Nanoporous Au Behavior in Methyl Orange Solutions
Abstract
1. Introduction
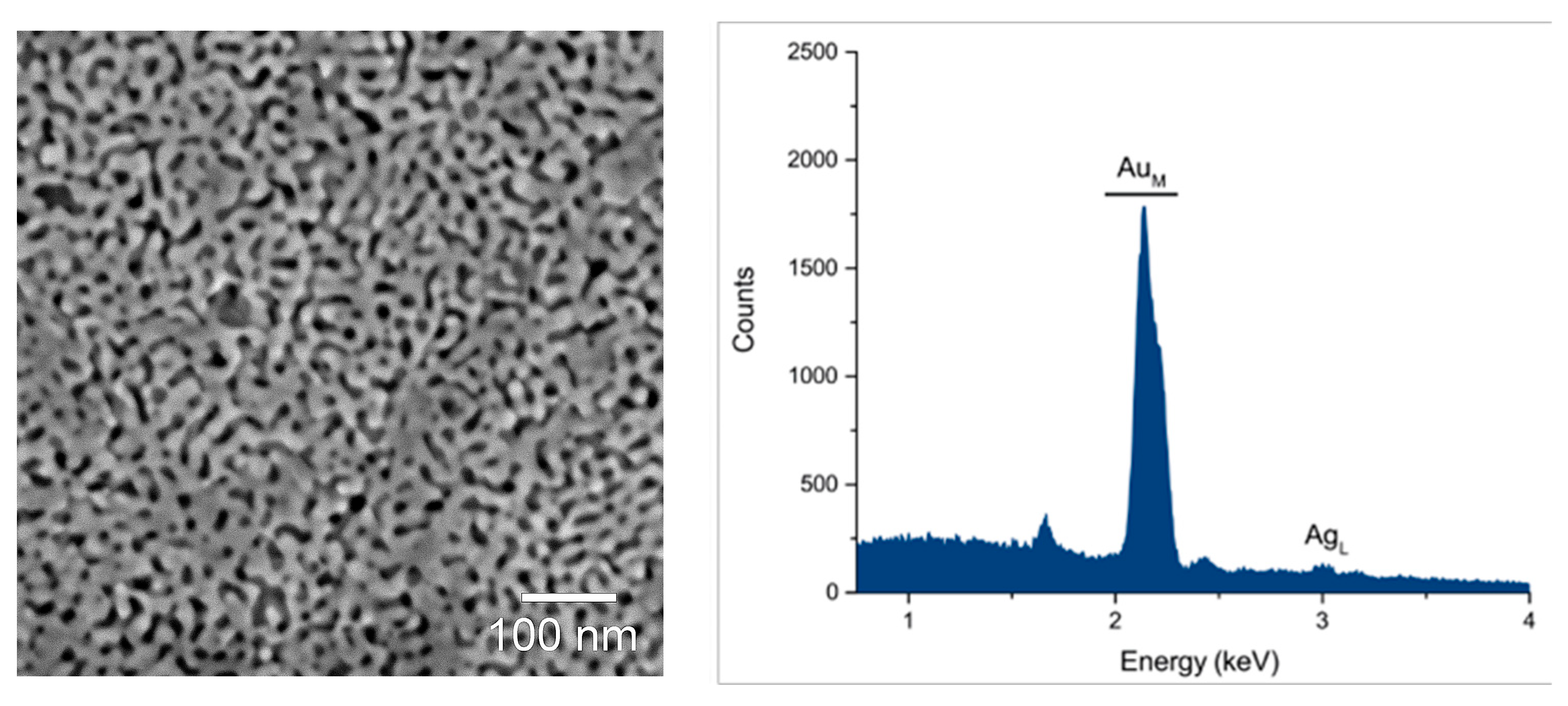
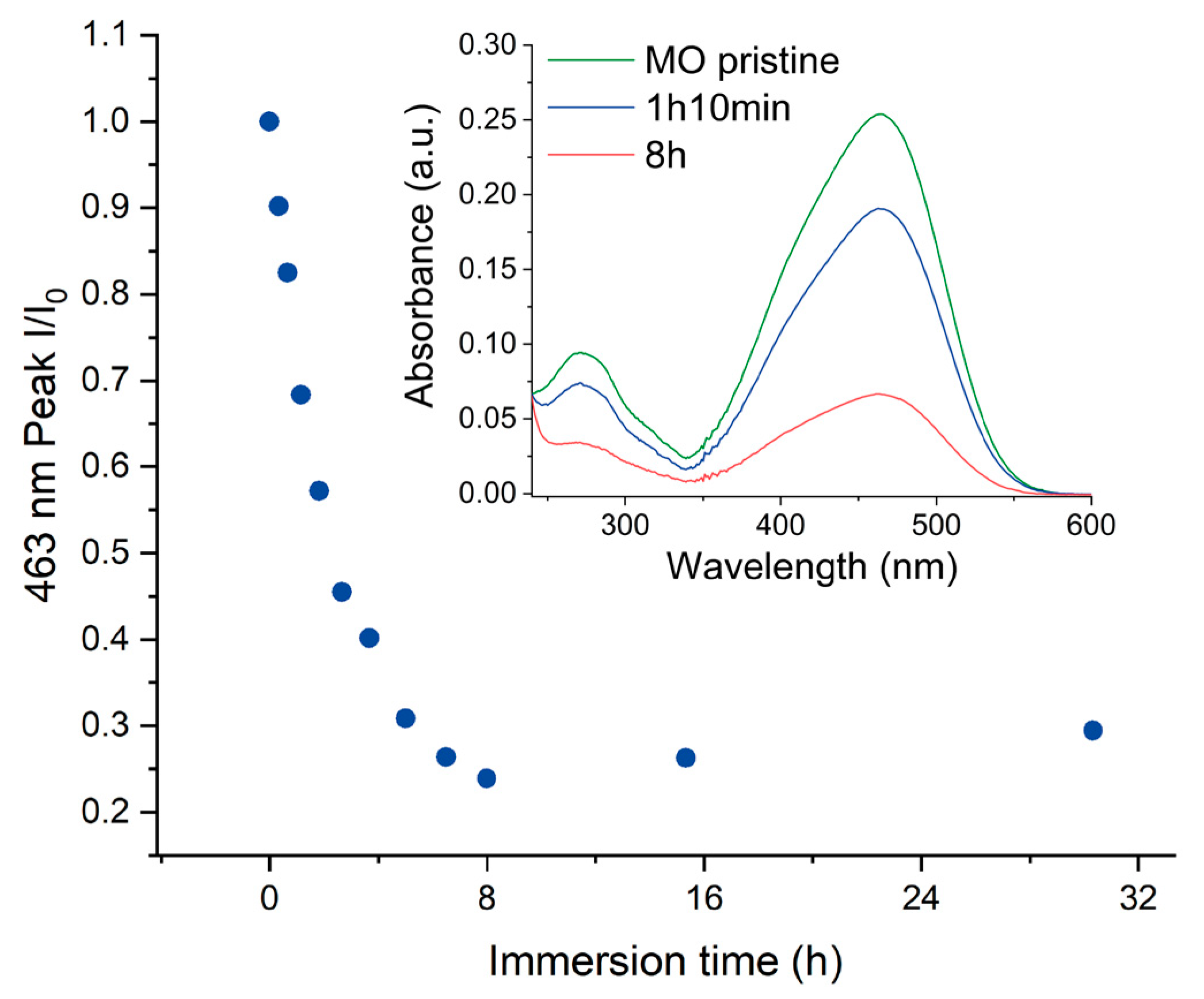
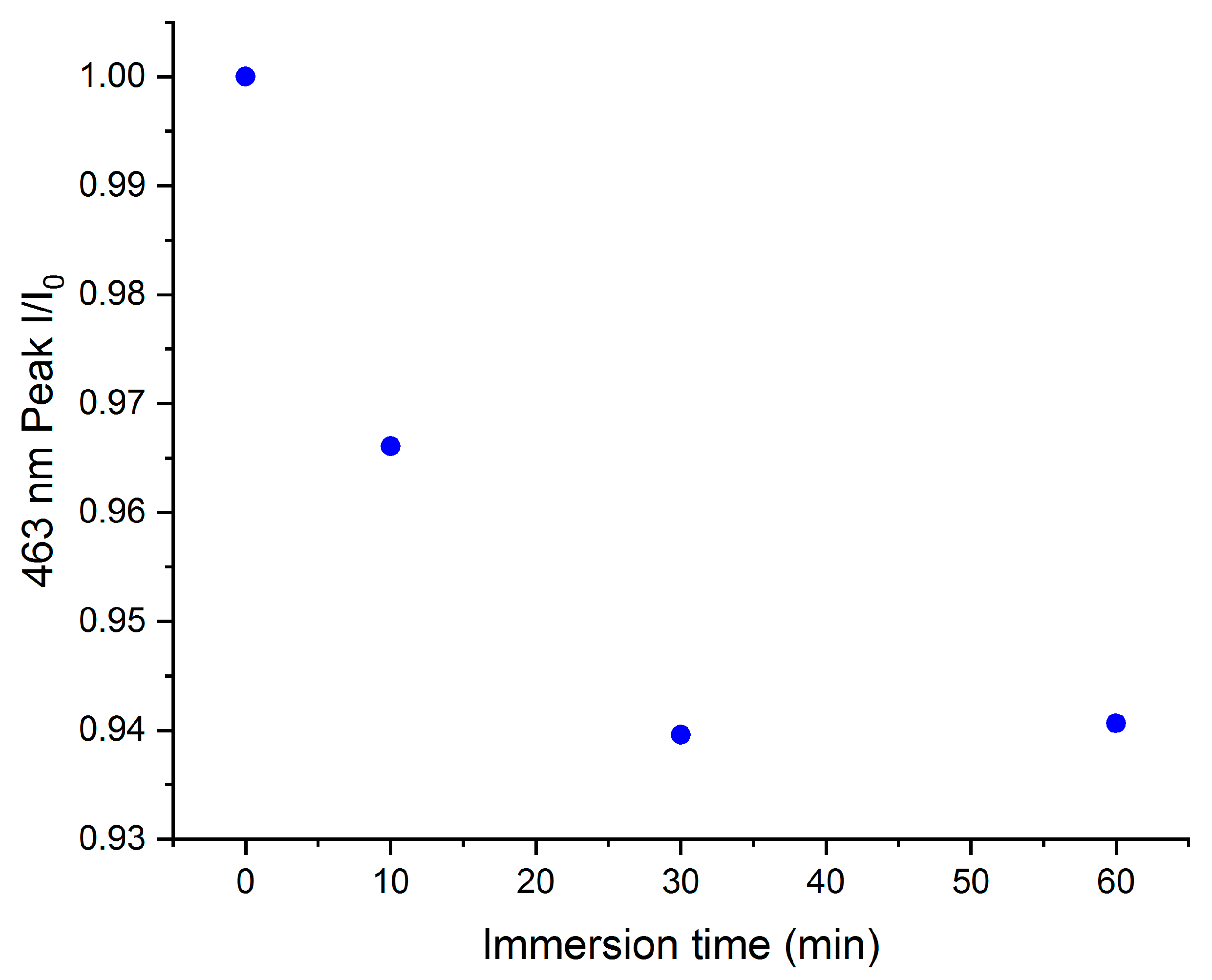
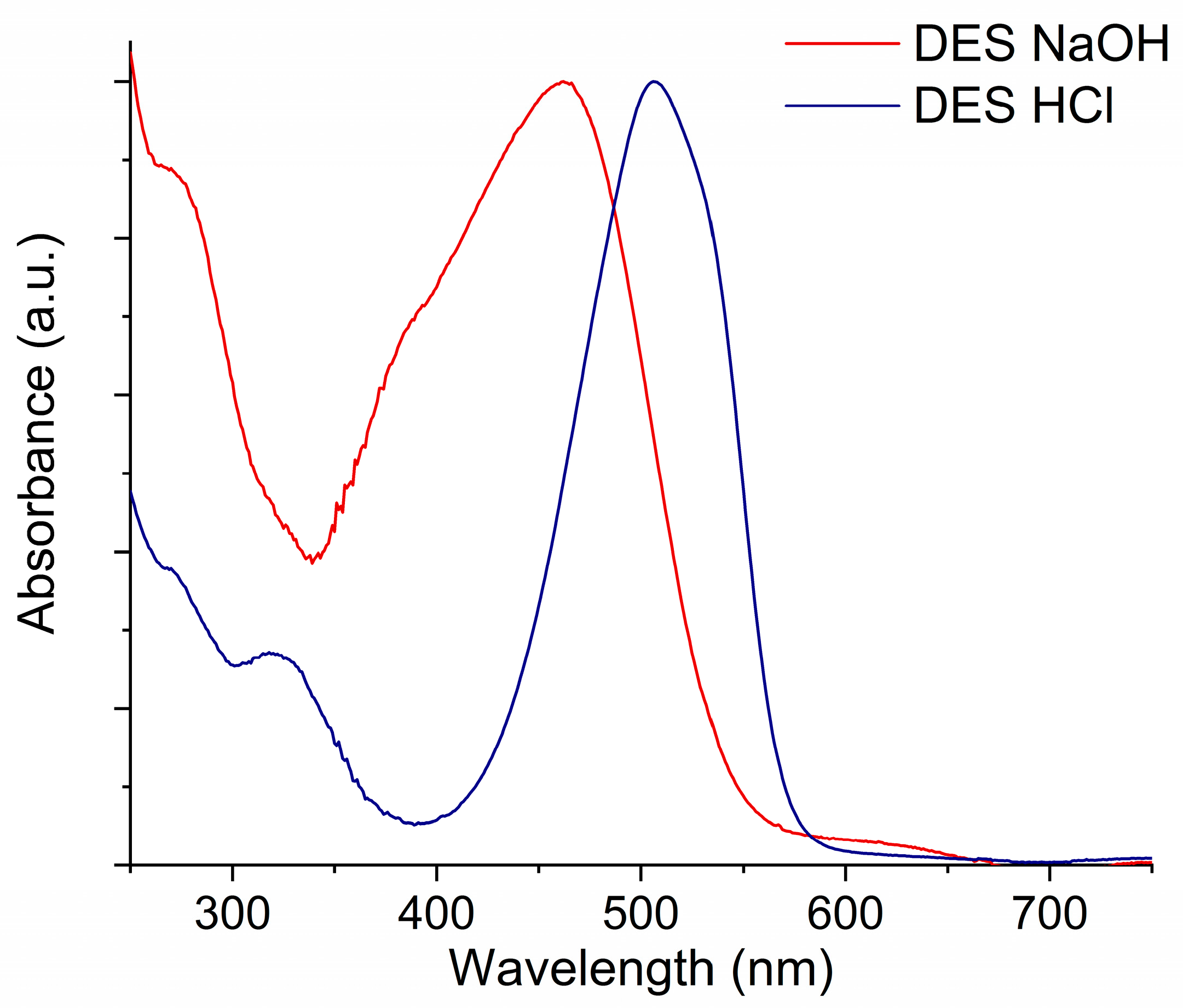
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. NP Au Fabrication
3.3. Immersion Tests in MO Solutions
3.3.1. Pellet Tests
3.3.2. Powder Tests
3.4. UV-Vis Measurements
3.5. High-Performance Liquid Chromatography
3.6. Scanning Electron Microscopy/Energy Dispersive Spectroscopy
3.7. N2 Adsorption–Desorption Isotherms
3.8. Electrochemical Measurements
3.9. X-ray Photoelectron Spectroscopy (XPS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Li, C.M. Nanoporous Metals: Fabrication Strategies and Advanced Electrochemical Applications in Catalysis, Sensing and Energy Systems. Chem. Soc. Rev. 2012, 41, 7016. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Pia, G.; Casula, M.F.; Delogu, F.; Sogne, E.; Falqui, A.; Pilia, L. Fabrication of Nanoporous Al by Vapor-Phase Dealloying: Morphology Features, Mechanical Properties and Model Predictions. Appl. Sci. 2021, 11, 6639. [Google Scholar] [CrossRef]
- Pia, G.; Delogu, F. Mechanical Properties of Nanoporous Au: From Empirical Evidence to Phenomenological Modeling. Metals 2015, 5, 1665–1694. [Google Scholar] [CrossRef]
- Seker, E.; Reed, M.L.; Begley, M.R. Nanoporous Gold: Fabrication, Characterization, and Applications. Materials 2009, 2, 2188–2215. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M. Nanoporous Metals for Catalytic and Optical Applications. MRS Bull. 2009, 34, 569–576. [Google Scholar] [CrossRef]
- Juarez, T.; Biener, J.; Weissmüller, J.; Hodge, A.M. Nanoporous Metals with Structural Hierarchy: A Review. Adv. Eng. Mater. 2017, 19, 1700389. [Google Scholar] [CrossRef]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Artmann, E.; Forschner, L.; Schüttler, K.M.; Al-Shakran, M.; Jacob, T.; Engstfeld, A.K. Nanoporous Au Formation on Au Substrates via High Voltage Electrolysis. ChemPhysChem 2023, 24, e202200645. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, F.; Torrisi, V.; Grillo, R.; Cacciato, G.; Zimbone, M.; Piccitto, G.; Grimaldi, M.G. Nanoporous Au Structures by Dealloying Au/Ag Thermal- or Laser-Dewetted Bilayers on Surfaces. Superlattices Microstruct. 2017, 103, 28–47. [Google Scholar] [CrossRef]
- Wittstock, G.; Bäumer, M.; Dononelli, W.; Klüner, T.; Lührs, L.; Mahr, C.; Moskaleva, L.V.; Oezaslan, M.; Risse, T.; Rosenauer, A.; et al. Nanoporous Gold: From Structure Evolution to Functional Properties in Catalysis and Electrochemistry. Chem. Rev. 2023, 123, 6716–6792. [Google Scholar] [CrossRef]
- Chen, Q.; Sieradzki, K. Spontaneous Evolution of Bicontinuous Nanostructures in Dealloyed Li-Based Systems. Nat. Mater. 2013, 12, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Chen-Wiegart, Y.K.; Harder, R.; Dunand, D.C.; McNulty, I. Evolution of Dealloying Induced Strain in Nanoporous Gold Crystals. Nanoscale 2017, 9, 5686–5693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Chu, Y.S.; Yi, J.; McNulty, I.; Shen, Q.; Voorhees, P.W.; Dunand, D.C. Morphological and Topological Analysis of Coarsened Nanoporous Gold by X-Ray Nanotomography. Appl. Phys. Lett. 2010, 96, 043122. [Google Scholar] [CrossRef][Green Version]
- Pia, G.; Delogu, F. Coarsening of Nanoporous Au: Relationship between Structure and Mechanical Properties. Acta Mater. 2015, 99, 29–38. [Google Scholar] [CrossRef]
- Pia, G.; Delogu, F. Nanoporous Au: Statistical Analysis of Morphological Features and Evaluation of Their Influence on the Elastic Deformation Behavior by Phenomenological Modeling. Acta Mater. 2015, 85, 250–260. [Google Scholar] [CrossRef]
- Gao, P.; Lv, Z.; Song, Y.; Song, M.; Qian, P. Evolution of Morphology and Microstructure of Coarsened Nanoporous Gold Studied by Automatic Thresholding and Image Recognition Algorithms. Scr. Mater. 2023, 226, 115256. [Google Scholar] [CrossRef]
- Satta, J.; Pinna, A.; Pia, G.; Pilia, L.; Carbonaro, C.M.; Chiriu, D.; Stagi, L.; Abdullah, Q.A.; Ricci, P.C. Stable CsPbBr3 Nanocrystals—Decorated Nanoporous Gold for Optoelectronic Applications. Crystals 2022, 12, 863. [Google Scholar] [CrossRef]
- van der Zalm, J.; Chen, S.; Huang, W.; Chen, A. Review—Recent Advances in the Development of Nanoporous Au for Sensing Applications. J. Electrochem. Soc. 2020, 167, 037532. [Google Scholar] [CrossRef]
- Kim, S.H. Nanoporous Gold: Preparation and Applications to Catalysis and Sensors. Curr. Appl. Phys. 2018, 18, 810–818. [Google Scholar] [CrossRef]
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Bäumer, M. Nanoporous Gold Catalysts for Selective Gas-Phase Oxidative Coupling of Methanol at Low Temperature. Science 2010, 327, 319–322. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, Y. Nanoporous Metals for Heterogeneous Catalysis: Following the Success of Raney Nickel. Chem.—A Eur. J. 2020, 26, 8845–8856. [Google Scholar] [CrossRef] [PubMed]
- Zielasek, V.; Jürgens, B.; Schulz, C.; Biener, J.; Biener, M.M.; Hamza, A.V.; Bäumer, M. Gold Catalysts: Nanoporous Gold Foams. Angew. Chem. Int. Ed. 2006, 45, 8241–8244. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Su, J.; Xu, X.; Liu, P.; Zhao, H.; Tian, F.; Ding, Y. Low Temperature CO Oxidation over Unsupported Nanoporous Gold. J. Am. Chem. Soc. 2007, 129, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, S.; Tsai, A.P. CO Oxidation Over a Fine Porous Gold Catalyst Fabricated by Selective Leaching from an Ordered AuCu3 Intermetallic Compound. Catal. Lett. 2008, 121, 337–341. [Google Scholar] [CrossRef]
- Wittstock, A.; Neumann, B.; Schaefer, A.; Dumbuya, K.; Kübel, C.; Biener, M.M.; Zielasek, V.; Steinrück, H.-P.; Gottfried, J.M.; Biener, J.; et al. Nanoporous Au: An Unsupported Pure Gold Catalyst? J. Phys. Chem. C 2009, 113, 5593–5600. [Google Scholar] [CrossRef]
- Demirci, C.; Marras, S.; Prato, M.; Pasquale, L.; Manna, L.; Colombo, M. Design of Catalytically Active Porous Gold Structures from a Bottom-up Method: The Role of Metal Traces in CO Oxidation and Oxidative Coupling of Methanol. J. Catal. 2019, 375, 279–286. [Google Scholar] [CrossRef]
- Abad, A.; Concepción, P.; Corma, A.; García, H. A Collaborative Effect between Gold and a Support Induces the Selective Oxidation of Alcohols. Angew. Chem. Int. Ed. 2005, 44, 4066–4069. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, B.; Egholm Christiansen, S.; Dahl Thomsen, M.L.; Christensen, C.H. Aerobic Oxidation of Aqueous Ethanol Using Heterogeneous Gold Catalysts: Efficient Routes to Acetic Acid and Ethyl Acetate. J. Catal. 2007, 251, 332–337. [Google Scholar] [CrossRef]
- Hughes, M.D.; Xu, Y.-J.; Jenkins, P.; McMorn, P.; Landon, P.; Enache, D.I.; Carley, A.F.; Attard, G.A.; Hutchings, G.J.; King, F.; et al. Tunable Gold Catalysts for Selective Hydrocarbon Oxidation under Mild Conditions. Nature 2005, 437, 1132–1135. [Google Scholar] [CrossRef]
- Ishida, T.; Haruta, M. Gold Catalysts: Towards Sustainable Chemistry. Angew. Chem. Int. Ed. 2007, 46, 7154–7156. [Google Scholar] [CrossRef]
- Wittstock, A.; Wichmann, A.; Biener, J.; Bäumer, M. Nanoporous Gold: A New Gold Catalyst with Tunable Properties. Faraday Discuss. 2011, 152, 87. [Google Scholar] [CrossRef] [PubMed]
- Hakamada, M.; Hirashima, F.; Mabuchi, M. Catalytic Decoloration of Methyl Orange Solution by Nanoporous Metals. Catal. Sci. Technol. 2012, 2, 1814. [Google Scholar] [CrossRef]
- Langhals, H. Color Chemistry. Synthesis, Properties and Applications of Organic Dyes and Pigments. 3rd Revised Edition. By Heinrich Zollinger. Angew. Chem. Int. Ed. 2004, 43, 5291–5292. [Google Scholar] [CrossRef]
- Farhan Hanafi, M.; Sapawe, N. A Review on the Water Problem Associate with Organic Pollutants Derived from Phenol, Methyl Orange, and Remazol Brilliant Blue Dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Li, H.; Jin, H.; Li, R.; Hua, J.; Zhang, Z.; Li, R. Magnetic Fe3O4@SiO2 Study on Adsorption of Methyl Orange on Nanoparticles. Sci. Rep. 2024, 14, 1217. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, Y.; Zhang, S.; Yu, C.; Ostrikov, K.; Zhang, Z. Overcoming the Permeability-Selectivity Challenge in Water Purification Using Two-Dimensional Cobalt-Functionalized Vermiculite Membrane. Nat. Commun. 2024, 15, 391. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jing, C.; Zhang, X.; Jiang, D.; Liu, X.; Dong, B.; Feng, L.; Li, S.; Zhang, Y. Acid-Salt Treated CoAl Layered Double Hydroxide Nanosheets with Enhanced Adsorption Capacity of Methyl Orange Dye. J. Colloid. Interface Sci. 2019, 548, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Vikrant, K.; Kim, K.-H.; Brown, R.J.C. Magnesium/Aluminum Layered Double Hydroxides Intercalated with Starch for Effective Adsorptive Removal of Anionic Dyes. J. Hazard. Mater. 2022, 424, 127454. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Ighalo, J.O.; Emenike, E.C.; Ogunfowora, L.A.; Igwegbe, C.A. Adsorption of Methyl Orange: A Review on Adsorbent Performance. Curr. Res. Green. Sustain. Chem. 2021, 4, 100179. [Google Scholar] [CrossRef]
- Mais, L.; Vacca, A.; Mascia, M.; Usai, E.M.; Tronci, S.; Palmas, S. Experimental Study on the Optimisation of Azo-Dyes Removal by Photo-Electrochemical Oxidation with TiO2 Nanotubes. Chemosphere 2020, 248, 125938. [Google Scholar] [CrossRef]
- Yen, C.W.; Mahmoud, M.A.; El-Sayed, M.A. Photocatalysis in Gold Nanocage Nanoreactors. J. Phys. Chem. A 2009, 113, 4340–4345. [Google Scholar] [CrossRef] [PubMed]
- Arabatzis, I. Characterization and Photocatalytic Activity of Au/TiO2 Thin Films for Azo-Dye Degradation. J. Catal. 2003, 220, 127–135. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Baiocchi, C.; Brussino, M.C.; Pramauro, E.; Prevot, A.B.; Palmisano, L.; Marcì, G.M. Characterization of Methyl Orange and Its Photocatalytic Degradation Products by HPLC/UV-VIS Diode Array and Atmospheric Pressure Ionization Quadrupole Ion. Int. J. Mass Spectrom. 2002, 214, 247–256. [Google Scholar] [CrossRef]
- Barbosa, J. INDICATORS|Acid–Base. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 360–371. [Google Scholar]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Rouya, E.; Cattarin, S.; Reed, M.L.; Kelly, R.G.; Zangari, G. Electrochemical Characterization of the Surface Area of Nanoporous Gold Films. J. Electrochem. Soc. 2012, 159, K97–K102. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000.


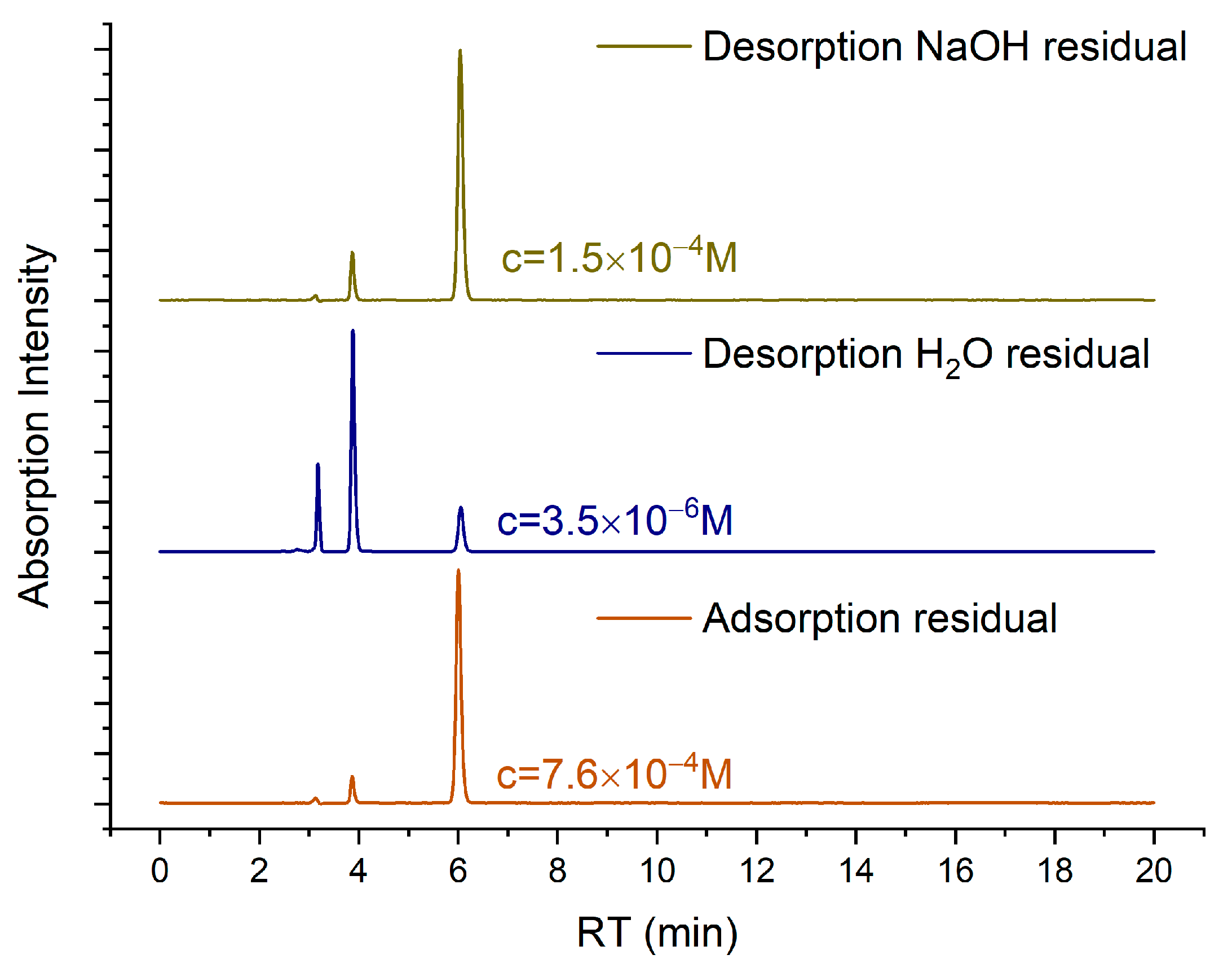
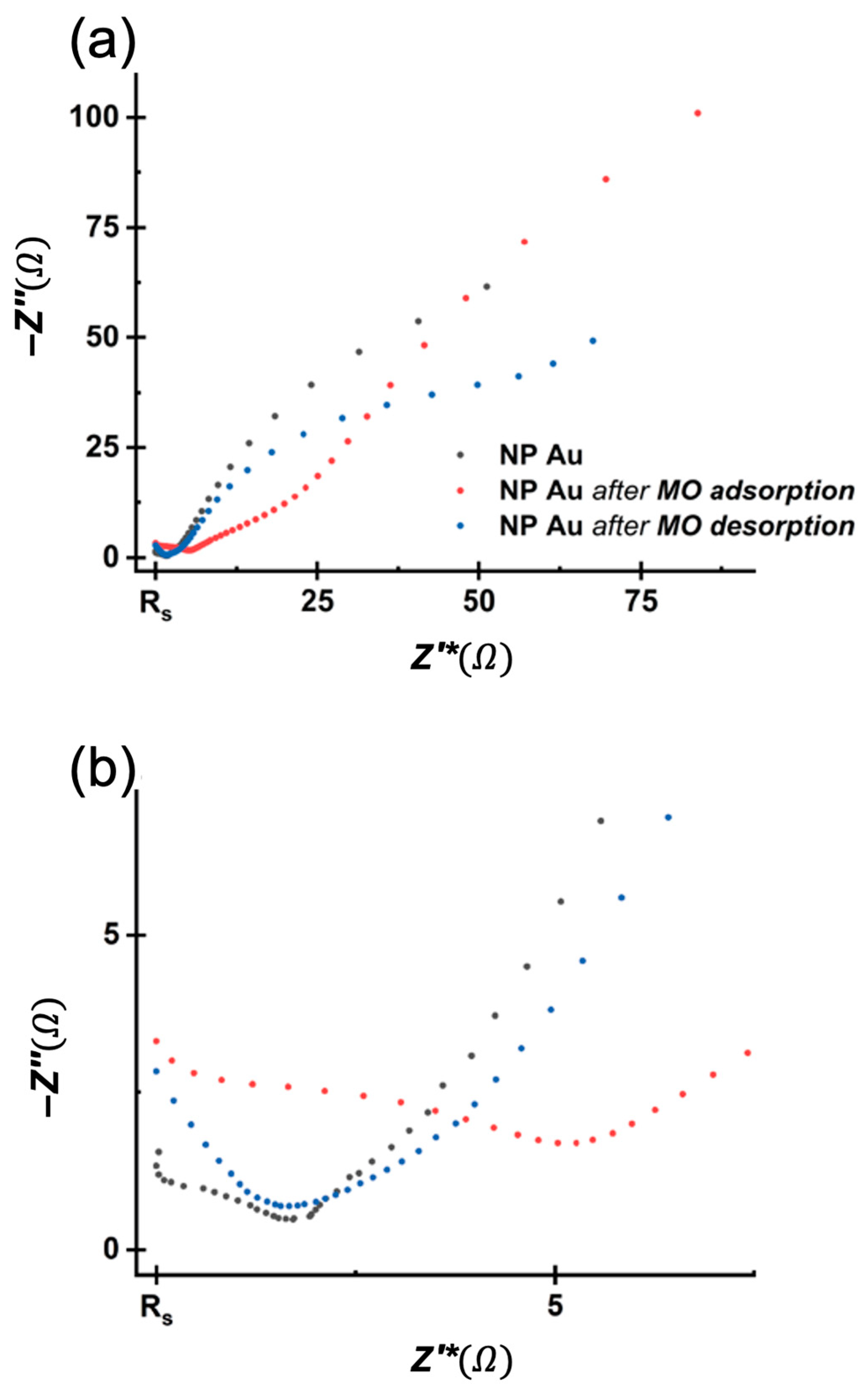
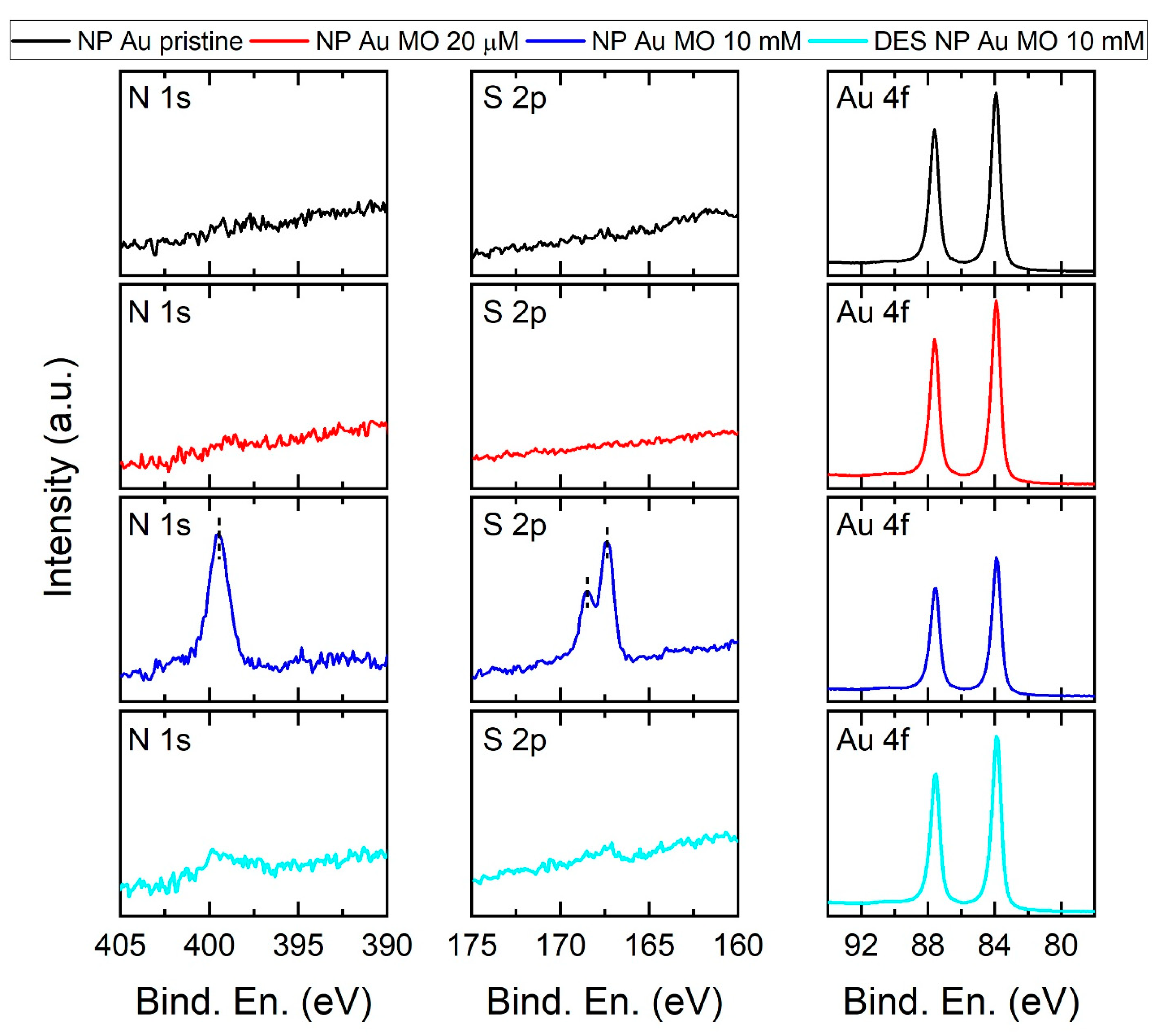
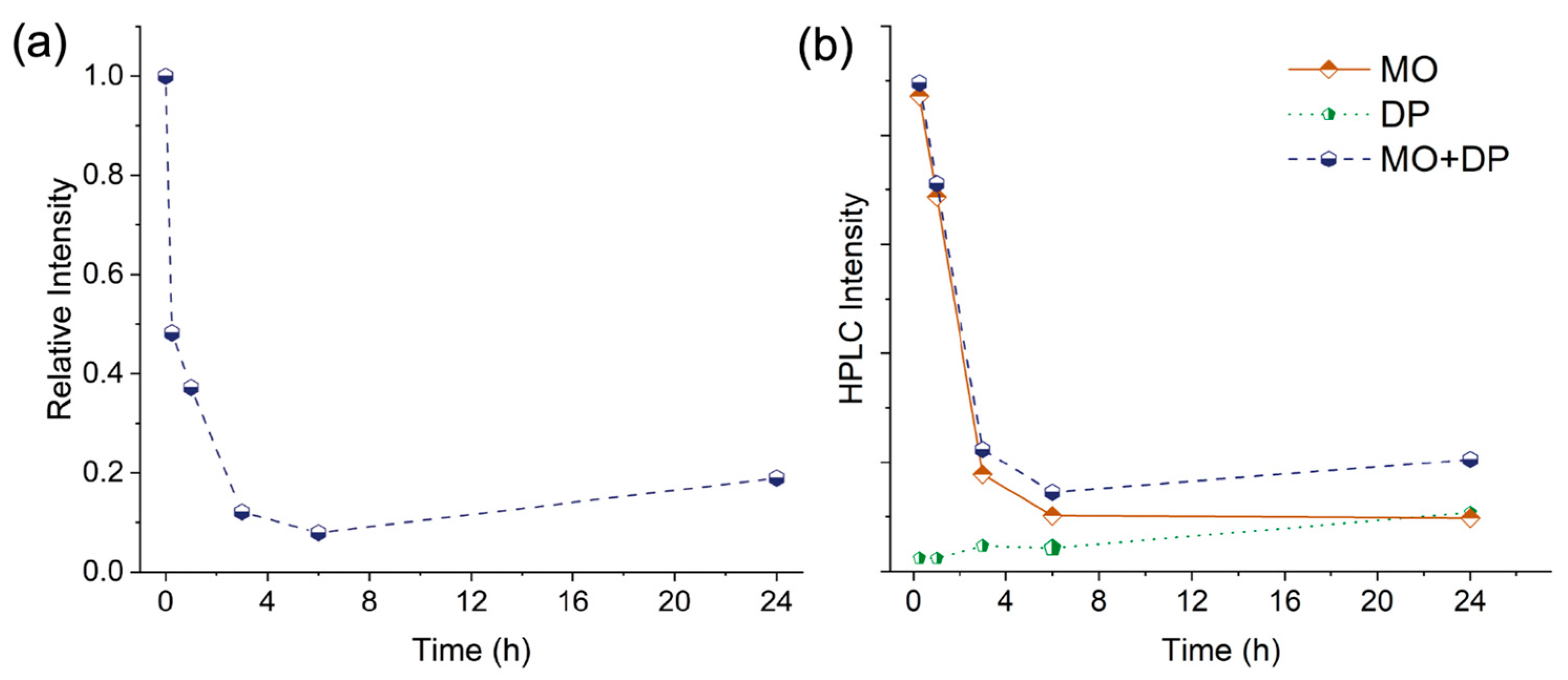
| Immersion Time (h) | Residual MO (%) | Recovered MO (%) |
|---|---|---|
| 0.25 | 48 | 98 |
| 1 | 37 | 95 |
| 3 | 10 | 87 |
| 6 | 6 | 79 |
| 24 | 5 | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinna, A.; Pia, G.; Melis, N.; Prato, M.; Cutrufello, M.G.; Sogne, E.; Falqui, A.; Pilia, L. Nanoporous Au Behavior in Methyl Orange Solutions. Molecules 2024, 29, 1950. https://doi.org/10.3390/molecules29091950
Pinna A, Pia G, Melis N, Prato M, Cutrufello MG, Sogne E, Falqui A, Pilia L. Nanoporous Au Behavior in Methyl Orange Solutions. Molecules. 2024; 29(9):1950. https://doi.org/10.3390/molecules29091950
Chicago/Turabian StylePinna, Andrea, Giorgio Pia, Nicola Melis, Mirko Prato, Maria Giorgia Cutrufello, Elisa Sogne, Andrea Falqui, and Luca Pilia. 2024. "Nanoporous Au Behavior in Methyl Orange Solutions" Molecules 29, no. 9: 1950. https://doi.org/10.3390/molecules29091950
APA StylePinna, A., Pia, G., Melis, N., Prato, M., Cutrufello, M. G., Sogne, E., Falqui, A., & Pilia, L. (2024). Nanoporous Au Behavior in Methyl Orange Solutions. Molecules, 29(9), 1950. https://doi.org/10.3390/molecules29091950











