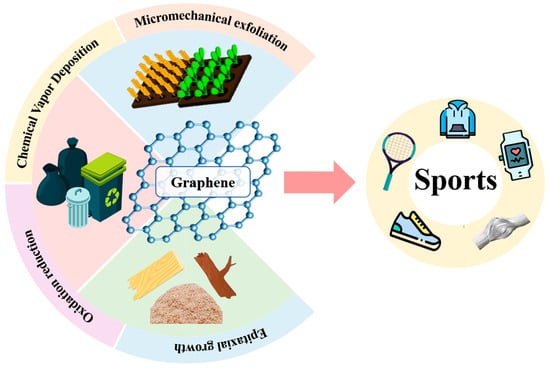
The Future of Graphene: Preparation from Biomass Waste and Sports Applications
Abstract
1. Introduction
2. Graphene Overview
2.1. Graphene Structure
2.2. Graphene Properties
2.3. Graphene Derivatives
3. Preparation Method of Graphene
3.1. Micromechanical Lift-Off
3.2. Chemical Vapor Deposition
3.3. Oxidation–Reduction Method
3.4. Epitaxial Growth Method
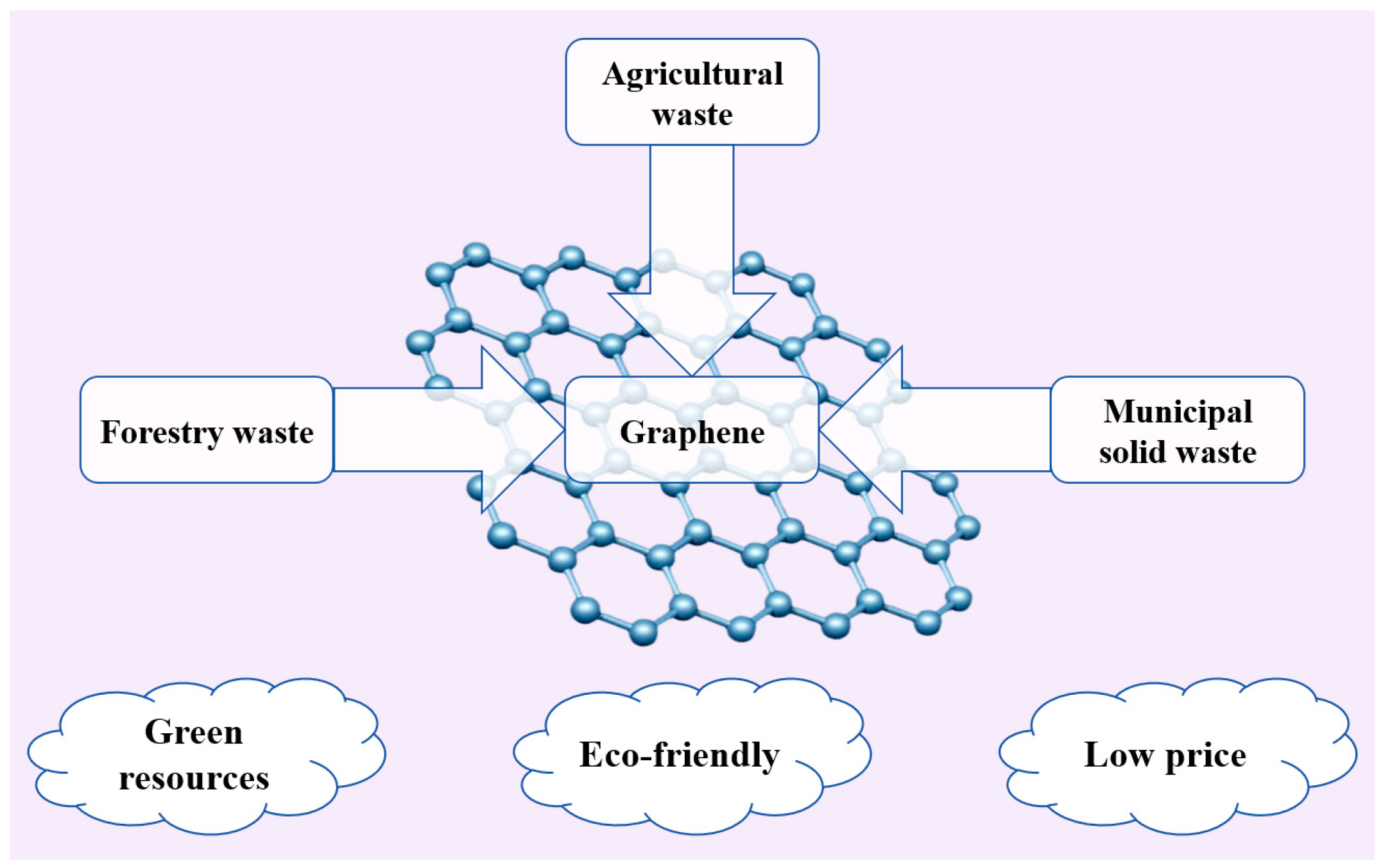
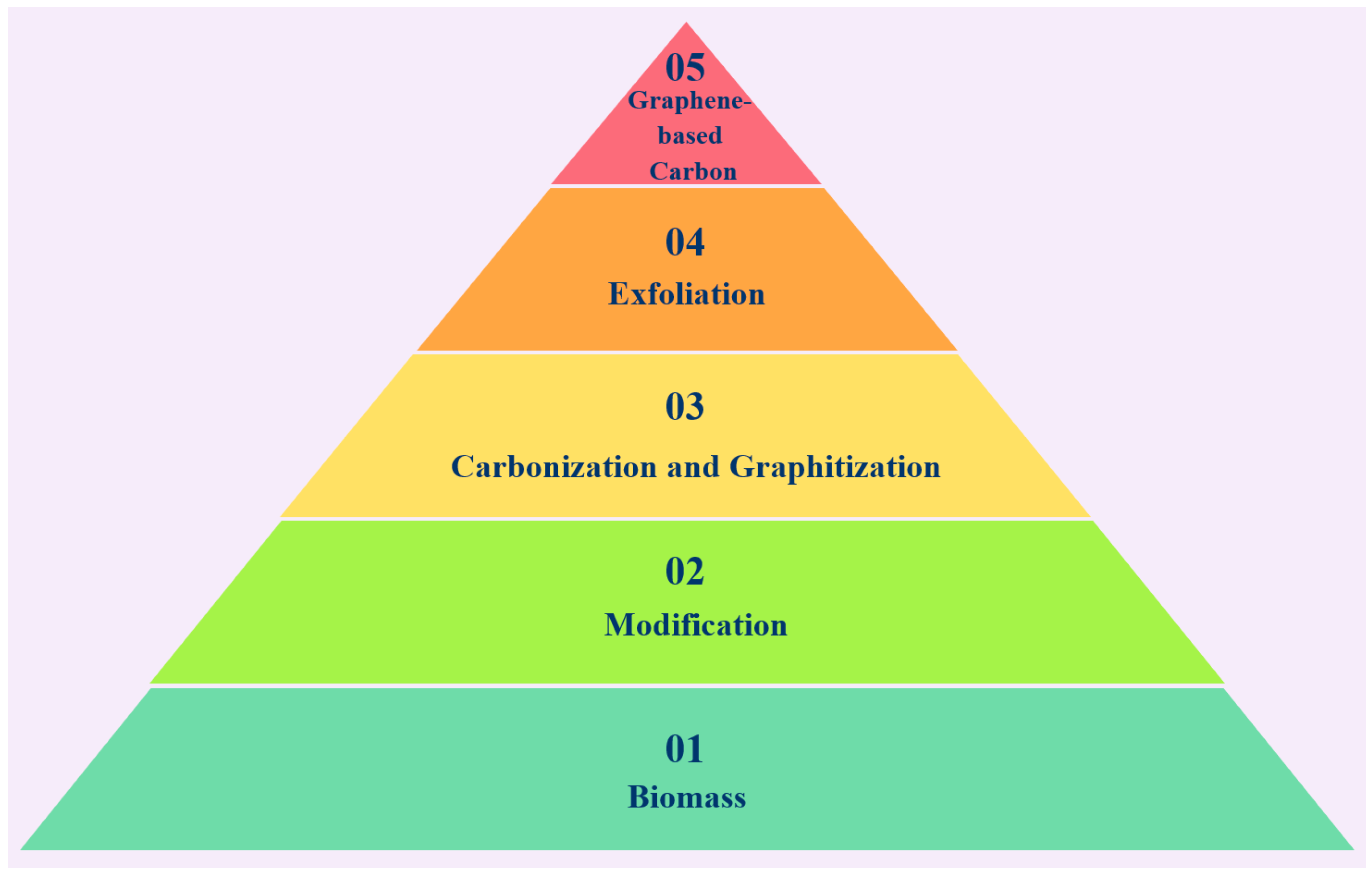
4. Biomass Waste-Derived Graphene
4.1. Agricultural Waste
4.2. Forestry Waste
4.3. Municipal Solid Waste
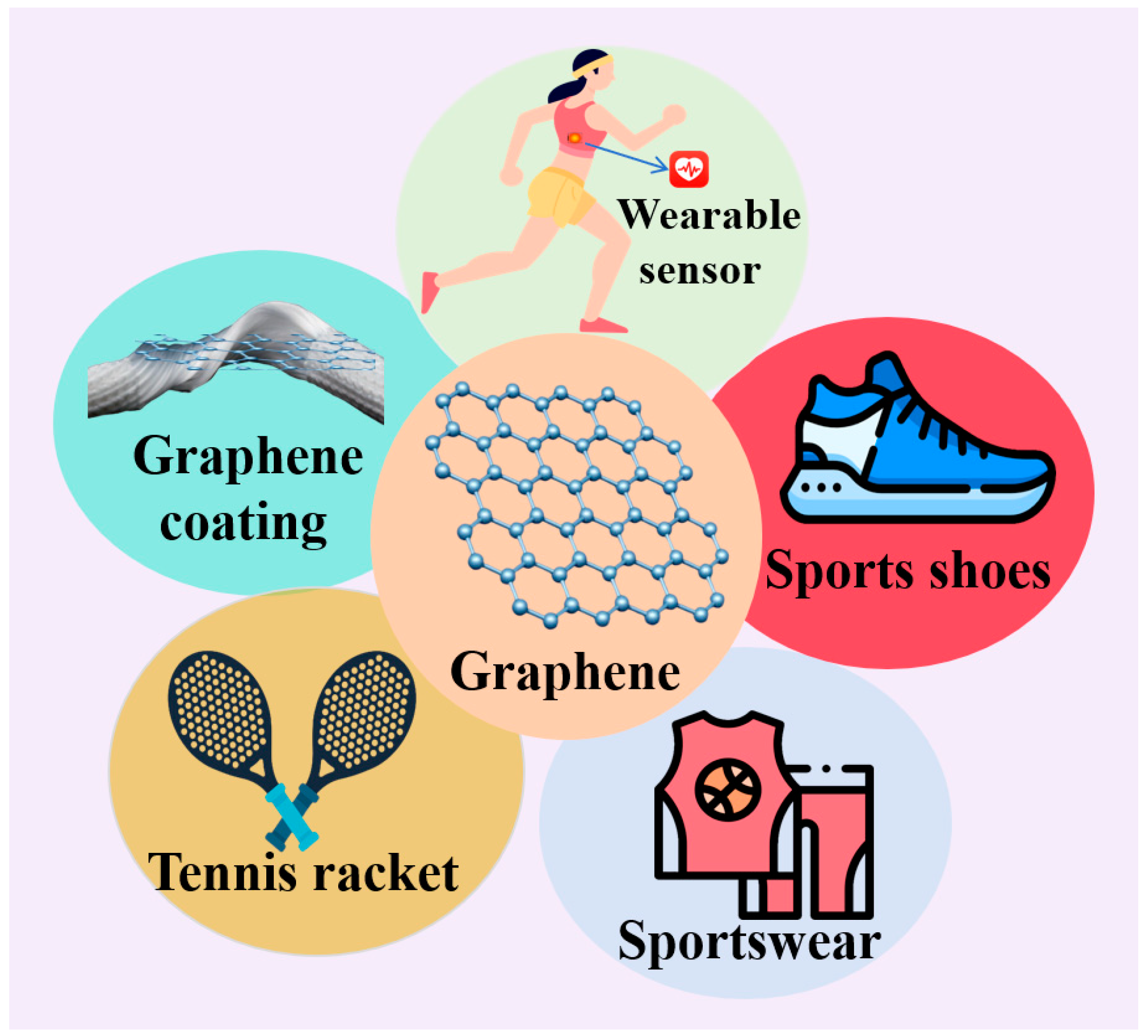
5. Graphene Applications in Sports Equipment
5.1. Graphene Wearable Sensors
5.2. Graphene Sneakers
5.3. Graphene Tennis Racket
5.4. Graphene Sportswear
5.5. Graphene Coating
5.6. Challenges of Biomass Waste Graphene in Sports Applications
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Luo, B.; Liu, S.; Zhi, L. Chemical approaches toward graphene–based nanomaterials and their applications in energy–related areas. Small 2012, 8, 630–646. [Google Scholar] [CrossRef]
- Di Matteo, P.; Petrucci, R.; Curulli, A. Not Only Graphene Two-Dimensional Nanomaterials: Recent Trends in Electrochemical (Bio) sensing Area for Biomedical and Healthcare Applications. Molecules 2023, 29, 172. [Google Scholar] [CrossRef]
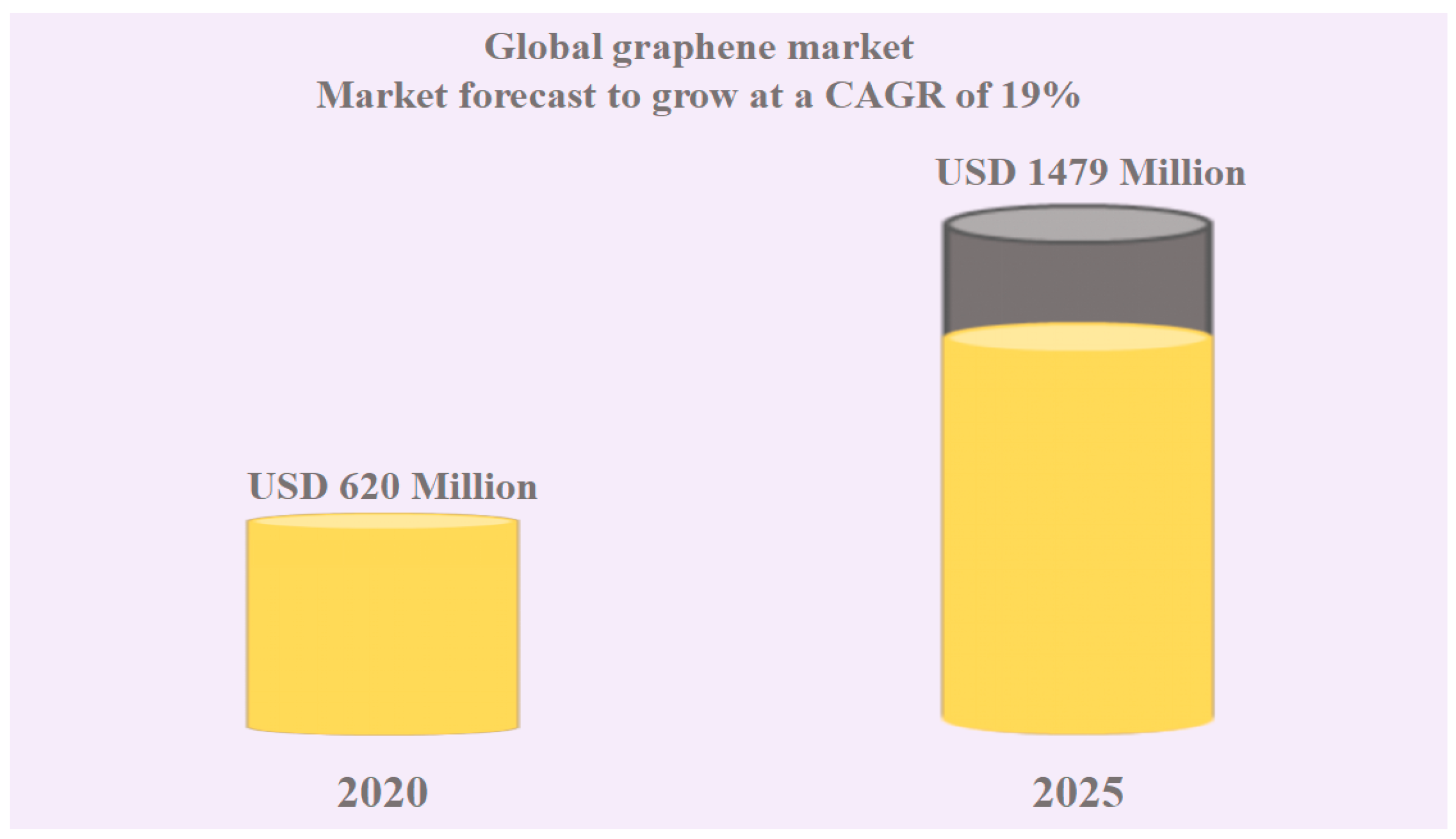
- GlobeNewswire. The Worldwide Graphene Industry Is Expected to Reach $1.4 Billion by 2025 at a CAGR of 19% from 2020. 2023. Available online: https://www.globenewswire.com/news-release/2021/04/02/2203853/28124/en/The-Worldwide-Graphene-Industry-is-Expected-to-Reach-1-4-Billion-by-2025-at-a-CAGR-of-19-from-2020.html (accessed on 12 January 2024).
- Acharya, P.B.; George, A.; Shrivastav, P.S.A. Status Update on the Development of Polymer and Metal-Based Graphene Electrochemical Sensors for Detection and Quantitation of Bisphenol A. Crit. Rev. Anal. Chem. 2022, 1–22. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Z.; Sun, Y.; Wang, X.; Liu, X.; Yang, Y.; Qiu, J. Activated coal-based graphene with hierarchical porous structures for ultra-high energy density supercapacitors. Diam. Relat. Mater. 2020, 106, 107827. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Wu, T.; Jin, B.; Yang, L.; Qiu, J. Synthesis, modification strategies and applications of coal-based carbon materials. Fuel Process. Technol. 2022, 230, 107203. [Google Scholar] [CrossRef]
- Srinivas, A.; Jivanlal Shaha, A.; Jakka, S.C.B. Studies on application of Petcoke-based graphene membranes for CO2 separation. Sep. Sci. Technol. 2023, 58, 2738–2747. [Google Scholar] [CrossRef]
- Sierra, U.; Álvarez, P.; Blanco, C.; Granda, M.; Santamaría, R.; Menéndez, R. Cokes of different origin as precursors of graphene oxide. Fuel 2016, 166, 400–403. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L. Green and facile production of high-quality graphene from graphite by the combination of hydroxyl radicals and electrical exfoliation in different electrolyte systems. RSC Adv. 2019, 9, 3693–3703. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Chia, W.Y.; Cheah, W.Y.; Munawaroh, H.S.H.; Ong, W.J. Abatement of hazardous materials and biomass waste via pyrolysis and co-pyrolysis for environmental sustainability and circular economy. Environ. Pollut. 2021, 278, 116836. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Y. Recent progress in the conversion of biomass wastes into functional materials for value-added applications. Sci. Technol. Adv. Mater. 2020, 21, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Kalak, T. Potential use of industrial biomass waste as a sustainable energy source in the future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Han, Y.; Ge, Y.; Chao, Y.; Wang, C.; Wallace, G.G. Recent progress in 2D materials for flexible supercapacitors. J. Energy Chem. 2018, 27, 57–72. [Google Scholar] [CrossRef]
- Li, G.; Luican, A.; Andrei, E.Y. Scanning tunneling spectroscopy of graphene on graphite. Phys. Rev. Lett. 2009, 102, 176804. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Dong, L.; Zhang, J.; Lu, H. Room-temperature intercalation and ~1000-fold chemical expansion for scalable preparation of high-quality graphene. Chem. Mater. 2016, 28, 2138–2146. [Google Scholar] [CrossRef]
- Kang, F.; Leng, Y.; Zhang, T.Y. Influences of H2O2 on synthesis of H2SO4-GICs. J. Phys. Chem. Solids 1996, 57, 889–892. [Google Scholar] [CrossRef]
- Tryba, B.; Morawski, A.W.; Inagaki, M. Preparation of exfoliated graphite by microwave irradiation. Carbon 2005, 43, 2417–2419. [Google Scholar] [CrossRef]
- Safian, M.T.U.; Haron, U.S.; Ibrahim, M.M. A review on bio-based graphene derived from biomass wastes. Bioresources 2020, 15, 9756. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, Z.F.; Zhang, Y.S.; Morozov, S.V.; Stormer, H.L.; Zeitler, U.; Maan, J.C.; Boebinger, G.S.; Kim, P.; Geim, A.K. Room-temperature quantum Hall effect in graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef] [PubMed]
- Fasolino, A.; Los, J.H.; Katsnelson, M.I. Intrinsic ripples in graphene. Nat. Mater. 2007, 6, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Shang, H.; Wang, W. Hypervelocity impact properties of graphene armor via molecular dynamics simulations. EPJ Web Conf. 2012, 26, 04027. [Google Scholar] [CrossRef]
- Lee, J.U.; Yoon, D.; Cheong, H. Estimation of Young’s modulus of graphene by Raman spectroscopy. Nano Lett. 2012, 12, 4444–4448. [Google Scholar] [CrossRef]
- Wallace, P.R. The band theory of graphite. Phys. Rev. 1947, 71, 622. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Chen, J.H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat. Nanotechnol. 2008, 3, 206–209. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.L.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Tian, C.; Girit, C.; Zettl, A.; Crommie, M.; Shen, Y.R. Gate-variable optical transitions in graphene. Science 2008, 320, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henriksen, E.A.; Jiang, Z.; Hao, Z.; Martin, M.C.; Kim, P.; Stormer, H.L.; Basov, D.N. Dirac charge dynamics in graphene by infrared spectroscopy. Nat. Phys. 2008, 4, 532–535. [Google Scholar] [CrossRef]
- Rana, F.; George, P.A.; Strait, J.H.; Dawlaty, J.; Shivaraman, S.; Chandrashekhar, M.; Spencer, M.G. Carrier recombination and generation rates for intravalley and intervalley phonon scattering in graphene. Phys. Rev. B 2009, 79, 115447. [Google Scholar] [CrossRef]
- Gokus, T.; Nair, R.R.; Bonetti, A.; Bohmler, M.; Lombardo, A.; Novoselov, K.S.; Geimt, A.K.; Ferrari, A.C.; Hartschuh, A. Making graphene luminescent by oxygen plasma treatment. ACS Nano 2009, 3, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Sofer, Z. Towards stoichiometric analogues of graphene: Graphane, fluorographene, graphol, graphene acid and others. Chem. Soc. Rev. 2017, 46, 4450–4463. [Google Scholar] [CrossRef]
- Ho, K.I.; Boutchich, M.; Su, C.Y.; Moreddu, R.; Marianathan, E.S.R.; Montes, L.; Lai, C.S. A self–aligned high–mobility graphene transistor: Decoupling the channel with fluorographene to reduce scattering. Adv. Mater. 2015, 27, 6519–6525. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A review on the mechanical properties of polymer composites reinforced by carbon nanotubes and graphene. Carbon Lett. 2021, 31, 149–165. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q. Molecular dynamic simulation of mechanical behaviour of RGO produced by thermal reduction method. Micro Nano Lett. 2017, 12, 638–642. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large–area thin–film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Moggio, B.C.; Bergamasco, R.; Andrade, C.M.G.; Aylon, L.B.R. On the Analysis of Cryogels and Xerogels Using Cellulose Nanofibers and Graphene Oxide. Polymers 2023, 15, 3833. [Google Scholar] [CrossRef]
- Andonegi, M.; Correia, D.M.; Pereira, N.; Costa, C.M.; Lanceros-Mendez, S.; Caba, K.; Guerrero, P. Sustainable Collagen Composites with Graphene Oxide for Bending Resistive Sensing. Polymers 2023, 15, 3855. [Google Scholar] [CrossRef]
- Yadav, S.; Singh Raman, A.P.; Meena, H.; Goswami, A.G.; Bhawna; Kumar, V.; Jain, P.; Kumar, G.; Sagar, M.; Rana, D.K.; et al. An update on graphene oxide: Applications and toxicity. ACS Omega 2022, 7, 35387–35445. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yan, B.; Li, T.; Long, Y.; Li, N.; Ye, M. Study on graphene-oxide-based polyacrylamide composite hydrogels. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1476–1481. [Google Scholar] [CrossRef]
- Barbosa, M.C.; Razzino, C.D.A.; Stocco, T.D.; Santana, M.D.V.; Ghosh, A.; Pereira, L.F.; Tierra-Criollo, C.J.; Lobo, A.O. Production of rGO-Based Electrospinning Nanocomposites Incorporated in Recycled PET as an Alternative Dry Electrode. Polymers 2022, 14, 4288. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly (sodium 4-styrenesulfonate). J. Mater. Chem. A 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, G.; Zheng, L.; Liu, Z.; Wang, B.; Wu, H.; He, Z.; Jin, Z.; Wang, G. High-Performance Near-Infrared Photodetector by Integration of PbS Quantum Dots with 3D-Graphene. IEEE Electron Device Lett. 2023, 44, 1240–1243. [Google Scholar] [CrossRef]
- Denis, P.A. Band gap opening of monolayer and bilayer graphene doped with aluminium, silicon, phosphorus, and sulfur. Chem. Phys. Lett. 2010, 492, 251–257. [Google Scholar] [CrossRef]
- García-Lastra, J.M. Strong dependence of band-gap opening at the Dirac point of graphene upon hydrogen adsorption periodicity. Phys. Rev. B 2010, 82, 235418. [Google Scholar] [CrossRef]
- Chen, S.; Duan, J.; Jaroniec, M.; Qiao, S.Z. Three–dimensional N–doped graphene hydrogel/NiCo double hydroxide electrocatalysts for highly efficient oxygen evolution. Angew. Chem. Int. Ed. 2013, 52, 13567–13570. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Botlhoko, O.J.; Setshedi, K.; Scriba, M.; Masukume, M.; Ray, S.S. Top-down synthesis of graphene: A comprehensive review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical vapour deposition of graphene—Synthesis, characterisation, and applications: A review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Gutiérrez-Cruz, A.; Ruiz-Hernández, A.R.; Vega-Clemente, J.F.; Luna-Gazcón, D.G.; Campos-Delgado, J. A review of top-down and bottom-up synthesis methods for the production of graphene, graphene oxide and reduced graphene oxide. J. Mater. Sci. 2022, 57, 14543–14578. [Google Scholar] [CrossRef]
- Wehling, T.O.; Novoselov, K.S.; Morozov, S.V.; Vdovin, E.E.; Katsnelson, M.I.; Geim, A.K.; Lichtenstein, A.I. Molecular Doping of Graphene. Nano Lett. 2008, 8, 173–177. [Google Scholar] [CrossRef]
- Tian, W.; Liu, X.; Yu, W. Research progress of gas sensor based on graphene and its derivatives: A review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef]
- Land, T.A.; Michely, T.; Behm, R.J.; Hemminger, J.C.; Comsa, G. STM investigation of single layer graphite structures produced on Pt (111) by hydrocarbon decomposition. Surf. Sci. 1992, 264, 261–270. [Google Scholar] [CrossRef]
- Suk, J.W.; Kitt, A.; Magnuson, C.W.; Hao, Y.; Ahmed, S.; An, J.; Swan, A.K.; Goldberg, B.B.; Ruoff, R.S. Transfer of CVD-grown monolayer graphene onto arbitrary substrates. ACS Nano 2011, 5, 6916–6924. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O‘Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Banerjee, B.C.; Hirt, T.J.; Walker, P.L. Pyrolytic carbon formation from carbon suboxide. Nature 1961, 192, 450–451. [Google Scholar] [CrossRef]
- Robertson, S.D. Graphite formation from low temperature pyrolysis of methane over some transition metal surfaces. Nature 1969, 221, 1044–1046. [Google Scholar] [CrossRef]
- Wang, R.; Hao, Y.; Wang, Z.; Gong, H.; Thong, J.T. Large-diameter graphene nanotubes synthesized using Ni nanowire templates. Nano Lett. 2010, 10, 4844–4850. [Google Scholar] [CrossRef]
- Juang, Z.Y.; Wu, C.Y.; Lu, A.Y.; Su, C.Y.; Leou, K.C.; Chen, F.R.; Tsai, C.H. Graphene synthesis by chemical vapor deposition and transfer by a roll-to-roll process. Carbon 2010, 48, 3169–3174. [Google Scholar] [CrossRef]
- Chen, S.; Bai, Q.; Wang, H.; Dou, Y.; Guo, W. Controlled growth of large-area monolayer graphene on Ni (110) facet: Insight from molecular dynamics simulation. Phys. E 2022, 144, 115465. [Google Scholar] [CrossRef]
- Orofeo, C.M.; Ago, H.; Hu, B.; Tsuji, M. Synthesis of large area, homogeneous, single layer graphene films by annealing amorphous carbon on Co and Ni. Nano Res. 2011, 4, 531–540. [Google Scholar] [CrossRef]
- Wang, H.; Yu, G. Direct CVD graphene growth on semiconductors and dielectrics for transfer–free device fabrication. Adv. Mater. 2016, 28, 4956–4975. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G. Graphene CVD growth on copper and nickel: Role of hydrogen in kinetics and structure. Phys. Chem. Chem. Phys. 2011, 13, 20836–20843. [Google Scholar] [CrossRef]
- Munoz, R.; Gómez–Aleixandre, C. Review of CVD synthesis of graphene. Chem. Vap. Depos. 2013, 19, 297–322. [Google Scholar] [CrossRef]
- Li, X.; Magnuson, C.W.; Venugopal, A.; Tromp, R.M.; Hannon, J.B.; Vogel, E.M.; Colombo, L.; Ruoff, R.S. Large-area graphene single crystals grown by low-pressure chemical vapor deposition of methane on copper. J. Am. Chem. Soc. 2011, 133, 2816–2819. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, X.; Yuan, Q.; Xue, J.; Lu, G.; Liu, Z.; Wang, H.; Wang, H.; Ding, F.; Yu, Q.; et al. Fast growth of inch-sized single-crystalline graphene from a controlled single nucleus on Cu–Ni alloys. Nat. Mater. 2016, 15, 43–47. [Google Scholar] [CrossRef]
- Park, H.J.; Meyer, J.; Roth, S.; Skákalová, V. Growth and properties of few-layer graphene prepared by chemical vapor deposition. Carbon 2010, 48, 1088–1094. [Google Scholar] [CrossRef]
- Brodie, B.C. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Endemann, H. Berichte der Deutschen Chemischen Gesellschaft. J. Am. Chem. Soc. 1880, 2, 366–371. [Google Scholar] [CrossRef][Green Version]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Wang, H.; Robinson, J.T.; Li, X.; Dai, H. Solvothermal reduction of chemically exfoliated graphene sheets. J. Am. Chem. Soc. 2009, 131, 9910–9911. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, Z.; Zhou, Q.; Gogotsi, Y.; Qiu, J. The role of microwave absorption on formation of graphene from graphite oxide. Carbon 2012, 50, 3267–3273. [Google Scholar] [CrossRef]
- Tateishi, H.; Koinuma, M.; Miyamoto, S.; Kamei, Y.; Hatakeyama, K.; Ogata, C.; Taniguchi, T.; Funatsu, A.; Matsumoto, Y. Effect of the electrochemical oxidation/reduction cycle on the electrochemical capacitance of graphite oxide. Carbon 2014, 76, 40–45. [Google Scholar] [CrossRef]
- Lung-Hao Hu, B.; Wu, F.Y.; Lin, C.T.; Khlobystov, A.N.; Li, L.J. Graphene-modified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Rong, P.; Yu, Q. Preparations, properties and applications of graphene in functional devices: A concise review. Ceram. Int. 2018, 44, 11940–11955. [Google Scholar] [CrossRef]
- Manoratne, C.H.; Rosa, S.R.D.; Kottegoda, I.R.M. XRD-HTA, UV visible, FTIR and SEM interpretation of reduced graphene oxide synthesized from high purity vein graphite. Mater. Sci. Res. India 2017, 14, 19–30. [Google Scholar] [CrossRef]
- Wu, W.; Yu, B. Corn flour nano-graphene prepared by the hummers redox method. ACS Omega 2020, 5, 30252–30256. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.C.; Patil, H.R.; Blakely, J.M. Equilibrium segregation of carbon to a nickel (111) surface: A surface phase transition. Surf. Sci. 1974, 43, 493–520. [Google Scholar] [CrossRef]
- Lahiri, J.; Miller, T.S.; Ross, A.J.; Adamska, L.; Oleynik, I.I.; Batzill, M. Graphene growth and stability at nickel surfaces. New J. Phys. 2011, 13, 025001. [Google Scholar] [CrossRef]
- Virojanadara, C.; Syväjarvi, M.; Yakimova, R.; Johansson, L.I.; Zakharov, A.A.; Balasubramanian, T. Homogeneous large-area graphene layer growth on 6 H-SiC (0001). Phys. Rev. B 2008, 78, 245403. [Google Scholar] [CrossRef]
- Hass, J.; Varchon, F.; Millan-Otoya, J.E.; Sprinkle, M.; Sharma, N.; Heer, W.A.; Berger, C.; First, P.N.; Magaud, L.; Conrad, E.H. Why multilayer graphene on 4 H–SiC (0001) behaves like a single sheet of graphene. Phys. Rev. Lett. 2008, 100, 125504. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Pan, Y.; Huang, L.; Hu, H.; Zhang, L.Z.; Guo, H.M.; Du, S.X.; Gao, H.J. Epitaxial growth and structural property of graphene on Pt (111). Appl. Phys. Lett. 2011, 98, 033101. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, W.; Zhang, Z. Contrasting behavior of carbon nucleation in the initial stages of graphene epitaxial growth on stepped metal surfaces. Phys. Rev. Lett. 2010, 104, 186101. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017, 35, 466–489. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol production from lignocellulosic biomass—Challenges and solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Kharangarh, P.R.; Ravindra, N.M.; Singh, G.; Umapathy, S. Synthesis of luminescent graphene quantum dots from biomass waste materials for energy–related applications—An Overview. Energy Storage 2023, 5, e390. [Google Scholar] [CrossRef]
- Zhou, Y.; He, J.; Chen, R.; Li, X. Recent advances in biomass-derived graphene and carbon nanotubes. Mater. Today Sustain. 2022, 18, 100138. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Dijk, C.; Doorn, W.; Alfen, B. Long term plant biomonitoring in the vicinity of waste incinerators in The Netherlands. Chemosphere 2015, 122, 45–51. [Google Scholar] [CrossRef]
- Prasad, R.; Abhijeet, S.; Garg, R.; Hosmane, G.B. Biomass fuel exposure and respiratory diseases in India. Biosci. Trends 2012, 6, 219–228. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2012, 38, 594–608. [Google Scholar] [CrossRef]
- Li, L.; Tang, Y.; Bao, Z.; Tu, W.; Peng, L.; Zou, L.; Zhao, C.; Li, Q. When graphene meets circular agriculture: Insights into agricultural sustainable development. Biosyst. Eng. 2024, 237, 92–117. [Google Scholar] [CrossRef]
- Hossain, S.S.; Mathur, L.; Roy, P.K. Rice husk/rice husk ash as an alternative source of silica in ceramics: A review. J. Asian Ceram. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- Bakar, R.A.; Yahya, R.; Gan, S.N. Production of high purity amorphous silica from rice husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar] [CrossRef]
- Allegretti, C.; Bellinetto, E.; D’Arrigo, P.; Ferro, M.; Griffini, G.; Rossato, L.A.M.; Ruffini, E.; Schiavi, L.; Serra, S.; Strini, A.; et al. Fractionation of Raw and Parboiled Rice Husks with Deep Eutectic Solvents and Characterization of the Extracted Lignins towards a Circular Economy Perspective. Molecules 2022, 27, 8879. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; et al. Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: A comprehensive utilization strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Lin, C.; Yang, W.; Meng, Y.; Guo, Y.; Li, M.; Xiao, D. Hierarchically porous nitrogen-rich carbon derived from wheat straw as an ultra-high-rate anode for lithium ion batteries. J. Mater. Chem. A 2012, 2, 9684–9690. [Google Scholar] [CrossRef]
- Chen, F.; Yang, J.; Bai, T.; Long, B.; Zhou, X. Facile synthesis of few-layer graphene from biomass waste and its application in lithium ion batteries. J. Electroanal. Chem. 2016, 768, 18–26. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, F.; Bai, T.; Long, B.; Liao, Q.; Ren, Y.; Yang, J. Interconnected highly graphitic carbon nanosheets derived from wheat stalk as high performance anode materials for lithium ion batteries. Green Chem. 2016, 18, 2078–2088. [Google Scholar] [CrossRef]
- Monties, B. Plant cell walls as fibrous lignocellulosic composites: Relations with lignin structure and function. Anim. Feed Sci. Technol. 1991, 32, 159–175. [Google Scholar] [CrossRef]
- Manchala, S.; Tandava, V.S.R.K.; Jampaiah, D.; Bhargava, S.K.; Shanker, V. Novel and highly efficient strategy for the green synthesis of soluble graphene by aqueous polyphenol extracts of eucalyptus bark and its applications in high-performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11612–11620. [Google Scholar] [CrossRef]
- Severo, L.S.; Thue, P.S.; Lima, D.R.; Didó, C.A.; Vasconcellos, M.A.; Armas, L.E.; Lima, E.C.; Benvenutti, E.V.; Menezes, E.W. 3D graphene sponge biomass-derived with high surface area applied as adsorbent for nitrophenols. J. Environ. Chem. Eng. 2023, 11, 109924. [Google Scholar] [CrossRef]
- Alfè, M.; Gargiulo, V.; Porto, M.; Migliaccio, R.; Le Pera, A.; Sellaro, M.; Pellegrino, C.; Abe, A.A.; Urciuolo, M.; Caputo, P.; et al. Pyrolysis and Gasification of a Real Refuse-Derived Fuel (RDF): The Potential Use of the Products under a Circular Economy Vision. Molecules 2022, 27, 8114. [Google Scholar] [CrossRef]
- Amaral, C.; Pedro, M.I.; Ferreira, D.C.; Marques, R.C. Performance and its determinants in the Portuguese municipal solid waste utilities. Waste Manag. 2022, 139, 70–84. [Google Scholar] [CrossRef]
- Harst, E.; Potting, J. A critical comparison of ten disposable cup LCAs. Environ. Impact Assess. Rev. 2012, 43, 86–96. [Google Scholar] [CrossRef]
- Ruan, G.; Sun, Z.; Peng, Z.; Tour, J.M. Growth of graphene from food, insects, and waste. ACS Nano 2011, 5, 7601–7607. [Google Scholar] [CrossRef]
- Rum, L.; Sten, O.; Vendrame, E.; Belluscio, V.; Camomilla, V.; Vannozzi, G.; Truppa, L.; Notarantonio, M.; Sciarra, T.; Lazich, A.; et al. Wearable sensors in sports for persons with disability: A systematic review. Sensors 2021, 21, 1858. [Google Scholar] [CrossRef]
- Pani, D.; Achilli, A.; Bonfiglio, A. Survey on textile electrode technologies for electrocardiographic (ECG) monitoring, from metal wires to polymers. Adv. Mater. Technol. 2018, 3, 1800008. [Google Scholar] [CrossRef]
- Ge, G.; Huang, W.; Shao, J.; Dong, X. Recent progress of flexible and wearable strain sensors for human-motion monitoring. J. Semicond. 2018, 39, 011012. [Google Scholar] [CrossRef]
- Zou, Y.; Zhong, M.; Li, S.; Qing, Z.; Xing, X.; Gong, G.; Zhou, C. Flexible Wearable Strain Sensors Based on Laser-Induced Graphene for Monitoring Human Physiological Signals. Polymers 2023, 15, 3553. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.I.; Kim, D.; Hyeon, T.; Kim, D.H. High-performance stretchable conductive nanocomposites: Materials, processes, and device applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef]
- Shathi, M.A.; Chen, M.; Khoso, N.A.; Rahman, M.T.; Bhattacharjee, B. Graphene coated textile based highly flexible and washable sports bra for human health monitoring. Mater. Des. 2020, 193, 108792. [Google Scholar] [CrossRef]
- Zheng, L.; Cheng, X.; Cao, D.; Wang, G.; Wang, Z.; Xu, D.; Xia, C.; Shen, L.; Yu, Y.; Shen, D. Improvement of Al2O3 films on graphene grown by atomic layer deposition with pre-H2O treatment. ACS Appl. Mater. Interfaces 2014, 6, 7014–7019. [Google Scholar] [CrossRef]
- Raza, T.; Tufail, M.K.; Ali, A.; Boakye, A.; Qi, X.; Ma, Y.; Ali, A.; Qu, L.; Tian, M. Wearable and flexible multifunctional sensor based on laser-induced graphene for the sports monitoring system. ACS Appl. Mater. Interfaces 2022, 14, 54170–54181. [Google Scholar] [CrossRef]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef]
- Shi, J.; Yang, J.; Zhou, J.; Ji, H.; Tang, X.; Gao, T. Effect of graphene on thermal stability and mechanical properties of ethylene-vinyl acetate: A molecular dynamics simulation. Mater. Res. Express 2020, 7, 035304. [Google Scholar] [CrossRef]
- Lunchev, A.V.; Kashcheev, A.; Lipik, V. Mechanical characteristics of poly (ethylene vinyl acetate) foams with graphene for the applications in sport footwear. Polym. Test. 2022, 113, 107688. [Google Scholar] [CrossRef]
- Tang, L.C.; Wan, Y.J.; Yan, D.; Pei, Y.B.; Zhao, L.; Li, Y.B.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Fu, B.; Gao, J. Study on the application of graphene polymer material in sports equipment. J. Phys. Conf. Ser. 2020, 1676, 012044. [Google Scholar] [CrossRef]
- HEAD Technology GMBH Ltd. Sporting Goods with Graphene Material. U.S. Patent 2013/0090193, 11 April 2013.
- Young, R.J.; Liu, M. The microstructure of a graphene-reinforced tennis racquet. J. Mater. Sci. 2016, 51, 3861–3867. [Google Scholar] [CrossRef]
- Kalaoglu-Altan, O.I.; Kayaoglu, B.K.; Trabzon, L. Improving thermal conductivities of textile materials by nanohybrid approaches. iScience 2022, 25, 103825. [Google Scholar] [CrossRef]
- Havenith, G.; Fiala, D. Thermal indices and thermophysiological modeling for heat stress. Compr. Physiol. 2011, 6, 255–302. [Google Scholar]
- Bhattacharjee, S.; Joshi, R.; Chughtai, A.A.; Macintyre, C.R. Graphene modified multifunctional personal protective clothing. Adv. Mater. Interfaces 2019, 6, 1900622. [Google Scholar] [CrossRef]
- Determining the Performance of Graphene-Enhanced Sportswear|Technical Textiles. Available online: https://www.technicaltextiles.net/node/76391 (accessed on 28 December 2023).
- Liu, T. Properties of graphene composite fiber seamless knitted fabric and its application in boxing training. Front. Mater. 2023, 10, 1098652. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Wang, S. Running compression garments design using biomass graphene modified fiber. J. Fiber Bioeng. Inform. 2019, 12, 155–166. [Google Scholar] [CrossRef]
- Widuchowski, W.; Widuchowski, J.; Koczy, B.; Szyluk, K. Untreated asymptomatic deep cartilage lesions associated with anterior cruciate ligament injury: Results at 10-and 15-year follow-up. Am. J. Sports Med. 2009, 37, 688–692. [Google Scholar] [CrossRef]
- Park, J.K.; Jung, J.; Subramaniam, P.; Shah, B.P.; Kim, C.; Lee, J.K.; Cho, J.H.; Lee, C.; Lee, K.B. Graphite-coated magnetic nanoparticles as multimodal imaging probes and cooperative therapeutic agents for tumor cells. Small 2011, 7, 1647. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Zheng, B.; He, J.; Liu, J.; Chen, C.; Lee, I.; Wang, X.; Liu, Y. Synergistic effects on incorporation of β-tricalcium phosphate and graphene oxide nanoparticles to silk fibroin/soy protein isolate scaffolds for bone tissue engineering. Polymers 2020, 12, 69. [Google Scholar] [CrossRef]
- Wang, C.H.; Guo, Z.S.; Pang, F.; Zhang, L.Y.; Yan, M.; Yan, J.H.; Li, K.W.; Li, X.J.; Li, Y.; Bi, L.; et al. Effects of graphene modification on the bioactivation of polyethylene-terephthalate-based artificial ligaments. ACS Appl. Mater. Interfaces 2015, 7, 15263–15276. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Cao, N.; Wang, Y.; Li, H.; Rooij, N.F.D.; Umar, A.; Feng, Y.; French, P.J.; Zhou, G. Three-dimensional graphene-based foams with “greater electron transferring areas” deriving high gas sensitivity. ACS Appl. Nano Mater. 2021, 4, 13234–13245. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Pan, D.; Wang, Y.; Noetzel, R.; Li, H.; Xie, P.; Pei, W.; Umar, A.; Jiang, L.; et al. Mimicking a dog’s nose: Scrolling graphene nanosheets. ACS Nano 2018, 12, 2521–2530. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Umar, A.; Wang, Y.; Li, H.; Zhou, G. Three-dimensional crumpled graphene-based nanosheets with ultrahigh NO2 gas sensibility. ACS Appl. Mater. 2017, 9, 11819–11827. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Larsen Andersen, M.; Talbro Barsberg, S. The nature of stable char radicals: An ESR and DFT study of structural and hydrogen bonding requirements. ChemPlusChem 2018, 83, 780–786. [Google Scholar] [CrossRef]
- Surup, G.R.; Nielsen, H.K.; Heidelmann, M.; Trubetskaya, A. Characterization and reactivity of charcoal from high temperature pyrolysis (800–1600 C). Fuel 2019, 235, 1544–1554. [Google Scholar] [CrossRef]
- Tamuly, J.; Bhattacharjya, D.; Saikia, B.K. Graphene/graphene derivatives from coal, biomass, and wastes: Synthesis, energy applications, and perspectives. Energy Fuels 2022, 36, 12847–12874. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, G.; Liu, R.; Qu, J.; Liu, H. Potential applications of solid waste-based geopolymer materials: In wastewater treatment and greenhouse gas emission reduction. J. Clean. Prod. 2024, 443, 141144. [Google Scholar] [CrossRef]

| Materials | Experimental Technique | Young’s Modulus | Tensile Strength | Refs. |
|---|---|---|---|---|
| Monolayer graphene | Nano-indentation in AFM | 1 TPa | 130 GPa | [41] |
| Free standing GO | Nano-indentation on a dynamic contact tool | 697 ± 15 GPa | 3–33 GPa | [41] |
| RGO paper | - | 41.8 GPa | 293.3 MPa | [42] |
| Method | Substrate | Temperature (°C) | Yield | Refs. |
|---|---|---|---|---|
| Micromechanical exfoliation | SiO2/Si | Room temperature | Difficult to generate production output | [62] |
| Chemical vapor deposition | Cu, Pt, Ni, Ru, Ir | >1000 | Can be produced on a large scale | [63] |
| Oxidation reduction | - | <500 | Can be produced on a large scale | [64] |
| Epitaxial growth | SiC | 1200–1600 | Suitable for small-scale production | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, Y.; Zhang, X. The Future of Graphene: Preparation from Biomass Waste and Sports Applications. Molecules 2024, 29, 1825. https://doi.org/10.3390/molecules29081825
Wu Y, Li Y, Zhang X. The Future of Graphene: Preparation from Biomass Waste and Sports Applications. Molecules. 2024; 29(8):1825. https://doi.org/10.3390/molecules29081825
Chicago/Turabian StyleWu, Yueting, Yanlong Li, and Xiangyang Zhang. 2024. "The Future of Graphene: Preparation from Biomass Waste and Sports Applications" Molecules 29, no. 8: 1825. https://doi.org/10.3390/molecules29081825
APA StyleWu, Y., Li, Y., & Zhang, X. (2024). The Future of Graphene: Preparation from Biomass Waste and Sports Applications. Molecules, 29(8), 1825. https://doi.org/10.3390/molecules29081825