Splenic Elemental Composition of Breast Cancer-Suffering Rats Supplemented with Pomegranate Seed Oil and Bitter Melon Extract
Abstract
1. Introduction
2. Results
2.1. Element Content in Fodder and Dietary Supplements
2.2. Element Content in Spleen Samples
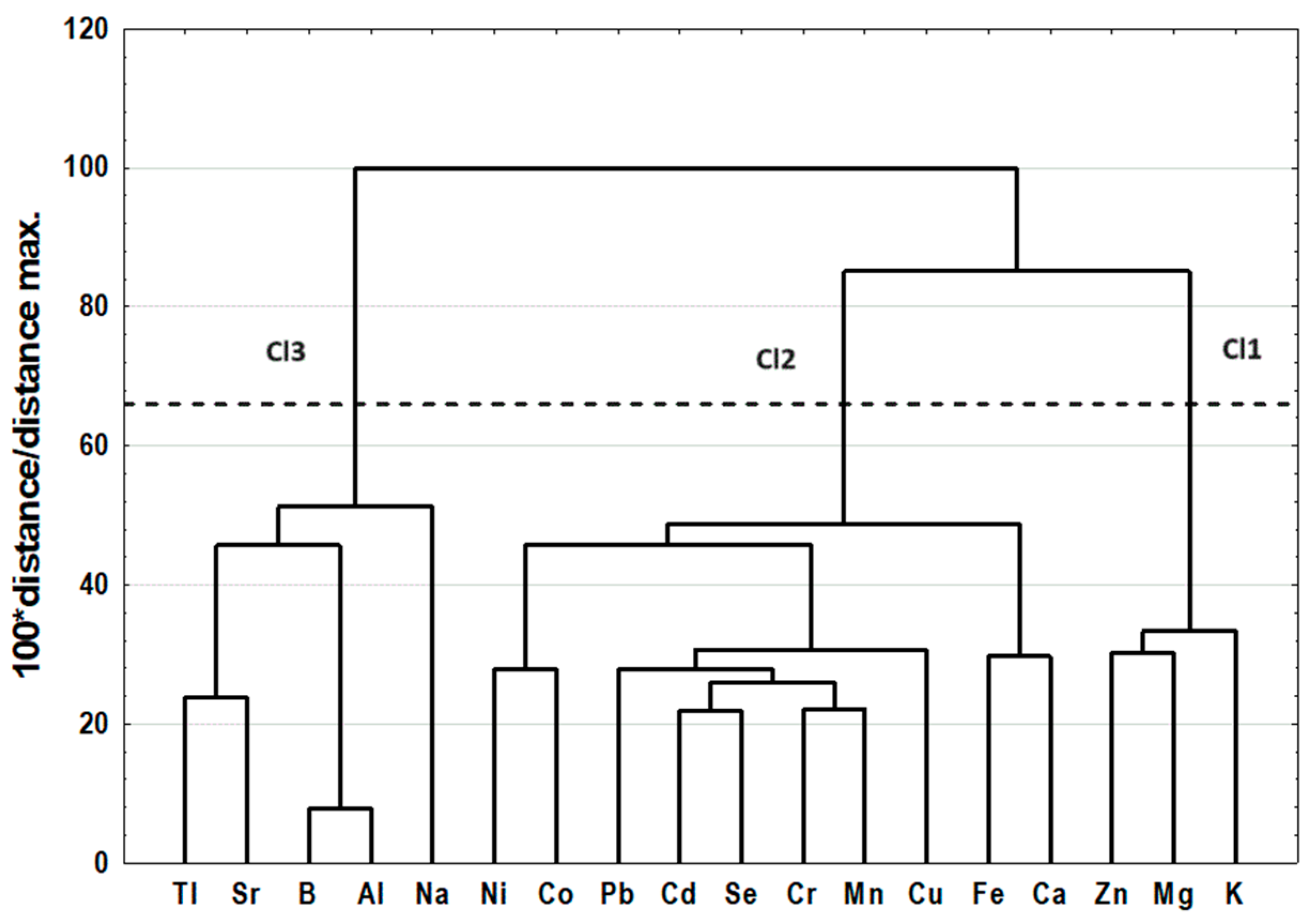
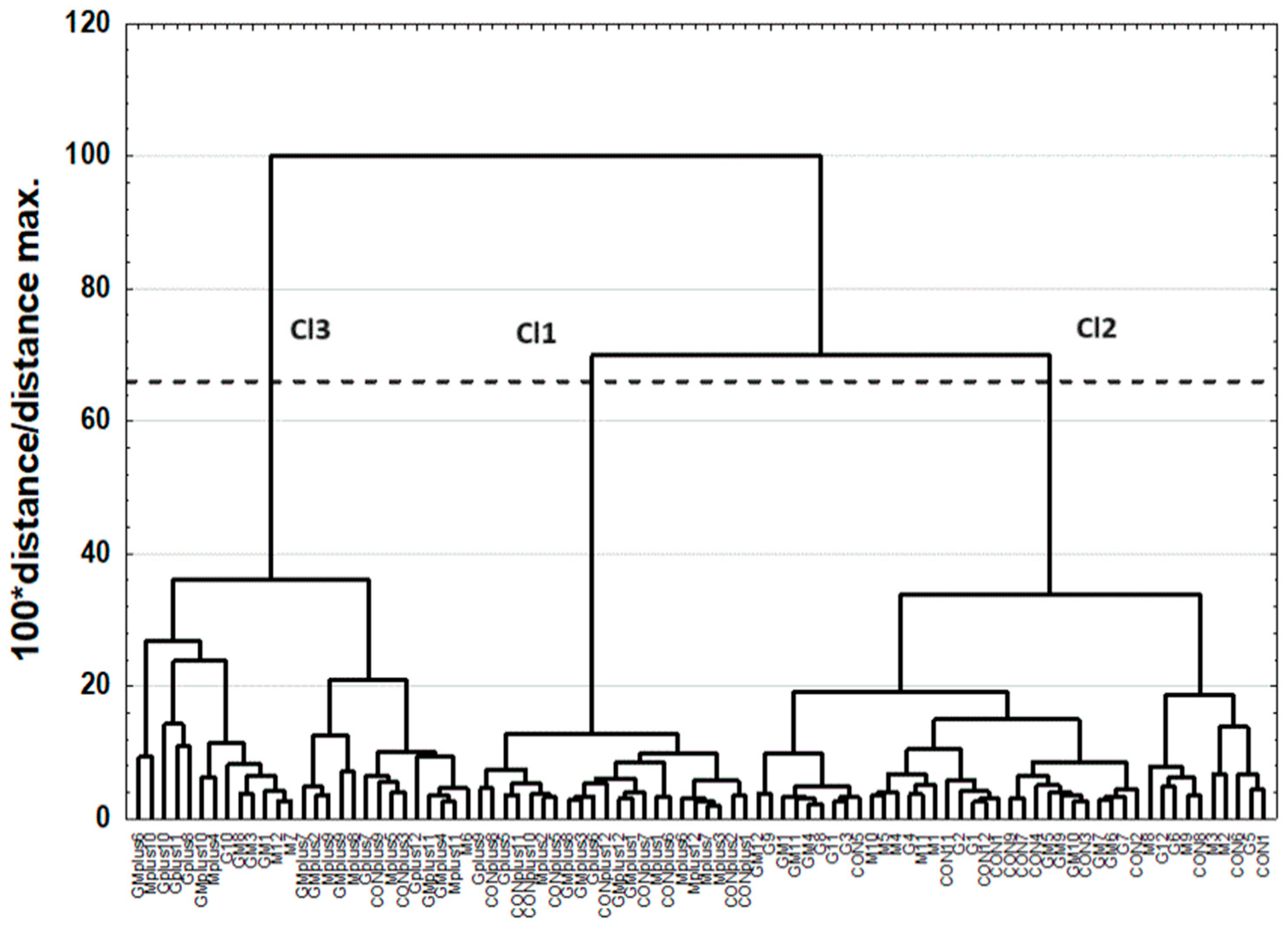
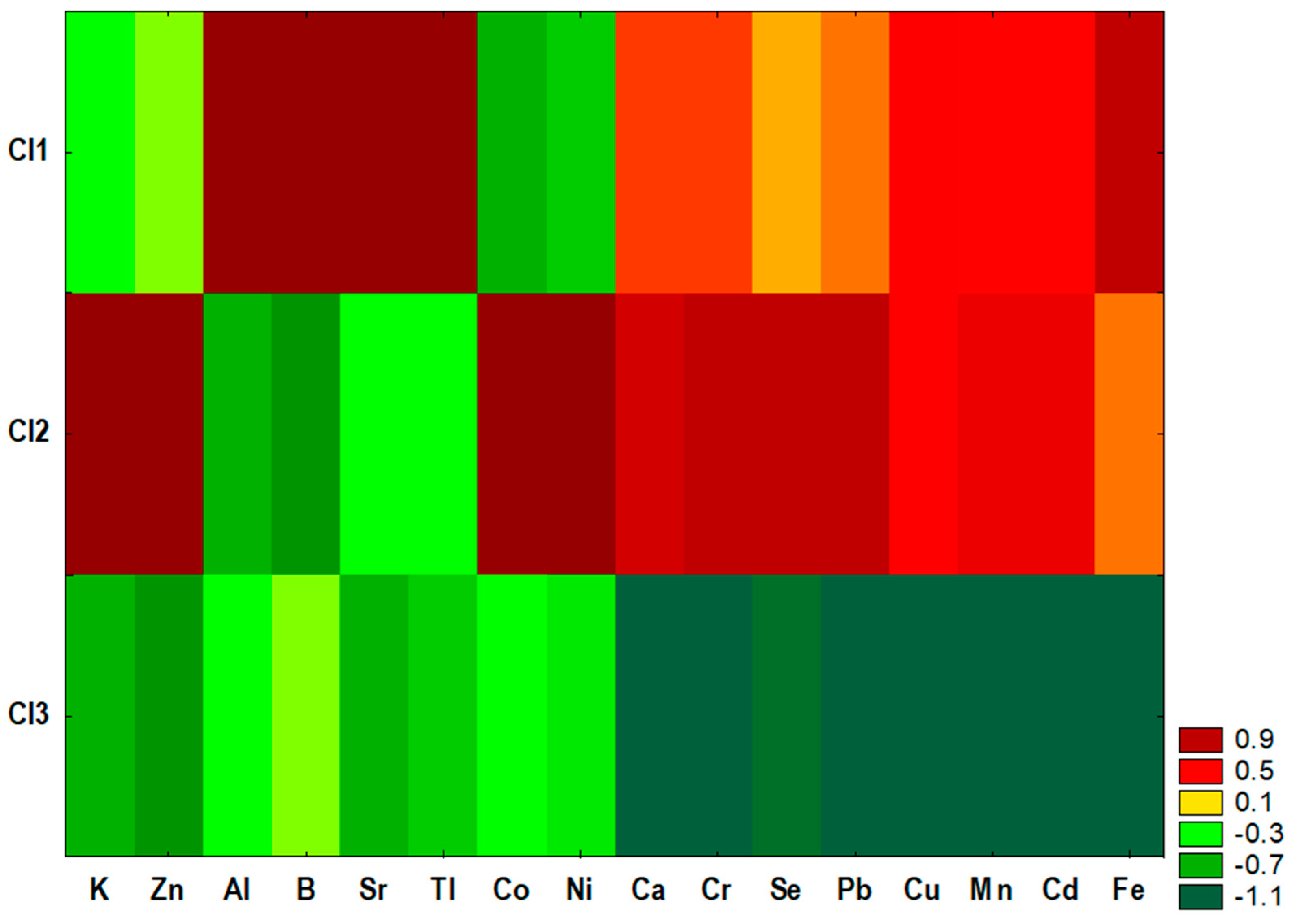
2.3. Cluster Analysis
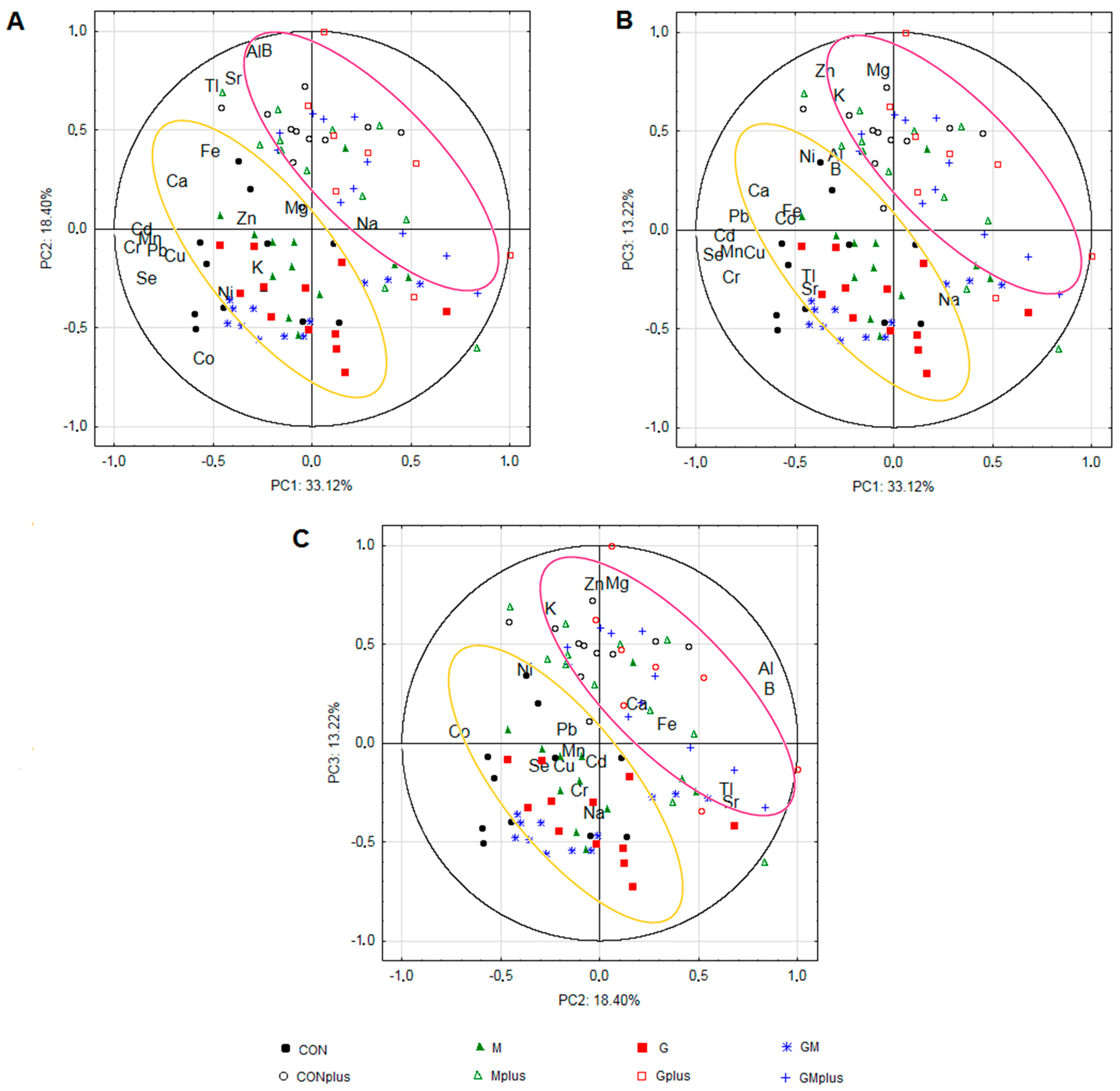
2.4. Principal Component Analysis (PCA)
2.5. Linear Discriminant Analysis (LDA)
3. Discussion
4. Materials and Methods
4.1. Dietary Ingredients
4.1.1. Bitter Melon Aqueous Extract (BME)
4.1.2. Pomegranate Seed Oil (PSO)
4.2. Animal Experiment
- -
- CON and CONplus—control groups without diet supplementation, fed a standard diet and tap water ad libitum;
- -
- M and Mplus—animals fed a standard diet supplemented with 1% BME ad libitum;
- -
- G and Gplus—animals fed a standard diet and water ad libitum and given 0.15 mL/day of PSO via gavage,
- -
- GM and GMplus—animals fed the standard diet and supplemented with both 0.15 mL/day of PSO via gavage and 1% BME ad libitum.
4.3. ICP-MS Element Determination
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catalano, O.A.; Soricelli, A.; Salvatore, M. Spleen anatomy, function and development. In Abdominal Imaging; Springer: Berlin/Heidelberg, Germany, 2013; Volume 9783642133275, pp. 1479–1494. ISBN 9783642133275. [Google Scholar]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Sajdikova, M.; Novakova, L. 5. Spleen—Functions of Cells and Human Body. Available online: https://fblt.cz/en/skripta/v-krev-a-organy-imunitniho-systemu/5-slezina/ (accessed on 22 November 2023).
- Cesta, M.F. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef]
- Nayak, B.N.; Buttar, H.S. Functions of spleen in health and disease. J. Pharm. Sci. Tech. MGMT 2015, 1, 1–7. [Google Scholar]
- Karakuchi, N.; Yanagawa, S.; Takei, D.; Kodama, S.; Takeshima, Y.; Sumimoto, K. A case of peritoneal dissemination and splenic metastasis after gastric cancer surgery that could be controlled with multidisciplinary treatment. Case Rep. Oncol. 2020, 13, 1164–1170. [Google Scholar] [CrossRef]
- Di Grande, A.; Peirs, S.; Donovan, P.D.; van Trimpont, M.; Morscio, J.; Lintermans, B.; Reunes, L.; Vandamme, N.; Goossens, S.; Nguyen, H.A.; et al. The spleen as a sanctuary site for residual leukemic cells following ABT-199 monotherapy in ETP-ALL. Blood Adv. 2021, 5, 1963–1976. [Google Scholar] [CrossRef]
- Velu, U.; Shetty, P.S.; Sharan, K.; Salins, S.; Singh, A. Spleen as an organ at risk in adjuvant chemoradiotherapy for gastric cancer: A retrospective dosimetric study. J. Radiother. Pract. 2023, 22, e57. [Google Scholar] [CrossRef]
- El Hadad, S.; Al Rowily, E.; Aldahlawi, A.; Alrahimi, J.; Hassoubah, S. The role of P53 and K-Ras in regulating spleen innate mediators in mice with colon cancer. Pharmacophore 2021, 12, 19–27. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Gridley, G.; Hoover, R.N.; Check, D.; Landgren, O. Long-term risks after splenectomy among 8149 cancer-free american veterans: A cohort study with up to 27 years follow-up. Haematologica 2014, 99, 392–398. [Google Scholar] [CrossRef]
- Noma, K.; Yamaguchi, Y.; Okita, R.; Matsuura, K.; Toge, T. The spleen plays an immunosuppressive role in patients with gastric cancer: Involvement of CD62L+ cells and TGF-Beta. Anticancer Res. 2005, 25, 645–650. [Google Scholar]
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.H.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Stöth, M.; Freire Valls, A.; Chen, M.; Hidding, S.; Knipper, K.; Shen, Y.; Klose, J.; Ulrich, A.; Ruiz de Almodovar, C.; Schneider, M.; et al. Splenectomy reduces lung metastases and tumoral and metastatic niche inflammation. Int. J. Cancer 2019, 145, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Jelińska, M.; Białek, M.; Lepionka, T.; Czerwonka, M.; Czauderna, M. The effect of diet supplementation with pomegranate and bitter melon on lipidomic profile of serum and cancerous tissues of rats with mammary tumours. Antioxidants 2020, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Leischner, C.; Helling, T.; Renner, O.; Burkard, M.; Marongiu, L. Minerals and cancer: Overview of the possible diagnostic value. Cancers 2022, 14, 1256. [Google Scholar] [CrossRef]
- Ames, B.N.; Wakimoto, P. Are vitamin and mineral deficiencies a major cancer risk? Nat. Rev. Cancer 2002, 2, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Velicer, C.M.; Ulrich, C.M. Vitamin and mineral supplement use among us adults after cancer diagnosis: A systematic review. J. Clin. Oncol. 2008, 26, 665–673. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Caballero, B.; Chang, S.; Alberg, A.J.; Semba, R.D.; Schneyer, C.R.; Wilson, R.F.; Cheng, T.-Y.; Vassy, J.; Prokopowicz, G.; et al. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: A systematic review for a national institutes of health state-of-the-science conference. Ann. Intern. Med. 2006, 145, 372–385. [Google Scholar] [CrossRef]
- Lepionka, T.; Białek, M.; Czauderna, M.; Białek, A. Pomegranate seed oil and bitter melon extract supplemented in diet influence the lipid profile and intensity of peroxidation in livers of SPRD rats exposed to a chemical carcinogen. Prostaglandins Other Lipid Mediat. 2021, 152, 106495. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Białek, M.; Lepionka, T.; Pachniewicz, P.; Czauderna, M. Oxysterols and lipidomic profile of myocardium of rats supplemented with pomegranate seed oil and/or bitter melon aqueous extract—Cardio-oncological animal model research. Chem. Phys. Lipids 2021, 235, 105057. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Białek, M.; Lepionka, T.; Ruszczyńska, A.; Bulska, E.; Czauderna, M. Cancer influences the elemental composition of the myocardium more strongly than conjugated linoleic acids-chemometric approach to cardio-oncological studies. Molecules 2021, 26, 7127. [Google Scholar] [CrossRef]
- Lepionka, T.; Białek, A.; Białek, M.; Czauderna, M.; Stawarska, A.; Wrzesień, R.; Bielecki, W.; Paśko, P.; Galanty, A.; Bobrowska-Korczak, B. Mammary cancer risk and serum lipid profile of rats supplemented with pomegranate seed oil and bitter melon extract. Prostaglandins Other Lipid Mediat. 2019, 142, 33–45. [Google Scholar] [CrossRef]
- Białek, M.; Białek, A.; Lepionka, T.; Paśko, P.; Galanty, A.; Tokarz, A.; Czauderna, M. Punica granatum (pomegranate) seed oil and Momordica charantia (bitter melon) extract affect the lipid’s profile and oxidative stability of femoral muscles of rats. Eur. J. Lipid Sci. Technol. 2019, 121, 1800420. [Google Scholar] [CrossRef]
- Amens, J.N.; Bahçecioglu, G.; Zorlutuna, P. Immune system effects on breast cancer. Cell. Mol. Bioeng. 2021, 14, 279–292. [Google Scholar] [CrossRef]
- Hao, S.; Fang, H.; Fang, S.; Zhang, T.; Zhang, L.; Yang, L. Changes in nuclear factor kappa b components expression in the ovine spleen during early pregnancy. J. Anim. Feed Sci. 2022, 31, 3–11. [Google Scholar] [CrossRef]
- Goff, S.L.; Danforth, D.N. The role of immune cells in breast tissue and immunotherapy for the treatment of breast cancer. Clin. Breast Cancer 2021, 21, e63–e73. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Cavallo, F.; Defilippi, P.; Conti, L. The crosstalk between tumor cells and the immune microenvironment in breast cancer: Implications for immunotherapy. Front. Oncol. 2021, 11, 610303. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, M.; Sun, J.; Li, X.; Shi, H.; Wang, X.; Liu, B.; Zhang, T.; Jiang, X.; Lin, L.; et al. Glycolytic neutrophils accrued in the spleen compromise anti-tumour T cell immunity in breast cancer. Nat. Metab. 2023, 5, 1408–1422. [Google Scholar] [CrossRef]
- Okuda, S.; Kubo, C.; Taniguchi, K.; Nomoto, K. Different susceptibility of various immune reactions to suppressive effect in the tumor-bearing state. Cancer Immunol. Immunother 1980, 9, 87–92. [Google Scholar] [CrossRef]
- Merdrignac, G.; Haddada, H.; De Vaux Saint Cyr, C.; Genetet, B. Spleen cell populations in normal and tumor-bearing hamsters. Immunol. Lett. 1985, 10, 81–86. [Google Scholar] [CrossRef]
- Yaqoob, P.; Calder, P.C. The effects of fatty acids on lymphocyte functions. Int. J. Biochem. 1993, 25, 1705–1714. [Google Scholar] [CrossRef]
- Jeffery, N.M.; Newsholme, E.A.; Calder, P.C. Level of polyunsaturated fatty acids and the n-6 to n-3 polyunsaturated fatty acid ratio in the rat diet alter serum lipid levels and lymphocyte functions. Prostaglandins Leukot. Essent. Fat. Acids 1997, 57, 149–160. [Google Scholar] [CrossRef]
- Peterson, L.; Jeffery, N.; Thies, F.; Sanderson, P.; Newsholme, E.; Calder, P. Eicosapentaenoic and docosahexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin e2 production but have different effect on lymphocyte functions and cell-mediated immunity. Lipids 1998, 33, 171–180. [Google Scholar] [CrossRef]
- Yamasaki, M.; Chujo, H.; Hirao, A.; Koyanagi, N.; Okamoto, T.; Tojo, N.; Oishi, A.; Iwata, T.; Yamauchi-Sato, Y.; Yamamoto, T.; et al. Immunoglobulin and cytokine production from spleen lymphocytes is modulated in C57BL/6J mice by dietary Cis-9, Trans-11 and Trans-10, Cis-12 conjugated linoleic acid. J. Nutr. 2003, 133, 784–788. [Google Scholar] [CrossRef]
- Lepionka, T.; Białek, M.; Czauderna, M.; Wojtak, W.; Maculewicz, E.; Białek, A. Exploring the influence of the selected conjugated fatty acids isomers and cancerous process on the fatty acids profile of spleen. Cancers 2024, 16, 479. [Google Scholar] [CrossRef]
- Saied, A.M.; Attia, A.I.; El-Kholy, M.S.; Reda, F.M.; EL Nagar, A.G. Effect of cinnamon oil supplementation into broiler chicken diets on growth, carcass traits, haemato-biochemical parameters, immune function, antioxidant status and caecal microbial count. J. Anim. Feed Sci. 2022, 31, 21–33. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Q.; Hou, J.; Gu, Y.; Zhang, Y.; Chen, Z.; Fan, J.; Zhou, W.; Qiu, S.; Zhang, Y.; et al. Tumor-induced generation of splenic erythroblast-like ter-cells promotes tumor progression. Cell 2018, 173, 634–648.e12. [Google Scholar] [CrossRef]
- Seban, R.D.; Rouzier, R.; Latouche, A.; Deleval, N.; Guinebretiere, J.M.; Buvat, I.; Bidard, F.C.; Champion, L. Total metabolic tumor volume and spleen metabolism on baseline [18F]-FDG PET/CT as independent prognostic biomarkers of recurrence in resected breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3560–3570. [Google Scholar] [CrossRef]
- Zaksas, N.P.; Nevinsky, G.A. Minor and trace elements in whole blood, tissues, proteins and immunoglobulins of mammals. In Trace Elements—Human Health and Environment; InTech: Houston, TX, USA, 2018. [Google Scholar]
- Wan, X.L.; Zheng, X.C.; Liang, J.R.; Xiao, X.; Yang, H.M.; Wang, Z.Y. Dietary vitamin a supplementation improves intestinal morphology and immune performance of goslings. J. Anim. Feed Sci. 2022, 31, 217–223. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, L.; Liu, P.; Ma, W.; Liu, Y.; Qiao, S.; Liu, Q.; Cai, J.; Zhang, Z. Editorial: The mechanism of trace elements on regulating immunity in prevention and control of human and animal diseases. Front. Immunol. 2023, 14, 1159289. [Google Scholar] [CrossRef]
- Failla, M.L. Trace elements and host defense: Recent advances and continuing challenges. J. Nutr. 2003, 133, 1443S–1447S. [Google Scholar] [CrossRef]
- Fernández-García, V.; González-Ramos, S.; Martín-Sanz, P.; Castrillo, A.; Boscá, L. Contribution of Extramedullary Hematopoiesis to Atherosclerosis. The spleen as a neglected hub of inflammatory cells. Front. Immunol. 2020, 11, 586527. [Google Scholar] [CrossRef]
- Saha, S.S.; Ghosh, M. Antioxidant effect of vegetable oils containing conjugated linolenic acid isomers against induced tissue lipid peroxidation and inflammation in rat model. Chem. Biol. Interact. 2011, 190, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hua, Q.; Zheng, L. Generation of myeloid cells in cancer: The spleen matters. Front. Immunol. 2020, 11, 1126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Xia, K.; Dai, J.; Huang, J.; Wang, Y.; Zhu, G.; Hu, Z.; Zeng, Z.; Jia, Y. Effects of dietary selenium on immune function of spleen in mice. J. Funct. Foods 2022, 89, 104914. [Google Scholar] [CrossRef]
- Moussaron, A.; Alexandre, J.; Chenard, M.P.; Mathelin, C.; Reix, N. Correlation between daily life aluminium exposure and breast cancer risk: A systematic review. J. Trace Elem. Med. Biol. 2023, 79, 127247. [Google Scholar] [CrossRef] [PubMed]
- Van Gerwen, M.; Alerte, E.; Alsen, M.; Little, C.; Sinclair, C.; Genden, E. The role of heavy metals in thyroid cancer: A meta-analysis. J. Trace Elem. Med. Biol. 2022, 69, 126900. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Thallium use, toxicity, and detoxification therapy: An overview. Appl. Sci. 2021, 11, 8322. [Google Scholar] [CrossRef]
- Chen, L.J.; Tang, L.Y.; He, J.R.; Su, Y.; Cen, Y.L.; Yu, D.D.; Wu, B.H.; Lin, Y.; Chen, W.Q.; Song, E.W.; et al. Urinary strontium and the risk of breast cancer: A case-control study in Guangzhou, China. Environ. Res. 2012, 112, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Białek, M.; Czauderna, M.; Krajewska, K.A.; Przybylski, W. Selected physiological effects of boron compounds for animals and humans. a review. J. Anim. Feed Sci. 2019, 28, 307–320. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Currie, L.A. Nomenclature in evaluation of analytical methods, including detect ion and quantification capabilities (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
| Labofeed H | PSO | Bitter Melon (Dry Matter) | BME (Fluid) | |
|---|---|---|---|---|
| mg/kg | µg/L | |||
| Macroelements | ||||
| K | 18,570 | 13.66 | 28,896 | 524,761 |
| Mg | 2930 | <LOQ | 2913 | 38,942 |
| Na | 2416 | <LOD | 180 | 14,018 |
| Ca | 7686 | <LOD | 1896 | 26,924 |
| Microelements | ||||
| Fe | 225.9 | 4.13 | 160.3 | 263.4 |
| Zn | 34.28 | <LOD | 15.61 | 399.3 |
| Cu | 9.88 | <LOD | 6.79 | 80.32 |
| Mn | 43.35 | <LOD | 239.8 | 1741 |
| Se | 0.16 | 0.05 | 0.11 | <LOD |
| Co | 0.23 | <LOD | 0.16 | 1.78 |
| Cr | 0.29 | 0.56 | 1.81 | 22.18 |
| Ni | 1.02 | <LOD | 2.61 | 47.25 |
| Al | 149.6 | <LOD | 527.1 | 3427 |
| Sr | 10.74 | <LOD | 17.0 | 120.8 |
| Pb | <LOD | <LOD | 1.41 | 6.90 |
| Cd | <LOD | <LOD | 0.06 | <LOD |
| B | <LOD | <LOD | <LOQ | 212.8 |
| Tl | <LOQ | <LOD | <LOD | <LOD |
| CON | M | G | GM | CONplus | Mplus | Gplus | GMplus | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Macroelements | |||||||||
| K [mg/kg] | 5206 ± 168 | 5098 ± 277 | 5102 ± 274 | 5091 ± 189 | 4908 ± 587 | 5024 ± 597 | 4269 ± 870 | 5091 ± 273 | 0.0815 |
| Mg [mg/kg] | 255.2 ± 22.9 | 244.7 ± 18.9 | 240.2 ± 32.3 | 232.1 ± 17.2 | 244.3 ± 26.2 | 257.3 ± 29.8 | 223.1 ± 36.5 | 258.4 ± 20.0 | 0.0276 |
| Na [mg/kg] | 19.53 ± 10.74 | 11.49 ± 5.87 a,b | 16.98 ± 10.48 | 17.65 ± 5.05 | 26.07 ± 10.16 a | 17.43 ± 11.29 | 51.60 (11.33–235.0) b | 13.85 ± 9.25 | 0.0048 |
| Ca [mg/kg] | 188.2 ± 38.0 a,b | 166.9 ± 37.9 c | 169.3 ± 30.9 d | 176.9 ± 28.5 e | 191.3 ± 25.0 f,g | 149.9 ± 35.4 | 128.9 ± 57.2 a,g | 115.6 ± 21.5 b,c,d,e,f | <0.0001 * |
| Microelements | |||||||||
| Fe [mg/kg] | 3939 ± 783 a,b | 3476 ± 478 | 2885 ± 496 | 3044 ± 155 | 3665 ± 561 c | 3925 ± 1734 d,e | 2420 ± 818 a,d | 2130 ± 1152 b,c,e | <0.0001 |
| Zn [mg/kg] | 22.98 ± 2.71 | 26.31 ± 7.89 | 22.91 ± 4.21 | 21.26 ± 2.09 | 21.27 ± 2.24 | 22.19 ± 1.67 | 20.34 ± 3.71 | 21.79 ± 0.91 | 0.2763 |
| Cu [mg/kg] | 3.38 ± 0.76 | 2.82 ± 0.77 | 2.86 ± 0.64 | 2.96 ± 0.71 | 2.98 ± 0.66 | 2.85 ± 0.49 | 2.45 ± 0.59 | 2.84 ± 0.46 | 0.1581 * |
| Mn [mg/kg] | 0.59 ± 0.11 | 0.53 ± 0.11 | 0.52 ± 0.11 | 0.55 ± 0.15 | 0.50 ± 0.08 | 0.51 ± 0.14 | 0.42 ± 0.10 | 0.50 ± 0.18 | 0.1937 |
| Se [mg/kg] | 0.73 ± 0.06 a,b,c,d | 0.63 ± 0.09 | 0.67 ± 0.12 e | 0.68 ± 0.11 f | 0.58 ± 0.10 a | 0.57 ± 0.14 b | 0.48 ± 0.09 c,d,f | 0.54 ± 0.07 d | <0.0001 |
| Co [mg/kg] | 0.054 ± 0.010 a,b,c,d | 0.046 ± 0.010 e,f | 0.059 ± 0.016 g,h,i,j | 0.064 ± 0.016 k,l,m,n | 0.010 ± 0.001 a,e,g,k | 0.010 ± 0.003 b,h,l | 0.009 ± 0.002 c,f,i,m | 0.010 ± 0.004 d,j,n | <0.0001 |
| Cr [mg/kg] | 0.51 ± 0.15 a,b | 0.38 ± 0.08 | 0.46 ± 0.10 | 0.50 ± 0.10 | 0.40 ± 0.07 | 0.36 ± 0.10 a | 0.39 ± 0.09 | 0.36 ± 0.11 b | 0.0019* |
| Ni [mg/kg] | 0.16 ± 0.06 a,b,c | 0.17 ± 0.14 d,e | 0.14 ± 0.12 f,g | 0.11 ± 0.08 | 0.03 ± 0.01 a,d,f | 0.05 ± 0.02 b | 0.09 ± 0.06 | 0.03 ± 0.01 c,e,g | <0.0001 |
| Al [mg/kg] | 49.33 ± 9.19 | 52.13 ± 10.33 | 42.02 ± 7.53 a | 41.29 ± 3.22 b,c | 57.80 ± 6.41 a,b | 51.87 ± 11.71 | 55.44 ± 16.06 | 53.82 ± 10.04 c | 0.0003 |
| Sr [mg/kg] | 0.20 ± 0.04 | 0.17 ± 0.02 a,b | 0.18 ± 0.06 c | 0.17 ± 0.04 d | 0.28 ± 0.07 a,c,d | 0.24 ± 0.07 | 0.25 ± 0.06 b | 0.21 ± 0.06 | <0.0001 |
| Pb [mg/kg] | 0.18 ± 0.05 a,b | 0.16 ± 0.05 | 0.14 ± 0.03 | 0.16 ± 0.06 | 0.14 ± 0.02 | 0.12 ± 0.03 a | 0.13 ± 0.03 | 0.11 ± 0.03 b | 0.0024 |
| Cd [μg/kg] | 3.76 ± 0.69 a,b,c | 2.77 ± 0.71 | 2.98 ± 0.74 | 3.27 ± 0.68 d,e | 3.02 ± 0.88 | 2.62 ± 1.11 a | 2.02 ± 0.53 b,d | 2.08 ± 0.74 c,e | <0.0001 * |
| B [mg/kg] | 72.34 ± 13.39 | 77.61 ± 15.63 | 64.27 ± 12.38 a | 63.74 ± 4.96 b | 87.17 ± 9.56 a,b | 76.22 ± 18.79 | 82.65 ± 24.57 | 83.47 ± 17.62 | 0.0018 |
| Tl [μg/kg] | 9.59 ± 2.82 | 6.88 ± 1.53 a,b,c | 8.03 ± 3.14 d | 7.46 ± 2.25 e,f | 11.99 ± 2.59 a,d | 10.85 ± 3.40 b,e | 9.37 ± 3.08 | 11.76 ± 3.99 c,f | <0.0001 * |
| Cl1 | Cl2 | Cl3 | p-Value | |
|---|---|---|---|---|
| Macroelements | ||||
| K (mg/kg) | 4919 ± 417 | 5152 ± 229 a | 4849 ± 724 a | 0.0255 |
| Mg (mg/kg) | 246.2 ± 22.0 | 245.9 ± 23.9 | 243.4 ± 35.3 | 0.9160 |
| Na (mg/kg) | 23.20 ± 10.86 | 16.75 ± 9.26 | 55.19 ± 142.96 | 0.1345 |
| Ca (mg/kg) | 168.0 ± 32.1 a | 178.5 ± 35.5 b | 133.6 ± 43.2 a,b | <0.0001 |
| Microelements | ||||
| Fe (mg/kg) | 3732 ± 1053 a | 3376 ± 691 b | 2586 ± 1263 a,b | 0.0002 |
| Zn (mg/kg) | 21.75 ± 1.72 | 23.91 ± 5.14 a | 20.93 ± 2.56 a | 0.0061 |
| Cu (mg/kg) | 3.17 ± 0.34 a | 3.20 ± 0.60 b | 2.26 ± 0.48 a,b | 0.0000 |
| Mn (mg/kg) | 0.56 ± 0.07 a | 0.58 ± 0.09 b | 0.39 ± 0.12 a,b | <0.0001 |
| Se (mg/kg) | 0.62 ± 0.07 a,b | 0.71 ± 0.07 a,c | 0.48 ± 0.08 b,c | <0.0001 |
| Co (mg/kg) | 0.011 ± 0.002 a | 0.059 ± 0.013 a,b | 0.016 ± 0.014 b | <0.0001 |
| Cr (mg/kg) | 0.44 ± 0.06 a | 0.48 ± 0.11 b | 0.31 ± 0.08 a,b | <0.0001 |
| Ni (mg/kg) | 0.04 ± 0.02 a | 0.16 ± 0.10 a,b | 0.05 ± 0.04 b | <0.0001 |
| Al (mg/kg) | 57.93 ± 7.00 a,b | 46.68 ± 8.92 a | 49.00 ± 12.93 b | 0.0002 |
| Sr (mg/kg) | 0.29 ± 0.04 a,b | 0.19 ± 0.03 a | 0.18 ± 0.06 b | <0.0001 |
| Pb (mg/kg) | 0.15 ± 0.02 a,b | 0.17 ± 0.05 a,c | 0.10 ± 0.02 b,c | <0.0001 |
| Cd (μg/kg) | 3.12 ± 0.70 a | 3.36 ± 0.70 b | 1.87 ± 0.53 a,b | <0.0001 |
| B (mg/kg) | 87.99 ± 10.94 a,b | 69.55 ± 12.66 a | 74.11 ± 20.41 b | 0.0001 |
| Tl (μg/kg) | 13.23 ± 2.37 a,b | 8.40 ± 2.52 a | 7.93 ± 2.82 b | <0.0001 |
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| K | −0.278 | −0.249 | 0.624 |
| Mg | −0.080 | 0.052 | 0.751 |
| Na | 0.279 | −0.029 | −0.408 |
| Ca | −0.682 | 0.186 | 0.144 |
| Fe | −0.517 | 0.338 | 0.042 |
| Zn | −0.348 | −0.028 | 0.751 |
| Cu | −0.702 | −0.178 | −0.170 |
| Mn | −0.818 | −0.103 | −0.162 |
| Se | −0.840 | −0.306 | −0.171 |
| Co | −0.550 | −0.709 | 0.002 |
| Cr | −0.824 | −0.104 | −0.293 |
| Ni | −0.435 | −0.379 | 0.315 |
| Al | −0.293 | 0.841 | 0.321 |
| Sr | −0.429 | 0.660 | −0.353 |
| Pb | −0.785 | −0.165 | 0.016 |
| Cd | −0.861 | −0.059 | −0.157 |
| B | −0.292 | 0.849 | 0.252 |
| Tl | −0.433 | 0.636 | −0.288 |
| Eigenvalue | 11.654 | ||
| Variance (%) | 33.12 | 18.40 | 13.22 |
| Cumulative (%) | 64.7 | ||
| Coefficients of Canonical Variables | |||||||
|---|---|---|---|---|---|---|---|
| DF1 | DF2 | DF3 | DF4 | DF5 | DF6 | DF7 | |
| Variables | 93.63% | 2.40% | 1.76% | 1.16% | 0.59% | 0.36% | 0.08% |
| Co | 6.96363 | 1.09279 | 0.128744 | 0.21518 | 0.23443 | 0.147156 | 0.88032 |
| Sr | −1.04424 | −0.29692 | 0.634247 | −0.28200 | −0.37835 | −0.702481 | 1.35566 |
| Mn | −0.93829 | 0.55813 | −0.890473 | 0.24603 | −0.52714 | 0.020905 | −1.34120 |
| Ni | 0.40805 | 0.19431 | 0.391771 | −0.99295 | 0.69071 | −0.294469 | 0.41979 |
| Ca | 0.70833 | −1.38438 | −0.594031 | −0.14685 | −0.87512 | 0.201752 | 0.05789 |
| B | −0.00917 | 2.01984 | −0.940549 | 1.15620 | −1.14442 | 0.269535 | 1.01068 |
| Cr | −0.16064 | −0.54411 | 1.663446 | 0.62522 | 0.19558 | −0.226185 | −0.76844 |
| Fe | 0.04448 | −0.33491 | −0.557203 | −0.84495 | 0.07122 | −0.695610 | −0.56046 |
| Cu | −0.12231 | 0.25923 | −0.959160 | −0.87929 | −0.10049 | 1.019662 | −0.23898 |
| Na | 0.22432 | −0.59006 | 0.533600 | 0.10718 | −0.00830 | 0.232868 | −0.08378 |
| Mg | 0.21959 | −0.74660 | 0.613614 | 0.37265 | 1.15938 | −0.062023 | 0.00759 |
| Tl | −0.16485 | −0.25790 | −0.142177 | 0.60750 | 0.79269 | −0.904604 | 0.74983 |
| K | 0.14740 | 0.16593 | −0.606916 | 0.08094 | 0.09168 | −0.231532 | 0.32261 |
| Cd | −0.00744 | −0.65129 | 0.197402 | 0.67524 | 0.65959 | 0.789825 | 0.55133 |
| Zn | −0.16766 | 0.00680 | −0.084258 | 0.22020 | −0.88783 | −0.739514 | 0.33817 |
| Al | −0.87280 | −0.09641 | 0.495011 | −1.72349 | 1.33040 | 1.441113 | −1.30752 |
| Average Values of Canonical Variables | |||||||
| CON | 6.33793 | −0.75139 | −0.01459 | −0.53807 | 1.250980 | 0.412474 | 0.040574 |
| M | 5.42223 | 1.80042 | −1.07040 | −1.20665 | −0.517138 | 0.096160 | −0.017901 |
| G | 7.95468 | −0.21525 | 0.46986 | 0.54690 | −0.207331 | −0.634582 | 0.385911 |
| GM | 8.61790 | −0.55439 | 0.44453 | 0.95220 | −0.471548 | 0.216111 | −0.379717 |
| CONplus | −8.32973 | −1.72443 | −0.71386 | −0.03044 | −0.658549 | 0.593416 | 0.203602 |
| Mplus | −7.50768 | −0.87100 | −0.70078 | −0.40368 | 0.233689 | −0.844036 | −0.233464 |
| Gplus | −8.18277 | 0.66246 | 2.88315 | −0.90017 | −0.162521 | 0.089866 | −0.024297 |
| GMplus | −8.43741 | 1.88802 | −0.43238 | 1.39343 | 0.461853 | 0.163634 | 0.037266 |
| Predicted Group Membership | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Actual Group | Correct Classification | CON | M | G | GM | CONplus | Mplus | Gplus | GMplus |
| CON | 83.33% | 10 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| M | 100.00% | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 |
| G | 58.33% | 1 | 0 | 7 | 4 | 0 | 0 | 0 | 0 |
| GM | 83.33% | 0 | 0 | 2 | 10 | 0 | 0 | 0 | 0 |
| CONplus | 90.91% | 0 | 0 | 0 | 0 | 10 | 1 | 0 | 0 |
| Mplus | 91.67% | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 1 |
| Gplus | 75.00% | 0 | 0 | 0 | 0 | 1 | 1 | 6 | 0 |
| GMplus | 90.91% | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 10 |
| Total | 84.44% | 11 | 12 | 11 | 14 | 11 | 14 | 6 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białek, M.; Lepionka, T.; Wojtak, W.; Ruszczyńska, A.; Bulska, E.; Czauderna, M.; Białek, A. Splenic Elemental Composition of Breast Cancer-Suffering Rats Supplemented with Pomegranate Seed Oil and Bitter Melon Extract. Molecules 2024, 29, 1942. https://doi.org/10.3390/molecules29091942
Białek M, Lepionka T, Wojtak W, Ruszczyńska A, Bulska E, Czauderna M, Białek A. Splenic Elemental Composition of Breast Cancer-Suffering Rats Supplemented with Pomegranate Seed Oil and Bitter Melon Extract. Molecules. 2024; 29(9):1942. https://doi.org/10.3390/molecules29091942
Chicago/Turabian StyleBiałek, Małgorzata, Tomasz Lepionka, Wiktoria Wojtak, Anna Ruszczyńska, Ewa Bulska, Marian Czauderna, and Agnieszka Białek. 2024. "Splenic Elemental Composition of Breast Cancer-Suffering Rats Supplemented with Pomegranate Seed Oil and Bitter Melon Extract" Molecules 29, no. 9: 1942. https://doi.org/10.3390/molecules29091942
APA StyleBiałek, M., Lepionka, T., Wojtak, W., Ruszczyńska, A., Bulska, E., Czauderna, M., & Białek, A. (2024). Splenic Elemental Composition of Breast Cancer-Suffering Rats Supplemented with Pomegranate Seed Oil and Bitter Melon Extract. Molecules, 29(9), 1942. https://doi.org/10.3390/molecules29091942








