Sesquiterpene Lactones Containing an α-Methylene-γ-Lactone Moiety Selectively Down-Regulate the Expression of Tumor Necrosis Factor Receptor 1 by Promoting Its Ectodomain Shedding in Human Lung Adenocarcinoma A549 Cells
Abstract
1. Introduction
2. Results
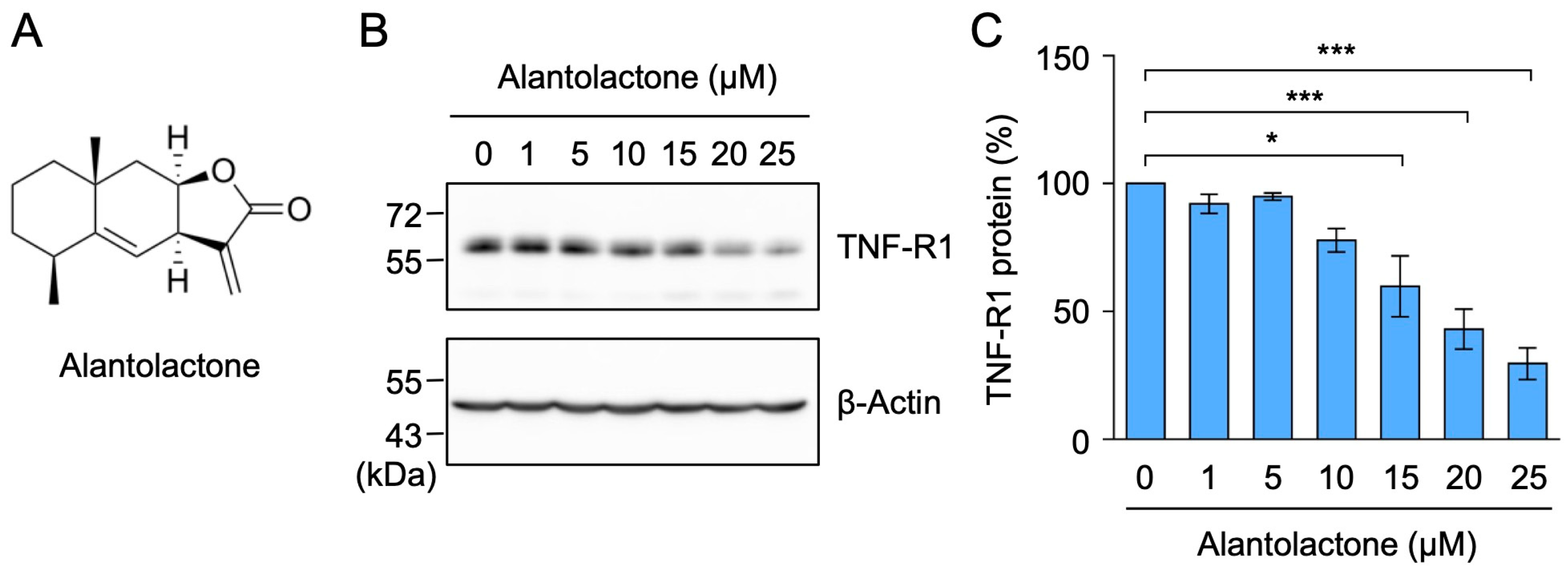
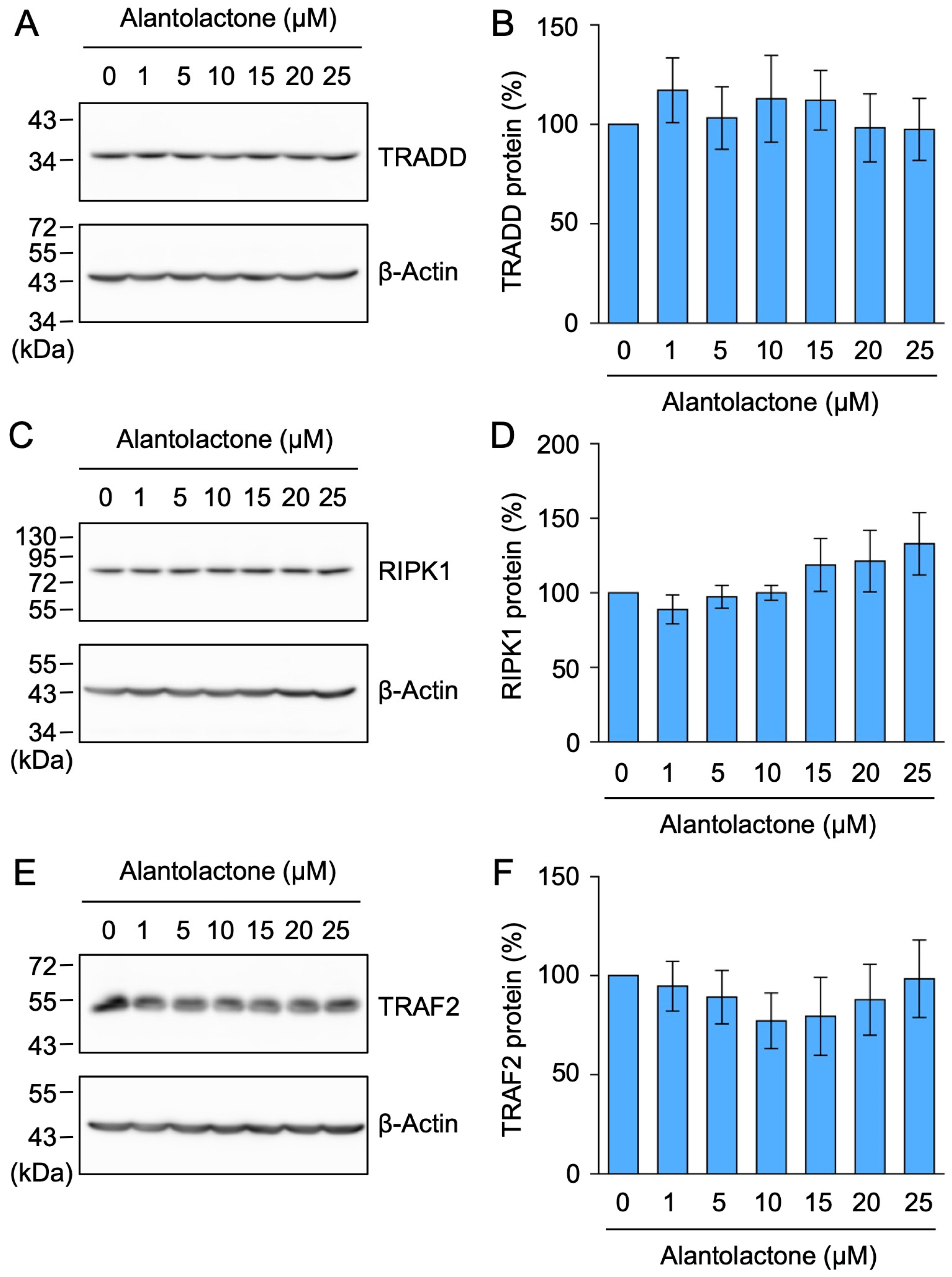
2.1. Alantolactone Selectively Down-Regulated the Expression of TNF-R1, but Not TRADD, RIPK1, or TRAF2
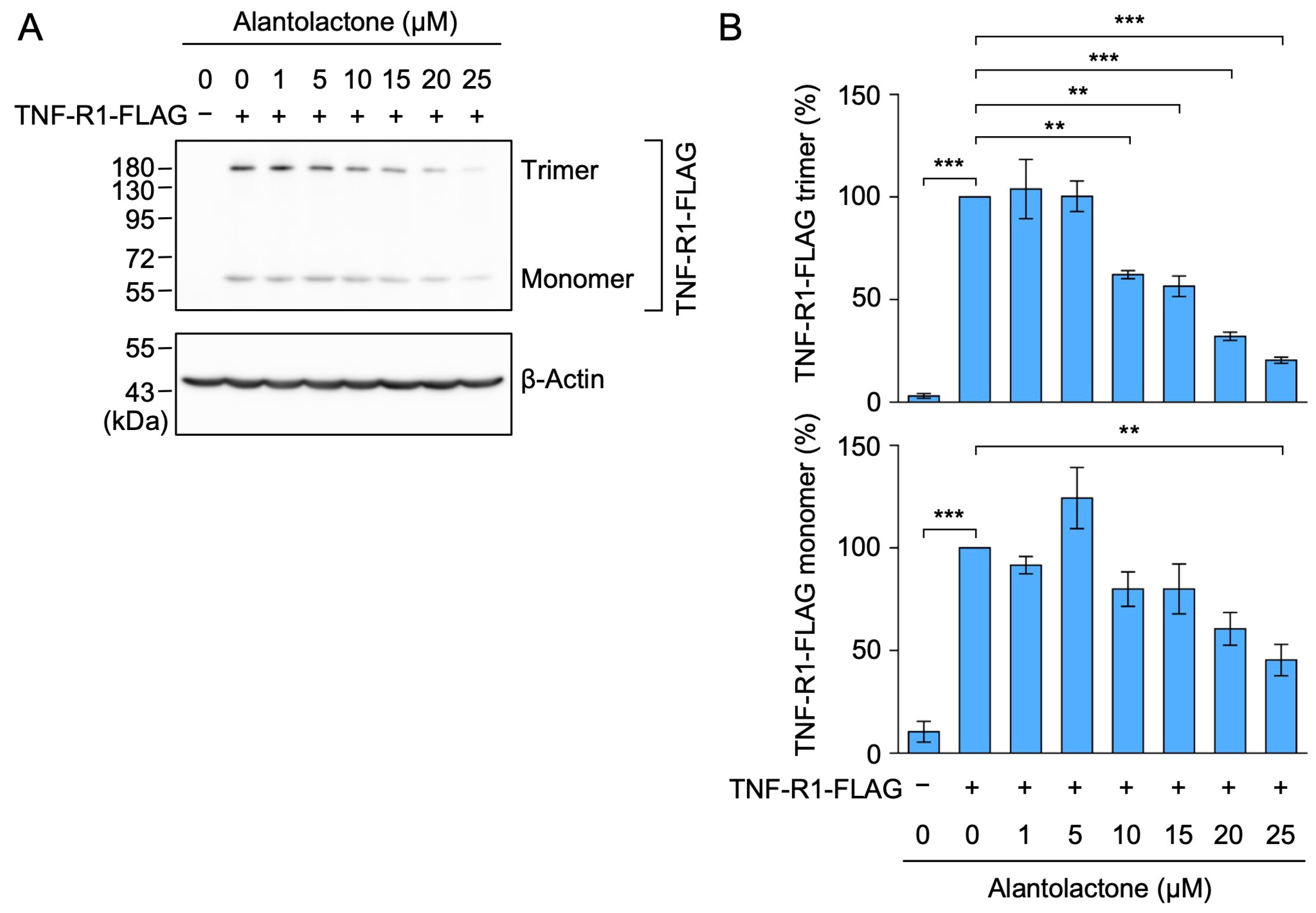
2.2. Alantolactone Down-Regulated the Expression of Transfected TNF-R1
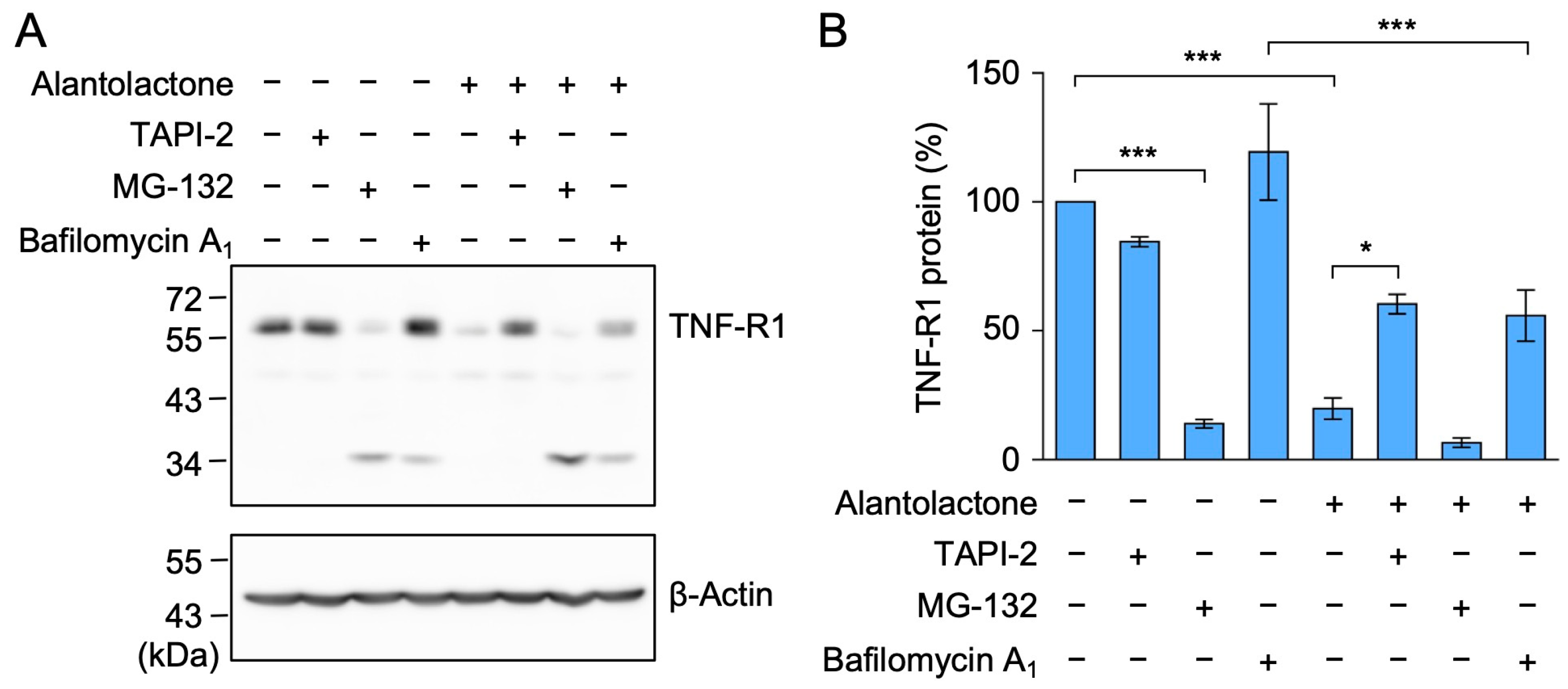
2.3. The TACE Inhibitor Reversed the Down-Regulation of TNF-R1 Expression by Alantolactone in A549 Cells
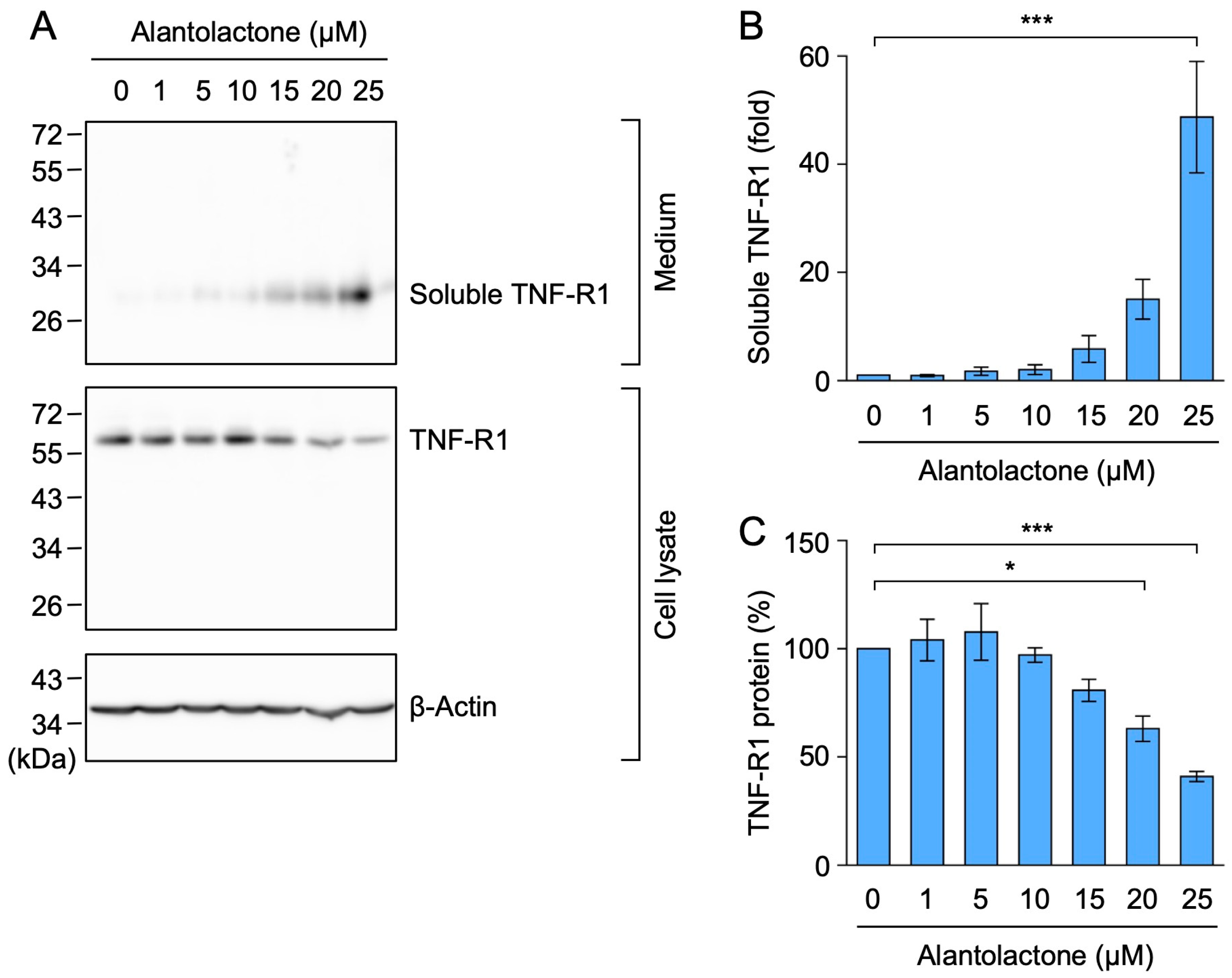
2.4. Alantolactone Promoted the Ectodomain Shedding of TNF-R1 in A549 Cells
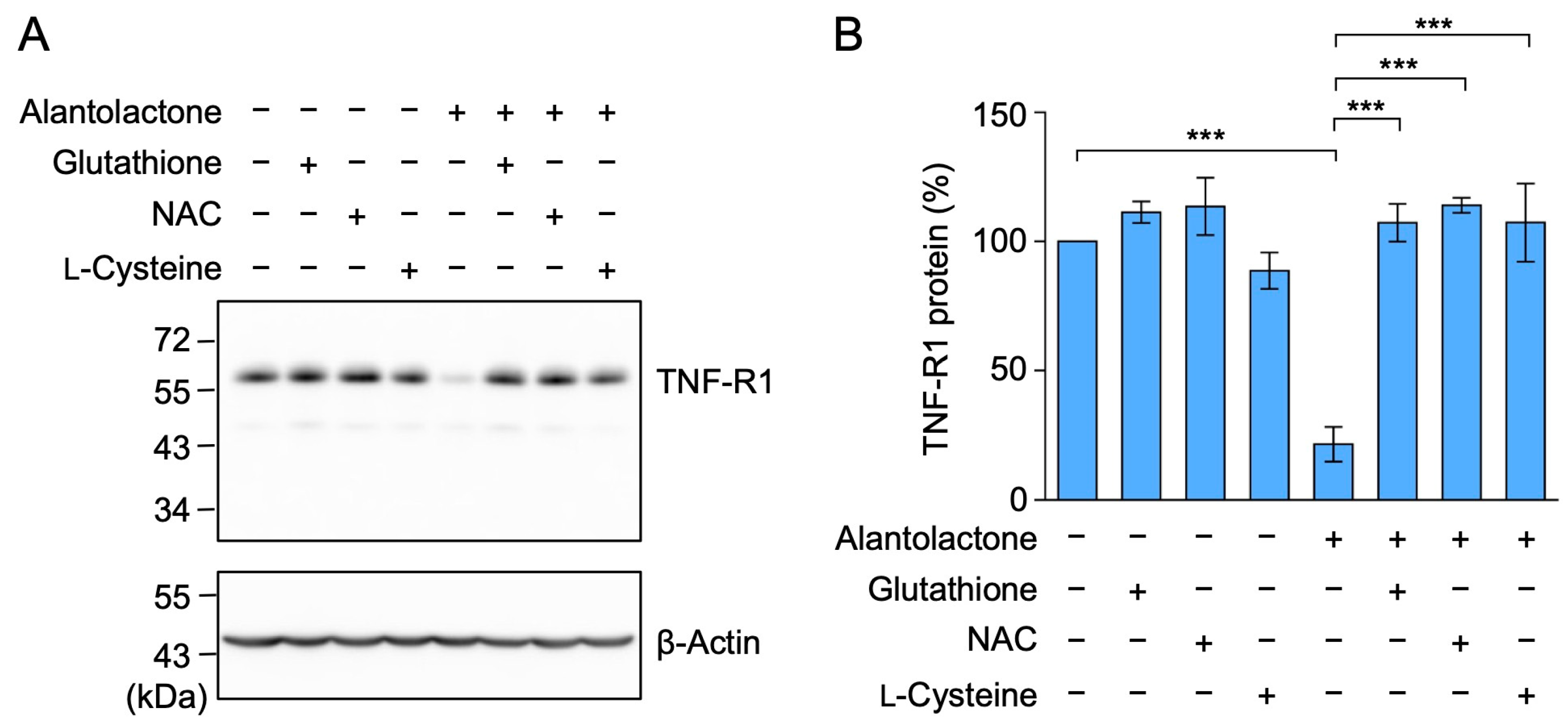
2.5. The Down-Regulation of TNF-R1 Expression by Alantolactone Was Suppressed by Glutathione, N-Acetyl-L-Cysteine (NAC), and L-Cysteine
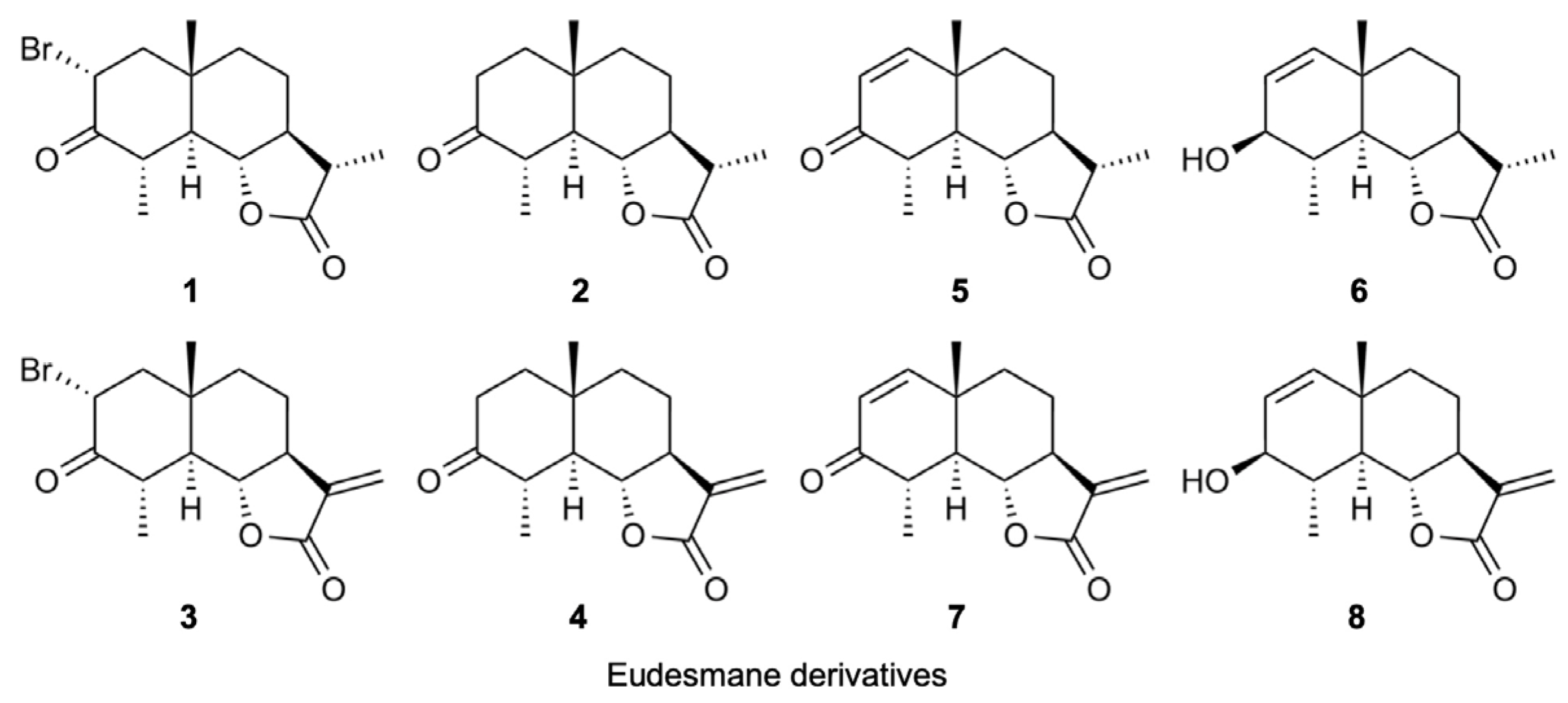
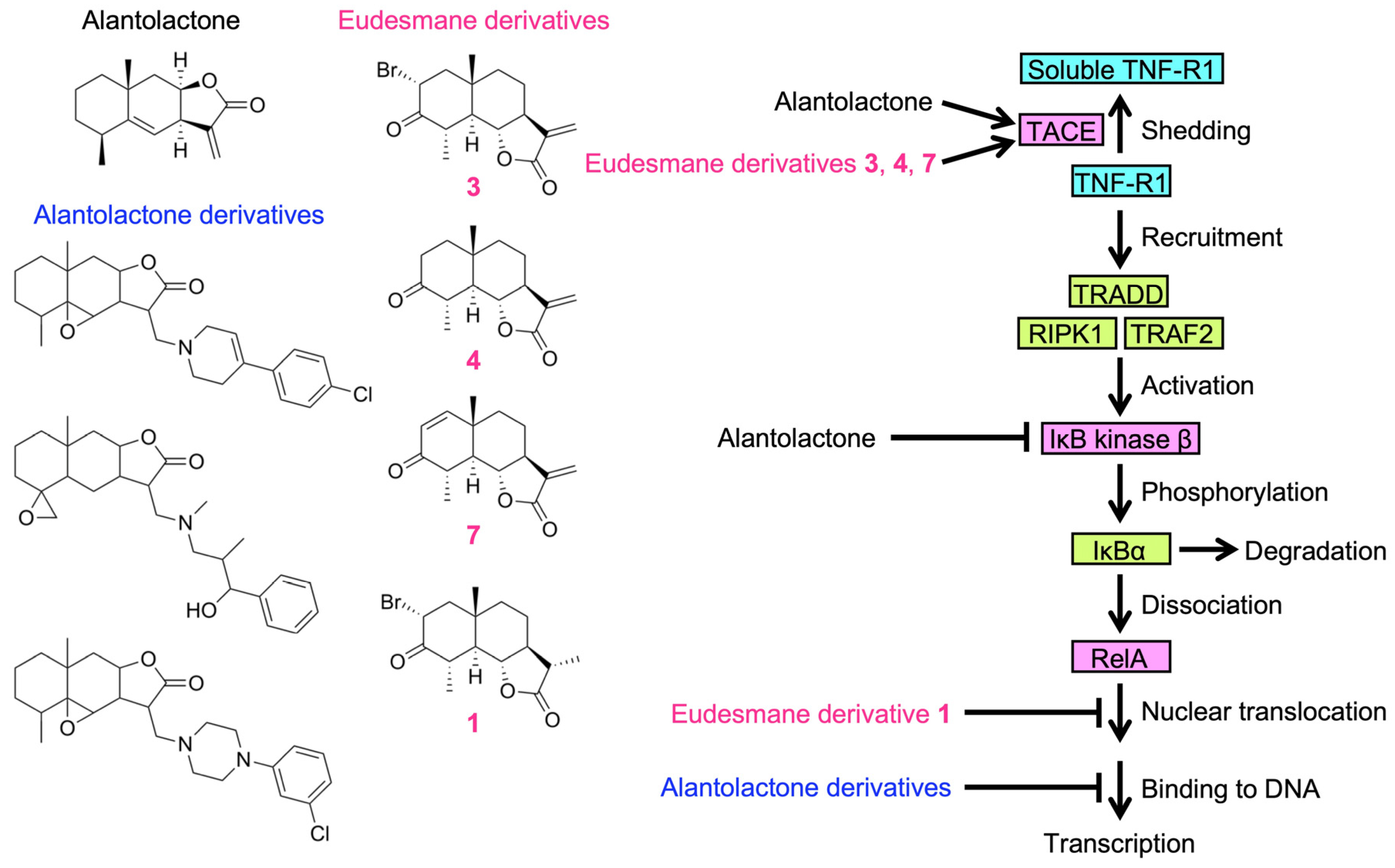
2.6. Structure–Activity Relationship of Eudesmane Derivatives: The α-Methylene-γ-Lactone Moiety of Eudesmane Derivatives Was Necessary to Promote the Ectodomain Shedding of TNF-R1
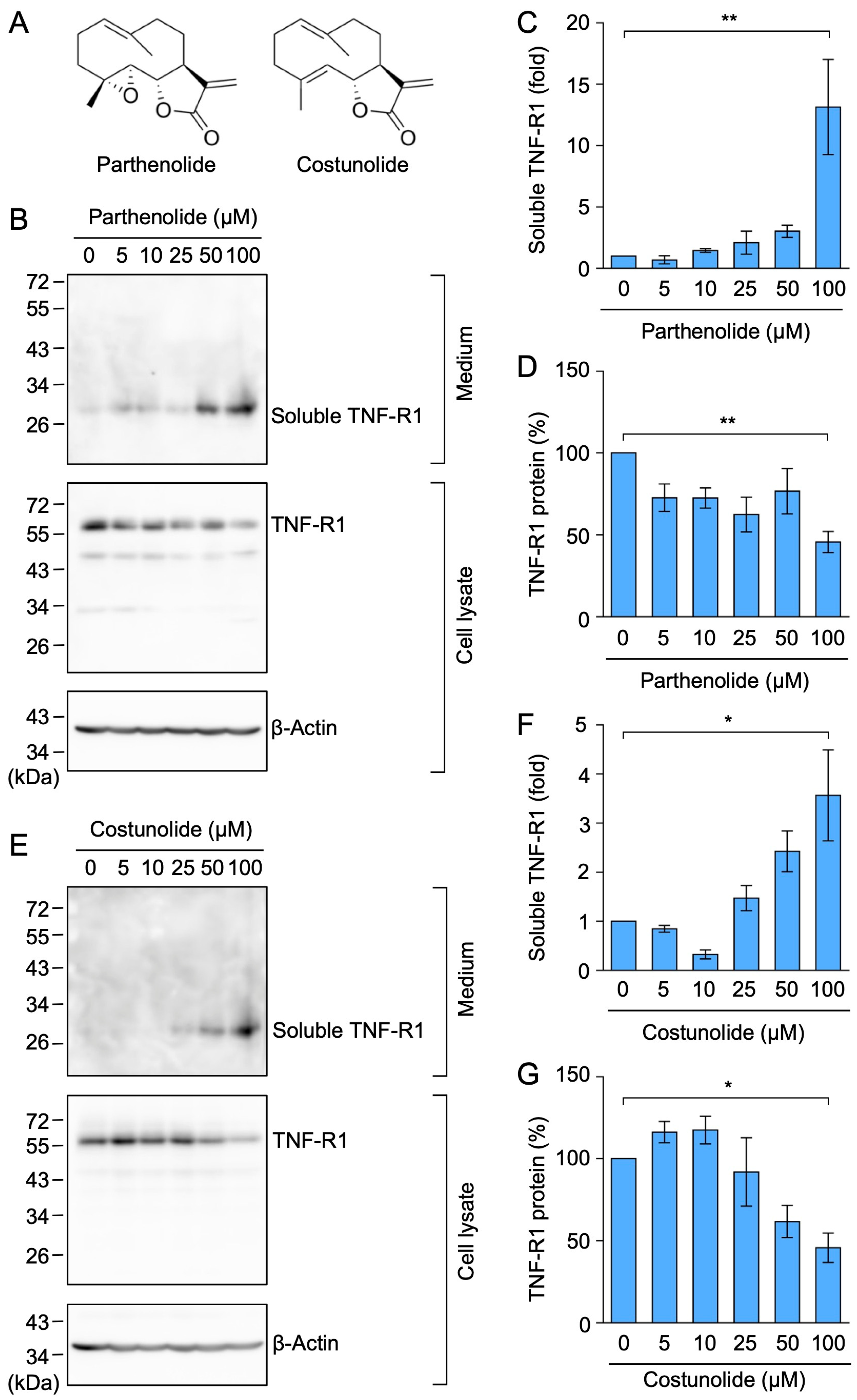
2.7. Parthenolide and Costunolide Promoted the Ectodomain Shedding of TNF-R1
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Reagents
4.3. Antibodies
4.4. Expression Vectors
4.5. Preparation of Cell Lysates and Medium Fractions
4.6. Western Blotting
4.7. Transfection
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallach, D. The tumor necrosis factor family: Family conventions and private idiosyncrasies. Cold Spring Harb. Perspect. Biol. 2018, 10, a028431. [Google Scholar] [CrossRef] [PubMed]
- Stow, J.L.; Murray, R.Z. Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 2013, 24, 227–239. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the control of the life and death balance of macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Atretkhany, K.N.; Gogoleva, V.S.; Drutskaya, M.S.; Nedospasov, S.A. Distinct modes of TNF signaling through its two receptors in health and disease. J. Leukoc. Biol. 2020, 107, 893–905. [Google Scholar] [CrossRef]
- Wajant, H.; Scheurich, P. TNFR1-induced activation of the classical NF-κB pathway. FEBS J. 2011, 278, 862–876. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Bhoj, V.G.; Chen, Z.J. Ubiquitylation in innate and adaptive immunity. Nature 2009, 458, 430–437. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef]
- Seals, D.F.; Courtneidge, S.A. The ADAMs family of metalloproteases: Multidomain proteins with multiple functions. Genes Dev. 2003, 17, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Gooz, M. ADAM-17: The enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 146–169. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Garbers, C.; Rose-John, S. ADAM17: A molecular switch to control inflammation and tissue regeneration. Trends. Immunol. 2011, 32, 380–387. [Google Scholar] [CrossRef]
- Peschon, J.J.; Slack, J.L.; Reddy, P.; Stocking, K.L.; Sunnarborg, S.W.; Lee, D.C.; Russell, W.E.; Castner, B.J.; Johnson, R.S.; Fitzner, J.N.; et al. An essential role for ectodomain shedding in mammalian development. Science 1998, 282, 1281–1284. [Google Scholar] [CrossRef]
- Horiuchi, K.; Kimura, T.; Miyamoto, T.; Takaishi, H.; Okada, Y.; Toyama, Y.; Blobel, C.P. TNF-α-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J. Immunol. 2007, 179, 2686–2689. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-α: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Kontermann, R.E.; Maier, O. Targeting sTNF/TNFR1 signaling as a new therapeutic strategy. Antibodies 2015, 4, 48–70. [Google Scholar] [CrossRef]
- Ruiz, A.; Palacios, Y.; Garcia, I.; Chavez-Galan, L. Transmembrane TNF and its receptors TNFR1 and TNFR2 in mycobacterial infections. Int. J. Mol. Sci. 2021, 22, 5461. [Google Scholar] [CrossRef]
- Matos, M.S.; Anastácio, J.D.; Nunes Dos Santos, C. Sesquiterpene lactones: Promising natural compounds to fight inflammation. Pharmaceutics 2021, 13, 991. [Google Scholar] [CrossRef]
- Paço, A.; Brás, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-inflammatory and immunoregulatory action of sesquiterpene lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene lactones from Artemisia genus: Biological activities and methods of analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef]
- Gilmore, T.D.; Herscovitch, M. Inhibitors of NF-κB signaling: 785 and counting. Oncogene 2006, 25, 6887–6899. [Google Scholar] [CrossRef]
- Kataoka, T. Chemical biology of inflammatory cytokine signaling. J. Antibiot. 2009, 62, 655–657. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Li, W.; Ma, X.C.; Qiu, F.; Sun, C.P. IκB kinase β (IKKβ): Structure, transduction mechanism, biological function, and discovery of its inhibitors. Int. J. Biol. Sci. 2023, 19, 4181–4203. [Google Scholar] [CrossRef]
- Babaei, G.; Gholizadeh-Ghaleh Aziz, S.; Rajabi Bazl, M.; Khadem Ansari, M.H. A comprehensive review of anticancer mechanisms of action of alantolactone. Biomed. Pharmacother. 2021, 136, 111231. [Google Scholar] [CrossRef]
- Cai, Y.; Gao, K.; Peng, B.; Xu, Z.; Peng, J.; Li, J.; Chen, X.; Zeng, S.; Hu, K.; Yan, Y. Alantolactone: A natural plant extract as a potential therapeutic agent for cancer. Front. Pharmacol. 2021, 12, 781033. [Google Scholar] [CrossRef]
- Liu, X.; Bian, L.; Duan, X.; Zhuang, X.; Sui, Y.; Yang, L. Alantolactone: A sesquiterpene lactone with diverse pharmacological effects. Chem. Biol. Drug Des. 2021, 98, 1131–1145. [Google Scholar] [CrossRef]
- Wei, W.; Huang, H.; Zhao, S.; Liu, W.; Liu, C.X.; Chen, L.; Li, J.M.; Wu, Y.L.; Yan, H. Alantolactone induces apoptosis in chronic myelogenous leukemia sensitive or resistant to imatinib through NF-κB inhibition and Bcr/Abl protein deletion. Apoptosis 2013, 18, 1060–1070. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Wang, C.; Cheng, W.; Tian, X.; Huo, X.; Wang, Y.; Sun, C.; Feng, L.; Xing, J.; et al. Alantolactone, a natural sesquiterpene lactone, has potent antitumor activity against glioblastoma by targeting IKKβ kinase activity and interrupting NF-κB/COX-2-mediated signaling cascades. J. Exp. Clin. Cancer Res. 2017, 36, 93. [Google Scholar] [CrossRef]
- Vu, Q.V.; Baba, K.; Sasaki, S.; Kawaguchi, K.; Hirano, H.; Osada, H.; Kataoka, T. Alantolactone derivatives inhibit the tumor necrosis factor α-induced nuclear factor κB pathway by a different mechanism from alantolactone. Eur. J. Pharmacol. 2024, 969, 176458. [Google Scholar] [CrossRef]
- Ogura, H.; Tsukumo, Y.; Sugimoto, H.; Igarashi, M.; Nagai, K.; Kataoka, T. Ectodomain shedding of TNF receptor 1 induced by protein synthesis inhibitors regulates TNF-α-mediated activation of NF-κB and caspase-8. Exp. Cell Res. 2008, 314, 1406–1414. [Google Scholar] [CrossRef]
- Quach, H.T.; Tanigaki, R.; Yokoigawa, J.; Yamada, Y.; Niwa, M.; Hirano, S.; Shiono, Y.; Kimura, K.; Kataoka, T. Allantopyrone A interferes with multiple components of the TNF receptor 1 complex and blocks RIP1 modifications in the TNF-α-induced signaling pathway. J. Antibiot. 2017, 70, 929–936. [Google Scholar] [CrossRef]
- Kusagawa, E.; Okuda, C.; Yamaguchi, R.; Nakano, K.; Miyake, Y.; Kataoka, T. Cucurbitacin B down-regulates TNF receptor 1 expression and inhibits the TNF-α-dependent nuclear factor κB signaling pathway in human lung adenocarcinoma A549 cells. Int. J. Mol. Sci. 2022, 23, 7130. [Google Scholar] [CrossRef]
- Tamura, R.; Chen, Y.; Shinozaki, M.; Arao, K.; Wang, L.; Tang, W.; Hirano, S.; Ogura, H.; Mitsui, T.; Taketani, S.; et al. Eudesmane-type sesquiterpene lactones inhibit multiple steps in the NF-κB signaling pathway induced by inflammatory cytokines. Bioorg. Med. Chem. Lett. 2012, 22, 207–211. [Google Scholar] [CrossRef]
- Kwok, B.H.B.; Koh, B.; Ndubuisi, M.I.; Elofsson, M.; Crews, C.M. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IκB kinase. Chem. Biol. 2001, 8, 759–766. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Luo, W.; Li, W.X.; Chen, P.; Wang, Z.; Chen, R.J.; Wang, Y.; Huang, W.J.; Liang, G. Costunolide alleviates atherosclerosis in high-fat diet-fed ApoE−/− mice through covalently binding to IKKβ and inhibiting NF-κB-mediated inflammation. Acta Pharmacol. Sin. 2023, 44, 58–70. [Google Scholar] [CrossRef]
- Chun, J.; Choi, R.J.; Khan, S.; Lee, D.S.; Kim, Y.C.; Nam, Y.J.; Lee, D.U.; Kim, Y.S. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int. Immunopharmacol. 2012, 14, 375–383. [Google Scholar] [CrossRef]
- Khan, M.; Yi, F.; Rasul, A.; Li, T.; Wang, N.; Gao, H.; Gao, R.; Ma, T. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life 2012, 64, 783–794. [Google Scholar] [CrossRef]
- Lei, J.C.; Yu, J.Q.; Yin, Y.; Liu, Y.W.; Zou, G.L. Alantolactone induces activation of apoptosis in human hepatoma cells. Food Chem. Toxicol. 2012, 50, 3313–3319. [Google Scholar] [CrossRef]
- Cui, L.; Bu, W.; Song, J.; Feng, L.; Xu, T.; Liu, D.; Ding, W.; Wang, J.; Li, C.; Ma, B.; et al. Apoptosis induction by alantolactone in breast cancer MDA-MB-231 cells through reactive oxygen species-mediated mitochondrion-dependent pathway. Arch. Pharm. Res. 2018, 41, 299–313. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Wang, S.; He, Y.; Huo, Y.; Yang, Z.; Cao, X. Alantolactone induces apoptosis and suppresses migration in MCF-7 human breast cancer cells via the p38 MAPK, NF-κB and Nrf2 signaling pathways. Int. J. Mol. Med. 2018, 42, 1847–1856. [Google Scholar] [CrossRef]
- He, Y.; Cao, X.; Kong, Y.; Wang, S.; Xia, Y.; Bi, R.; Liu, J. Apoptosis-promoting and migration-suppressing effect of alantolactone on gastric cancer cell lines BGC-823 and SGC-7901 via regulating p38MAPK and NF-κB pathways. Hum. Exp. Toxicol. 2019, 38, 1132–1144. [Google Scholar] [CrossRef]
- Ren, Y.; Yue, B.; Ren, G.; Yu, Z.; Luo, X.; Sun, A.; Zhang, J.; Han, M.; Wang, Z.; Dou, W. Activation of PXR by alantolactone ameliorates DSS-induced experimental colitis via suppressing NF-κB signaling pathway. Sci. Rep. 2019, 9, 16636. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Wang, Y.; Tan, R. Anti-neuroinflammatory effect of alantolactone through the suppression of the NF-κB and MAPK signaling pathways. Cells 2019, 8, 739. [Google Scholar] [CrossRef]
- Dang, X.; He, B.; Ning, Q.; Liu, Y.; Guo, J.; Niu, G.; Chen, M. Alantolactone suppresses inflammation, apoptosis and oxidative stress in cigarette smoke-induced human bronchial epithelial cells through activation of Nrf2/HO-1 and inhibition of the NF-κB pathways. Respir. Res. 2020, 21, 95. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, Y.; Wang, X. Alantolactone mitigates renal injury induced by diabetes via inhibition of high glucose-mediated inflammatory response and macrophage infiltration. Immunopharmacol. Immunotoxicol. 2020, 42, 84–92. [Google Scholar] [CrossRef]
- Chuo, W.H.; Tung, Y.T.; Wu, C.L.; Bracci, N.R.; Chang, Y.K.; Huang, H.Y.; Lin, C.C. Alantolactone suppresses proliferation and the inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced skin lesions in a psoriasis-like mouse model. Life 2021, 11, 616. [Google Scholar] [CrossRef]
- Scheidereit, C. IκB kinase complexes: Gateways to NF-κB activation and transcription. Oncogene 2006, 25, 6685–6705. [Google Scholar] [CrossRef]
- Solt, L.A.; May, M.J. The IκB kinase complex: Master regulator of NF-κB signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 years of NF-κB: A blossoming of relevance to human pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef]
- Liu, L.; Hua, Y.; Wang, D.; Shan, L.; Zhang, Y.; Zhu, J.; Jin, H.; Li, H.; Hu, Z.; Zhang, W. A sesquiterpene lactone from a medicinal herb inhibits proinflammatory activity of TNF-α by inhibiting ubiquitin-conjugating enzyme UbcH5. Chem. Biol. 2014, 21, 1341–1350. [Google Scholar] [CrossRef]
- Yun, H.J.; Lee, H.Y. The novel TAK1 inhibitor handelin inhibits NF-κB and AP-1 activity to alleviate elastase-induced emphysema in mice. Life Sci. 2023, 319, 121388. [Google Scholar] [CrossRef]
- Tamura, R.; Morimoto, K.; Hirano, S.; Wang, L.; Zhao, M.; Ando, M.; Kataoka, T. Santonin-related compound 2 inhibits the nuclear translocation of NF-κB subunit p65 by targeting cysteine 38 in TNF-α-induced NF-κB signaling pathway. Biosci. Biotechnol. Biochem. 2012, 76, 2360–2363. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Su, C. Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone. Int. J. Mol. Sci. 2015, 16, 10888–10906. [Google Scholar] [CrossRef]
- Freund, R.R.A.; Gobrecht, P.; Fischer, D.; Arndt, H.D. Advances in chemistry and bioactivity of parthenolide. Nat. Prod. Rep. 2020, 37, 541–565. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xie, Y.; Hu, H. Antitumor activity and mechanism of costunolide and dehydrocostus lactone: Two natural sesquiterpene lactones from the Asteraceae family. Biomed. Pharmacother. 2020, 125, 109955. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, E.; Montero, J.C.; Esparís-Ogando, A.; Yuste, L.; Pandiella, A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor α-converting enzyme at threonine 735: A potential role in regulated shedding. Mol. Biol. Cell 2002, 13, 2031–2044. [Google Scholar] [CrossRef]
- Soond, S.M.; Everson, B.; Riches, D.W.H.; Murphy, G. ERK-mediated phosphorylation of Thr735 in TNFα-converting enzyme and its potential role in TACE protein trafficking. J. Cell Sci. 2005, 118, 2371–2380. [Google Scholar] [CrossRef]
- Liu, C.; Xu, P.; Lamouille, S.; Xu, J.; Derynck, R. TACE-mediated ectodomain shedding of the type I TGF-β receptor downregulates TGF-β signaling. Mol. Cell 2009, 35, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Derynck, R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol. Cell 2010, 37, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Vind, A.C.; Genzor, A.V.; Bekker-Jensen, S. Ribosomal stress-surveillance: Three pathways is a magic number. Nucleic Acids Res. 2020, 48, 10648–10661. [Google Scholar] [CrossRef]
- Kataoka, T. Translation inhibitors and their unique biological properties. Eur. J. Pharmacol. 2012, 676, 1–5. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Huang, H.; Yuan, X.; Zhang, P.; Ye, C.; Wei, M.; Huang, Y.; Luo, X.; Luo, J. Alantolactone inhibits proliferation, metastasis and promotes apoptosis of human osteosarcoma cells by suppressing Wnt/β-catenin and MAPKs signaling pathways. Genes Dis. 2022, 9, 466–478. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cao, P.; Xia, Y.; Hong, L.; Zhang, T.; Shen, X.; Zheng, P.; Shen, H.; Zhao, Y.; Zou, P. Potent inhibition of gastric cancer cells by a natural compound via inhibiting TrxR1 activity and activating ROS-mediated p38 MAPK pathway. Free Radic. Res. 2019, 53, 104–114. [Google Scholar] [CrossRef]
- Ren, Y.; Lv, C.; Zhang, J.; Zhang, B.; Yue, B.; Luo, X.; Yu, Z.; Wang, H.; Ren, J.; Wang, Z. Alantolactone exhibits antiproliferative and apoptosis-promoting properties in colon cancer model via activation of the MAPK-JNK/c-Jun signaling pathway. Mol. Cell. Biochem. 2021, 476, 4387–4403. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Wada, T.; Kusaka, H.; Takase, K.; Hirata, N.; Yanagi, Y. Studies on the syntheses of sesquiterpene lactones. 10. Improved syntheses of (+)-tuberiferin and the related α-methylene-γ-lactones and their biological activities. J. Org. Chem. 1987, 52, 4792–4796. [Google Scholar] [CrossRef]
- Kawai, S.; Kataoka, T.; Sugimoto, H.; Nakamura, A.; Kobayashi, T.; Arao, K.; Higuchi, Y.; Ando, M.; Nagai, K. Santonin-related compound 2 inhibits the expression of ICAM-1 in response to IL-1 stimulation by blocking the signaling pathway upstream of IκB degradation. Immunopharmacol 2000, 48, 129–135. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, Q.V.; Sayama, S.; Ando, M.; Kataoka, T. Sesquiterpene Lactones Containing an α-Methylene-γ-Lactone Moiety Selectively Down-Regulate the Expression of Tumor Necrosis Factor Receptor 1 by Promoting Its Ectodomain Shedding in Human Lung Adenocarcinoma A549 Cells. Molecules 2024, 29, 1866. https://doi.org/10.3390/molecules29081866
Vu QV, Sayama S, Ando M, Kataoka T. Sesquiterpene Lactones Containing an α-Methylene-γ-Lactone Moiety Selectively Down-Regulate the Expression of Tumor Necrosis Factor Receptor 1 by Promoting Its Ectodomain Shedding in Human Lung Adenocarcinoma A549 Cells. Molecules. 2024; 29(8):1866. https://doi.org/10.3390/molecules29081866
Chicago/Turabian StyleVu, Quy Van, Shinsei Sayama, Masayoshi Ando, and Takao Kataoka. 2024. "Sesquiterpene Lactones Containing an α-Methylene-γ-Lactone Moiety Selectively Down-Regulate the Expression of Tumor Necrosis Factor Receptor 1 by Promoting Its Ectodomain Shedding in Human Lung Adenocarcinoma A549 Cells" Molecules 29, no. 8: 1866. https://doi.org/10.3390/molecules29081866
APA StyleVu, Q. V., Sayama, S., Ando, M., & Kataoka, T. (2024). Sesquiterpene Lactones Containing an α-Methylene-γ-Lactone Moiety Selectively Down-Regulate the Expression of Tumor Necrosis Factor Receptor 1 by Promoting Its Ectodomain Shedding in Human Lung Adenocarcinoma A549 Cells. Molecules, 29(8), 1866. https://doi.org/10.3390/molecules29081866





