Research Progress on the Mechanism of the Antitumor Effects of Cannabidiol
Abstract
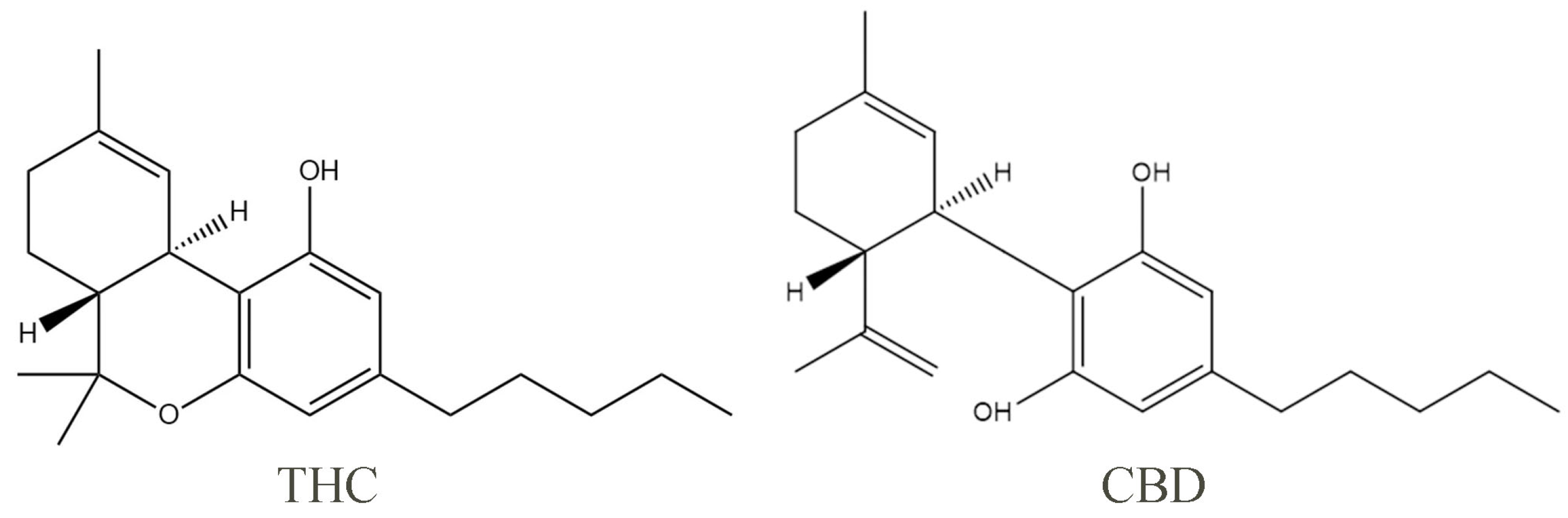
1. Introduction
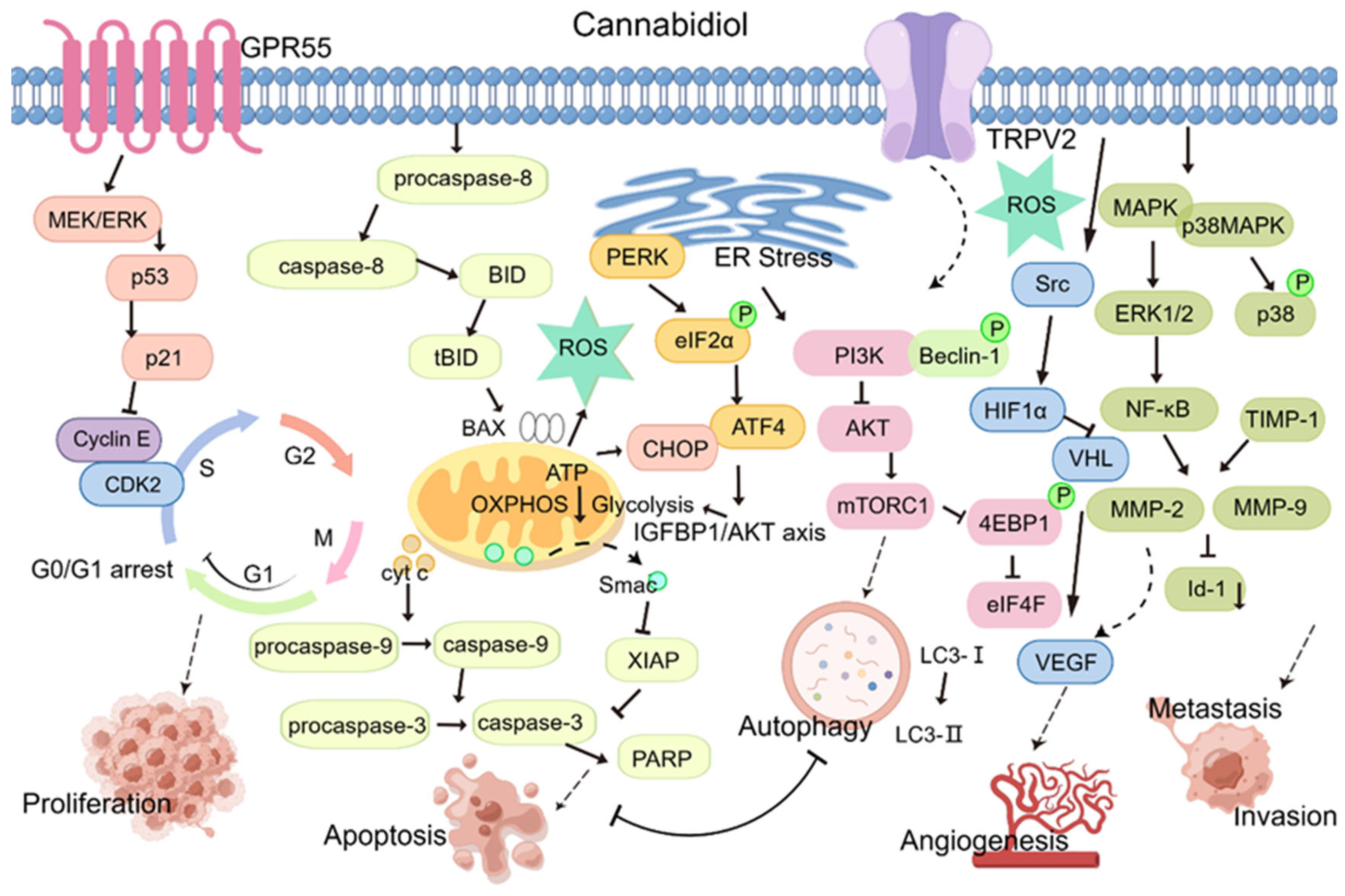
2. Antitumor Mechanisms of CBD
2.1. CBD Regulates the Tumor Cell Cycle
2.2. Multiple CBD-Associated Pathways Can Induce Tumor Cell Apoptosis
2.3. CBD Induces Tumor Cell Autophagy
2.4. CBD Inhibits Tumor Cell Angiogenesis
2.5. CBD Suppresses Tumor Cell Invasion and Migration
2.6. CBD Affects Energy Metabolism in Tumor Cells
3. Synergistic Sensitization of Antitumor Drugs and Reversal of Drug Resistance
4. Clinical Application for the Prevention and Treatment of Tumors
5. Summary and Prospect
Funding
Conflicts of Interest
References
- Hussain, S.A.; Zhou, R.; Jacobson, C.; Weng, J.; Cheng, E.; Lay, J.; Hung, P.; Lerner, J.T.; Sankar, R. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: A potential role for infantile spasms and lennox-gastaut syndrome. Epilepsy Behav. 2015, 47, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.M.; Quigley, H.; Quattrone, D.; Englund, A.; Di Forti, M. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: Increasing risk for psychosis. World Psychiatry 2016, 15, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, B.R.; Levin, F.R. Treatment of cannabis use disorders: A review of the literature. Am. J. Addict. 2007, 16, 331–342. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (cbd) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Hudson, R.; Renard, J.; Norris, C.; Rushlow, W.J.; Laviolette, S.R. Cannabidiol counteracts the psychotropic side-effects of δ-9-tetrahydrocannabinol in the ventral hippocampus through bidirectional control of erk1-2 phosphorylation. J. Neurosci. 2019, 39, 8762–8777. [Google Scholar] [CrossRef]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; Martín, D.L.J.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e228909. [Google Scholar] [CrossRef]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol-from plant to human body: A promising bioactive molecule with multi-target effects in cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, H.; Liu, C.; Liu, M.; Shangguan, F.; Liu, Y.; Yang, S.; Li, H.; An, J.; Song, S.; et al. A novel mechanism of cannabidiol in suppressing ovarian cancer through lair-1 mediated mitochondrial dysfunction and apoptosis. Environ. Toxicol. 2023, 38, 1118–1132. [Google Scholar] [CrossRef]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef]
- Ferro, R.; Adamska, A.; Lattanzio, R.; Mavrommati, I.; Edling, C.E.; Arifin, S.A.; Fyffe, C.A.; Sala, G.; Sacchetto, L.; Chiorino, G.; et al. Gpr55 signalling promotes proliferation of pancreatic cancer cells and tumour growth in mice, and its inhibition increases effects of gemcitabine. Oncogene 2018, 37, 6368–6382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q.; et al. Cannabidiol induces cell cycle arrest and cell apoptosis in human gastric cancer sgc-7901 cells. Biomolecules 2019, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Attardi, L.D. The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer 2005, 5, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Mckallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and nox4 expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Vaccani, A.; Bianchessi, S.; Costa, B.; Macchi, P.; Parolaro, D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell. Mol. Life Sci. 2006, 63, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Kim, B.R.; Kim, D.Y.; Jeong, Y.A.; Jeong, S.; Na, Y.J.; Park, S.H.; Yun, H.K.; Jo, M.J.; Kim, B.G.; et al. Cannabidiol enhances the therapeutic effects of TRAIL by upregulating DR5 in colorectal cancer. Cancers 2019, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Jo, M.J.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jeong, Y.A.; Kim, B.G.; et al. Cannabidiol promotes apoptosis via regulation of XIAP/Smac in gastric cancer. Cell Death Dis. 2019, 10, 846. [Google Scholar] [CrossRef]
- Sreevalsan, S.; Joseph, S.; Jutooru, I.; Chadalapaka, G.; Safe, S.H. Induction of apoptosis by cannabinoids in prostate and colon cancer cells is phosphatase dependent. Anticancer Res. 2011, 31, 3799–3807. [Google Scholar]
- Haustein, M.; Ramer, R.; Linnebacher, M.; Manda, K.; Hinz, B. Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of icam-1. Biochem. Pharmacol. 2014, 92, 312–325. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Tan, J.; Gong, Y.; Dai, H.; Chen, H.; Xu, X.; Yang, A.; Zhang, Y.; Bie, P. Microrna-216b-5p functions as a tumor-suppressive rna by targeting tpt1 in pancreatic cancer cells. J. Cancer 2017, 8, 2854–2865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alharris, E.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. Role of mirna in the regulation of cannabidiol-mediated apoptosis in neuroblastoma cells. Oncotarget 2019, 10, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Takamura, A.; Komatsu, M.; Hara, T.; Sakamoto, A.; Kishi, C.; Waguri, S.; Eishi, Y.; Hino, O.; Tanaka, K.; Mizushima, N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011, 25, 795–800. [Google Scholar] [CrossRef]
- Wei, M.F.; Chen, M.W.; Chen, K.C.; Lou, P.J.; Lin, S.Y.; Hung, S.C.; Hsiao, M.; Yao, C.J.; Shieh, M.J. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy 2014, 10, 1179–1192. [Google Scholar] [CrossRef]
- Duffy, A.; Le, J.; Sausville, E.; Emadi, A. Autophagy modulation: A target for cancer treatment development. Cancer Chemother. Pharmacol. 2015, 75, 439–447. [Google Scholar] [CrossRef]
- Koay, L.C.; Rigby, R.J.; Wright, K.L. Cannabinoid-induced autophagy regulates suppressor of cytokine signaling-3 in intestinal epithelium. Am. J. Physiol. Gastroint. Liver Physiol. 2014, 307, G140–G148. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Kim, N.Y.; Mohan, C.D.; Sethi, G.; Ahn, K.S. Cannabidiol activates mapk pathway to induce apoptosis, paraptosis, and autophagy in colorectal cancer cells. J. Cell. Biochem. 2024, 125, 30537. [Google Scholar] [CrossRef]
- Huang, T.; Xu, T.; Wang, Y.; Zhou, Y.; Yu, D.; Wang, Z.; He, L.; Chen, Z.; Zhang, Y.; Davidson, D.; et al. Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating trpv4. Autophagy 2021, 17, 3592–3606. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Sinha, S.; Kroemer, G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy 2008, 4, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Nabissi, M.; Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Ricci-Vitiani, L.; Pallini, R.; Santoni, G. Cannabidiol stimulates aml-1a-dependent glial differentiation and inhibits glioma stem-like cells proliferation by inducing autophagy in a trpv2-dependent manner. Int. J. Cancer 2015, 137, 1855–1869. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.G.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; et al. Cannabidiol overcomes oxaliplatin resistance by enhancing nos3- and sod2-induced autophagy in human colorectal cancer cells. Cancers 2019, 11, 781. [Google Scholar] [CrossRef]
- Nareshkumar, R.N.; Sulochana, K.N.; Coral, K. Inhibition of angiogenesis in endothelial cells by human lysyl oxidase propeptide. Sci. Rep. 2018, 8, 10426. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in u87-mg and t98g glioma cells through a multitarget effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De, L.R.C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Semenza, G.L. Oxygen sensing and homeostasis. Physiology 2015, 30, 340–348. [Google Scholar] [CrossRef]
- Jo, M.J.; Kim, B.G.; Kim, W.Y.; Lee, D.H.; Yun, H.K.; Jeong, S.; Park, S.H.; Kim, B.R.; Kim, J.L.; Kim, D.Y.; et al. Cannabidiol suppresses angiogenesis and stemness of breast cancer cells by downregulation of hypoxia-inducible factors-1α. Cancers 2021, 13, 5667. [Google Scholar] [CrossRef]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson, W.R.; et al. Modulation of the tumor microenvironment and inhibition of egf/egfr pathway: Novel anti-tumor mechanisms of cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Kanugula, A.K.; Adapala, R.K.; Jamaiyar, A.; Lenkey, N.; Guarino, B.D.; Liedtke, W.; Yin, L.; Paruchuri, S.; Thodeti, C.K. Endothelial trpv4 channels prevent tumor growth and metastasis via modulation of tumor angiogenesis and vascular integrity. Angiogenesis 2021, 24, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, G.; Scollo, M.; Lempereur, L.; Saccani-Jotti, G.; Basile, F.; Bernardini, R. Endocannabinoids inhibit release of nerve growth factor by inflammation-activated mast cells. Biochem. Pharmacol. 2011, 82, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Zhu, L.; Jie, J.; Yang, P.; Sheng, N.; Chen, X.; Chen, X. Cannabidiol inhibits invasion and metastasis in colorectal cancer cells by reversing epithelial-mesenchymal transition through the Wnt/β-catenin signaling pathway. J. Cancer Res. Clin. Oncol. 2023, 149, 3587–3598. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, L.; Castillo, A.M.; Tapia, R.J.; Zamudio-Meza, H.; Domínguez-Robles, M.; Meza, I. CBD reverts the mesenchymal invasive phenotype of breast cancer cells induced by the inflammatory cytokine IL-1β. Int. J. Mol. Sci. 2020, 21, 2429. [Google Scholar] [CrossRef]
- Stahl, A.; Mueller, B.M. Melanoma cell migration on vitronectin: Regulation by components of the plasminogen activation system. Int. J. Cancer 1997, 71, 116–122. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Ling, M.T.; Wang, X.; Zhang, X.; Wong, Y.C. The multiple roles of id-1 in cancer progression. Differentiation 2006, 74, 481–487. [Google Scholar] [CrossRef]
- Mcallister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Allison, J.; Almanza, C.; Pakdel, A.; Lee, J.; Limbad, C.; et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 2011, 129, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Murase, R.; Limbad, C.; Singer, E.; Allison, J.; Adrados, I.; Kawamura, R.; Pakdel, A.; Fukuyo, Y.; Nguyen, D.; et al. Id-1 is a key transcriptional regulator of glioblastoma aggressiveness and a novel therapeutic target. Cancer Res. 2013, 73, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Bublitz, K.; Freimuth, N.; Merkord, J.; Rohde, H.; Haustein, M.; Borchert, P.; Schmuhl, E.; Linnebacher, M.; Hinz, B. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. Faseb J. 2012, 26, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Murase, R.; Kawamura, R.; Singer, E.; Pakdel, A.; Sarma, P.; Judkins, J.; Elwakeel, E.; Dayal, S.; Martinez-Martinez, E.; Amere, M.; et al. Targeting multiple cannabinoid anti-tumour pathways with a resorcinol derivative leads to inhibition of advanced stages of breast cancer. Br. J. Pharmacol. 2014, 171, 4464–4477. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. Lond. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. The chemical constitution of respiration ferment. Science 1928, 68, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Teslaa, T.; Teitell, M.A. Techniques to monitor glycolysis. Methods Enzymol. 2014, 542, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, F.; Zhou, H.; Ma, N.; Wu, S.; Huang, H.; Jin, G.; Wu, S.; Hong, W.; Zhuang, W.; Xia, H.; et al. A novel mechanism of cannabidiol in suppressing hepatocellular carcinoma by inducing gsdme dependent pyroptosis. Front. Cell. Dev. Biol. 2021, 9, 697832. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, L.; Wang, Y.; Deng, G.; Cao, X.; Ke, B.; Wu, X.; Gu, Y.; Cheng, H.; Xu, Q.; et al. Single-cell analyses reveal cannabidiol rewires tumor microenvironment via inhibiting alternative activation of macrophage and synergizes with anti-PD-1 in colon cancer. J. Pharm. Anal. 2023, 13, 726–744. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Valle-Reyes, J.S.; Hernández-Cruz, A.; Pottosin, I.; Dobrovinskaya, O. Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis. 2019, 10, 779. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Kostrzewa, M.; Marolda, V.; Cerasuolo, M.; Maccarinelli, F.; Coltrini, D.; Rezzola, S.; Giacomini, A.; Mollica, M.P.; Motta, A.; et al. Cannabidiol alters mitochondrial bioenergetics via vdac1 and triggers cell death in hormone-refractory prostate cancer. Pharmacol. Res. 2023, 189, 106683. [Google Scholar] [CrossRef]
- Deng, L.; Ng, L.; Ozawa, T.; Stella, N. Quantitative analyses of synergistic responses between cannabidiol and dna-damaging agents on the proliferation and viability of glioblastoma and neural progenitor cells in culture. J. Pharmacol. Exp. Ther. 2017, 360, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Valenti, M.; Vaccani, A.; Gasperi, V.; Perletti, G.; Marras, E.; Fezza, F.; Maccarrone, M.; Parolaro, D. 5-lipoxygenase and anandamide hydrolase (faah) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J. Neurochem. 2008, 104, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Safety and tolerability of trail receptor agonists in cancer treatment. Eur. J. Clin. Pharmacol. 2015, 71, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Nkune, N.W.; Kruger, C.A.; Abrahamse, H. Possible enhancement of photodynamic therapy (pdt) colorectal cancer treatment when combined with cannabidiol. Anti-Cancer Agents Med. Chem. 2021, 21, 137–148. [Google Scholar] [CrossRef]
- Mokoena, D.; George, B.P.; Abrahamse, H. Cannabidiol combination enhances photodynamic therapy effects on MCF-7 breast cancer cells. Cells 2024, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Kim, T.; Kwon, H.; Kim, J.K.; Park, Y.T.; Ham, J.; Kim, Y.J. Cannabidiol enhances cabozantinib-induced apoptotic cell death via phosphorylation of p53 regulated by ER stress in hepatocellular carcinoma. Cancers 2023, 15, 3987. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.; Judkins, J.; Salomonis, N.; Matlaf, L.; Soteropoulos, P.; Mcallister, S.; Soroceanu, L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015, 6, e1601. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Morelli, M.B.; Tomassoni, D.; Marinelli, O.; Aguzzi, C.; Zeppa, L.; Nabissi, M.; Santoni, G.; Amantini, C. The effects of cannabidiol via trpv2 channel in chronic myeloid leukemia cells and its combination with imatinib. Cancer Sci. 2022, 113, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, O.; Milletsever, A.; Tasan, S.; Selcuk, E.; Savran, M. The effects of cannabidiol against methotrexate-induced lung damage. Basic Clin. Pharmacol. Toxicol. 2024, 134, 695–703. [Google Scholar] [CrossRef]
- Tajik, T.; Baghaei, K.; Moghadam, V.E.; Farrokhi, N.; Salami, S.A. Extracellular vesicles of cannabis with high cbd content induce anticancer signaling in human hepatocellular carcinoma. Biomed. Pharmacother. 2022, 152, 113209. [Google Scholar] [CrossRef] [PubMed]
- Kosgodage, U.S.; Mould, R.; Henley, A.B.; Nunn, A.V.; Guy, G.W.; Thomas, E.L.; Inal, J.M.; Bell, J.D.; Lange, S. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front. Pharmacol. 2018, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Miao, L.; Gao, W.; Chen, Z.; Mchugh, K.J.; Sun, Y.; Tochka, Z.; Tomasic, S.; Sadtler, K.; Hyacinthe, A.; et al. Engineered plga microparticles for long-term, pulsatile release of sting agonist for cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaaz6606. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Delie, F.; Cohen, M.; Martin-Sabroso, C.; Mezzanzanica, D.; Figini, M.; Satta, A.; Torres-Suárez, A.I. Enhancing ovarian cancer conventional chemotherapy through the combination with cannabidiol loaded microparticles. Eur. J. Pharm. Biopharm. 2020, 154, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Sun, Y.; Fan, J.; Nguyen, W.; Chen, S.; Long, Y.; Chen, W.; Zhu, A.; Liu, B. Engineering cannabidiol synergistic carbon monoxide nanocomplexes to enhance cancer therapy via excessive autophagy. Acta Pharm. Sin. B 2023, 13, 4591–4606. [Google Scholar] [CrossRef] [PubMed]
- Iffland, K.; Grotenhermen, F. An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.; Liu, W.; Dalgleish, A. Report of objective clinical responses of cancer patients to pharmaceutical-grade synthetic cannabidiol. Anticancer Res. 2018, 38, 5831–5835. [Google Scholar] [CrossRef]
- Barrie, A.M.; Gushue, A.C.; Eskander, R.N. Dramatic response to laetrile and cannabidiol (cbd) oil in a patient with metastatic low grade serous ovarian carcinoma. Gynecol. Oncol. Rep. 2019, 29, 10–12. [Google Scholar] [CrossRef]
- Sulé-Suso, J.; Watson, N.A.; van Pittius, D.G.; Jegannathen, A. Striking lung cancer response to self-administration of cannabidiol: A case report and literature review. Sage Open Med. Case Rep. 2019, 7, 1–4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Liu, M.; Liu, C.; Zhang, H.; Yang, S.; An, J.; Qu, G.; Song, S.; Cao, Q. Research Progress on the Mechanism of the Antitumor Effects of Cannabidiol. Molecules 2024, 29, 1943. https://doi.org/10.3390/molecules29091943
Ma L, Liu M, Liu C, Zhang H, Yang S, An J, Qu G, Song S, Cao Q. Research Progress on the Mechanism of the Antitumor Effects of Cannabidiol. Molecules. 2024; 29(9):1943. https://doi.org/10.3390/molecules29091943
Chicago/Turabian StyleMa, Li, Mengke Liu, Chuntong Liu, Huachang Zhang, Shude Yang, Jing An, Guiwu Qu, Shuling Song, and Qizhi Cao. 2024. "Research Progress on the Mechanism of the Antitumor Effects of Cannabidiol" Molecules 29, no. 9: 1943. https://doi.org/10.3390/molecules29091943
APA StyleMa, L., Liu, M., Liu, C., Zhang, H., Yang, S., An, J., Qu, G., Song, S., & Cao, Q. (2024). Research Progress on the Mechanism of the Antitumor Effects of Cannabidiol. Molecules, 29(9), 1943. https://doi.org/10.3390/molecules29091943






