Niu, F.-J.; Li, K.-W.; Wang, Y.-Y.; Wang, J.; Zhou, C.-Z.; Gao, L.-N. Chamomile: A Review of Its Traditional Uses, Chemical Constituents, Pharmacological Activities and Quality Control Studies. Molecules 2022, 28, 133. (In English) [Google Scholar] [CrossRef]
- Mileva, M.; Ilieva, Y.; Jovtchev, G.; Gateva, S.; Zaharieva, M.M.; Georgieva, A.; Dimitrova, L.; Dobreva, A.; Angelova, T.; Vilhelmova-Ilieva, N.; et al. Rose Flowers—A Delicate Perfume or a Natural Healer? Biomolecules 2021, 11, 127. (In English) [Google Scholar] [CrossRef]
- Smith, A.G.; Miles, V.N.; Holmes, D.T.; Chen, X.; Lei, W. Clinical Trials, Potential Mechanisms, and Adverse Effects of Arnica as an Adjunct Medication for Pain Management. Medicines 2021, 8, 58. (In English) [Google Scholar] [CrossRef]
- Casas, M.P.; Conde, E.; Ribeiro, D.; Fernandes, E.; Domínguez, H.; Torres, M.D. Bioactive properties of Acacia dealbata flowers extracts. Waste Biomass Valorization 2020, 11, 2549–2557. [Google Scholar] [CrossRef]
- Dasalukunte Ananda, K.; Halappa, K. Evaluation and conversion of temple waste flowers into incense sticks in tumakuru district of karnataka, India. Holist. Approach Environ. 2023, 13, 10–21. [Google Scholar] [CrossRef]
- Katekar, V.P.; Rao, A.B.; Sardeshpande, V.R. A cleaner and ecological rosewater production technology based on solar energy for rural livelihood. Clean. Circ. Bioeconomy 2022, 2, 100022. [Google Scholar] [CrossRef]
- Park, Y.-S.; Kim, I.-D.; Dhungana, S.K.; Park, E.-J.; Park, J.-J.; Kim, J.-H.; Shin, D.-H. Quality Characteristics and Antioxidant Potential of Lemon (Citrus limon Burm. f.) Seed Oil Extracted by Different Methods. Front. Nutr. 2021, 8, 644406. (In English) [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.; Rodrigues, R.; Gaspar, M.; Braga, M.; Quina, M. Integrated management of residues from tomato production: Recovery of value-added compounds and biogas production in the biorefinery context. J. Environ. Manag. 2021, 299, 113505. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.O.; Honfoga, J.N.B.; Medeiros, L.L.D.; Madruga, M.S.; Bezerra, T.K.A. Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods. Molecules 2021, 26, 46. [Google Scholar] [CrossRef]
- Mamiru, D.; Girma, G. Extraction and characterization of pectin from watermelon rind using acetic acid. Heliyon 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, R.B.; Machado, B.A.S.; Barreto, G.d.A.; Nascimento, R.Q.; Corrêa, L.C.; Leal, I.L.; Tavares, P.P.L.G.; Ferreira, E.d.S.; Umsza-Guez, M.A. Syrah Grape Skin Residues Has Potential as Source of Antioxidant and Anti-Microbial Bioactive Compounds. Biology 2021, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- Plazzotta, S.; Ibarz, R.; Manzocco, L.; Martín-Belloso, O. Optimizing the antioxidant biocompound recovery from peach waste extraction assisted by ultrasounds or microwaves. Ultrason. Sonochem. 2020, 63, 104954. [Google Scholar] [CrossRef] [PubMed]
- Grassino, A.N.; Djaković, S.; Bosiljkov, T.; Halambek, J.; Zorić, Z.; Dragović-Uzelac, V.; Petrović, M.; Brnčić, S.R. Valorisation of Tomato Peel Waste as a Sustainable Source for Pectin, Polyphenols and Fatty Acids Recovery Using Sequential Extraction. Waste Biomass Valorization 2020, 11, 4593–4611. [Google Scholar] [CrossRef]
- Santangelo, E.; Carnevale, M.; Migliori, C.; Picarella, M.; Dono, G.; Mazzucato, A.; Gallucci, F. Evaluation of tomato introgression lines diversified for peel color as a source of functional biocompounds and biomass for energy recovery. Biomass Bioenergy 2020, 141, 105735. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Zuorro, A. Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures. Molecules 2020, 25, 2038. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.d.S.; Nunes, I.L.; Block, J.M. Ultrasound-Assisted Extraction for the Recovery of Carotenoids from Guava’s Pulp and Waste Powders. Plant Foods Hum. Nutr. 2020, 75, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Karbuz, P.; Tugrul, N. Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 2021, 58, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Akter, K.; Islam, M.A.; Rasul, S.; Sumi, M.J.; Yeasmin, A.; Biswash, T.; Uddin, M.N.; Fakir, M.S.A. Comparative Morpho-Agronomic and Biochemical Profiling of Different Roselle Morphotypes Based on their Growth and Yield Associated Attributes. J. Agric. Crops 2023, 9, 514–523. [Google Scholar] [CrossRef]
- Mahasawat, P.; Boukaew, S.; Prasertsan, P. Exploring the potential of Crotalaria juncea flower extracts as a source of antioxidants, antimicrobials, and cytoprotective agents for biomedical applications. BioTechnologia 2023, 104, 359–370. (In English) [Google Scholar] [CrossRef]
- Trendafilova, A.; Staleva, P.; Petkova, Z.; Ivanova, V.; Evstatieva, Y.; Nikolova, D.; Rasheva, I.; Atanasov, N.; Topouzova-Hristova, T.; Veleva, R.; et al. Phytochemical Profile, Antioxidant Potential, Antimicrobial Activity, and Cytotoxicity of Dry Extract from Rosa damascena Mill. Molecules 2023, 28, 7666. [Google Scholar] [CrossRef]
- Mohammadi, M.; Aelaei, M.; Saidi, M. Antibacterial properties of Scrophularia striata Boiss. (Tashenehdari) extract on vase life improvement in “Stanza” and “Pink Elegance” gerbera cut flowers. Biocatal. Agric. Biotechnol. 2020, 28, 101738. [Google Scholar] [CrossRef]
- Zumahi, S.A.-A.; Arobi, N.; Taha, H.; Hossain, K.; Kabir, H.; Matin, R.; Bashar, M.; Ahmed, F.; Hossain, A.; Rahman, M.M. Extraction, optical properties, and aging studies of natural pigments of various flower plants. Heliyon 2020, 6, e05104. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Pacheco, B.; Cruz-Moreno, B.A.; Aguirre-Becerra, H.; García-Trejo, J.F.; Feregrino-Pérez, A.A. Bioactive Compounds from Organic Waste. Molecules 2024, 29, 2243. https://doi.org/10.3390/molecules29102243
Parra-Pacheco B, Cruz-Moreno BA, Aguirre-Becerra H, García-Trejo JF, Feregrino-Pérez AA. Bioactive Compounds from Organic Waste. Molecules. 2024; 29(10):2243. https://doi.org/10.3390/molecules29102243
Chicago/Turabian StyleParra-Pacheco, Benito, Byanka A. Cruz-Moreno, Humberto Aguirre-Becerra, Juan Fernando García-Trejo, and Ana Angélica Feregrino-Pérez. 2024. "Bioactive Compounds from Organic Waste" Molecules 29, no. 10: 2243. https://doi.org/10.3390/molecules29102243
APA StyleParra-Pacheco, B., Cruz-Moreno, B. A., Aguirre-Becerra, H., García-Trejo, J. F., & Feregrino-Pérez, A. A. (2024). Bioactive Compounds from Organic Waste. Molecules, 29(10), 2243. https://doi.org/10.3390/molecules29102243







