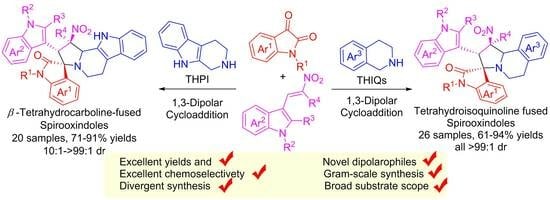
4. Experimental Section
4.1. General Information
Reagents were of the highest commercial grade (Adamas), the solvents were AR, and were used without further purification. NMR spectra were recorded on a Bruker DRX 400 (
1H: 400 MHz,
13C: 100 MHz) with TMS as the internal standard. Chemical shifts (
δ) were expressed in ppm,
J values were given in Hz, and deuterated DMSO-
d6 was used as a solvent. IR spectra were recorded on an FT-IR Thermo Nicolet Avatar 360 using KBr pellet. The mass spectroscopic data were obtained from an Agilient 6550 Q-TOF & Thermo Fisher-QE spectrometer. Melting points were determined with an SGWX-4 melting-point apparatus. The reactions were monitored by thin-layer chromatography (TLC) with silica gel GF
254, and all compounds were visualized by UV and sprayed with H
2SO
4 (10%) in ethanol, followed by heating. A suitable single crystal was selected and analyzed with Rigaku XtaLab Synergy. The
1H and
13C NMR spectras for compounds
4,
6,
8, and single crystal X-ray diffraction study data of compound
4c,
6b were included in
Supplementary Materials.
4.2. General Procedure for the Synthesis of Compounds 4
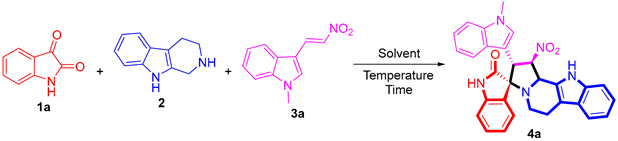
A mixture of isatins (1, 0.5 mmol), THPI (2, 0.5 mmol), 3-(2-nitrovinyl)-indoles (3, 0.5 mmol) in MeOH was stirred under reflux conditions for 12 h and indicated by TLC. Then, the reaction mixture was allowed to cool to room temperature and extracted with dichloromethane (3 × 50 mL). The combined organic layers were washed with saturated brine solution (20 mL), followed by drying over MgSO4 and evaporating in vacuo. The crude product was purified by column chromatography to give the pure title compounds.
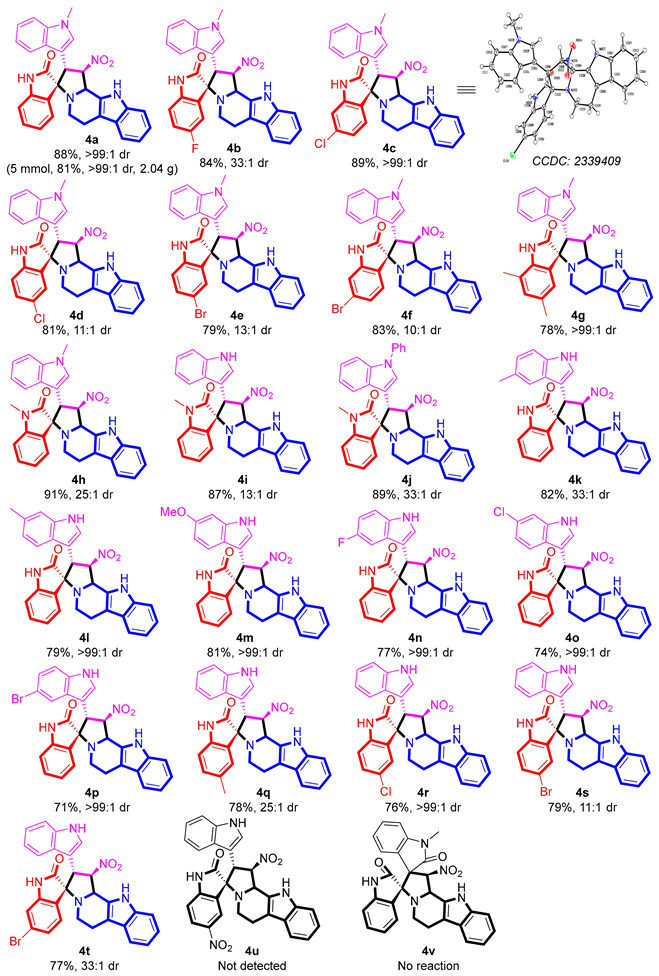
2′-(1-Methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4a). Yellow solid; 88% yield; >99: 1 dr; mp 258.7–258.9 °C; IR(KBr) 741, 1011, 1192, 1315, 1335, 1450, 1472, 1553, 1711, 2818, 3057, 3337 cm−1; HRMS(ESI) calcd for C30H25N5O3 [M+H]+ 504.2030, found 504.2024; 1H-NMR (400 MHz, DMSO-d6): δ 10.85 (s, 1H, NH), 10.17 (s, 1H, NH), 7.72 (d, J = 7.2 Hz, 1H, ArH), 7.44–7.39 (m, 3H, ArH), 7.35–7.31 (m, 2H, ArH), 7.30–7.18 (m, 1H, ArH), 7.23–7.06 (m, 2H, ArH), 7.04–6.98 (m, 1H, ArH), 6.81–6.75 (m, 2H, ArH), 6.66 (d, J = 7.6 Hz, 1H, ArH), 6.09–6.07 (m, 1H, CH), 5.97 (d, J = 6.8 Hz, 1H, CH), 4.84 (d, J = 4.0 Hz, 1H, CH), 3.76 (s, 3H, CH2), 2.80–2.73 (m, 2H, CH2), 2.68–2.64 (m, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 177.2, 143.6, 136.6, 136.5, 130.3, 128.6, 128.5, 127.4, 126.9, 124.9, 122.9, 121.8, 121.5, 119.3, 119.1, 118.4, 118.3, 112.0, 110.3, 110.1, 109.5, 108.9, 92.2, 74.8, 60.6, 51.7, 43.3, 33.0, 22.4.
5-Fluoro-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4b). Light yellow solid; 84% yield; 33: 1 dr; mp 269–270 °C; IR(KBr) 741, 814, 1121, 1169, 1321, 1458, 1487, 1557, 1717, 2887, 3308, 3368 cm−1; HRMS(ESI) calcd for C30H24FN5O3 [M+H]+ 522.1936, found 522.1940; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 10.88 (s, 1H, NH), 10.21 (s, 1H, NH), 7.56–7.54 (m, 1H, ArH), 7.43 (d, J = 8.0 Hz, 3H, ArH), 7.40–7.34 (m, 1H, ArH), 7.17–7.06 (m, 3H, ArH), 7.00 (d, J = 14.8 Hz, 1H, ArH), 6.88–6.82 (m, 2H, ArH), 6.68–6.64 (m, 1H, ArH), 6.10–6.07 (m, 1H, CH), 5.95 (d, J = 6.4 Hz, 1H, CH), 4.84 (d, J = 4.0 Hz, 1H, CH), 3.76 (s, 3H, CH3), 2.81–2.65 (m, 4H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 177.1, 160.1, 157.7, 139.8, 136.6, 136.5, 130.5, 130.4, 130.1, 128.8, 127.3, 126.9, 121.8, 121.5, 119.4, 119.1, 118.4, 118.3, 116.9, 112.0, 111.1, 110.3, 109.4, 107.8, 91.9, 75.1, 60.7, 51.6, 43.4, 33.0, 22.4.
6-Chloro-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4c). Chartreuse solid; 89% yield; >99: 1 dr; mp 268–269 °C; IR(KBr) 568, 733, 918, 1128, 1177, 1319, 1483, 1545, 1618, 1699, 1719, 2893, 3312, 3377 cm−1; HRMS(ESI) calcd for C30H24ClN5O3 [M+H]+ 538.1640, found 538.1644; 1H-NMR (400 MHz, DMSO-d6): δ 10.86 (s, 1H, NH), 10.32 (s, 1H, NH), 7.73 (d, J = 8.0 Hz, 1H, ArH), 7.44–7.35 (m, 4H, ArH), 7.27 (d, J = 8.0 Hz, 1H, ArH), 7.09 (t, J = 12.4 Hz, 2H, ArH), 7.00 (t, J = 14.8 Hz, 1H, ArH), 6.85 (d, J = 3.6 Hz, 2H, ArH), 6.68 (s, 1H, ArH), 6.08 (t, J = 10.8 Hz, 1H, CH), 5.93 (d, J = 6.4 Hz, 1H, CH), 4.83 (d, J = 4.0 Hz, 1H, CH), 3.77 (s, 3H, CH3), 2.79–2.65 (m, 4H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 177.1, 145.0, 136.6, 136.5, 134.5, 130.1, 128.8, 127.5, 127.3, 126.8, 126.5, 122.8, 121.9, 121.5, 119.4, 119.1, 118.3, 112.0, 110.4, 110.2, 109.4, 107.7, 91.9, 74.5, 60.7, 51.6, 43.3, 33.0, 22.4.
5-Chloro-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4d). Chartreuse solid; 81% yield; 11: 1 dr; mp 242–243 °C; IR(KBr) 733, 741, 1045, 1194, 1238, 1323, 1476, 1545, 1618, 1697, 1740, 3300, 3366 cm-1; HRMS(ESI) calcd for C30H24ClN5O3 [M+H]+ 538.1640, found 538.1644; 1H-NMR (400 MHz, DMSO-d6): δ 10.87 (s, 1H, NH), 10.29 (s, 1H, NH), 7.71 (s, 1H, ArH), 7.43 (d, J = 8.0 Hz, 5H, ArH), 7.01–7.00 (m, 3H, ArH), 6.85 (s, 2H, ArH), 6.68 (s, 1H, ArH), 6.09 (s, 1H, CH), 5.93 (s, 1H, CH), 4.85 (s, 1H, CH), 3.77 (s, 3H, CH3), 2.77–2.66 (m, 4H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 176.8, 142.5, 136.7, 136.5, 130.9, 130.2, 130.0, 128.8, 127.2, 126.9, 124.8, 121.9, 121.5, 119.4, 119.1, 118.4, 118.3, 112.0, 111.7, 110.3, 109.5, 107.8, 91.8, 74.8, 60.2, 51.7, 43.3, 33.0, 22.4.
5-Bromo-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4e). Light yellow solid; 79% yield; 13: 1 dr; mp 235–236 °C; IR(KBr) 428, 733, 820, 1045, 1173, 1194, 1238, 1323, 1474, 1545, 1618, 1697, 1740, 1816, 3300, 3362 cm−1; HRMS(ESI) calcd for C30H24BrN5O3 [M]+ 581.1063, found 581.1070; 1H-NMR (400 MHz, DMSO-d6): δ 10.87 (s, 1H, NH), 10.29 (s, 1H, NH), 7.83 (d, J = 2.0 Hz, 1H, ArH), 7.51 (d, J = 2.0 Hz, 1H, ArH), 7.49–7.42 (m, 2H, ArH), 7.40–7.35 (m, 2H, ArH), 7.10–7.04 (m, 2H, ArH), 7.00 (t, J = 14.8 Hz, 1H, ArH), 6.84 (d, J = 3.6 Hz, 2H, ArH), 6.64 (d, J = 8.4 Hz, 1H, ArH), 6.10–6.08 (m, 1H, CH), 5.92 (d, J = 6.4 Hz, 1H, CH), 4.84 (d, J = 3.6 Hz, 1H, CH), 3.76 (s, 3H, CH3), 2.79–2.65 (m, 4H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 176.6, 143.0, 136.7, 136.5, 133.1, 131.3, 130.0, 128.8, 127.6, 127.2, 126.9, 121.9, 121.6, 119.4, 119.1, 118.4, 118.3, 114.5, 112.2, 110.1, 110.3, 109.5, 107.9, 91.7, 74.8, 60.2, 51.7, 43.4, 33.9, 22.4.
6-Bromo-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4f). Yellow solid; 83% yield; 10: 1 dr; mp 242–243 °C; IR(KBr) 743, 912, 1329, 1483, 1551, 1611, 1676, 1709, 2820, 3383, 3464 cm−1; HRMS(ESI) calcd for C30H24BrN5O3 [M+H]+ 582.1135, found 582.1139; 1H-NMR (400 MHz, DMSO-d6): δ 10.87 (s, 1H, NH), 10.32 (s, 1H, NH), 7.66 (d, J = 8.0 Hz, 7H, ArH), 7.43–7.35 (m, 5H, ArH), 7.09 (t, J = 13.2 Hz, 2H, ArH), 7.00 (t, J = 15.2 Hz, 1H, ArH), 6.84 (t, J = 17.6 Hz, 3H, ArH), 6.08–6.06 (m, 1H, CH), 5.91 (d, J = 6.4 Hz, 1H, CH), 4.82 (d, J = 4.0 Hz, 1H, CH), 3.77 (s, 3H, CH3), 2.78–2.64 (m, 4H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 177.0, 145.2, 136.6, 136.5, 130.1, 128.8, 127.2, 126.9, 126.7, 122.9, 121.9, 121.5, 119.4, 119.1, 118.3, 112.9, 112.0, 110.4, 109.4, 107.7, 91.9, 74.6, 60.7, 51.5, 33.1, 21.3.
5, 7-Dimethyl-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4g). Light yellow solid; 78% yield; >99: 1 dr; mp 274–275 °C; IR(KBr) 737, 1178, 1338, 1489, 1553, 1689, 2910, 3057, 3336, 3385, 3423, 3649, 3711, 3734, 3820 cm−1; HRMS(ESI) calcd for C32H29N5O3 [M+H]+ 532.2343, found 532.2353; 1H-NMR (400 MHz, DMSO-d6): δ 10.86 (s, 1H, NH), 10.16 (s, 1H, NH), 7.42 (s, 2H, ArH), 7.36 (s, 3H, ArH), 7.09 (s, 2H, ArH), 7.00 (s, 1H, ArH), 6.93 (s, 1H, ArH), 6.82 (s, 2H, ArH), 6.07 (s, 1H, CH), 5.97 (s, 1H, CH), 4.81 (s, 1H, CH), 3.76 (s, 3H, CH3), 2.77 (s, 1H, CH2), 2.67 (s, 2H, CH2), 2.51 (s, 1H, CH2), 2.38 (s, 3H, CH3), 1.99 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 177.7, 139.8, 136.6, 132.0, 131.7, 130.3, 128.6, 128.3, 127.4, 127.0, 122.6, 121.8, 121.5, 119.3, 119.1, 118.7, 118.3, 110.0, 110.2, 109.4, 108.2, 92.2, 75.0, 60.5, 51.7, 43.3, 33.0, 22.4, 21.3, 16.5.
Methyl-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4h). Yellow solid; 91% yield; 25: 1 dr; mp 259.4–262.2 °C; IR(KBr) 1190, 1334, 1493, 1687, 2916, 3066, 3341, 3387, 3433, 3650 cm−1; HRMS(ESI) calcd for C31H27N5O3 [M+H]+ 517.2114, found 517.2121; 1H-NMR (400 MHz, DMSO-d6): δ 10.85 (s, 1H, NH), 10.17 (s, 1H, NH), 7.72 (d, J = 7.2 Hz, 1H, ArH), 7.43–7.31 (m, 3H, ArH), 7.29–7.27 (m, 2H, ArH), 7.22 (d, J = 7.6 Hz, 1H, ArH), 7.11 (s, 2H, ArH), 7.09–6.64 (m, 1H, ArH), 6.08 (d, J = 4.4 Hz, 1H, ArH), 6.06 (d, J = 4.4 Hz, 1H, ArH), 4.83 (d, J = 4.4 Hz, 1H, CH), 3.76 (s, 3H, CH2), 2.79–2.64 (m, 4H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 177.2, 143.6, 136.6, 130.3, 128.5, 127.4, 126.8, 124.8, 122.9, 121.7, 119.3, 118.3, 111.9, 110.2, 109.5, 107.9, 92.2, 74.8, 60.6, 51.6, 43.3, 33.0, 22.4.
2′-(1H-Indol-3-yl)-1-methyl-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4i). Yellow solid; 87% yield; 13: 1 dr; mp 267.4–268.2 °C; IR(KBr) 1198, 1365, 1478, 1689, 2962, 3040, 3348, 3390, 3456, 3647 cm−1; HRMS(ESI) calcd for C30H25N5O3 [M+H]+ 504.2030, found 504.2037; 1H-NMR (400 MHz, DMSO-d6): δ 10.87 (s, 1H, NH), 10.18 (s, 1H, NH), 7.72 (d, J = 7.2 Hz, 1H, ArH), 7.30 (d, J = 8.0 Hz, 3H, ArH), 7.27 (s, 2H, ArH), 7.22 (t, J = 14.8 Hz, 1H, ArH), 7.11 (t, J = 15.2 Hz, 2H, ArH), 7.05 (d, J = 6.8 Hz, 1H, ArH), 6.80–6.74 (m, 2H, ArH), 6.65 (d, J = 7.6 Hz, 1H, ArH), 6.09–6.07 (m, 1H, ArH), 5.96 (d, J = 6.4 Hz, 1H, ArH), 4.84 (d, J = 4.4 Hz, 1H, CH), 3.76 (s, 3H, CH2), 2.53 (d, J = 6.0 Hz, 2H, CH2), 2.73 (s, 2H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 177.2, 143.6, 136.6, 130.3, 128.6, 127.4, 126.8, 124.8, 122.9, 121.8, 119.3, 118.4, 111.9, 110.3, 109.5, 107.9, 92.9, 74.8, 60.6, 51.7, 43.3, 33.0, 22.4.
Methyl-1′-nitro-2′-(1-phenyl-1H-indol-3-yl)-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3, 3′-indolizino[8, 7-b]indol]-2-one (4j). Yellow solid; 89% yield; 33: 1 dr; mp 237.8–242.2 °C; IR(KBr) 1188, 1340, 1355, 1457, 1491, 1545, 1629, 2967, 3337, 3443 cm−1; HRMS(ESI) calcd for C36H29N5O3 [M+H]+ 580.2343, found 580.2351; 1H-NMR (400 MHz, DMSO-d6): δ 10.87 (s, 1H, NH), 7.87–7.85 (m, 1H, ArH), 7.47–7.44 (m, 3H, ArH), 7.40–7.28 (m, 6H, ArH), 7.12–7.06 (m, 2H, ArH), 7.00 (t, J = 8.0 Hz, 1H, ArH), 6.74 (t, J = 12.0 Hz, 1H, ArH), 6.56–6.51 (m, 4H, ArH), 6.19–6.16 (m, 1H, ArH), 5.82 (d, J = 6.4 Hz, 1H, ArH), 4.95 (d, J = 3.6 Hz, 1H, CH), 3.77 (s, 3H, CH2), 2.93 (t, J = 8.0 Hz, 1H, CH2), 2.78–2.74 (m, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 174.7, 144.8, 136.7, 133.8, 130.3, 129.8, 128.5, 127.2, 126.6, 124.9, 121.9, 119.3, 118.4, 112.0, 110.2, 109.6, 108.1, 91.3, 74.8, 61.3, 52.8, 43.3, 33.1, 22.5.
2′-(5-Methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3, 3′-indolizino[8,7-b]indol]-2-one (4k). Yellow solid; 82% yield; 33: 1 dr; mp 233.7–235.6 °C; IR(KBr) 588, 746, 1179, 1192, 1323, 1362, 1450, 1472, 1551, 1622, 1701, 2900, 3331, 3431 cm−1; HRMS(ESI) calcd for C30H25N5O3 [M+H]+ 504.2030, found 504.2037; 1H-NMR (400 MHz, DMSO-d6): δ 11.00 (d, J = 2.0 Hz, 1H, NH), 10.82 (s, 1H, NH), 10.12 (s, 1H, NH), 7.73 (d, J = 7.2 Hz, 1H, ArH), 7.44–7.40 (m, 2H, ArH), 7.33–7.30 (m, 2H, ArH), 7.26–7.17 (m, 1H, ArH), 7.09 (t, J = 14.8 Hz, 1H, ArH), 7.00 (t, J = 14.8 Hz, 1H, ArH), 6.81 (d, J = 7.6 Hz, 1H, ArH), 6.66 (d, J = 7.6 Hz, 1H, ArH), 6.37 (s, 1H, ArH), 6.13–6.10 (m, 1H, CH), 5.95 (d, J = 6.8 Hz, 1H, CH), 4.55 (d, J =4.0 Hz, 1H, CH), 2.81–2.78 (m, 2H, CH2), 2.73 (s, 1H, CH2), 2.66 (t, J = 11.2 Hz, 2H, CH2), 2.12 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 177.1, 143.8, 136.5, 134.6, 130.3, 130.1, 128.9, 127.5, 127.3, 126.9, 124.9, 124.2, 123.3, 122.8, 121.5, 119.0, 118.3, 118.0, 112.0, 111.5, 110.1, 109.5, 108.4, 91.9, 74.7, 60.7, 52.1, 43.3, 22.4, 21.6.
2′-(6-Methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4l). Tangerine solid; 79% yield; >99: 1 dr; mp 224.1–225.7 °C; IR(KBr) 451, 737, 804, 1186, 1271, 1329, 1357, 1471, 1541, 1701, 2818, 3350, 3419, 3437 cm−1; HRMS(ESI) calcd for C30H25N5O3 [M+H]+ 504.2030, found 504.2024; 1H-NMR (400 MHz, DMSO-d6): δ 10.98 (s, 1H, NH), 10.97 (s, 1H, NH), 10.81 (s, 1H, NH), 7.71 (s, 1H, ArH), 7.69 (s, 2H, ArH), 7.42 (t, J = 16.8 Hz, 2H, ArH), 7.30–7.27 (m, 1H, ArH), 7.22–7.07 (m, 2H, ArH), 7.02–6.98 (m, 1H, CH), 5.95 (d, J = 6.4 Hz, 1H, CH), 4.82 (d, J = 4.0 Hz, 1H, CH), 2.79–2.51 (m, 4H, CH2), 2.31 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 177.2, 143.7, 136.6, 136.5, 130.8, 130.3, 130.2, 128.7, 126.9, 125.0, 124.8, 123.6, 122.9, 121.5, 120.9, 119.0, 118.3, 117.9, 112.0, 111.7, 110.1, 109.4, 108.7, 92.2, 74.8, 60.7, 51.9, 43.3, 22.4, 21.7.
2′-(6-Methoxy-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8,7-b]indol]-2-one (4m). Yellow solid; 81% yield; >99: 1 dr; mp 246.7–248.0 °C; IR(KBr) 742, 750, 1175, 1281, 1359, 1452, 1487, 1551, 1618, 1699, 2829, 3354, 3435 cm−1; HRMS(ESI) calcd for C30H25N5O4 [M+H]+ 520.1979, found 520.1986; 1H-NMR (400 MHz, DMSO-d6): δ 11.00 (s, 1H, NH), 10.99 (s, 1H, NH), 10.81 (s, 1H, NH), 7.78 (d, J = 7.2 Hz, 1H, ArH), 7.45–7.37 (m, 3H, ArH), 7.32–7.23 (m, 1H, ArH), 7.20 (t, J = 14.8 Hz, 2H, ArH), 7.08 (t, J = 14.8 Hz, 1H, ArH), 7.00 (t, J = 14.8 Hz, 1H, ArH), 6.68 (d, J = 7.6 Hz, 1H, ArH), 6.62–6.60 (m, 1H, ArH), 6.15–6.12 (m, 1H, ArH), 6.09 (d, J = 2.4 Hz, 1H, CH), 5.94 (d, J = 6.8 Hz, 1H, CH), 4.83 (d, J =4.0 Hz, 1H, CH), 3.42 (d, J = 8.0 Hz, 3H, CH3), 2.78–2.63 (m, 2H, CH2), 2.51 (t, J = 3.2 Hz, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 177.0, 153.5, 143.8 136.6, 132.2, 130.3, 130.2, 129.0, 127.3, 126.9, 124.9, 124.8, 121.9, 121.5, 119.0, 118.3, 112.7, 112.3, 112.0, 110.1, 109.5, 108.8, 99.3, 91.9, 74.6, 60.7, 55.2, 51.8, 43.3, 22.4.
2′-(5-Fluoro-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4n). Light yellow solid; 77% yield; >99:1 dr; mp 228.9–231.2 °C; IR(KBr) 754, 939, 1041, 1165, 1321, 1364, 1472, 1558, 1618, 1701, 2916, 3057, 3309, 3453 cm−1; HRMS(ESI) calcd for C29H22FN5O3 [M+H]+ 508.1779, found 508.1786; 1H-NMR (400 MHz, DMSO-d6): δ 11.26 (s, 1H, NH), 10.81 (s, 1H, NH), 10.14 (s, 1H, NH), 7.74 (d, J = 7.2 Hz, 1H, ArH), 7.40 (d, J = 11.2 Hz, 3H, ArH), 7.39–7.31 (m, 2H, ArH), 7.29–7.21 (m, 1H, ArH), 7.09 (t, J = 14.8 Hz, 1H, ArH), 6.99 (t, J = 14.8 Hz, 1H, ArH), 6.86–6.82 (m, 1H, ArH), 6.67 (d, J = 7.6 Hz, 1H, ArH), 6.39 (d, J = 10.4 Hz, 1H, ArH), 6.12 (t, J = 10.8 Hz, 1H, CH), 5.95 (d, J = 6.4 Hz, 1H, CH), 4.78 (d, J =4.0 Hz, 1H, CH), 2.79–2.77 (m, 1H, CH2), 2.72 (s, 1H, CH2), 2.67 (s, 1H, CH2), 2.64 (s, 1H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 177.1, 143.6, 136.5, 132.9, 130.3, 130.2, 128.6, 127.2, 126.9, 126.6, 125.0, 122.9, 121.5, 119.1, 118.3, 113.0, 112.0, 110.1, 109.8, 109.4, 109.1, 103.2, 103.0, 91.7, 74.7, 60.7, 51.9, 43.3, 22.4.
2′-(1H-Indol-3-yl)-1′-methyl-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8,7-b]indol]-2-one (4o). Light yellow solid; 74% yield; >99: 1 dr; mp 273–274 °C; IR(KBr) 746, 1180, 1192, 1250, 1321, 1335, 1369, 1452, 1472, 1549, 1618, 1707, 1730, 3213, 3418 cm−1; HRMS(ESI) calcd for C29H22ClN5O3 [M+H]+ 524.1484, found 524.1489; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 11.31 (d, J = 2.0 Hz, 1H, NH), 10.83 (s, 1H, NH), 10.15 (s, 1H, NH), 7.72 (d, J = 7.2 Hz, 1H, ArH), 7.44–7.40 (m, 4H, ArH), 7.37 (d, J = 2.0 Hz, 1H, ArH), 7.21 (t, J = 14.8 Hz, 1H, ArH), 7.09 (t, J = 14.8 Hz, 1H, ArH), 6.99 (t, J = 14.8 Hz, 1H, ArH), 7.76–7.73 (m, 1H, ArH2), 6.69–6.65 (m, 2H, ArH), 6.13–6.11 (m, 1H, CH), 5.95 (d, J = 6.8 Hz, 1H, CH), 4.82 (d, J = 4.0 Hz, 1H, CH), 7.79–7.72 (m, 4H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 177.1, 143.6, 136.6, 136.5, 130.3, 130.2, 128.5, 126.9, 126.4, 125.8, 125.7, 124.9, 123.0, 121.5, 119.6, 119.4, 119.0, 118.3, 112.0, 111.6, 110.1, 109.4, 109.2, 91.8, 74.7, 60.3, 51.6, 43.3, 22.4.
2′-(5-Bromo-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8, 7-b]indol]-2-one (4p). Brown solid; 71% yield; >99: 1 dr; mp 254–255 °C; IR(KBr) 457, 746, 1190, 1327, 1471, 1553, 1622, 1697, 3057, 3647, 3724, 3799, 3819, 3838 cm−1; HRMS(ESI) calcd for C29H22BrN5O3 [M+H]+ 568.0979, found 568.0970; 1H-NMR (400 MHz, DMSO-d6): δ 11.35 (s, 1H, NH), 10.81 (s, 1H, NH), 10.13 (s, 1H, NH), 7.74 (d, J = 7.2 Hz, 1H, ArH), 7.45–7.40 (m, 3H, ArH), 7.33–7.29 (m, 1H, ArH), 7.27–7.22 (m, 2H, ArH), 7.11–7.07 (m, 2H, ArH), 6.99 (t, J = 7.6 Hz, 1H, ArH), 6.75 (s, 1H, ArH), 6.68 (d, J = 7.6 Hz, 1H, ArH), 6.16–6.13 (m, 1H, CH), 5.94 (d, J = 6.4 Hz, 1H, CH), 4.80 (d, J = 4.0 Hz, 1H, CH), 2.80 (d, J = 4.8 Hz, 1H, CH2), 2.78 (d, J = 3.6 Hz, 1H, CH2), 2.67 (d, J = 11.2 Hz, 1H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 177.1, 143.6, 136.6, 136.5, 135.3, 130.4, 130.2, 128.6, 126.9, 126.1, 125.0, 124.2, 122.9, 121.5, 120.9, 119.0, 118.3, 113.9, 112.0, 111.9, 110.1, 109.5, 108.7, 91.4, 74.7, 60.7, 51.9, 42.3, 22.4.
5-Methyl-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8,7-b]indol]-2-one (4q). Yellow solid; 78% yield; 25:1 dr; mp 242.1–242.3 °C; IR(KBr) 733, 814, 1045, 1171, 1198, 1240, 1323, 1358, 1495, 1543, 1694, 1738, 2814, 3335, 3354 cm−1; HRMS(ESI) calcd for C31H27N5O3 [M+H]+ 518.2187, found 518.2192; 1H-NMR (400 MHz, DMSO-d6): δ 10.85 (s, 1H, NH), 10.07 (s, 1H, NH), 7.54 (s, 1H, ArH), 7.43–7.33 (m, 4H, ArH), 7.00 (t, J = 14.4 Hz, 4H, ArH), 6.82 (s, 2H, ArH), 6.55 (d, J = 8.0 Hz, 1H, ArH), 6.06 (d, J = 4.8 Hz, 1H, CH), 5.96 (d, J = 5.6 Hz, 1H, CH), 4.81 (d, J = 3.2 Hz, 1H, CH), 3.76 (s, 3H, CH3), 2.78–2.65 (m, 4H, CH2), 2.41 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 177.2, 141.1, 136.6, 136.5, 131.8, 130.5, 130.3, 128.6, 128.5, 127.4, 126.9, 125.3, 121.8, 121.5, 119.3, 119.0, 118.5, 118.3, 112.0, 110.2, 109.9, 109.4, 108.1, 92.2, 74.9, 60.6, 51.8, 43.3, 33.0, 22.4, 21.4.
5-Chloro-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8,7-b]indol]-2-one (4r). Brown solid; 76% yield; >99:1 dr; mp 257–258 °C; IR(KBr) 584, 744, 1176, 1319, 1436, 1473, 1686, 1734, 2360, 3178, 3246, 3277, 3566, 3689, 3726, 3840 cm−1; HRMS(ESI) calcd for C29H22ClN5O3 [M+H]+ 524.1484, found 524.1494; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 11.19 (s, 1H, NH), 10.85 (s, 1H, NH), 10.28 (s, 1H, NH), 7.71 (s, 1H, ArH), 7.45–7.32 (m, 5H, ArH), 7.10 (t, J = 14.8 Hz, 1H, ArH), 7.00 (t, J = 14.4 Hz, 2H, ArH), 6.80 (d, J = 6.0 Hz, 2H, ArH), 6.69 (d, J = 8.4 Hz, 1H, ArH), 6.14–6.11 (m, 1H, CH), 5.76 (d, J = 6.0 Hz, 1H, CH), 4.86 (d, J = 3.6 Hz, 1H, CH), 2.77–2.65 (m, 4H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 176.8, 142.6, 136.5, 136.2, 131.1, 130.2, 130.1, 126.9, 126.8, 126.7, 124.9, 124.6, 121.7, 121.5, 119.2, 119.1, 118.3, 118.1, 112.1, 112.0, 111.6, 109.4, 108.7, 91.8, 74.8, 60.9, 51.4, 43.3, 22.4.
5-Bromo-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8,7-b]indol]-2-one (4s). Brown solid; 79% yield; 11:1 dr; mp 261–263 °C; IR(KBr) 743, 822, 1098, 1177, 1184, 1474, 1547, 1701, 2369, 2819, 3334, 3406 cm−1; HRMS(ESI) calcd for C29H22BrN5O3 [M+H]+ 568.0979, found 568.0987; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 11.19 (s, 1H, NH), 10.85 (s, 1H, NH), 10.28 (s, 1H, NH), 7.84 (s, 1H, ArH), 7.50 (d, J = 8.0 Hz, 1H, ArH), 7.45–7.38 (m, 3H, ArH), 7.33 (d, J = 8.0 Hz, 1H, ArH), 7.00 (t, J = 14.4 Hz, 1H, ArH), 7.01 (t, J = 14.0 Hz, 2H, ArH), 6.78 (t, J = 13.2 Hz, 2H, ArH), 6.65 (d, J = 8.0 Hz, 1H, ArH), 6.13 (t, J = 8.8 Hz, 1H, CH), 5.93 (d, J = 5.2 Hz, 1H, CH), 4.87 (d, J = 2.8 Hz, 1H, CH), 2.76 (t, J = 11.2 Hz, 2H, CH2), 2.72 (d, J = 4.8 Hz, 1H, CH2), 2.67 (d, J = 11.2 Hz, 1H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 176.6, 143.0, 136.5, 136.2, 133.0, 131.5, 130.1, 127.6, 126.9, 125.6, 121.7, 121.5, 119.2, 119.1, 118.3, 118.1, 114.4, 112.1, 112.0, 109.5, 108.7, 71.8, 74.7, 60.9, 51.8, 43.3, 22.4.
6-Bromo-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,11′,11b’-hexahydrospiro[indoline-3,3′-indolizino[8,7-b]indol]-2-one (4t). Yellow solid; 77% yield; 33: 1 dr; mp 266–267 °C; IR(KBr) 484, 910, 1126, 1182, 1273, 1379, 1445, 1553, 1608, 1699, 3396, 3421, 3437 cm−1; HRMS(ESI) calcd for C29H22BrN5O3 [M+H]+ 568.0979, found 568.0971; 1H-NMR (400 MHz, DMSO-d6): δ 11.19 (s, 1H, NH), 11.10 (s, 1H, NH), 10.53 (s, 1H, NH), 7.67 (d, J = 7.6 Hz, 1H, ArH), 7.42 (t, J = 11.6 Hz, 4H, ArH), 7.40–7.31 (m, 1H, ArH), 7.33 (d, J = 8.4 Hz, 1H, ArH), 7.11–6.98 (m, 2H, ArH), 6.86–6.79 (m, 3H, ArH), 6.13–6.10 (m, 1H, CH3), 5.94 (d, J = 6.4 Hz, 1H, CH), 4.85 (d, J = 4.0 Hz, 1H, CH), 2.80–2.77 (m, 2H, CH2), 2.73 (t, J = 13.6 Hz, 1H, CH2), 2.68–2.63 (m, 1H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 177.0, 145.2, 136.5, 136.2, 130.1, 128.1, 126.9, 126.8, 125.6, 124.6, 122.8, 121.8, 121.5, 119.2, 119.1, 118.3, 118.1, 113.0, 112.1, 112.0, 109.4, 108.6, 92.0, 74.6, 60.8, 51.3, 43.3, 22.4.
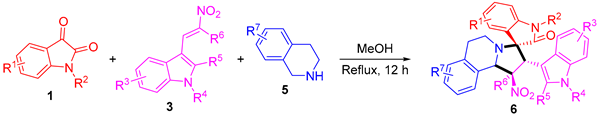
4.3. General Procedure for the Synthesis of Compounds 6
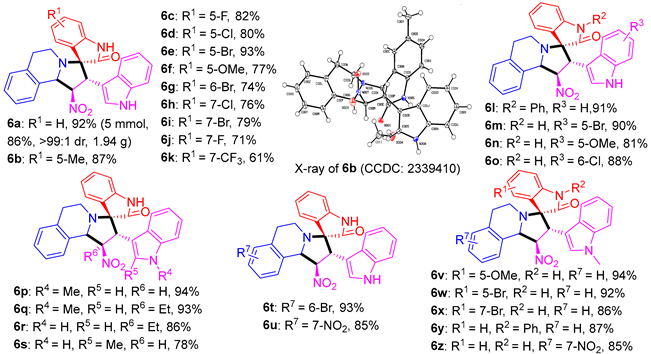
A mixture of isatins (1, 0.5 mmol), THIQs (5, 0.5 mmol), and 3-(2-nitrovinyl)-indoles (3, 0.5 mmol) in MeOH was stirred under reflux conditions for 12 h. The resulting precipitate was collected by filtrating and washing with cold MeOH 2–3 times. The crude product was further purified by recrystallization (for products 6a, 6b, 6e, 6l, 6m, 6o, 6p, 6q, 6r, 6t, 6v, 6w, and 6y) in MeOH or column chromatography (for products 6c, 6d, 6f, 6g, 6h, 6i, 6j, 6k, 6n, 6s, 6u, 6x, 6z, the mixtures of petroleum ether and ethyl acetate were used as eluents) to afford the pure corresponding compound.
2′-(Indolin-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6a). Yellow solid; 92% yield; >99: 1 dr; mp 288–289 °C; IR (KBr) 908, 1057, 1188, 1339, 1472, 1547, 1620, 1705, 2810, 2945, 3337 cm−1; HRMS(ESI) calcd for C27H22N4O3 [M+H]+ 451.1765, found 451.1769; 1H-NMR (400 MHz, DMSO-d6): δ 10.82 (s, 1H, NH), 9.56 (s, 1H, NH), 8.25–7.75 (m, 1H, ArH), 7.74 (d, J = 7.6 Hz, 1H, ArH), 7.72 (s, 1H, ArH), 7.72–7.68 (m, 3H, ArH), 7.67 (s, 1H, ArH), 7.66–7.63 (m, 2H, ArH), 7.46–7.42 (m, 1H, ArH), 7.29 (s, 1H, ArH), 7.27 (s, 1H, ArH), 7.22 (d, J = 0.8 Hz, 1H, CH), 7.21–7.15 (m, 1H, CH), 7.14 (d, J = 6.8 Hz, 1H, CH), 6.78–6.75 (m, 1H, CH), 3.53 (t, J = 17.6 Hz, 1H, CH2), 3.39 (t, J = 12.8 Hz, 1H, CH2), 3.30–3.17 (m, 1H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 177.1, 143.8, 136.6, 135.8, 134.1, 130.2, 129.6, 128.7, 127.7, 127.2, 126.2, 125.1, 124.9, 124.6, 123.0, 121.9, 119.4, 118.4, 111.7, 111.6, 110.1, 109.5, 94.1, 63.6, 52.9, 43.1, 29.6.
2′-(1H-Indol-3-yl)-5-methyl-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6b). Yellow solid; 87% yield; >99: 1 dr; mp 263–264 °C; IR(KBr) 419, 739, 1458, 1491, 1555, 1705, 3385, 3421, 3523, 3736, 3821, 3838 cm−1; HRMS(ESI) calcd for C28H24N4O3 [M+H]+ 465.1921, found 465.1930; 1H-NMR (400 MHz, DMSO-d6): δ 11.16 (d, J = 1.6 Hz, 1H, NH), 10.01 (s, 1H, NH), 7.55 (d, J = 2.4 Hz, 1H, ArH), 7.48 (s, 1H, ArH), 7.28 (d, J = 8 Hz, 2H, ArH), 7.21–7.15 (m, 3H, ArH), 7.07 (d, J = 7.6 Hz, 1H, ArH), 6.96 (t, J = 15.2 Hz, 1H, ArH), 6.68 (t, J = 14.8 Hz, 1H, ArH), 6.61 (d, J = 8 Hz, 1H, ArH), 6.50 (d, J = 8 Hz, 1H, ArH), 6.41–6.38 (m, 1H, ArH), 5.80 (d, J = 6.8 Hz, 1H, CH), 4.66 (d, J = 4.4 Hz, 1H, CH), 4.18–4.14 (m, 1H, CH), 4.69 (d, J = 4.4 Hz, 1H, CH), 2.98–2.93 (m, 1H, CH2), 2.71–2.67 (m, 1H, CH2), 2.62 (t, J = 11.2 Hz, 2H, CH2), 2.40 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 176.9, 141.2, 136.1, 135.4, 133.8, 131.7, 130.4, 129.5, 128.4, 127.2, 127.1, 126.1, 125.2, 125.1, 125.0, 121.5, 119.1, 118.1, 111.9, 109.8, 108.8, 93.3, 75.0, 63.3, 52.6, 49.1, 42.8, 21.3.
5-Fluoro-2′-(1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6c). Brown solid; 82% yield; >99: 1 dr; mp 225–229 °C; IR(KBr) 727, 748, 1186, 1204, 1477, 1544, 1616, 1697, 2857, 2926, 3235 cm−1; HRMS(ESI) calcd for C27H21FN4O3 [M+H]+ 469.1670, found 469.1679; 1H-NMR (400 MHz, DMSO-d6): δ 11.29 (s, 1H, NH), 10.23 (s, 1H, NH), 7.66 (d, J = 2.0 Hz, 1H, ArH), 7.59 (d, J = 2.4 Hz, 1H, ArH), 7.36–7.31 (m, 3H, ArH), 7.29–7.15 (m, 3H, ArH), 6.98 (t, J = 14.8 Hz, 1H, ArH), 6.73 (t, J = 15.2 Hz, 1H, ArH), 6.65 -6.62(m, 1H, ArH), 6.44–6.41 (m, 1H, ArH), 5.77 (d, J = 6.8 Hz, 1H, CH), 4.70 (d, J = 4.4 Hz, 1H, CH), 4.18–4.15 (m, 1H, CH), 3.18 (d, J = 5.2 Hz, 2H, CH2), 3.02–2.94 (m, 1H, CH2), 2.69 (t, J = 14.4 Hz, 1H, CH2), 2.64–2.61 (m, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 177.7, 143.5, 135.5, 135.4, 134.9, 133.7, 130.2, 129.6, 128.3, 127.3, 126.2, 125.3, 124.7, 122.7, 120.7, 119.0, 111.0, 110.0, 103.1, 91.0, 63.7, 60.3, 42.8, 40.1, 29.9, 21.2, 14.6, 11.8.
5-Chloro-2′-(1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6d). Yellow solid; 80% yield; >99: 1 dr; mp 256–258 °C; IR(KBr) 743, 1175, 1139, 1373, 1458, 1489, 1553, 1695, 3198, 3309, 3368, 3649 cm−1; HRMS(ESI) calcd for C27H21ClN4O3 [M+H]+ 485.1375, found 485.1368; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 10.96 (s, 1H, NH), 9.98 (s, 1H, NH), 7.43 (d, J = 2.4 Hz, 1H, ArH), 7.29–7.22 (m, 1H, ArH), 7.18–7.13 (m, 3H, ArH), 7.00–6.95 (m, 2H, ArH), 6.76–6.72 (m, 2H, ArH), 6.59–6.55 (m, 1H, ArH), 6.22–6.18 (m, 1H, CH), 5.85 (d, J = 6.8 Hz, 1H, CH), 4.70 (t, J = 4.8 Hz, 1H, CH), 4.01–3.97 (m, 1H, CH), 3.26 (m, 2H, CH2), 3.07 (s, 1H, CH2), 2.76(t, J = 14.4 Hz, 1H, CH2), 2.76 (t, J = 14.4 Hz, 1H, CH2), 2.68 (s, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 177.1, 160.2, 157.8, 139.6, 136.1, 135.3, 133.3, 130.2, 130.1, 129.3, 127.0, 126.9, 125.9, 124.9, 124.5, 121.5, 119.1, 117.9, 116.3, 116.1, 112.1, 111.8, 111.7, 110.8, 110.7, 108.4, 93.2, 75.2, 63.3, 52.6, 49.4, 42.7, 29.9.
5-Bromo-2′-(1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6e). Yellow solid; 93% yield; >99: 1 dr; mp 280–282 °C; IR(KBr) 733, 1132, 1190, 1319, 1338, 1375, 1418, 1545, 1614, 1711, 3323, 3566 cm−1; HRMS(ESI) calcd for C27H21BrN4O3 [M+H]+ 529.0870, found 529.0877; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 11.14 (d, J = 2.4 Hz, 1H, NH), 10.20 (s, 1H, ArH), 7.60 (d, J = 8.0 Hz, 1H, ArH), 7.53 (d, J = 2.4 Hz, 1H, ArH), 7.37–7.34 (m, 1H, ArH), 7.30–7.25 (m, 2H, ArH), 7.19–7.13 (m, 3H, ArH), 6.99–6.95 (m, 1H, ArH), 6.75–6.71 (m, 2H, ArH), 6.66 (d, J = 8.0 Hz, 1H, ArH), 6.36–6.33 (m, 1H, CH), 5.78 (d, J = 6.8 Hz, 1H, CH), 4.67 (d, J = 4.4 Hz, 1H, CH), 3.02–2.93 (m, 1H, CH2), 2.72 (t, J = 11.6 Hz, 1H, CH2), 2.67–2.61(m, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 176.7, 145.2, 136.1, 135.3, 133.5, 129.4, 127.8, 127.1, 126.9, 126.5, 126.1, 125.4, 125.1, 125.0, 122.8, 121.5, 119.1, 117.9, 112.9, 111.9, 108.4, 93.1, 74.7, 63.3, 52.4, 42.8, 29.9.
2′-(1H-Indol-3-yl)-5-methoxy-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6f). Light yellow solid; 77% yield; >99: 1 dr; mp 233–236 °C; IR(KBr) 743, 762, 1030, 1205, 1300, 1335, 1490, 1545, 1690, 2829, 2935, 3442 cm−1; HRMS(ESI) calcd for C28H24N4O4 [M+H]+ 481.1870, found 481.1861; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 10.92 (s, 1H, NH), 9.81 (s, 1H, NH), 7.42 (s, 1H, ArH), 7.26 (t, J = 15.6 Hz, 2H, ArH), 7.16 (t, J = 7.2 Hz, 4H, ArH), 6.98–6.95 (m, 1H, ArH), 7.77 (t, J = 8.4 Hz, 3H, ArH), 6.53 (s, 1H, CH), 6.19–6.16 (m, 1H, CH), 5.85 (d, J = 6.8 Hz, 1H, CH), 4.70 (d, J = 4.8 Hz, 1H, CH), 4.00 (d, J = 4.8 Hz, 1H, CH2), 3.85 (d, J = 1.6 Hz, 3H, CH2), 3.10 (s, 1H, CH2), 2.77 (t, J = 18.8 Hz, 1H, CH2), 2.71 (s, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 177.1, 156.0, 136.7, 136.1, 135.3, 133.5, 129.5, 129.4, 127.0, 126.9, 125.9, 124.7, 124.4, 121.5, 119.1, 118.1, 114.5, 111.7, 111.0, 110.4, 108.6, 93.5, 75.3, 63.2, 55.9, 52.7, 49.4, 42.7, 29.9.
6-Bromo-2′-(1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3, 3′-pyrrolo[2, 1-a]isoquinolin]-2-one (6g). Yellow solid; 74% yield; >99: 1 dr; mp 282–284 °C; IR(KBr) 1543, 1616, 1715, 1734, 2343, 2359, 3337, 3628, 3714, 3820 cm−1; HRMS(ESI) calcd for C27H21BrN4O3 [M+H]+ 529.0870, found 529.0878; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 11.08 (s, 1H, NH), 10.15 (s, 1H, NH), 7.58 (t, J = 8.0 Hz, 1H, ArH), 7.49 (s, 1H, ArH), 7.40 (d, J = 8.4 Hz, 1H, ArH), 7.36–7.21 (m, 2H, ArH), 7.20–7.14 (m, 3H, ArH), 7.07 (s, 1H, ArH), 6.97–6.67 (m, 3H, ArH), 6.31–6.28 (m, 1H, CH), 5.78 (d, J = 6.0 Hz, 1H, CH), 4.67 (d, J = 4.0 Hz, 1H, CH), 2.98 (s, 1H, CH2), 2.70 (s, 1H, CH2), 2.65 (d, J = 10.0 Hz, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 176.8, 145.2, 136.1, 135.3, 133.5, 129.4, 127.7, 127.1, 126.9, 126.3, 126.0, 125.4, 125.0, 124.9, 122.8, 121.5, 119.1, 117.9, 112.9, 111.8, 108.4, 93.1, 74.7, 62.3, 52.4, 42.8, 29.9.
7-Chloro-2′-(1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6h). Yellow solid; 76% yield; >99: 1 dr; mp 262–264 °C; IR(KBr) 739, 1177, 1339, 1458, 1545, 1556, 1622, 1714, 3421, 3566, 3751, 3853 cm−1; HRMS(ESI) calcd for C27H21ClN4O3 [M+H]+ 485.1375, found 485.1384; 1H-NMR (400 MHz, DMSO-d6): δ 11.22 (d, J = 2.0 Hz, 1H, NH), 10.58 (s, 1H, NH), 7.65 (d, J = 7.2 Hz, 1H, ArH), 7.60 (d, J = 2.4 Hz, 1H, ArH), 7.37 (d, J = 7.6 Hz, 1H, ArH), 7.25 (d, J = 7.6 Hz, 2H, ArH), 7.21 (d, J = 3.2 Hz, 4H, ArH), 6.98 (t, J =14.8 Hz, 1H, ArH), 6.69 (t, J = 14.8 Hz, 1H, ArH), 6.63 (d, J = 8 Hz, 1H, ArH), 6.44–6.43 (m, 1H, ArH), 5.77 (d, J = 6.8 Hz, 1H, CH), 4.69 (d, J = 4.4 Hz, 1H, CH), 4.19–4.15 (m, 1H, CH), 3.18 (d, J = 5.2 Hz, 2H, CH2), 2.96 (t, J = 16.8 Hz, 1H, CH2), 2.92 (s, 1H, CH2), 2.90 (s, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 176.9, 141.4, 136.2, 135.3, 133.5, 130.5, 130.2, 129.5, 127.3, 126.9, 126.2, 125.2, 125.1, 124.3, 123.4, 121.7, 119.1, 117.9, 114.2, 112.0, 108.5, 92.9, 75.5, 63.4, 52.8, 49.1, 42.9.
7-Bromo-2′-(1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6i). Light yellow solid; 79% yield; >99: 1 dr; mp 288–290 °C; IR(KBr) 419, 745, 1338, 1473, 1549, 1684, 1715, 3244, 3309, 3503, 3628, 3734, 3819 cm−1; HRMS(ESI) calcd for C27H21BrN4O3 [M+H]+ 529.0870, found 529.0878; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 11.02 (s, 1H, NH), 10.29 (s, 1H, NH), 7.67 (d, J = 7.6 Hz, 1H, ArH), 7.46 (d, J = 2.0 Hz, 1H, ArH), 7.40 (d, J = 8.0 Hz, 1H, ArH), 7.29–7.25 (m, 2H, ArH), 7.19–7.10 (m, 4H, ArH), 6.98 (t, J = 29.6 Hz, 1H, ArH), 6.74 (t, J = 14.8 Hz, 1H, ArH), 6.66 (d, J = 8.0 Hz, 1H, ArH), 6.27–6.24 (m, 1H, CH), 5.83 (d, J = 6.8 Hz, 2H, CH), 4.71 (t, J = 4.8 Hz, 1H, CH), 3.07–3.01 (m, 1H, CH2), 2.76 (t, J = 16.4 Hz, 2H, CH2), 2.70 (d, J = 14.8 Hz, 1H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 177.0, 123.0, 136.2, 135.3, 133.3, 132.8, 130.3, 129.3, 127.0, 126.8, 126.0, 124.9, 124.6, 124.2, 123.5, 121.6, 119.1, 118.0, 111.8, 108.3, 102.5, 93.1, 75.7, 63.2, 52.8, 42.7, 29.9.
7-Fluoro-2′-(1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6j). Yellow solid; 71% yield; >99: 1 dr; mp 279–281 °C; IR(KBr) 583, 735, 1196, 1339, 1474, 1556, 1645, 1713, 3176, 3367, 3385, 3566, 3736 cm−1; HRMS(ESI) calcd for C27H21FN4O3 [M+H]+ 469.1670, found 469.1673; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 11.11 (d, J = 2.0 Hz, 1H, NH), 10.54 (s, 1H, NH), 7.54 (t, J = 10.4 Hz, 2H, ArH), 7.27 (t, J = 14.4 Hz, 2H, ArH), 7.22–7.19 (m, 5H, ArH), 7.18 (t, J = 5.2 Hz, 1H, ArH), 6.98–6.94 (m, 1H, ArH), 6.71–6.67 (m, 1H, ArH), 6.54 (d, J = 8.0 Hz, 1H, ArH), 5.82 (d, J = 6.8 Hz, 1H, CH), 4.70 (d, J = 4.8 Hz, 1H, CH), 4.08–4.04 (m, 1H, CH), 3.21 (d, J = 5.2 Hz, 2H, CH2), 3.04 (d, J = 5.2 Hz, 1H, CH2), 2.97 (d, J = 6.8 Hz, 1H, CH2), 2.75 (d, J = 10.4 Hz, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 176.7, 147.7, 145.2, 136.2, 135.3, 133.5, 131.6, 131.5, 130.8, 130.6, 129.4, 127.1, 126.9, 126.0, 125.1, 124.9, 123.6, 123.5, 121.5, 120.5, 119.0, 117.8, 117.1, 117.0, 111.8, 108.4, 93.0, 75.1, 75.0, 63.2, 52.9, 49.2, 42.8, 29.9.
2′-(1H-Indol-3-yl)-1′-nitro-7-(trifluoromethyl)-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6k). White solid; 61% yield; >99: 1 dr; mp >320 °C; IR (KBr) 737, 1099, 1119, 1171, 1341, 1458, 1558, 1628, 1726, 2835, 2918, 3397 cm−1; HRMS(ESI) calcd for C28H21F3N4O3 [M+H]+ 519.1639, found 519.1647; 1H-NMR (400 MHz, DMSO-d6): δ 11.21 (d, J = 2 Hz, 1H, NH), 10.58 (s, 1H, NH), 7.97 (s, J = 7.2 Hz, 1H, ArH), 7.64 (d, J = 2.4 Hz, 1H, ArH), 7.62 (s, 1H, ArH), 7.60 (s, 1H, ArH), 7.42 (d, J = 7.6 Hz, 1H, ArH), 7.36 (t, J = 15.2 Hz, 1H, ArH), 7.19–7.15 (m, 2H, ArH), 6.98–6.94 (m, 1H, ArH), 6.66–6.62 (m, 1H, ArH), 6.49 (t, J = 6.4 Hz, 1H, ArH), 6.41 (s, 1H, CH), 5.76 (d, J = 6.8 Hz, 1H, CH), 5.85 (d, J = 6.8 Hz, 1H, CH), 4.72 (d, J = 4 Hz, 1H, CH), 2.98 (s, 1H, CH2), 2.71 (s, 1H, CH2), 2.66–2.61 (m, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 177.2, 141.2, 136.3, 135.4, 133.4, 130.8, 129.5, 128.8, 127.3, 126.8, 126.2, 125.4, 125.1, 123.2, 122.3, 121.6, 119.0, 117.8, 111.9, 111.3, 111.0, 108.5, 92.5, 63.6, 52.9, 40.1, 39.9, 39.3.
2′-(1H-Indol-3-yl)-1′,9′-dinitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6l). White solid; 91% yield; >99: 1 dr; mp 290–292 °C; IR (KBr) 743, 1302, 1371, 1497, 1553, 1595, 1612, 1686, 2830, 2928, 3065, 3291 cm−1; HRMS(ESI) calcd for C33H26N4O3 [M+H]+ 527.2078, found 527.2084; 1H-NMR (400 MHz, DMSO-d6): δ 11.01 (d, J = 2 Hz, 1H, NH), 7.83 (d, J = 1.6 Hz, 1H, ArH), 7.81 (s, 1H, ArH), 7.52–7.30 (m, 2H, ArH), 7.29–7.21 (m, 5H, ArH), 7.20–7.15 (m, 3H, ArH), 6.97 (t, J = 14.8 Hz, 1H, ArH), 6.66 (d, J = 7.6 Hz, 1H, ArH), 6.43 (t, J = 8 Hz, 3H, ArH), 6.39–6.36 (m, 2H, CH), 5.85 (d, J = 6.8 Hz, 1H, CH), 4.80 (d, J = 4.4 Hz, 1H, CH), 3.13–3.06 (m, 1H, CH2), 3.93 (s, 1H, CH2), 2.80 (d, J = 3.2 Hz, 1H, CH2), 2.77(s, 1H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 174.6, 145.0, 136.3, 135.4, 133.9, 133.4, 130.0, 129.5, 129.4, 128.1, 128.0, 127.1, 126.8, 126.5, 126.0, 125.3, 124.6, 124.5, 124.0, 121.5, 118.9, 118.0, 111.6, 109.3, 108.6, 92.5, 79.1, 63.9, 53.9, 40.3, 40.1, 39.5, 30.0.
2′-(5-Bromo-1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6m). Brown solid; 90% yield; >99: 1 dr; mp 264–266 °C; IR(KBr) 752, 1184, 1321, 1339, 1458, 1471, 1549, 1618, 1705, 2810, 2947, 3298 cm−1; HRMS(ESI) calcd for C27H21BrN4O3 [M+H]+ 529.0870, found 529.0863; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 11.22 (d, J = 1.2 Hz, 1H, NH), 9.98 (s, 1H, NH), 7.69 (d, J = 7.2 Hz, 1H, ArH), 7.56 (d, J = 2.0 Hz, 1H, ArH), 7.29 (d, J = 0.8 Hz, 2H, ArH), 7.14 (t, J = 8.8 Hz, 5H, ArH), 7.03–7.01 (m, 1H, ArH), 6.62 (d, J = 7.6 Hz, 1H, ArH), 6.57 (d, J = 1.2 Hz, 1H, ArH), 6.34–6.31 (m, 1H, CH), 5.82 (d, J = 7.2 Hz, 1H, CH), 4.63 (d, J = 4.8 Hz, 1H, CH), 3.06 (d, J = 5.2 Hz, 1H, CH2), 2.77 (t, J = 16 Hz, 1H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 176.9, 143.6, 135.3, 134.9, 133.5, 130.2, 129.4, 128.6, 128.2, 127.0, 126.1, 125.9, 125.0, 124.5, 124.0, 122.7, 120.9, 113.4, 111.9, 110.0, 108.5, 92.5, 74.8, 63.3, 52.8, 42.7, 29.9.
2′-(5-Methoxy-1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6n). Brown solid; 81% yield; >99: 1 dr; mp 283–285 °C; IR(KBr) 1173, 1217, 1472, 1549, 1622, 1699, 2995, 3178, 3230, 3462, 3566, 3726, 3840 cm−1; HRMS(ESI) calcd for C28H24N4O4 [M+H]+ 481.1870, found 481.1878; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 10.98 (d, J = 2.0 Hz, 1H, NH), 10.03 (s, 1H, NH), 7.71 (d, J = 6.8 Hz, 1H, ArH), 7.57 (d, J = 2.4 Hz, 1H, ArH), 7.31–7.25 (m, 2H, ArH), 7.21–7.13 (m, 5H, ArH), 6.63 (d, J = 7.6 Hz, 1H, ArH), 6.57–6.54 (m, 1H, ArH), 6.43–6.40 (m, 1H, ArH), 5.92 (d, J = 2.4 Hz, 1H, CH), 5.78 (d, J = 6.8 Hz, 1H, CH), 4.65 (d, J = 4.4 Hz, 1H, CH), 3.38 (d, J = 6.4 Hz, 3H, CH2), 3.00–2.92 (m, 1H, CH2), 2.70–2.60 (m, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 176.7, 153.5, 143.8, 135.4, 133.7, 131.2, 130.1, 129.5, 128.8, 127.3, 127.1, 126.1, 125.4, 125.2, 124.7, 122.7, 112.5, 112.2, 110.1, 108.7, 99.1, 92.9, 79.4, 74.8, 63.3, 55.2, 52.7, 42.8.
2′-(6-Chloro-1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6o). Yellow solid; 88% yield; >99: 1 dr; mp 258–260 °C; IR(KBr) 418, 752, 1190, 1339, 1456, 1544, 1695, 3209, 3354, 3523, 3649, 3749, 3802 cm−1; HRMS(ESI) calcd for C27H21ClN4O3 [M+H]+ 485.1375, found 485.1369; 1H-NMR (400 MHz, DMSO-d6): δ 10.82 (s, 1H, NH), 9.56 (s, 1H, NH), 8.24 (d, J = 7.2 Hz, 1H, ArH), 8.02 (d, J = 3.2 Hz, 1H, ArH), 7.79–7.71 (m, 4H, ArH), 7.70–7.63 (m, 3H, ArH), 7.46–7.42 (m, 1H, ArH), 7.28 (d, J = 8.0 Hz, 1H, ArH), 7.22 (t, J = 7.6 Hz, 1H, ArH), 7.19–7.13 (m, 1H, ArH), 6.78–6.75 (m, 1H, CH), 6.38 (d, J = 7.2 Hz, 1H, CH), 5.34 (d, J = 4.8 Hz, 1H, ArH), 3.53 (d, J = 6.0 Hz, 1H, CH2), 3.30 (d, J = 1.2 Hz, 1H, CH2), 3.27–3.25 (m, 1H, CH2), 3.17 (d, J = 3.2 Hz, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6): δ 176.8, 143.6, 136.6, 135.4, 133.7, 130.3, 129.5, 128.3, 127.2, 126.2, 126.1, 125.8, 125.2, 124.7, 122.9, 119.5, 119.3, 111.5, 110.1, 109.1, 92.8, 74.9, 63.3, 52.5, 49.1, 42.8, 29.8.
2′-(1-Methyl-1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6p). Yellow solid; 94% yield; >99: 1 dr; mp 265–267 °C; IR (KBr) 741, 1125, 1190, 1331, 1458, 1551, 1628, 1699, 2835, 2918, 3200, 3221 cm−1; HRMS(ESI) calcd for C28H24N4O3 [M+H]+ 465.1921, found 465.1927; 1H-NMR (400 MHz, DMSO-d6): δ 10.10 (s, 1H, NH), 7.66 (d, J = 6.8 Hz, 1H, ArH), 7.56 (s, 1H, ArH), 7.33 (s, 1H, ArH), 7.29 (s, 1H, ArH), 7.28–7.20 (m, 1H, ArH), 7.19–7.15 (m, 3H, ArH), 7.01 (t, J = 14.8 Hz, 1H, ArH), 6.70 (s, J = 14.8 Hz, 1H, ArH), 6.59 (s, 1H, ArH), 6.53 (s, 1H, ArH), 6.37–6.34 (m, 1H, CH), 5.78 (d, J = 6.8 Hz, 1H, CH), 4.65 (d, J = 4.8 Hz, 1H, CH), 4.06 (s, 1H, CH), 3.75 (s, 3H, CH2), 2.95 (d, J = 6 Hz, 1H, CH2), 2.67 (s, 1H, CH3), 2.65 (s, 1H, CH3), 2.59 (t, J = 36.4 Hz, 1H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 176.9, 143.6, 136.6, 135.4, 133.7, 130.3, 129.6, 129.1, 128.3, 127.4, 127.2, 126.2, 125.0, 124.7, 122.9, 121.7, 119.2, 118.2, 110.2, 110.1, 108.0, 93.2, 63.2, 52.4, 40.2, 40.0, 39.5, 39.3.
1′-Ethyl-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6q). Yellow solid; 93% yield; >99: 1 dr; mp 220–224 °C; IR(KBr) 737, 1339, 1474, 1535, 1622, 1697, 3447, 3481, 3566, 3587, 3614 cm−1; HRMS(ESI) calcd for C30H28N4O3 [M+H]+ 493.2234, found 493.2240; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 10.58 (s, 1H, NH), 7.44 (d, J = 7.2 Hz, 1H, ArH), 7.29 (d, J = 8.0 Hz, 1H, ArH), 7.22 (s, 1H, ArH), 7.19–7.07 (m, 7H, ArH), 6.99–6.93 (m, 2H, ArH), 6.72 (d, J = 7.6 Hz, 1H, ArH), 5.27 (s, 1H, CH), 5.25 (s, 1H, CH), 3.74 (s, 3H, CH2), 3.21 (s, 3H, CH2), 3.04–2.91 (m, 1H, CH2), 2.73–2.52 (m, 4H, CH2), 2.13–2.04 (m, 1H, CH3), 0.81 (t, J = 14.0 Hz, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 178.4, 143.5, 135.9, 135.6, 135.2, 129.9, 129.7, 129.2, 128.7, 128.4, 127.0, 125.8, 124.5, 123.9, 122.8, 122.1, 119.8, 117.4, 110.1, 110.0, 105.6, 100.6, 73.9, 67.9, 55.0, 49.2, 33.2, 30.1, 29.5, 9.9.
1′-Ethyl-2′-(1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6r). Brown solid; 86% yield; >99: 1 dr; mp 230–234 °C; IR(KBr) 735, 746, 1339, 1473, 1533, 1697, 1734, 3197, 3275, 3523, 3712, 3853 cm−1; HRMS(ESI) calcd for C29H26N4O3 [M+H]+ 479.2078, found 479.2084; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 10.88 (s, 1H, NH), 10.42 (s, 1H, NH), 7.43 (d, J = 7.2 Hz, 1H, ArH), 7.25 (t, J = 7.6 Hz, 2H, ArH), 7.13–7.10 (m, 6H, ArH), 7.00–6.01 (m, 2H, ArH), 6.87 (t, J = 14.8 Hz, 1H, ArH), 6.67 (d, J = 7.6 Hz, 1H, ArH), 5.27 (d, J = 9.2 Hz, 2H, CH), 3.03–2.94 (m, 1H, CH2), 2.69–2.62 (m, 4H, CH2), 2.09–2.04 (m, 1H, CH2), 0.79 (t, J = 14.4 Hz, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 178.6, 143.3, 135.5, 135.4, 135.2, 129.7, 129.5, 128.7, 128.4, 126.8, 125.6, 124.4, 123.8, 122.7, 121.8, 119.5, 117.2, 111.7, 109.9, 106.4, 100.6, 74.0, 68.0, 60.1, 55.4, 42.7, 30.1, 29.5, 21.1, 14.4, 9.8.
2′-(2-Methyl-1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6s). Brown solid; 78% yield; >99: 1 dr; mp 215–224 °C; IR(KBr) 741, 1339, 1458, 1554, 1683, 1705, 3367, 3524, 3711, 3751, 3820 cm-1; HRMS(ESI) calcd for C28H24N4O3 [M+H]+ 465.1921, found 465.1926; 1H-NMR (400 MHz, DMSO-d6): δ 10.95 (s, 1H, NH), 10.06 (s, 1H, NH), 7.59 (d, J = 7.2 Hz, 1H, ArH), 7.51 (s, 2H, ArH), 7.32–7.14 (m, 5H, ArH), 6.98 (t, J = 14.4 Hz, 1H, ArH), 6.90 (d, J = 6.8 Hz, 1H, ArH), 6.66 (d, J = 7.6 Hz, 1H, ArH), 6.46 (t, J = 11.2 Hz, 1H, ArH), 5.99 (d, J = 6.4 Hz, 1H, CH), 4.54 (d, J = 4.4 Hz, 1H, CH), 4.07–4.00 (m, 1H, CH), 3.00–2.94 (m, 1H, CH2), 2.71 (d, J = 6.8 Hz, 3H, CH2), 2.51 (s, 1H, CH3), 1.83 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 177.7, 143.6, 135.6, 135.5, 134.9, 133.7, 130.2, 129.6, 128.3, 127.3, 126.2, 125.3, 124.7, 122.7, 120.7, 119.0, 111.0, 110.0, 103.1, 91.0, 74.9, 63.7, 60.3, 54.3, 42.8, 21.2, 14.6, 11.8.
8′-Bromo-2′-(1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6t). Yellow solid; 93% yield; >99: 1 dr; mp >320 °C; IR(KBr) 754, 1338, 1489, 1552, 1683, 2827, 2927, 3047, 3197, 3286, 3348, 3462, 3566, 3711, 3801 cm−1; HRMS(ESI) calcd for C27H21BrN4O3 [M+H]+ 529.0870, found 529.0862; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 11.04 (s, 1H, NH), 11.02 (s, 1H, NH), 7.66 (d, J = 7.2 Hz, 1H, ArH), 7.49 (d, J = 2.4 Hz, 1H, ArH), 7.34 (d, J = 6.4 Hz, 2H, ArH), 7.27–7.21 (m, 3H, ArH), 7.18–7.15 (m, 1H, ArH), 6.94 (t, J = 14.8 Hz, 1H, ArH), 6.67 (t, J = 15.2 Hz, 1H, ArH), 6.60 (d, J = 7.6 Hz, 2H, ArH), 6.32–6.30 (m, 1H, CH), 5.77 (d, J = 6.8 Hz, 1H, CH), 4.69 (d, J = 4.4 Hz, 1H, CH), 3.04–2.95 (m, 1H, CH2), 2.72 (s, 1H, CH2), 2.70–2.64 (m, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 176.9, 143.6, 138.2, 136.1, 133.0, 132.0, 130.0, 128.9, 128.2, 127.2, 127.0, 124.7, 124.5, 122.7, 121.4, 120.3, 119.0, 118.1, 111.7, 110.0, 108.6, 93.1, 74.8, 62.8, 52.5, 42.3, 29.8.
2′-(1H-Indol-3-yl)-1′, 9′-dinitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6u). Yellow solid; 85% yield; >99: 1 dr; mp 287–289 °C; IR(KBr) 1350, 1472, 1519, 1683, 1717, 2320, 3365, 3444, 3566, 3674, 3734, 3799, 3838 cm−1; HRMS(ESI) calcd for C27H21N5O5 [M+H]+ 496.1615, found 496.1621; 1H-NMR (400 MHz, DMSO-d6+CDCl3): δ 10.83 (s, 1H, NH), 9.93 (s, 1H, NH), 8.22 (s, 1H, ArH), 8.03 (t, J = 8.4 Hz, 1H, ArH), 8.02 (s, 1H, ArH), 7.86 (s, 1H, ArH), 7.70–7.16 (m, 4H, ArH), 6.97 (t, J = 14.8 Hz, 1H, ArH), 6.74–6.60 (m, 3H, ArH), 6.41–6.38 (m, 1H, ArH), 5.95 (d, J = 6.4 Hz, 1H, CH), 4.79 (d, J = 4.4 Hz, 1H, CH), 3.18–3.13 (m, 1H, CH2), 2.83 (d, J = 10.0 Hz, 1H, CH2), 2.78 (d, J = 8.6 Hz, 2H, CH2); 13C-NMR (100 MHz, DMSO-d6+CDCl3): δ 177.0, 145.9, 143.8, 143.5, 136.1, 135.0, 130.5, 130.0, 127.8, 126.9, 124.4, 122.7, 121.8, 121.5, 120.4, 119.1, 118.1, 111.6, 110.1, 108.4, 92.9, 74.7, 62.8, 52.4, 41.9, 30.3.
1′-Ethyl-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6v). White solid; 94% yield; >99: 1 dr; mp 271–273 °C; IR (KBr) 741, 1030, 1204, 1302, 1375, 1491, 1551, 1699, 2839, 2920, 3347, 3431 cm−1; HRMS(ESI) calcd for C29H26N4O4 [M+H]+ 495.2027, found 495.2033; 1H-NMR (400 MHz, DMSO-d6): δ 9.96 (s, 1H, NH), 7.55 (s, 1H, ArH), 7.33 (d, J = 8.4 Hz, 1H, ArH), 7.26 (s, 1H, ArH), 7.24–7.20 (m, 3H, ArH), 7.18 (t, J = 9.2 Hz, 1H, ArH), 7.02–7.01 (m, 1H, ArH), 6.86–6.83 (m, 1H, ArH), 6.77 (t, J = 8.0 Hz, 1H, ArH), 6.67 (s, 1H, ArH), 6.54 (s, 1H, CH), 6.36–6.33 (m, 1H, CH), 5.77 (d, J = 6.8 Hz, 1H, CH), 4.65 (d, J = 4.8 Hz, 1H, CH), 3.83 (s, 3H, CH3), 3.75 (s, 3H, CH2), 3.01 (s, 1H, CH2), 2.70 (s, J = 5.2 Hz, 1H, CH3), 2.67 (s, 1H, CH3), 2.63 (s, 1H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 176.8, 155.9, 136.8, 136.6, 135.4, 133.7, 129.6, 129.1, 127.4, 127.2, 126.2, 125.0, 121.7, 119.3, 118.4, 115.0, 111.1, 110.6, 110.2, 108.0, 93.2, 63.2, 60.3, 56.1, 52.4, 40.1, 40.0, 39.5, 33.1.
5-Bromo-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6w). Orange solid; 92% yield; >99: 1 dr; mp 264–266 °C; IR (KBr) 741, 908, 1204, 1375, 1481, 1558, 1612, 1718, 2839, 2920, 3246, 3358 cm−1; HRMS(ESI) calcd for C28H23BrN4O3 [M+H]+ 543.1026, found 543.1020; 1H-NMR (400 MHz, DMSO-d6): δ 10.29 (s, 1H, NH), 7.62 (d, J = 8 Hz, 1H, ArH), 7.56 (s, 1H, ArH), 7.41–7.36 (m, 1H, ArH), 7.27 (s, 1H, ArH), 7.26–7.21 (m, 1H, ArH), 7.20–7.15 (m, 3H, ArH), 7.05 (t, J = 15.2 Hz, 1H, ArH), 6.79 (s, 1H, ArH), 6.76 (t, J = 6.8 Hz, 1H, ArH), 6.65 (s, 1H, CH), 6.38–6.35 (m, 1H, CH), 5.76 (d, J = 6.8 Hz, 1H, CH), 4.67 (d, J = 4.8 Hz, 1H, CH), 3.76 (s, 3H, CH2), 2.97–2.90 (m, 1H, CH2), 2.71 (d, J = 4.4 Hz, 1H, CH3), 2.65 (s, 1H, CH3), 2.62 (t, J = 3.2 Hz, 1H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 176.6, 145.3, 136.6, 135.3, 133.5, 129.6, 129.3, 127.7, 126.7, 126.2, 125.6, 125.1, 122.9, 121.8, 119.3, 118.1, 112.9, 110.3, 107.7, 93.0, 63.3, 60.2, 52.2, 42.8, 40.2, 40.0, 39.3, 33.1.
7-Bromo-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3, 3′-pyrrolo[2, 1-a]isoquinolin]-2-one (6x). Orange solid; 86% yield; >99: 1 dr; mp 285–287 °C; IR (KBr) 741, 1125, 1190, 1331, 1458, 1549, 1628, 1699, 2835, 2918, 3200, 3221 cm−1; HRMS(ESI) calcd for C28H23BrN4O3 [M+H]+ 543.1026, found 543.1031; 1H-NMR (400 MHz, DMSO-d6): δ 10.12 (s, 1H, NH), 7.66 (s, 1H, ArH), 7.54 (s, 1H, ArH), 7.43 (s, 1H, ArH), 7.41 (d, J = 5.2 Hz, 1H, ArH), 7.31 (s, 1H, ArH), 7.29 (s, J = 0.8 Hz, 1H, ArH), 7.26 (s, 1H, ArH), 7.23–7.18 (m, 1H, ArH), 7.02 (t, J = 15.2 Hz, 1H, ArH), 6.72 (s, 1H, ArH), 6.60 (s, J = 7.6 Hz, 1H, ArH), 6.54 (d, J = 8 Hz, 1H, CH), 6.40–6.37 (m, 1H, CH), 5.73 (d, J = 6.8 Hz, 1H, CH), 4.65 (d, J = 4.4 Hz, 1H, CH), 3.74 (s, 3H, CH2), 2.97–2.89 (m, 1H, CH2), 2.73 (s, 1H, CH3), 2.67 (t, J = 10.4 Hz, 1H, CH3), 2.63 (t, J = 7.6 Hz, 1H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 176.8, 143.6, 138.4, 136.6, 133.1, 132.1, 130.3, 129.1, 128.1, 127.4, 124.7, 122.9, 121.7, 120.3, 119.2, 118.2, 110.2, 110.1, 107.9, 93.0, 62.9, 52.4, 40.2, 40.0, 39.6, 33.1, 29.6, 14.6.
2′-(1-Methyl-1H-indol-3-yl)-1′-nitro-1-phenyl-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3, 3′-pyrrolo[2, 1-a]isoquinolin]-2-one (6y). Orange solid; 87% yield; >99: 1 dr; mp 277–279 °C; IR (KBr) 741, 908, 1203, 1302, 1375, 1551, 1612, 1701, 2810, 2920, 3246, 3358 cm−1; HRMS(ESI) calcd for C34H28N4O3 [M+H]+ 541.2234, found 541.2240; 1H-NMR (400 MHz, DMSO-d6): δ 7.81–7.79 (m, 1H, ArH), 7.63 (s, 1H, ArH), 7.39 (s, Hz, 1H, ArH), 7.38–7.33 (m, 4H, ArH), 7.31 (s, 1H, ArH), 7.30 (m, 1H, ArH), 7.25 (s, 1H, ArH), 7.24–7.18 (m, 1H, ArH), 7.06 (s, 1H, ArH), 6.68 (s, 1H, ArH), 6.64 (s, 1H, ArH), 6.48 (s, 1H, ArH), 6.47 (t, J = 6.4 Hz, 1H, ArH), 6.45 (t, J = 2.8 Hz, 1H, ArH), 6.43–6.41 (m, 1H, CH), 6.34 (s, 1H, CH), 5.79 (d, J = 6.8 Hz, 1H, CH), 4.76 (d, J = 4 Hz, 1H, CH), 3.76 (s, 3H, CH2), 2.99 (s, 1H, CH2), 2.85 (s, 1H, CH3), 2.75 (s, 1H, CH3), 2.73 (s, 1H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 174.5, 145.0, 136.8, 135.4, 133.9, 133.4, 130.4, 129.9, 129.6, 129.0, 128.5, 127.9, 127.3, 127.2, 126.6, 126.2, 125.4, 124.8, 124.3, 121.8, 119.1, 118.1, 110.1, 109.4, 108.0, 92.3, 63.9, 53.6, 42.8, 40.1, 39.9, 39.5, 33.1, 29.9.
2′-(1-Methyl-1H-indol-3-yl)-1′,9′-dinitro-1′,5′,6′,10b’-tetrahydro-2′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (6z). Orange solid; 85% yield; >99: 1 dr; mp >320 °C; IR (KBr) 741, 1125, 1190, 1341, 1458, 1518, 1557, 1628, 1699, 2835, 2918, 3110 cm−1; HRMS(ESI) calcd for C28H23N5O5 [M+H]+ 510.1772, found 510.1779; 1H-NMR (400 MHz, DMSO-d6): δ 10.11 (s, 1H, NH), 8.32 (s, 1H, ArH), 8.21 (d, J = 86 Hz, 1H, ArH), 8.08 (s, 1H, ArH), 8.07 (s, 1H, ArH), 7.67 (d, J = 7.2 Hz, 1H, ArH), 7.28 (s, 1H, ArH), 7.23 (s, 1H, ArH), 7.19 (s, 1H, ArH), 7.04–7.00 (m, 1H, ArH), 6.72–6.78 (m, 1H, ArH), 6.62 (s, 1H, ArH), 6.61–6.59 (m, 1H, CH), 6.50 (s, 1H, CH), 5.85 (d, J = 6.8 Hz, 1H, CH), 4.69 (d, J = 4.4 Hz, 1H, CH), 3.76 (s, 3H, CH2), 3.07–3.04 (m, 1H, CH2), 2.84 (s, 1H, CH3), 2.73 (s, 1H, CH3), 2.66 (t, J = 11.2 Hz, 1H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 176.6, 145.9, 144.2, 143.7, 136.6, 135.2, 131.1, 130.4, 129.2, 128.2, 127.3, 124.7, 123.0, 122.1, 121.7, 120.9, 119.2, 118.3, 110.2, 110.1, 108.0, 92.6, 62.9, 52.3, 40.2, 40.0, 39.6, 39.4.
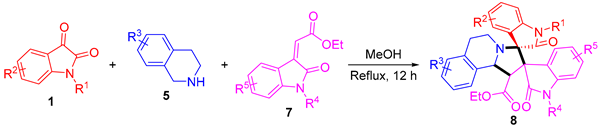
4.4. General Procedure for the Synthesis of Compounds 8
A mixture of isatins (1, 0.5 mmol), THIQs (5, 0.5 mmol), and ethyl (Z)-2-(2-oxoindolin-3-ylidene) acetates (7, 0.5 mmol) in MeOH was stirred under reflux conditions for 12 h and indicated by TLC. Then, the reaction mixture was allowed to cool to room temperature and extracted with dichloromethane (3×50 mL). The combined organic layers were washed with saturated brine solution (20 mL), followed by drying over MgSO4 and evaporating in vacuo. The crude product was purified by column chromatography to give the pure title compounds.
Ethyl 2,2″-dioxo-1′,5′,6′,10b’-tetrahydrodispiro[indoline-3,2′-pyrrolo[2,1-a]isoquinoline-3′,3″-indoline]-1′-carboxylate (8a). Orange solid; 66% yield; 25: 1 dr; mp > 320 °C; IR (KBr) 1190, 1292, 1341, 1474, 1618, 1709, 2345, 2372, 3210 cm−1; HRMS(ESI) calcd for C29H25N3O4 [M+H]+ 480.1918, found 480.1914; 1H-NMR (400 MHz, DMSO-d6): δ 10.77 (s, 1H, NH), 10.60 (s, 1H, NH), 7.95 (d, J = 7.2 Hz, 1H, ArH), 7.32 (s, 1H, ArH), 7.30 (t, J = 15.2 Hz, 3H, ArH), 7.27 (s, 1H, ArH), 7.08–7.03 (m, 3H, ArH), 6.97–6.92 (m, 2H, ArH), 6.89 (d, J = 8.0 Hz, 1H, ArH), 6.34 (d, J = 7.6 Hz, 1H, ArH), 5.51 (s, 1H, CH2), 4.06 (s, 1H, CH), 3.50–3.45 (m, 2H, CH2), 2.83 (d, J = 6.4 Hz, 1H, CH), 2.65 (t, J = 16.0 Hz, 2H, CH2), 1.21 (s, 1H, CH3), 0.44 (t, J = 14.4 Hz, 2H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 179.4, 177.3, 167.9, 144.0, 142.9, 135.7, 135.0, 132.0, 130.3, 129.4, 129.2, 127.5, 126.8, 126.1, 125.8, 124.6, 123.3, 122.4, 110.0, 109.8, 70.5, 68.6, 62.7, 60.5, 57.9, 42.2, 40.5, 29.7, 13.3.
1″-Methyl-2, 2″-dioxo-1′,5′,6′,10b’-tetrahydrodispiro[indoline-3, 2′-pyrrolo[2,1-a]isoquinoline-3′,3″-indoline]-1′-carboxylate (8b). Pale yellow solid; 85% yield; >99: 1 dr; mp 294–296 °C; IR (KBr) 1211, 1352, 1373, 1471, 1616, 1653, 1699, 1717, 1869, 2345 cm−1; HRMS(ESI) calcd for C30H27N3O4 [M+H]+ 494.2074, found 494.2083; 1H-NMR (400 MHz, DMSO-d6): δ 10.78 (s, 1H, NH), 7.99 (s, 1H, ArH), 7.97–7.39 (m, 1H, ArH), 7.35–7.28 (m, 2H, ArH), 2.28–7.13 (m, 1H, ArH), 7.10–7.04 (m, 4H, ArH), 6.95 (t, J = 15.6 Hz, 2H, ArH), 6.35 (d, J = 7.6 Hz, 1H, ArH), 5.54 (s, 1H, CH), 4.09 (s, 1H, CH), 3.47–3.39 (m, 2H, CH2), 3.22 (s, 3H, CH3), 2.87–2.80 (m, 1H, CH2), 2.78–2.50 (m, 3H, CH2), 0.43 (t, J = 14.4 Hz, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 179.3, 175.5, 167.9, 145.3, 142.9, 135.6, 134.9, 131.9, 130.5, 129.5, 129.3, 126.9, 126.7, 126.2, 125.8, 124.3, 123.4, 123.2, 122.5, 109.9, 109.1, 70.4, 68.8, 63.0, 60.6, 58.0, 42.2, 29.6, 26.4, 13.3.
2, 2″-Dioxo-1″-phenyl-1′,5′,6′,10b’-tetrahydrodispiro[indoline-3,2′-pyrrolo[2,1-a]isoquinoline-3′,3″-indoline]-1′-carboxylate (8c). Pale yellow solid; 74% yield; >99: 1 dr; mp > 300 °C; IR (KBr) 1200, 1369, 1458, 1508, 1616, 1647, 1707, 1734, 2345, 3736 cm−1; HRMS(ESI) calcd for C35H29N3O4 [M+H]+ 556.2231, found 556.2234; 1H-NMR (400 MHz, DMSO-d6): δ 10.84 (s, 1H, NH), 7.88 (s, 1H, ArH), 7.66 (t, J = 16.0 Hz, 1H, ArH), 7.53 (t, J = 14.0 Hz, 3H, ArH), 7.49–7.44 (m, 1H, ArH), 7.38–7.32 (m, 2H, ArH), 7.30–7.20 (m, 1H, ArH), 7.09–7.04 (m, 3H, ArH), 7.02–6.95 (m, 2H, ArH), 6.81 (m, 1H, ArH), 6.37 (d, J = 8.0 Hz, 1H, ArH), 5.53 (s, 1H, CH), 4.18 (d, J = 6.4 Hz, 1H, CH), 3.54–3.41 (m, 2H, CH2), 2.93–2.83 (m, 2H, CH2), 2.80–2.51 (m, 2H, CH2), 0.45 (t, J = 14.0 Hz, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 179.3, 175.0, 168.2, 145.0, 142.9, 135.6, 135.0, 134.7, 131.8, 130.6, 130.2, 129.5, 129.3, 128.6, 127.2, 126.9, 126.6, 126.2, 125.6, 124.9, 123.9, 123.3, 122.5, 110.0, 109.5, 70.3, 69.0, 63.7, 60.8, 57.8, 42.2, 40.5, 40.1, 29.7, 13.4.
5″-Methyl-2,2″-dioxo-1′,5′,6′,10b’-tetrahydrodispiro[indoline-3,2′-pyrrolo[2,1-a]isoquinoline-3′,3″-indoline]-1′-carboxylate (8d). Pale yellow solid; 64% yield; 34: 1 dr; mp > 300 °C; IR (KBr) 1196, 1474, 1497, 1616, 1707, 1736, 2345, 2371, 3198, 3566 cm−1; HRMS(ESI) calcd for C30H27N3O4 [M+H]+ 494.2074, found 494.2081; 1H-NMR (400 MHz, DMSO-d6): δ 10.77 (s, 1H, NH), 10.49 (d, J = 8.0 Hz, 1H, NH), 7.95 (d, J = 7.2 Hz, 1H, ArH), 7.29 (t, J = 15.2 Hz, 2H, ArH), 7.10 (s, 3H, ArH), 7.09 (s, 2H, ArH), 7.06–7.02 (m, 1H, ArH), 6.94 (t, J = 16.0 Hz, 1H, ArH), 7.77 (d, J = 8.0 Hz, 1H, ArH), 5.50 (s, 1H, ArH), 4.03 (t, J = 12.4 Hz, 1H, CH2), 2.88–2.80 (m, 1H, CH), 2.65 (d, J = 10.8 Hz, 2H, CH3), 2.63–2.56 (m, 2H, CH2), 2.55–2.51 (m, 2H, CH3), 2.30 (s, 3H, CH2), 0.44 (t, J = 14.0 Hz, 2H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 179.5, 177.2, 167.9, 142.9, 141.6, 135.8, 135.0, 132.0, 131.2, 130.6, 129.4, 129.2, 127.6, 127.5, 126.8, 126.1, 125.9, 125.1, 123.3, 122.4, 109.8, 70.5, 68.6, 62.7, 60.4, 57.8, 42.2, 29.7, 21.1, 13.3.
5″-Methoxy-2,2″-dioxo-1′,5′,6′,10b’-tetrahydrodispiro[indoline-3,2′-pyrrolo[2,1-a]isoquinoline-3′,3″-indoline]-1′-carboxylate (8e). Brown solid; 71% yield; 40: 1 dr; mp 297–299 °C; IR (KBr) 1190, 1288, 1491, 1616, 1701, 1734, 2345, 3167, 3190, 3736 cm−1; HRMS(ESI) calcd for C30H27N3O5 [M+H]+ 510.2023, found 510.2018; 1H-NMR (400 MHz, DMSO-d6): δ 10.78 (s, 1H, NH), 10.42 (s, 1H, NH), 7.96 (d, J = 7.2 Hz, 1H, ArH), 7.31–7.02 (m, 3H, ArH), 6.96–6.89 (m, 2H, ArH), 6.87–6.82 (m, 1H, ArH), 6.80 (s, 2H, ArH), 6.35 (d, J = 7.6 Hz, 1H, ArH), 5.49 (s, 1H, ArH), 3.74 (s, 3H, CH3), 3.49–3.46 (m, 2H, CH), 3.42 (d, J = 9.6 Hz, 2H, CH2), 2.90–2.81 (m, 1H, CH2), 2.66–2.51 (m, 3H, CH2), 0.45 (t, J = 14.4 Hz, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 179.4, 177.1, 167.8, 155.4, 142.9, 137.2, 135.8, 135.0, 132.0, 129.5, 129.2, 138.9, 126.8, 126.1, 125.9, 123.3, 122.4, 115.1, 110.9, 110.6, 109.8, 70.8, 68.6, 62.7, 60.4, 57.7, 55.9, 40.5, 29.7, 13.3.
5″-Fluoro-2,2″-dioxo-1′,5′,6′,10b′-tetrahydrodispiro[indoline-3,2′-pyrrolo[2,1-a]isoquinoline-3′,3″-indoline]-1′-carboxylate (8f). Pale yellow solid; 77% yield; 25: 1 dr; mp > 300 °C; IR (KBr) 1192, 1211, 1296, 1474, 1491, 1616, 1705, 1734, 3210, 3566 cm−1; HRMS(ESI) calcd for C29H24FN3O4 [M+H]+ 498.1824, found 498.1828; 1H-NMR (400 MHz, DMSO-d6): δ 10.79 (s, 1H, NH), 10.64 (s, 1H, NH), 7.94–7.28 (m, 1H, ArH), 7.18–7.13 (m, 1H, ArH), 7.08–7.03 (m, 2H, ArH), 6.97–6.93 (m, 3H, ArH), 6.91–6.87 (m, 2H, ArH), 6.34 (d, J = 8.0 Hz, 1H, ArH), 5.48 (s, 1H, ArH), 4.04 (s, 2H, CH), 3.50–3.42 (m, 2H, CH2), 2.90–2.82 (m, 1H, CH2), 2.67–2.51 (m, 3H, CH2), 0.45 (t, J = 14.0 Hz, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 179.3, 177.2, 167.8, 160.0, 157.3, 142.9, 140.2, 135.6, 134.9, 131.9, 129.5, 129.3, 129.2, 126.8, 123.3, 122.4, 116.9, 116.7, 112.4, 112.1, 111.0, 110.9, 109.9, 70.8, 68.5, 62.6, 60.5, 57.7, 13.3.
6″-Bromo-2,2″-dioxo-1′,5′,6′,10b′-tetrahydrodispiro[indoline-3,2′-pyrrolo[2,1-a]isoquinoline-3′,3″-indoline]-1′-carboxylate (8g). Pale yellow solid; 61% yield; >99: 1 dr; mp > 300 °C; IR (KBr) 1190, 1341, 1474, 1616, 1709, 1717, 1734, 2345, 3370, 3566 cm−1; HRMS(ESI) calcd for C29H24BrN3O4 [M+H]+ 557.0950, found 557.0955; 1H-NMR (400 MHz, DMSO-d6): δ 10.77 (d, J =8.8 Hz, 2H, NH), 7.91 (s, 1H, ArH), 7.89–7.28 (m, 2H, ArH), 7.27–7.24 (m, 3H, ArH), 7.22–7.03 (m, 1H, ArH), 6.97–6.94 (m, 1H, ArH), 6.34 (d, J =7.6 Hz, 1H, ArH), 5.46 (s, 1H, ArH), 4.04 (s, 1H, ArH), 3.51–3.41 (m, 2H, ArH), 2.85 (s, 2H, CH), 2.83 (t, J =15.6 Hz, 1H, CH2), 2.68 (m, 1H, CH2), 2.64–2.56 (m, 2H, CH2), 2.54–2.50 (m, 2H, CH2), 0.45 (t, J =14.4 Hz, 1H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 179.3, 177.1, 167.8, 145.6, 142.9, 135.6, 134.9, 131.8, 129.5, 129.3, 126.9, 126.6, 126.2, 125.7, 125.2, 123.3, 123.0, 122.5, 112.8, 110.0, 70.3, 68.6, 62.6, 60.6, 57.7, 42.2, 29.7, 21.1, 13.3.
10b′-Methyl-2,2″-dioxo-1′,5′,6′,10b′-tetrahydrodispiro[indoline-3,2′-pyrrolo[2,1-a]isoquinoline-3′,3″-indoline]-1′-carboxylate (8h). Purple solid; 60% yield; >99: 1 dr; mp > 300 °C; IR (KBr) 1213, 1323, 1458, 1474, 1616, 1653, 1676, 1719, 2345, 3566 cm−1; HRMS(ESI) calcd for C30H27N3O4 [M+H]+ 494.2074, found 494.2069; 1H-NMR (400 MHz, DMSO-d6): δ 11.02 (s, 1H, NH), 10.29 (s, 1H, NH), 8.07 (s, 1H, ArH), 7.68 (t, J = 16.3 Hz, 1H, ArH), 7.46–7.39 (m, 1H, ArH), 7.29–7.19 (m, 1H, ArH), 7.18–7.10 (m, 4H, ArH), 6.98 (t, J = 15.2 Hz, 1H, ArH), 6.76 (s, 1H, ArH), 7.74–6.65 (m, 1H, ArH), 6.27–6.24 (m, 1H, ArH), 5.83 (d, J = 6.8 Hz, 1H, ArH), 4.71 (t, J = 4.8 Hz, 1H, CH), 3.36 (d, J = 1.6 Hz, 3H, CH2), 3.07–3.01 (m, 1H, CH2), 2.76 (t, J = 16.4 Hz, 2H, CH2), 2.72–2.54 (m, 6H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 176.6, 142.6, 135.8, 134.9, 132.9, 132.5, 130.0, 129.0, 126.7, 126.5, 125.6, 124.6, 124.2, 123.8, 123.1, 121.2, 118.8, 117.6, 111.4, 108.0, 102.2, 92.7, 79.0, 78.6, 78.3, 75.3, 62.9, 52.5, 42.4, 29.6.
8′,9′-Dimethoxy-2,2″-dioxo-1′,5′,6′,10b′-tetrahydrodispiro[indoline-3,2′-pyrrolo[2,1-a]isoquinoline-3′,3″-indoline]-1′-carboxylate (8i). Yellow solid; 71% yield; >99: 1 dr; mp 280–282 °C; IR (KBr) 1105, 1142, 1215, 1341, 1373, 1474, 1522, 1618, 1701, 2345 cm−1; HRMS(ESI) calcd for C31H29N3O6 [M+H]+ 540.2129, found 540.2133; 1H-NMR (400 MHz, DMSO-d6): δ 10.72 (s, 1H, NH), 10.58 (s, 1H, NH), 7.93 (s, 1H, ArH), 7.91–7.08, (m, 3H, ArH), 7.08–7.04 (m, 2H, ArH), 6.94 (d, J = 7.6 Hz, 2H, ArH), 6.89 (d, J = 7.6 Hz, 1H, ArH), 6.62 (s, 1H, ArH), 5.86 (s, 1H, ArH), 5.44 (s, 1H, ArH), 4.07 (s, 1H, CH), 3.65–3.51 (m, 3H, CH3), 3.50–3.46 (m, 1H, CH2), 3.45 (t, J = 18.4 Hz, 1H, CH), 3.36 (s, 3H, CH2), 2.75–2.67 (m, 1H, CH2), 2.65–2.47 (m, 2H, CH2), 0.43 (t, J = 14.0 Hz, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 179.6, 177.4, 168.0, 147.5, 146.8, 144.0, 142.9, 132.1, 130.3, 129.2, 127.6, 127.1, 126.9, 125.8, 124.8, 122.5, 122.3, 112.4, 110.0, 110.0, 106.7, 70.7, 68.8, 62.1, 60.4, 58.3, 55.7, 54.9, 42.2, 29.2, 13.3.
5,7-Dimethyl-2,2″-dioxo-1′,5′,6′,10b′-tetrahydrodispiro[indoline-3, 2′-pyrrolo[2,1-a]isoquinoline-3′,3”-indoline]-1′-carboxylate (8j). Pale yellow solid; 83% yield; >99: 1 dr; mp > 300 °C; IR (KBr) 752, 1032, 1159, 1184, 1204, 1285, 1346, 1474, 1622, 1716 cm−1; HRMS(ESI) calcd for C31H29N3O4 [M+H]+ 508.2231, found 508.2236; 1H-NMR (400 MHz, DMSO-d6): δ 10.70 (s, 1H, NH), 10.52 (s, 1H, NH), 7.61 (s, 1H, ArH), 7.30 (d, J = 8.0 Hz, 2H, ArH), 7.18 (d, J = 7.6 Hz, 2H, ArH), 7.05–6.94 (m, 2H, ArH), 6.93–6.88 (m, 1H, ArH), 6.36 (d, J = 8.0 Hz, 1H, ArH), 5.47 (s, 1H, ArH), 4.17–4.13 (m, 1H, CH2), 4.05 (d, J = 9.2 Hz, 1H, CH2), 3.51–3.43 (m, 2H, CH), 3.41 (d, 1H, CH2), 3.17 (d, J = 5.2 Hz, CH2), 2.87 (s, 2H, CH2), 2.84–2.63 (m, 3H, CH3), 2.58–2.24 (m, 3H, CH3), 0.43 (t, J = 14.0 Hz, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 179.9, 177.2, 167.9, 144.0, 139.1, 135.9, 134.9, 131.7, 131.0, 130.6, 130.3, 129.4, 127.7, 126.7, 126.1, 124.6, 123.5, 122.4, 118.7, 110.0, 70.4, 68.6, 62.9, 60.3, 58.1, 49.1, 42.2, 29.7, 21.4, 16.9, 13.2.