Optimization of Phytochemical-Rich Citrus maxima Albedo Extract Using Response Surface Methodology
Abstract
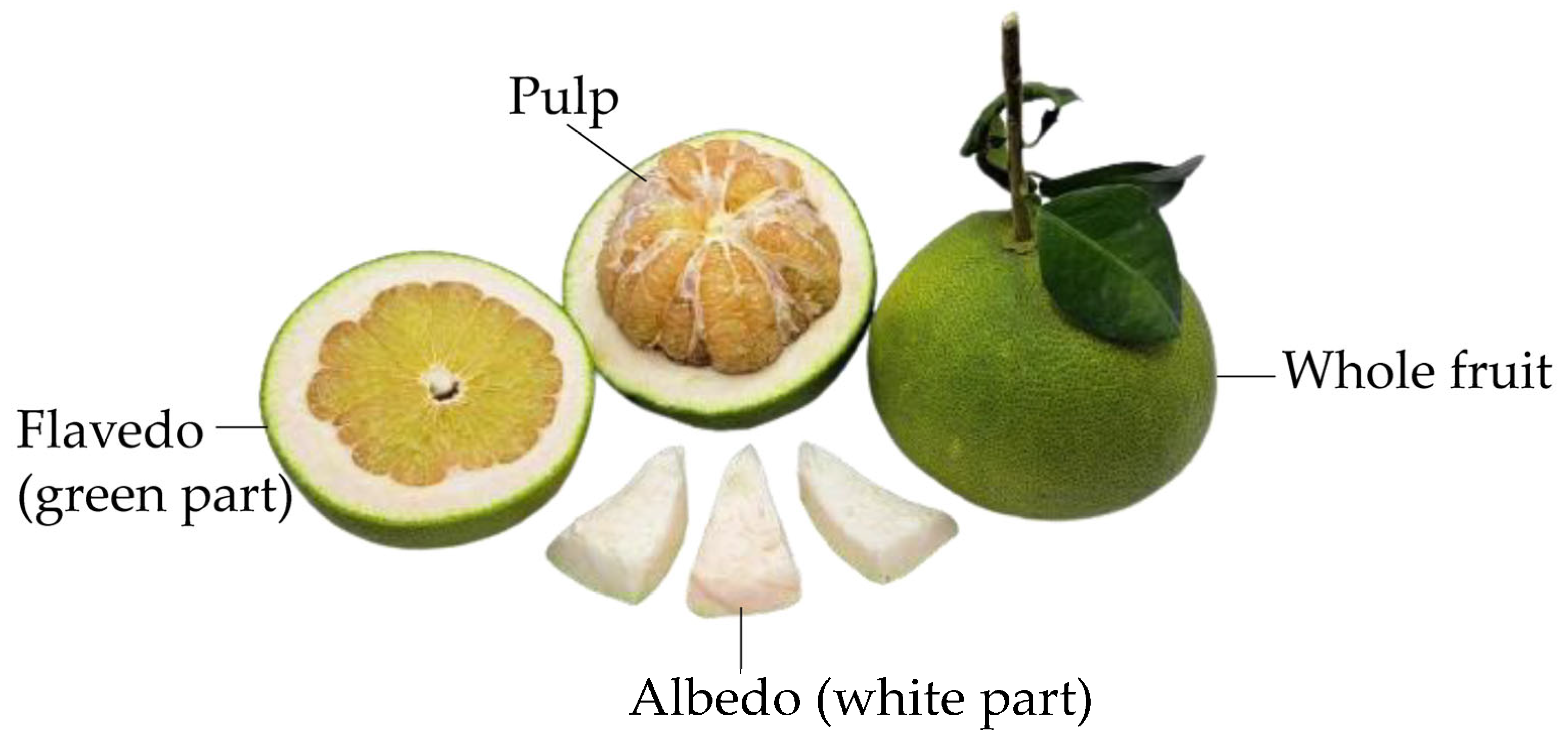
1. Introduction
2. Results
2.1. Optimization of Extraction Conditions Using Response Surface Methodology
2.1.1. Model Fitting, Analysis of Variance, and Validation
2.1.2. The Effect of Extraction Conditions on Total Flavonoid Contents
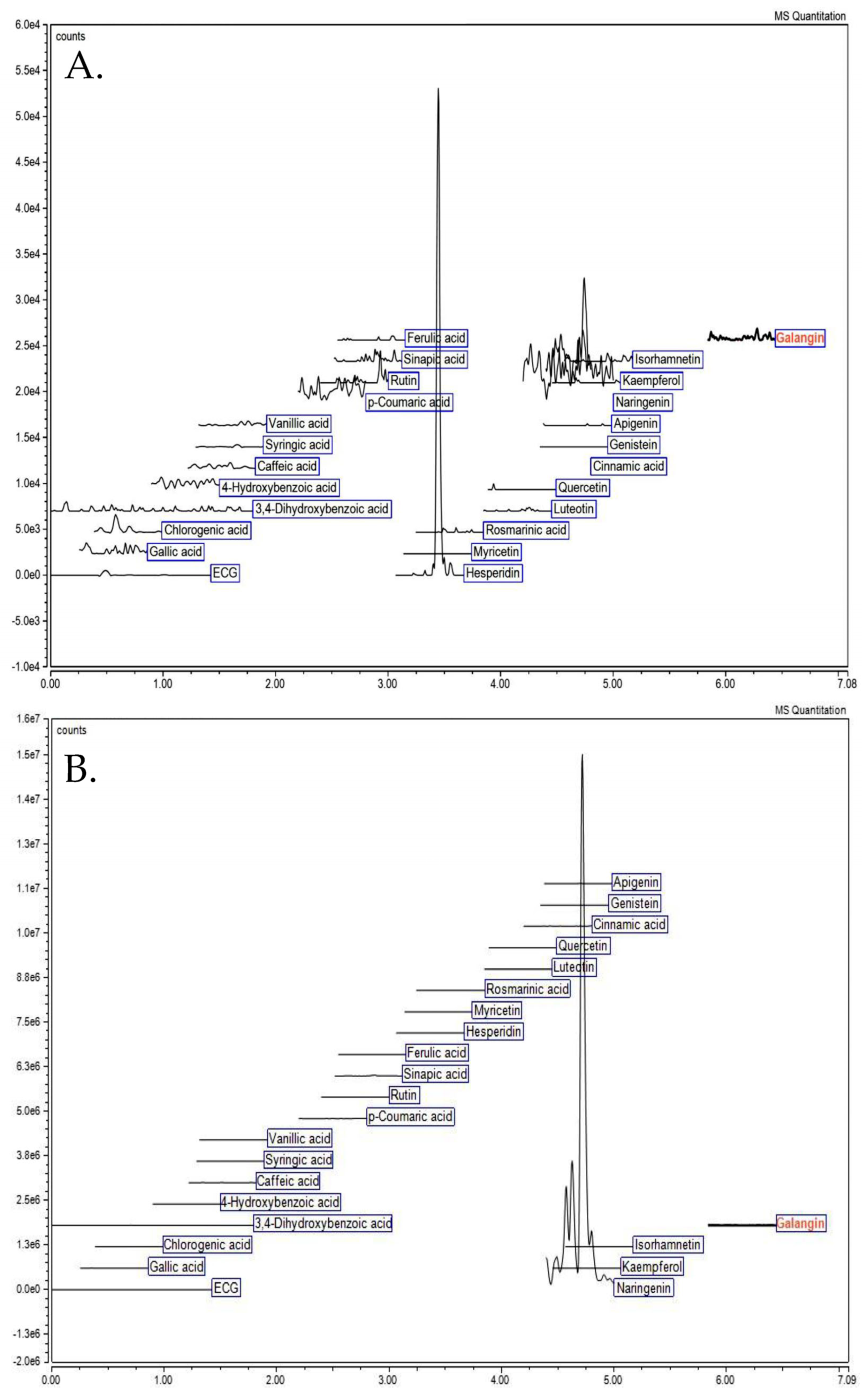
2.2. Analysis of Phytochemical Profile
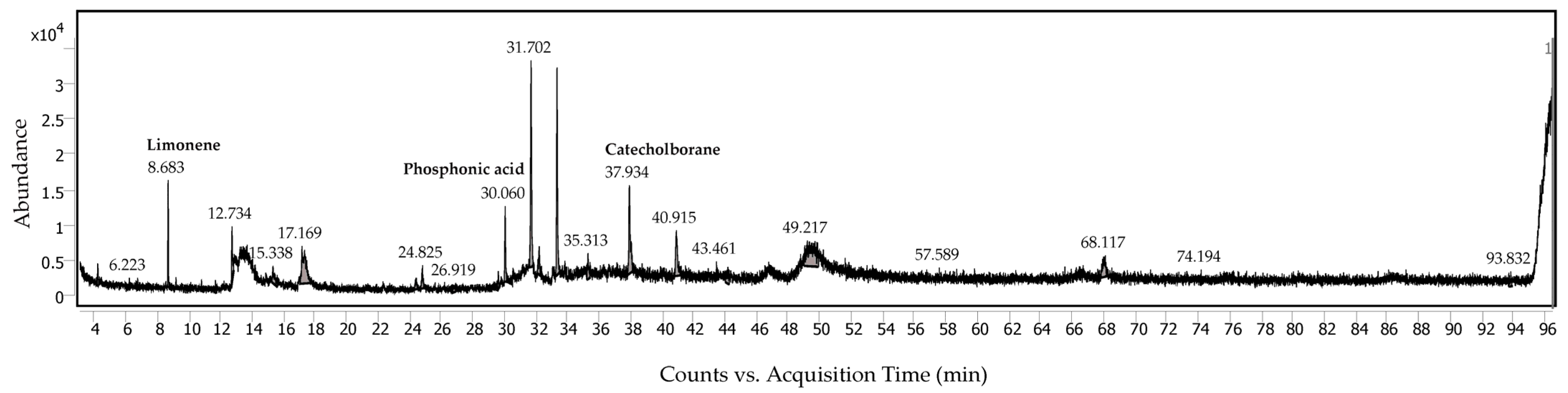
2.3. Analysis of Volatile Compounds
2.4. TPCs, TFCs, Antioxidant Activities, and Enzyme Inhibitory Activities
2.5. Genotoxicity Testing Using Bacterial Reverse Mutation Test (Ames Test)
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Selection, Preparation, and Extraction
4.3. Determination of Total Flavonoid Contents
4.4. Determination of Total Phenolic Contents
4.5. Determination of Antioxidant Properties
4.6. Determination of Enzyme Inhibitory Activities
4.7. Determination of Phytochemical Profiles Utilizing Liquid Chromatography–Electrospray Ionization–Tandem Mass Spectrometry (LC–ESI–MS/MS)
4.8. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
4.9. Bacterial Reverse Mutation Test (Ames Test)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Thielen, M.; Speck, T.; Seidel, R. Viscoelasticity and compaction behaviour of the foam-like pomelo (Citrus maxima) peel. J. Mater. Sci. 2013, 48, 3469–3478. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Khan, U.M.; Sameen, A.; Aadil, R.M.; Shahid, M.; Sezen, S.; Zarrabi, A.; Ozdemir, B.; Sevindik, M.; Kaplan, D.N.; Selamoglu, Z.; et al. Citrus Genus and Its Waste Utilization: A Review on Health-Promoting Activities and Industrial Application. Evid. Based Complement. Altern. Med. 2021, 2021, 2488804. [Google Scholar] [CrossRef] [PubMed]
- Phucharoenrak, P.; Muangnoi, C.; Trachootham, D. A Green Extraction Method to Achieve the Highest Yield of Limonin and Hesperidin from Lime Peel Powder (Citrus aurantifolia). Molecules 2022, 27, 820. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Tran, N.Y.T.; Le, T.D.; Dao, P.T.; Bach, G.L.; Huynh, P.X.; Tran, Q.N. Evaluation of different extraction methods on the polyphenols yield, flavonoids yield, and antioxidant activity of the pomelo flavedo extract from Da Xanh Citrus maxima [burm] merr.) variety. Food Sci. Technol. 2022, 42, 1–9. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- OECD. Test No. 471: Bacterial Reverse Mutation Test; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Cheong, M.-W.; Loke, X.-Q.; Liu, S.-Q.; Pramudya, K.; Curran, P.; Yu, B. Characterization of Volatile Compounds and Aroma Profiles of Malaysian Pomelo (Citrus grandis (L.) Osbeck) Blossom and Peel. J. Essent. Oil Res. 2011, 23, 34–44. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef]
- Thorne, D.; Kilford, J.; Hollings, M.; Dalrymple, A.; Ballantyne, M.; Meredith, C.; Dillon, D. The mutagenic assessment of mainstream cigarette smoke using the Ames assay: A multi-strain approach. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 782, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ani, P.N.; Abel, H.C. Nutrient, phytochemical, and antinutrient composition of Citrus maxima fruit juice and peel extract. Food Sci. Nutr. 2018, 6, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Zarina, Z.; Tan, S.Y. Determination of flavonoids in Citrus grandis (Pomelo) peels and their inhibition activity on lipid peroxidation in fish tissue. Int. Food Res. J. 2013, 20, 313. [Google Scholar]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Tanghiranrat, J.; Itsarasook, K.; Promphamorn, P.; Imsathian, N.; Sriburin, N. Evaluation of antioxidant properties and bioactive compound from pomelo (Citrus grandis (L.) osbeck) peel in antimicrobial for cosmetic products. Phranakhon Rajabhat Res. J. Sci. Technol. 2019, 15, 69–85. [Google Scholar]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant Activity of Pink-Flesh Guava (Psidium guajava L.): Effect of Extraction Techniques and Solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Gertenbach, D.D. Solid-Liquid Extraction Technologies for Manufacturing Nutraceuticals. In Functional Foods: Bioachemical and Processing Aspects; Shi, J., Mazza, G., Maguer, M.L., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 332–365. [Google Scholar]
- Puri, M.; Kaur, L.; Marwaha, S.-S. Partial Purification and Characterization of Limonoate Dehydrogenase from Rhodococcus fascians for the Degradation of Limonin. J. Microbiol. Biotechnol. 2002, 12, 669–673. [Google Scholar]
- Binkowska, I. Hesperidin: Synthesis and characterization of bioflavonoid complex. SN Appl. Sci. 2020, 2, 445. [Google Scholar] [CrossRef]
- Xiao, J. Recent advances on the stability of dietary polyphenols. eFood 2022, 3, e21. [Google Scholar] [CrossRef]
- Ha, T.T.; Mai, T.N.P.; Tran, T.T.; Nguyen, N.H.K.; Le, T.D.; Nguyen, V.M. Antioxidant activity and inhibitory efficacy of Citrus grandis peel extract against carbohydrate digestive enzymes in vitro. Food Sci. Technol. 2022, 42, 1–7. [Google Scholar] [CrossRef]
- Pichaiyongvongdee, S.; Rattanapun, B.; Haruenkit, R. Total Polyphenol Content and Antioxidant Properties in Different Tissues of Seven Pomelo (Citrus grandis (L.) Osbeck) Cultivars. Agric. Nat. Resour. 2014, 48, 989–996. [Google Scholar]
- Zhao, Z.; He, S.; Hu, Y.; Yang, Y.; Jiao, B.; Fang, Q.; Zhou, Z. Fruit flavonoid variation between and within four cultivated Citrus species evaluated by UPLC-PDA system. Sci. Hortic. 2017, 224, 93–101. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Bernatoniene, J. Optimization of Naringin and Naringenin Extraction from Citrus x paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics 2022, 14, 890. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, X.; Chen, X.; Rao, S.; Ju, T.; Li, J.; Yang, Z. Extraction and recovery of bioactive soluble phenolic compounds from brocade orange (Citrus sinensis) peels: Effect of different extraction methods thereon. LWT 2023, 173, 114337. [Google Scholar] [CrossRef]
- Li, P.; Yao, X.; Zhou, Q.; Meng, X.; Zhou, T.; Gu, Q. Citrus Peel Flavonoid Extracts: Health-Beneficial Bioactivities and Regulation of Intestinal Microecology in vitro. Front. Nutr. 2022, 9, 888745. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Suttisansanee, U.; Thiyajai, P.; Chalermchaiwat, P.; Wongwathanarat, K.; Pruesapan, K.; Charoenkiatkul, S.; Temviriyanukul, P. Phytochemicals and In Vitro Bioactivities of Aqueous Ethanolic Extracts from Common Vegetables in Thai Food. Plants 2021, 10, 1563. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Justin Thenmozhi, A.; Raja, T.R.; Janakiraman, U.; Manivasagam, T. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem. Res. 2015, 40, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liu, L.; Wang, Q.; Gao, Y. Naringenin alleviates cognition deficits in high-fat diet-fed SAMP8 mice. J. Food Biochem. 2020, 44, e13375. [Google Scholar] [CrossRef]
- Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones. Molecules 2018, 23, 1509. [Google Scholar] [CrossRef]
- Adewole, K.E.; Ishola, A.A. BACE1 and cholinesterase inhibitory activities of compounds from Cajanus cajan and Citrus reticulata: An in silico study. Silico Pharm. 2021, 9, 14. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Kittibunchakul, S.; Trisonthi, P.; Inthachat, W.; Siriwan, D.; Suttisansanee, U. Analysis of Phytonutrients, Anti-Mutagenic and Chemopreventive Effects of Tropical Fruit Extracts. Foods 2021, 10, 2600. [Google Scholar] [CrossRef]
- Wannasaksri, W.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Charoenkiatkul, S.; Suttisansanee, U. In Vitro Phytotherapeutic Properties of Aqueous Extracted Adenia viridiflora Craib. towards Civilization Diseases. Molecules 2021, 26, 1082. [Google Scholar] [CrossRef] [PubMed]
- Luu, L.K.; Thangsiri, S.; Sahasakul, Y.; Aursalung, A.; Inthachat, W.; Temviriyanukul, P.; On-Nom, N.; Chupeerach, C.; Suttisansanee, U. Nutrients, Phytochemicals and In Vitro Disease Prevention of Nephelium hypoleucum Kurz Fruit. Nutrients 2023, 15, 950. [Google Scholar] [CrossRef]
- Sirichai, P.; Kittibunchakul, S.; Thangsiri, S.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Inthachat, W.; Nuchuchua, O.; Aursalung, A.; Sahasakul, Y. Impact of drying processes on phenolics and in vitro health-related activities of indigenous plants in Thailand. Plants 2022, 11, 294. [Google Scholar] [CrossRef]
- Wang, W.Q.; Duan, H.X.; Pei, Z.T.; Xu, R.R.; Qin, Z.T.; Zhu, G.C.; Sun, L.W. Evaluation by the Ames Assay of the Mutagenicity of UV Filters Using Benzophenone and Benzophenone-1. Int. J. Environ. Res. Public Health 2018, 15, 1907. [Google Scholar] [CrossRef]
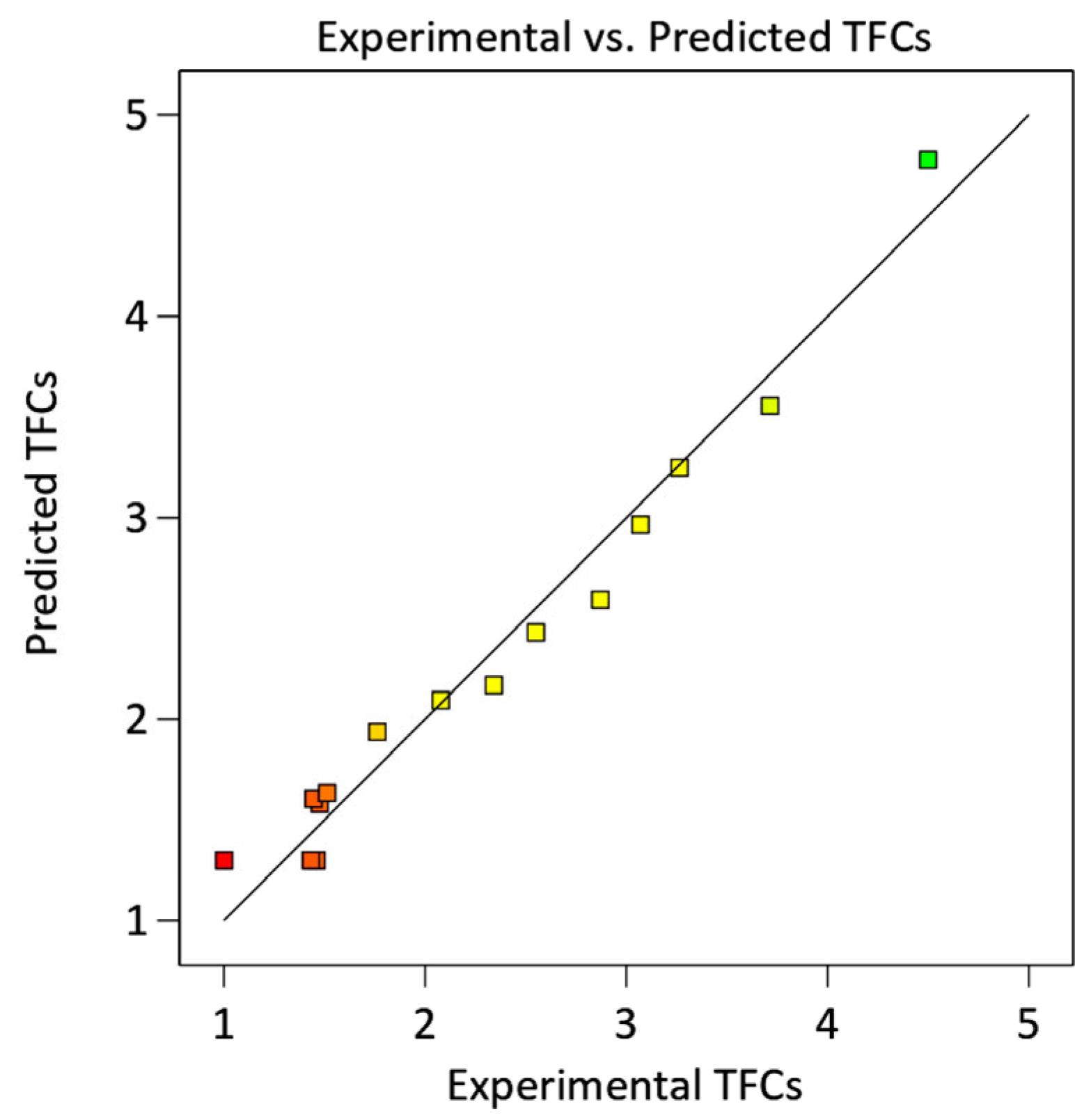
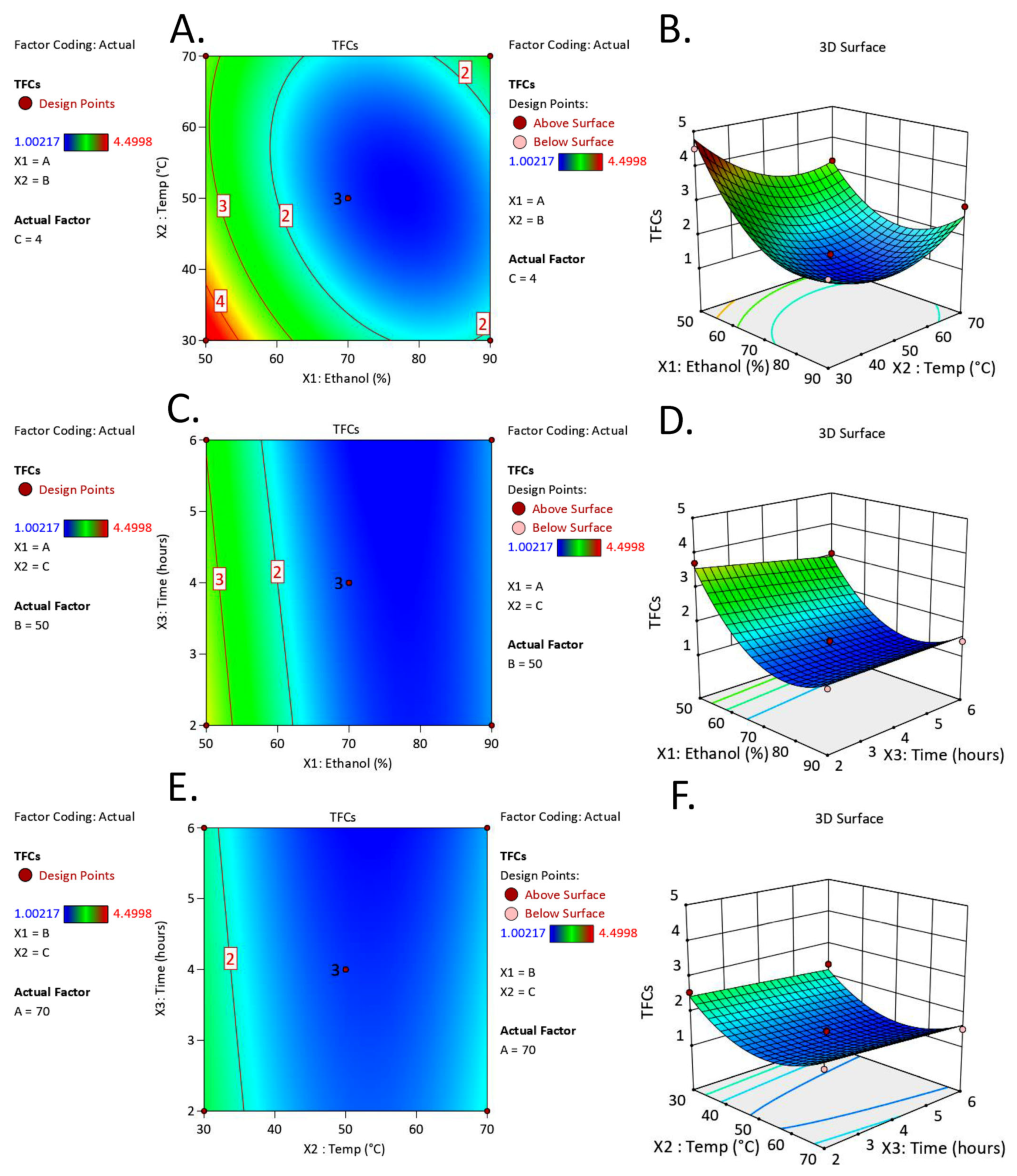
| Run | X1: Ethanol (% v/v) | X2: Temperature (°C) | X3: Time (h) | TPCs (mg GAE/g DW) | TFCs (mg QE/g DW) | |||
|---|---|---|---|---|---|---|---|---|
| Coded | Uncoded | Coded | Uncoded | Coded | Uncoded | Experimental | Experimental | |
| 1 | −1 | 50 | 1 | 70 | 0 | 4 | 13.02 ± 0.03 f | 3.26 ± 0.01 c |
| 2 | −1 | 50 | 0 | 50 | 1 | 6 | 1.84 ± 0.17 k | 3.07 ± 0.01 d |
| 3 | 0 | 70 | 1 | 70 | −1 | 2 | 20.35 ± 0.01 c | 1.76 ± 0.02 i |
| 4 | 0 | 70 | 1 | 70 | 1 | 6 | 20.59 ± 0.04 b | 1.51 ± 0.01 j |
| 5 | 0 | 70 | 0 | 50 | 0 | 4 | 11.39 ± 0.01 i | 1.43 ± 0.02 k |
| 6 | 0 | 70 | 0 | 50 | 0 | 4 | 11.34 ± 0.00 i | 1.40 ± 0.01 k |
| 7 | 1 | 90 | 1 | 70 | 0 | 4 | 12.16 ± 0.01 h | 2.87 ± 0.02 e |
| 8 | 0 | 70 | 0 | 50 | 0 | 4 | 11.38 ± 0.03 i | 1.46 ± 0.02 k |
| 9 | −1 | 50 | 0 | 50 | −1 | 2 | 4.47 ± 0.029 j | 3.71 ± 0.03 b |
| 10 | 1 | 90 | −1 | 30 | 0 | 4 | 15.46 ± 0.03 e | 2.08 ± 0.05 h |
| 11 | 0 | 70 | −1 | 30 | 1 | 6 | 11.36 ± 0.01 i | 2.34 ± 0.02 g |
| 12 | 1 | 90 | 0 | 50 | 1 | 6 | 11.34 ± 0.02 i | 1.45 ± 0.04 k |
| 13 | −1 | 50 | −1 | 30 | 0 | 4 | 15.79 ± 0.03 d | 4.50 ± 0.01 a |
| 14 | 1 | 90 | 0 | 50 | −1 | 2 | 21.88 ± 0.04 a | 1.48 ± 0.02 jk |
| 15 | 0 | 70 | −1 | 30 | −1 | 2 | 12.92 ± 0.03 g | 2.55 ± 0.05 f |
| Source | TPCs | TFCs | ||||
|---|---|---|---|---|---|---|
| Coefficient | p-Value | Significance | Coefficient | p-Value | Significance | |
| Model | 11.38 | 0.4440 | ns | 1.30 | 0.0030 | ** |
| X1 | 0.7000 | 0.7033 | −0.8347 | 0.0005 | ** | |
| X2 | 3.24 | 0.1085 | −0.2574 | 0.0590 | ||
| X3 | −1.16 | 0.5326 | −0.1415 | 0.2386 | ||
| X1X2 | −0.1325 | 0.9591 | 0.5069 | 0.0195 | * | |
| X1X3 | −0.7980 | 0.7584 | 0.1534 | 0.3520 | ||
| X2X3 | −1.92 | 0.4669 | −0.0102 | 0.9482 | ||
| 5.17 | 0.0711 | 1.13 | 0.0008 | *** | ||
| −2.44 | 0.3494 | 0.7478 | 0.0049 | ** | ||
| 1.00 | 0.6919 | −0.0038 | 0.9817 | |||
| R2 | 0.5923 | 0.9686 | ||||
| R2 adjusted | 0.0703 | 0.9122 | ||||
| Lack of fit | 1.59 | <0.0001 | **** | 0.4086 | ns | |
| Phenolics | Amount (µg/g DW) |
|---|---|
| Non-acid hydrolysis | |
| Hesperidin | 161.03 ± 5.03 |
| Acid hydrolysis | |
| Apigenin | 41.96 ± 1.29 |
| Naringenin | 3430.41 ± 135.02 |
| Sinapic acid | 142.32 ± 11.92 |
| Peak Number with Possibility Score of More Than 50% | Retention Time (min) | Name | Formula | Area (%) |
|---|---|---|---|---|
| 1 | 8.683 | Limonene | C10H16 | 4.05 |
| 2 | 30.060 | Phosphonic acid, (phydroxyphenyl)- | C6H7O4P | 4.28 |
| 3 | 37.934 | Catecholborane | C6H5BO2 | 9.26 |
| Types | Analyses | Values |
|---|---|---|
| Antioxidant activities | DPPH (µmol TE/g DW) | 15.35 ± 0.47 |
| FRAP (µmol TE/g DW) | 9.36 ± 0.06 | |
| ORAC (µmol TE/g DW) | 675.34 ± 22.16 | |
| Enzyme inhibitions | AChE (% inhibition) # | 14.57 ± 1.17 |
| BChE (% inhibition) # | 26.76 ± 1.17 | |
| BACE-1 (% inhibition) $ | 54.61 ± 5.08 | |
| Lipase (% inhibition) # | 20.89 ± 0.35 | |
| α-Glucosidase (% inhibition) # | 45.40 ± 1.73 | |
| α-Amylase (% inhibition) # | 35.38 ± 2.24 |
| Doses (µg/Plate) | TA98 | TA100 | TA102 | TA1535 | TA1537 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | |
| Neg | 87.67 ± 4.61 | 1.00 (−) | 71.17 ± 2.73 | 1.00 (−) | 373.33 ± 4.46 | 1.00 (−) | 9.67 ± 1.11 | 1.00 (−) | 8.67 ± 0.94 | 1.00 (−) |
| 10 | 85.00 ± 3.00 | 0.97 (−) | 69.50 ± 3.15 | 0.98 (−) | 374.50 ± 6.80 | 1.00 (−) | 11.00 ± 1.15 | 1.14 (−) | 9.17 ± 0.37 | 1.06 (−) |
| 100 | 88.33 ± 4.85 | 1.01 (−) | 71.50 ± 2.99 | 1.00 (−) | 376.50 ± 4.79 | 1.01 (−) | 10.33 ± 0.94 | 1.07 (−) | 9.33 ± 0.75 | 1.08 (−) |
| 500 | 83.67 ± 3.14 | 0.95 (−) | 73.17 ± 5.34 | 1.03 (−) | 374.67 ± 4.50 | 1.00 (−) | 10.00 ± 1.00 | 1.03 (−) | 8.67 ± 1.37 | 1.00 (−) |
| 1000 | 86.17 ± 2.41 | 0.98 (−) | 71.33 ± 2.36 | 1.00 (−) | 373.67 ± 4.03 | 1.00 (−) | 9.83 ± 1.07 | 1.02 (−) | 9.17 ± 1.34 | 1.06 (−) |
| 2000 | 85.50 ± 4.19 | 0.98 (−) | 69.00 ± 3.27 | 0.97 (−) | 374.00 ± 7.85 | 1.00 (−) | 10.67 ± 1.11 | 1.10 (−) | 10.00 ± 0.82 | 1.15 (−) |
| 4-NQO | 1047.33 ± 32.20 | 11.95 (+) | ||||||||
| NaN3 | 1146.67 ± 22.82 | 16.11 (+) | 271.17 ± 5.81 | 28.05 (+) | ||||||
| MMC | 1108.67 ± 19.92 | 2.97 (+) | ||||||||
| 9-AA | 776.67 ± 33.02 | 89.62 (+) | ||||||||
| Doses (µg/plate) | TA98 | TA100 | TA102 | TA1535 | TA1537 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | |
| Neg | 89.83 ± 3.72 | 1.00 (−) | 85.83 ± 3.29 | 1.00 (−) | 375.00 ± 4.00 | 1.00 (−) | 10.00 ± 1.15 | 1.00 (−) | 9.50 ± 0.96 | 1.00 (−) |
| 10 | 92.00 ± 3.27 | 1.02 (−) | 84.50 ± 2.57 | 0.98 (−) | 380.33 ± 5.65 | 1.01 (−) | 11.33 ± 1.11 | 1.13 (−) | 9.67 ± 0.94 | 1.02 (−) |
| 100 | 94.17 ± 3.58 | 1.05 (−) | 82.00 ± 2.38 | 0.96 (−) | 374.33 ± 4.35 | 1.00 (−) | 10.33 ± 1.11 | 1.03 (−) | 9.33 ± 0.94 | 0.98 (−) |
| 500 | 95.17 ± 2.54 | 1.06 (−) | 81.50 ± 3.64 | 0.95 (−) | 383.50 ± 5.62 | 1.02 (−) | 10.67 ± 1.37 | 1.07 (−) | 10.67 ± 1.25 | 1.12 (−) |
| 1000 | 90.17 ± 1.46 | 1.00 (−) | 81.83 ± 4.41 | 0.95 (−) | 374.00 ± 3.70 | 1.00 (−) | 10.83 ± 1.57 | 1.08 (−) | 11.17 ± 0.90 | 1.18 (−) |
| 2000 | 90.33 ± 3.54 | 1.01 (−) | 82.83 ± 6.79 | 0.97 (−) | 374.83 ± 5.01 | 1.00 (−) | 11.17 ± 0.69 | 1.12 (−) | 10.83 ± 1.07 | 1.14 (−) |
| 2-AA | 1134.67 ± 53.80 | 12.63 (+) | 1006.67 ± 40.24 | 11.73 (+) | 1118.00 ± 23.20 | 2.98 (+) | 369.83 ± 8.39 | 36.98 (+) | 200.83 ± 6.44 | 21.14 (+) |
| Assay | Assay Components | ||||
|---|---|---|---|---|---|
| Enzyme | Substrate | Indicator | Extract | Detection Wavelength | |
| AChE | 100 μL of 0.25 µg/mL AChE 1 | 50 μL of 0.32 mM ACh | 10 µL of 16 mM DTNB | 40 µL | 412 nm |
| BChE | 100 μL of 1.5 µg/mL BChE 2 | 50 μL of 0.4 mM BCh | |||
| Lipase | 100 µL of 5 µg/mL lipase 3 | 50 μL of 0.2 mM DMPTB | |||
| BACE-1 | BACE-1 FRET assay kit (Sigma-Aldrich, St. Louis, MO, USA) following manufacturer’s recommendations | λex = 320 nm λem = 405 nm | |||
| α-Amylase | 100 µL of 50 mg/mL α-amylase 4 | 50 µL of 30 mM pNPM | 50 µL | 405 nm | |
| α-Glucosidase | 100 µL of 0.1 U/mL α-glucosidase 5 | 50 µL of 2 mM pNPG + 160 µL KPB (pH 7) | 50 µL | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inthachat, W.; Temviriyanukul, P.; On-Nom, N.; Kanoongon, P.; Thangsiri, S.; Chupeerach, C.; Suttisansanee, U. Optimization of Phytochemical-Rich Citrus maxima Albedo Extract Using Response Surface Methodology. Molecules 2023, 28, 4121. https://doi.org/10.3390/molecules28104121
Inthachat W, Temviriyanukul P, On-Nom N, Kanoongon P, Thangsiri S, Chupeerach C, Suttisansanee U. Optimization of Phytochemical-Rich Citrus maxima Albedo Extract Using Response Surface Methodology. Molecules. 2023; 28(10):4121. https://doi.org/10.3390/molecules28104121
Chicago/Turabian StyleInthachat, Woorawee, Piya Temviriyanukul, Nattira On-Nom, Panyaporn Kanoongon, Sirinapa Thangsiri, Chaowanee Chupeerach, and Uthaiwan Suttisansanee. 2023. "Optimization of Phytochemical-Rich Citrus maxima Albedo Extract Using Response Surface Methodology" Molecules 28, no. 10: 4121. https://doi.org/10.3390/molecules28104121
APA StyleInthachat, W., Temviriyanukul, P., On-Nom, N., Kanoongon, P., Thangsiri, S., Chupeerach, C., & Suttisansanee, U. (2023). Optimization of Phytochemical-Rich Citrus maxima Albedo Extract Using Response Surface Methodology. Molecules, 28(10), 4121. https://doi.org/10.3390/molecules28104121





