Multicomponent Reactions between Heteroatom Compounds and Unsaturated Compounds in Radical Reactions
Abstract
:1. Introduction
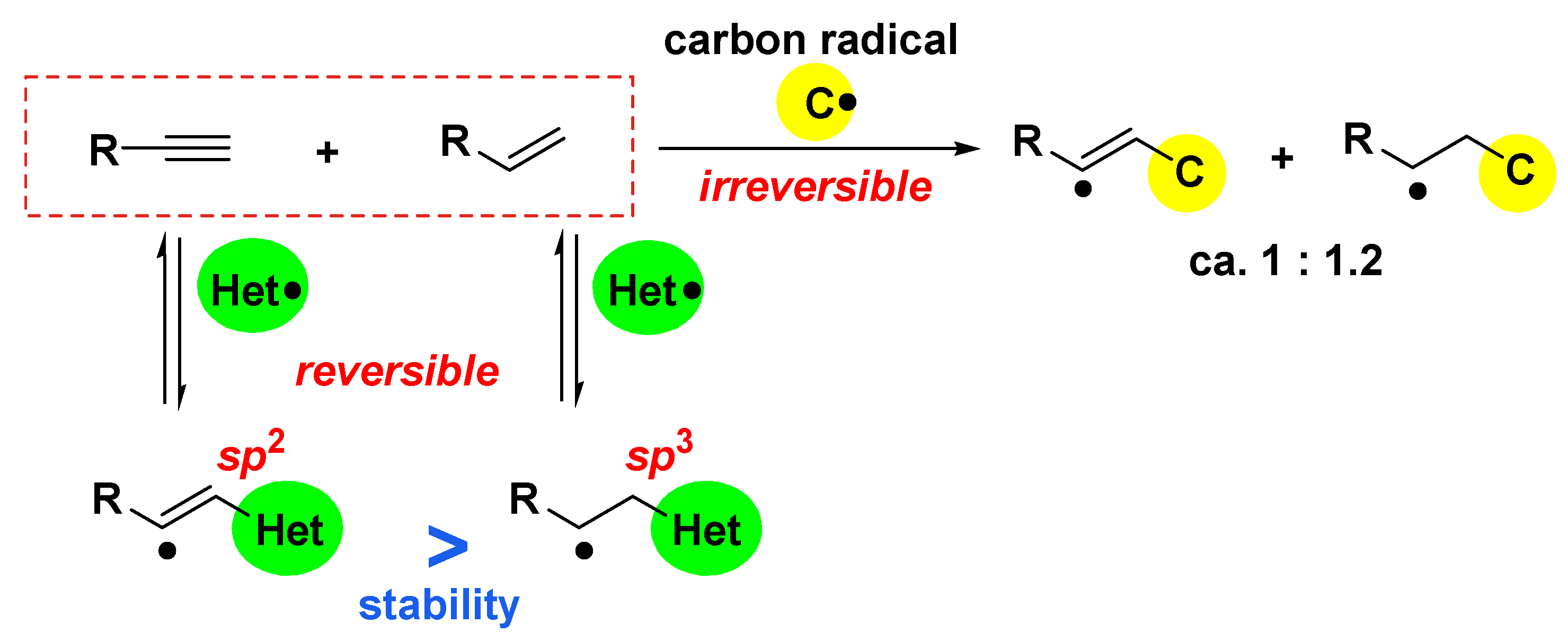
2. General Concept for Photoinduced Radical Addition of Interelement Compounds to Unsaturated Bonds
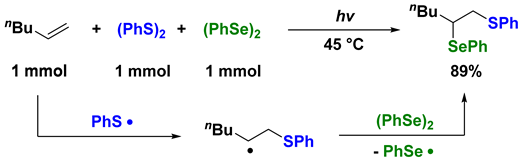
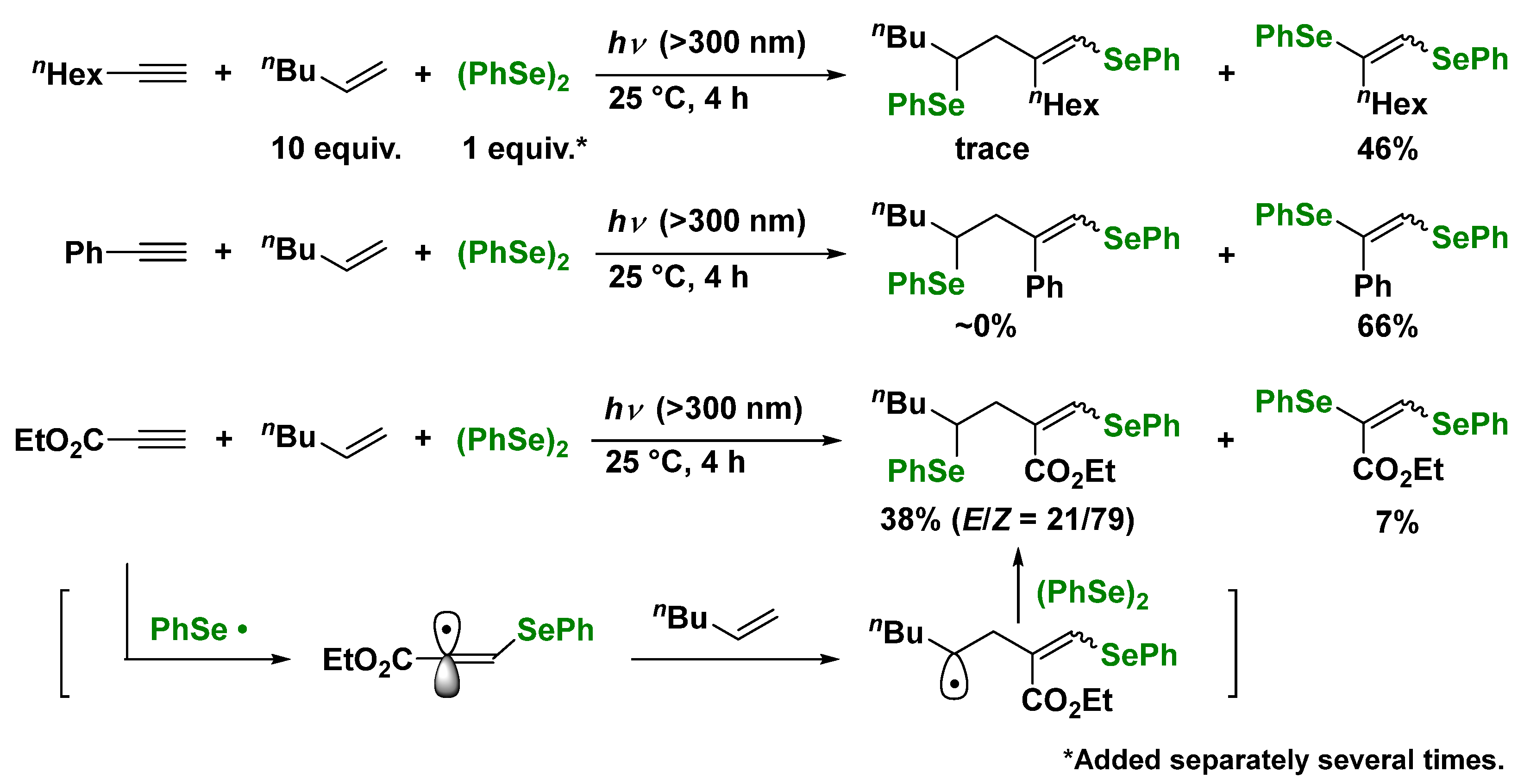
3. Photoinduced Radical Addition to Unsaturated Bonds Using Mixed Systems of Interelement Compounds
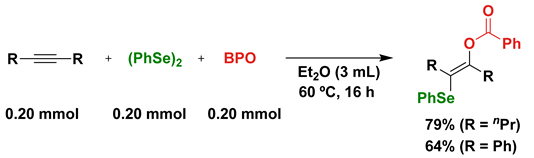
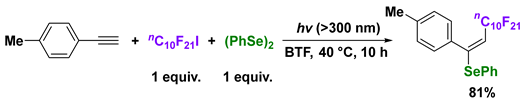

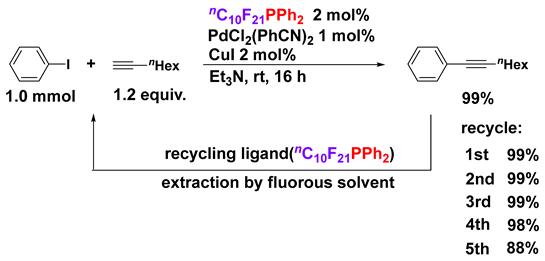
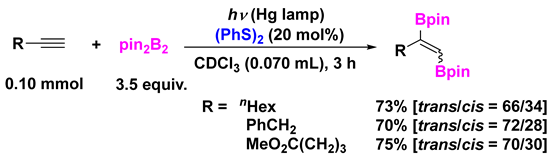

4. Photoinduced Radical Addition of Interelement Compounds to Several Unsaturated Compounds
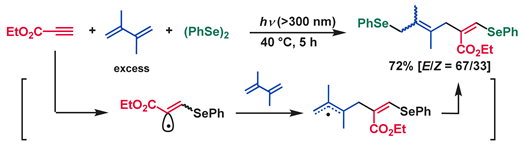

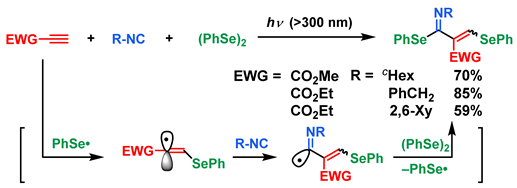
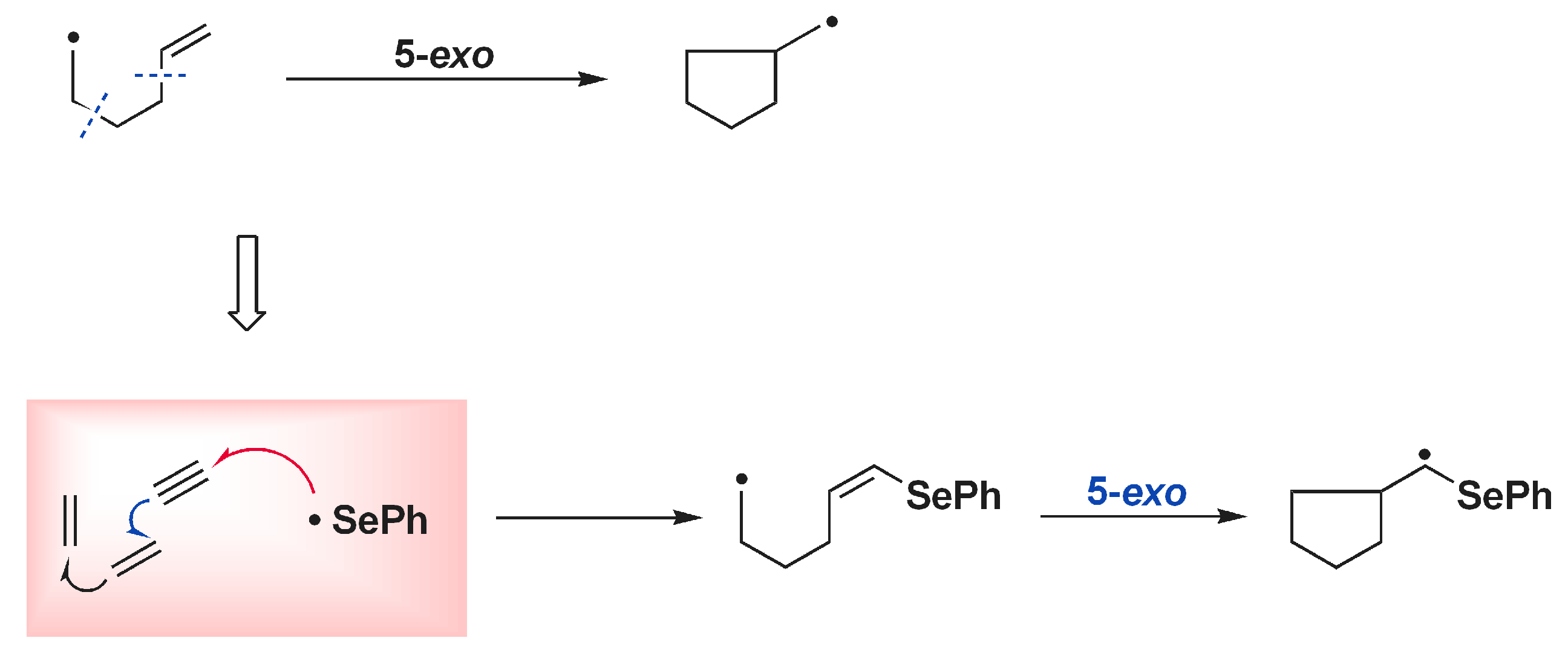
5. Photoinduced Multicomponent Reactions of Interelement Compounds Involving Cyclization Process
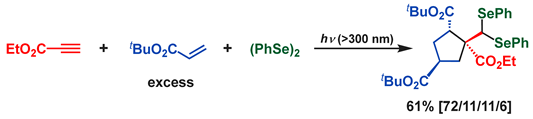
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, J.; Bienaymé, E. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005; pp. 1–468. [Google Scholar]
- Qiu, G.; Ding, Q.; Wu, J. Recent advances in isocyanide insertion chemistry. Chem.Soc. Rev. 2013, 42, 5257–5269. [Google Scholar] [CrossRef]
- Ryu, I.; Sonoda, N.; Curran, D.P. Tandem Radical Reactions of Carbon Monoxide, Isocyanides, and Other Reagent Equivalents of the Geminal Radical Acceptor/Radical Precursor Synthon. Chem. Rev. 1996, 96, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.R. Intermolecular olefin functionalization involving aryl radicals generated from arenediazonium salts. Chem. Eur. J. 2009, 15, 820–833. [Google Scholar] [CrossRef]
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Godineau, E.; Landais, Y. Radical and Radical-Ionic Multicomponent Processes. Chem. Eur. J. 2009, 15, 3044–3055. [Google Scholar] [CrossRef] [PubMed]
- Wille, U. Radical cascades initiated by intermolecular radical addition to alkynes and related triple bond systems. Chem. Rev. 2013, 113, 813–853. [Google Scholar] [CrossRef] [PubMed]
- Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Carbonylation reactions of alkyl iodides through the interplay of carbon radicals and Pd catalysts. Acc. Chem. Res. 2014, 47, 1563–1574. [Google Scholar] [CrossRef]
- Sumino, S.; Ryu, I. Bromine-Radical-Mediated Bromoallylation of C-C unsaturated Bonds: A Facile Access to 1,4-, 1,5-, 1,6-, and 1,7-Dienes and Related Compounds. Synlett 2023, 34, 1001–1011. [Google Scholar]
- Trowbridge, A.; Reich, D.; Gaunt, M.J. Multicomponent synthesis of tertiary alkylamines by photocatalytic olefin-hydroaminoalkylation. Nature 2018, 561, 522–527. [Google Scholar] [CrossRef]
- Coppola, G.A.; Pillitteri, S.; Van der Eycken, E.V.; You, S.-L.; Sharm, U.K. Multicomponent reactions and photo/electrochemistry join forces: Atom economy meets energy efficiency. Chem. Soc. Rev. 2022, 51, 2313–2382. [Google Scholar] [CrossRef]
- Garbarino, S.; Ravelli, D.; Protti, S.; Basso, A. Photoinduced multicomponent reactions. Angew. Chem. Int. Ed. 2016, 55, 15476–15484. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Brunelli, F.; Tron, G.C.; Giustiniano, M. Isocyanide-Based Multicomponent Reactions Promoted by Visible Light Photoredox Catalysis. Chem. Eur. J. 2023, 29, e202203150. [Google Scholar] [CrossRef]
- Wu, J.; Xia, H.; Pan, X. Recent advances in photoinduced trifluoromethylation and difluoroalkylation. Org. Chem. Front. 2016, 3, 1163–1185. [Google Scholar]
- Oh, E.H.; Kim, H.J.; Han, S.B. Recent Developments in Visible-Light-Catalyzed Multicomponent Trifluoromethylation of Unsaturated Carbon-Carbon Bonds. Synthesis 2018, 50, 3346–3358. [Google Scholar]
- Ogawa, A.; Tanaka, H.; Yokoyama, H.; Obayashi, R.; Yokoyama, K.; Sonoda, N. A highly selective thioselenation of olefins using disulfide-diselenide mixed system. J. Org. Chem. 1992, 57, 111–115. [Google Scholar] [CrossRef]
- Toru, T.; Seko, T.; Maekawa, T. Addition of S-Benzoyl Phenylselenosulfide to Olefins: Selenothiocarboxylation. Tetrahedron Lett. 1985, 26, 3263–3266. [Google Scholar] [CrossRef]
- Toru, T.; Seko, T.; Maekawa, T.; Ueno, Y. Reaction of Olefins with Se-Phenyl (Selenothioperoxy)benzoate: A New Anti-Markownikoff Benzeneselenenylation. J. Chem. Soc. Perkin Trans. I. 1988, 26, 575–581. [Google Scholar] [CrossRef]
- Denes, F.; Pichowicz, M.; Povie, G.; Renaud, P. Thiyl radicals in organic synthesis. Chem. Rev. 2014, 114, 2587–2693. [Google Scholar] [CrossRef]
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of Vinyl Selenides. Chem. Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef]
- Procter, D.J. The synthesis of thiols, selenols, sulfides, selenides, sulfoxides, selenoxides, sulfones and selenones. J. Chem. Soc. Perkin Transactions 1 1999, 6, 641–668. [Google Scholar] [CrossRef]
- Comasseto, J.V.; Ling, L.W.; Petragnani, N.; Stefani, H.A. Vinylic selenides and tellurides—Preparation, reactivity and synthetic applications. Synthesis 1997, 1997, 373–403. [Google Scholar] [CrossRef]
- Curran, D.P.; Eichenberger, E.; Collis, M.; Roepel, M.G.; Thoma, G. Group Transfer Addition Reactions of Methyl(phenylseleno)malononitrile to Alkenes. J. Am. Chem. Soc. 1994, 116, 4279–4288. [Google Scholar] [CrossRef]
- Zong, Y.; Lang, Y.; Yang, M.; Li, X.; Fan, X.; Wu, J. Synthesis of β-Sulfonyl Amides through a Multicomponent Reaction with the Insertion of Sulfur Dioxide under Visible Light Irradiation. Org. Lett. 2019, 21, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Tlahuext-Aca, A.; Garza-Sanchez, A.R.; Glorius, F. Multicomponent Oxyalkylation of Styrenes Enabled by Hydrogen-Bond-Assisted Photoinduced Electron Transfer. Angew. Chem. Int. Ed. 2017, 56, 3708–3711. [Google Scholar] [CrossRef] [PubMed]
- Hofman, K.; Liu, N.-W.; Manolikakes, G. Radicals and Sulfur Dioxide: A Versatile Combination for the Construction of Sulfonyl-Containing Molecules. Chem. Eur. J. 2018, 24, 11852–11863. [Google Scholar] [CrossRef]
- Ogawa, A.; Obayashi, R.; Ine, H.; Tsuboi, Y.; Sonoda, N.; Hirao, T. Highly regioselective thioselenation of acetylenes by using a (PhS)2-(PhSe)2 binary system. J. Org. Chem. 1998, 63, 881–884. [Google Scholar] [CrossRef]
- Ogawa, A.; Ogawa, I.; Obayashi, R.; Umezu, K.; Doi, M.; Hirao, T. Highly Selective Thioselenation of Vinylcyclopropanes with a (PhS)2−(PhSe)2 Binary System and Its Application to Thiotelluration. J. Org. Chem. 1999, 64, 86–92. [Google Scholar] [CrossRef]
- Taniguchi, T.; Fujii, T.; Idota, A.; Ishibashi, H. Reductive Addition of the Benzenethiyl Radical to Alkynes by Amine-Mediated Single Electron Transfer Reaction to Diphenyl Disulfide. Org. Lett. 2009, 11, 3298–3301. [Google Scholar] [CrossRef]
- Yu, J.; Mao, R.; Wanga, Q.; Wu, J. Synthesis of β-keto sulfones via a multicomponent reaction through sulfonylation and decarboxylation. Org. Chem. Front. 2017, 4, 617–621. [Google Scholar] [CrossRef]
- Zeni, G.; Lüdtke, D.S.; Penatieri, R.B.; Braga, A.L. Vinylic Tellurides: From Preparation to Their Applicability in Organic Synthesis. Chem. Rev. 2006, 106, 1032–1076. [Google Scholar] [CrossRef]
- Kippo, T.; Hamaoka, K.; Ryu, I. Bromine Radical-Mediated Sequential Radical Rearrangement and Addition Reaction of Alkylidenecyclopropanes. J. Am. Chem. Soc. 2013, 135, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Saeki, T.; Mihara, K.; Higashimae, S.; Kawaguchi, S.; Sonoda, M.; Nomoto, A.; Ogawa, A. A Benzoyl Peroxide/Diphenyl Diselenide Binary System for Functionalization of Alkynes Leading to Alkenyl and Alkynyl Selenides. J. Org. Chem. 2017, 82, 12477–12484. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yuan, Y.; Yao, M.; Wang, H.; Wang, D.; Gao, M.; Chen, Y.-H.; Lei, A. Electrochemical Aminoselenation and Oxyselenation of Styrenes with Hydrogen Evolution. Org. Lett. 2019, 21, 1297–1300. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Sonawane, R.A.; Ninomiya, M.; Koketsu, M. Synthesis of Seleno-Heterocycles via Electrophilic/RadicalCyclization of Alkyne Containing Heteroatoms. Adv. Synth. Catal. 2020, 362, 3485–3515. [Google Scholar] [CrossRef]
- Schweitzer-Chaput, B.; Demaerel, J.; Engler, H.; Klussmann, M. Acid-Catalyzed Oxidative Radical Addition of Ketones to Olefins. Angew. Chem. Int. Ed. 2014, 53, 8737–8740. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Kawaguchi, S.; Nomoto, A.; Ogawa, A. Photoinduced highly selective thiophosphination of alkynes using a (PhS)2/(Ph2P)2 binary system. Tetrahedron Lett. 2008, 49, 4043–4046. [Google Scholar] [CrossRef]
- Wada, T.; Kondoh, A.; Yorimitsu, H.; Oshima, K. Intermolecular Radical Addition of Alkylthio- and Arylthiodiphenylphosphines to Terminal Alkynes. Org. Chem. 2008, 10, 1155–1157. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Shirai, T.; Ohe, T.; Nomoto, A.; Sonoda, M.; Ogawa, A. Highly regioselective simultaneous introduction of phosphino and seleno groups into unsaturated bonds by the novel combination of (Ph2P)2 and (PhSe)2 upon photoirradiation. J. Org. Chem. 2009, 74, 1751–1754. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Ohe, T.; Shirai, T.; Nomoto, A.; Sonoda, M.; Ogawa, A. Highly selective phosphinotelluration of terminal alkynes using a (Ph2P)2-(PhTe)2 mixed system upon visible light irradiation: Straightforward access to 1-phosphino-2-telluro-alkenes. Organometallics 2010, 29, 312–316. [Google Scholar] [CrossRef]
- Pan, X.-Q.; Zou, J.-P.; Yi, W.-B.; Zhang, W. Recent advances in sulfur- and phosphorous-centered radical reactions for the formation of Se–C and P–C bonds. Tetrahedron 2015, 71, 7481–7529. [Google Scholar] [CrossRef]
- Yorimitsu, H. Homolytic substitution at phosphorus for C–P bond formation in organic synthesis. Beilstein J. Org. Chem. 2013, 9, 1269–1277. [Google Scholar] [CrossRef]
- Lamas, M.-C.; Studer, A. Radical Alkylphosphanylation of Olefins with Stannylated or Silylated Phosphanes and Alkyl Iodides. Org. Lett. 2011, 13, 2236–2239. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Miyabe, H.; Sugino, H.; Miyata, O.; Naito, T. Tandem Radical-Addition–Aldol-Type Reactionof an α,β-Unsaturated Oxime Ether. Angew. Chem. Int. Ed. 2005, 44, 6190–6193. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, X.; Chen, L.; Maa, Y.; Wu, G. The copper-catalyzed radical aminophosphinoylation of maleimides with anilines and diarylphosphine oxides. Org. Chem. Front. 2022, 9, 2471–2476. [Google Scholar] [CrossRef]
- Tamai, T.; Nomoto, A.; Tsuchii, K.; Minamida, Y.; Mitamura, T.; Sonoda, M.; Ogawa, A. Highly selective perfluoroalkylchalcogenation of alkynes by the combination of iodoperfluoroalkanes and organic dichalcogenides upon photoirradiation. Tetrahedron 2012, 68, 10516–10522. [Google Scholar] [CrossRef]
- Postigo, A. Electron Donor-Acceptor Complexes in Perfluoroalkylation Reactions. Eur. J. Org. Chem. 2018, 2018, 6391–6404. [Google Scholar] [CrossRef]
- Ye, J.-H.; Zhu, L.; Yan, S.-S.; Miao, M.; Zhang, X.-C.; Zhou, W.-J.; Li, J.; Lan, Y.; Yu, D.-G. Radical Trifluoromethylative Dearomatization of Indoles and Furans with CO2. ACS Catalysis 2017, 7, 8324–8330. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Minamida, Y.; Ohe, T.; Nomoto, A.; Sonoda, M.; Ogawa, A. Synthesis and properties of perfluoroalkyl phosphine ligands: Photoinduced reaction of diphosphines with perfluoroalkyl iodides. Angew. Chem. Int. Ed. 2013, 52, 1748–1752. [Google Scholar] [CrossRef]
- Horváth, I.T.; Rábai, J. Facile Catalyst Separation without Water: Fluorous Biphase Hydroformylation of Olefins. Science 1994, 266, 72–75. [Google Scholar] [CrossRef]
- Betzemeier, B.; Knochel, P. Palladium-Catalyzed Cross-Coupling of Organozinc Bromides with Aryl Iodides in Perfluorinated Solvents. Angew. Chem. Int. Ed. Engl. 1997, 36, 2623–2624. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Minamida, Y.; Okuda, T.; Sato, Y.; Saeki, T.; Yoshimura, A.; Nomoto, A.; Ogawa, A. Photoinduced Synthesis of P-Perfluoroalkylated Phosphines from Triarylphosphines and Their Application in the Copper-Free Cross-Coupling of Acide Clorides and Terminal Alkynes. Adv. Synth. Catal. 2015, 357, 2509–2519. [Google Scholar] [CrossRef]
- Gladysz, J.A.; Curran, D.P.; Horváth, I.T. Handbook of Fluorous Chemistry; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Yoshimura, A.; Takamachi, Y.; Han, L.-B.; Ogawa, A. Organosulfide-Catalyzed Diboration of Terminal Alkynes under Light. Chem. Eur. J. 2015, 21, 13930–13933. [Google Scholar] [CrossRef] [PubMed]
- Neeve, E.C.; Geier, S.J.; Mkhalid, I.A.I.; Westcott, S.A.; Marder, T.B. Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse. Chem. Rev. 2016, 116, 9091–9161. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, A.B.; Shishido, R.; Ito, H.; Fernández, E. Transition-metal-free B–B and B–interelement reactions with organic molecules. Chem. Soc. Rev. 2017, 46, 415–430. [Google Scholar] [CrossRef]
- Cheng, Y.; Mück-Lichtenfeld, C.; Studer, A. Transition Metal-Free 1,2-Carboboration of Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 6221–6225. [Google Scholar] [CrossRef]
- Tian, Y.-M.; Guo, X.-N.; Braunschweig, H.; Radius, U.; Marder, T.B. Photoinduced Borylation for the Synthesis of Organoboron Compounds. Chem. Rev. 2021, 121, 3561–3597. [Google Scholar] [CrossRef]
- Ren, S.-C.; Zhang, F.-L.; Qi, J.; Huang, Y.-S.; Xu, A.-Q.; Yan, H.-Y.; Wang, Y.-F. Radical Borylation/Cyclization Cascade of 1,6-Enynes for the Synthesis of Boron-Handled Hetero- and Carbocycles. J. Am. Chem. Soc. 2017, 139, 6050–6053. [Google Scholar] [CrossRef]
- Friese, F.W.; Studer, A. New avenues for C–B bond formation via radical intermediates. Chem. Sci. 2019, 10, 8503–8518. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ogawa, A. Metal-Free One-Pot Multi-Functionalization of Unsaturated Compounds with Interelement Compounds by Radical Process. Molecules 2023, 28, 787. [Google Scholar] [CrossRef]
- Back, T.G.; Krishna, M.V. Free-radical addition of diselenides to dimethyl acetylenedicarboxylate, methyl propiolate, and dimethyl maleate. J. Org. Chem. 1988, 53, 2533–2536. [Google Scholar] [CrossRef]
- Ogawa, A.; Obayashi, R.; Doi, M.; Sonoda, N.; Hirao, T. A Novel Photoinduced Thioselenation of Allenes by Use of a Disulfide−Diselenide Binary System. J. Org. Chem. 1998, 63, 4277–4281. [Google Scholar] [CrossRef]
- Liu, L.; Ward, R.M.; Schomaker, J.M. Mechanistic Aspects and Synthetic Applications of Radical Additions to Allenes. Chem. Rev. 2019, 119, 12422–12490. [Google Scholar] [CrossRef]
- Ogawa, A.; Obayashi, R.; Sonoda, N.; Hirao, T. Diphenyl diselenide-assisted dithiolation of 1,3-dienes with diphenyl disulfide upon irradiation with near-UV light. Tetrahedron Lett. 1998, 39, 1577–1578. [Google Scholar] [CrossRef]
- Tsuchii, K.; Tsuboi, Y.; Kawaguchi, S.-i.; Takahashi, J.; Sonoda, N.; Nomoto, A.; Ogawa, A. Highly Selective Double Chalcogenation of Isocyanides with Disulfide−Diselenide Mixed Systems. J. Org. Chem. 2007, 72, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G.C.; Zhu, J. To each his own: Isonitriles for all flavors. Functionalized isocyanides as valuable tools in organic synthesis. Chem. Soc. Rev. 2017, 46, 1295–1357. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Huang, J.; Zhu, Q. Recent progress in imidoyl radical-involved reactions. Org. Biomol. Chem. 2016, 14, 2593–2602. [Google Scholar] [CrossRef]
- Lygin, A.V.; De Meijere, A. Isocyanides in the synthesis of nitrogen heterocycles. Angew. Chem. Int. Ed. 2010, 49, 9094–9124. [Google Scholar] [CrossRef]
- Ogawa, A.; Doi, M.; Ogawa, I.; Hirao, T. Highly selective three-component coupling of ethyl propiolate, alkenes, and diphenyl diselenide under visible-light irradiation. Angew. Chem. Int. Ed. 1999, 38, 2027–2029. [Google Scholar] [CrossRef]
- Ogawa, A.; Ogawa, I.; Sonoda, N. A novel three-component coupling of alkynes, vinylcyclopropanes, and diphenyl diselenide under visible-light irradiation. J. Org. Chem. 2000, 65, 7682–7685. [Google Scholar] [CrossRef]
- Ogawa, A.; Doi, M.; Tsuchii, K.; Hirao, T. Selective sequential addition of diphenyl diselenide to ethyl propiolate and isocyanides upon irradiation with near-UV light. Tetrahedron Lett. 2001, 42, 2317–2319. [Google Scholar] [CrossRef]
- Tsuchii, K.; Doi, M.; Hirao, T.; Ogawa, A. Highly selective sequential addition and cyclization reactions involving of diphenyl diselenide, an alkyne, and alkenes under visible-light irradiation. Angew. Chem. Int. Ed. 2003, 42, 3490–3493. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Hur, C.U.; Rhee, Y.H.; Park, Y.C.; Kim, S.Y. Propiolate-Olefin-Olefin Three-Component Annulation Mediated by the Addition of Stannyl Radicals. J. Chem. Soc.. Chem. Commun. 1993, 19, 1466–1468. [Google Scholar] [CrossRef]
- Bowman, W.R.; Bridge, C.F.; Brookes, P. Synthesis of heterocycles by radical cyclisation. J. Chem. Soc. Perkin Trans. 1 2000, 1, 1–14. [Google Scholar] [CrossRef]
- Naito, T. Heteroatom radical addition-cyclization and its synthetic application. Heterocycles 1999, 50, 505–541. [Google Scholar] [CrossRef]
- Kerry Gilmore, K.; Alabugin, I.V. Cyclizations of Alkynes: Revisiting Baldwin’s Rules for Ring Closure. Chem. Rev. 2011, 111, 6513–6556. [Google Scholar] [CrossRef]
- Godineau, E.; Landais, Y. Multicomponent Radical Processes: Synthesis of Substituted Piperidinones. J. Am. Chem. Soc. 2007, 129, 12662–12663. [Google Scholar] [CrossRef]
- Rueping, M.; Vila, C. Visible Light Photoredox-Catalyzed Multicomponent Reactions. Org. Lett. 2013, 15, 2092–2095. [Google Scholar] [CrossRef]
- Joseph, D.; Idris, M.A.; Chen, J.; Lee, S. Recent Advances in the Catalytic Synthesis of Arylsulfonyl Compounds. ACS Catal. 2021, 11, 4169–4204. [Google Scholar] [CrossRef]
- Ryu, I.; Tani, A.; Fukuyama, T.; Ravelli, D.; Fagnoni, M.; Albini, A. Atom-Economical Synthesis of Unsymmetrical Ketones through Photocatalyzed C-H Activation of Alkanes and Coupling with CO and Electrophilic Alkenes. Angew. Chem. 2011, 123, 1909–1912. [Google Scholar] [CrossRef]
- Lipp, B.; Kammer, L.M.; Kücükdisli, M.; Luque, A.; Kühlborn, J.; Pusch, S.; Matulevičiūtė, G.; Schollmeyer, D.; Šačkus, A.; Opatz, T. Visible Light-Induced Sulfonylation/Arylation of Styrenes in a Double Radical Three-Component Photoredox Reaction. Chem. Eur. J. 2019, 25, 8965–8969. [Google Scholar] [CrossRef]
- Liautard, V.; Robert, F.; Landais, Y. Free-Radical Carboalkynylation and Carboalkenylation of Olefins. Org. Lett. 2011, 13, 2658–2661. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, T.; Iwata, K.; Ogawa, A. Photoinduced intramolecular cyclization of o-ethenylaryl isocyanides with organic disulfides mediated by diphenyl ditelluride. J. Org. Chem. 2011, 76, 3880–3887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Daniliuc, C.G.; Studer, A. 6-Phosphorylated Phenanthridines from 2-Isocyanobiphenyls via Radical C-P and C-C Bond Formation. Org. Lett. 2014, 16, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, S.; Heravi, M.M.; Nazari, N. Isocyanide-based multicomponent reactions in the synthesis of heterocycles. RSC Adv. 2016, 6, 53203–53272. [Google Scholar] [CrossRef]
- Zhang, B.; Studer, A. 2-Trifluoromethyated Indoles via Radical Trifluoromethylation of Isonitriles. Org. Lett. 2014, 16, 1216–1219. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, Y.; Wang, R.; Zheng, M.; Zhang, Y.; Yu, S. Synthesis of 6-alkylated phenanthridine derivatives using photoredox neutral somophilic isocyanide insertion. Angew. Chem. Int. Ed. 2013, 52, 13289–13292. [Google Scholar] [CrossRef]
- An, X.-D.; Yu, S. Visible-Light-Promoted and One-Pot Synthesis of Phenanthridines and Quinolines from Aldehydes and O-Acyl Hydroxylamine. Org. Lett. 2015, 17, 2692–2695. [Google Scholar] [CrossRef]
- Zamudio-Medina, A.; García-González, M.C.; Padilla, J.; González-Zamora, E. Synthesis of a tetracyclic lactam system of Nuevamine by four-component reaction and free radical cyclization. Tetrahedron Lett. 2010, 51, 4837–4839. [Google Scholar] [CrossRef]
- Shen, Z.-J.; Wu, Y.-N.; He, C.-L.; He, L.; Hao, W.-J.; Wang, A.-F.; Tu, S.-J.; Jiang, B. Stereoselective synthesis of sulfonated 1-indenones via radical-triggered multi-component cyclization of β-alkynyl propenones. Chem. Commun. 2018, 54, 445–448. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, Z.-L.; Xu, P.; Wang, S.-Y.; Ji, S.-J. Aerobic radical-cascade cycloaddition of isocyanides, selenium and imidamides: Facile access to 1,2,4-selenadiazoles under metal-free conditions. Green Chem. 2017, 19, 1613–1618. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Yu, X.-Y.; Chen, J.-R.; Qiao, M.-M.; Qi, X.; Shi, D.-Q.; Xiao, W.-J. Visible-Light-Driven Aza-ortho-quinone Methide Generation for the Synthesis of Indoles in a Multicomponent Reaction Angew. Chem. Int. Ed. 2017, 56, 9527–9531. [Google Scholar] [CrossRef] [PubMed]
- Kaicharla, T.; Thangaraj, M.; Biju, A.T. Practical Synthesis of Phthalimides and Benzamides by a Multicomponent Reaction Involving Arynes, Isocyanides, and CO2/ H2O. Org. Lett. 2014, 16, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-B.; Ye, J.-H.; Zhou, W.-J.; Zhang, Y.-H.; Ding, L.; Gui, Y.-Y.; Yan, S.-S.; Li, J.; Yu, D.-G. Oxy-Difluoroalkylation of Allylamines with CO2 via Visible-Light Photoredox Catalysis. Org. Lett. 2018, 20, 190–193. [Google Scholar] [CrossRef]
- Rossi, B.; Pastori, N.; Clerici, A.; Punta, C. Free-radical hydroxymethylation of ketimines generated in situ: A one-pot multicomponent synthesis of β,β-disubstituted-β-aminoalcohols. Tetrahedron 2012, 68, 10151–10156. [Google Scholar] [CrossRef]
- Matcha, K.; Antonchick, A.P. Cascade Multicomponent Synthesis of Indoles, Pyrazoles, and Pyridazinones by Functionalization of Alkenes. Angew. Chem. Int. Ed. 2014, 53, 11960–11964. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Z.-Q. An Intermolecular Azidoheteroarylation of Simple Alkenes via Free-Radical Multicomponent Cascade Reactions. Org. Lett. 2017, 29, 5649–5652. [Google Scholar] [CrossRef]
- Beniazza, R.; Liautard, V.; Poittevin, C.; Ovadia, B.; Mohammed, S.; Robert, F.; Landais, Y. Free-Radical Carbo-Alkenylation of Olefins: Scope, Limitations and Mechanistic Insights. Chem. Eur. J. 2017, 23, 2439–2447. [Google Scholar] [CrossRef]
- Kanazawa, J.; Maeda, K.; Uchiyama, M. Radical Multicomponent Carboamination of [1.1.1]Propellane. J. Am. Chem. Soc. 2017, 139, 17791–17794. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.-J.; Cheng, L.; Wang, D.; Liu, L. TEMPO promoted direct multi-functionalization of terminal alkynes with 2-oxindoles/benzofuran-2(3H)-one. Org. Biomol. Chem. 2018, 16, 5228–5231. [Google Scholar] [CrossRef]
- Liu, H.; Fang, Y.; Wang, S.-Y.; Ji, S.-J. TEMPO-Catalyzed Aerobic Oxidative Selenium Insertion Reaction: Synthesis of 3-Selenylindole Derivatives by Multicomponent Reaction of Isocyanides, Selenium Powder, Amines, and Indoles under Transition-Metal-Free Conditions. Org. Lett. 2018, 20, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ding, R.; Tang, H.; Pan, Y.; Xu, Y.; Chen, Y. Simultaneous Construction of C−Se And C−S Bonds via the Visible-Light-Mediated Multicomponent Cascade Reaction of Diselenides, Alkynes, and SO2. Chem. Asian J. 2019, 14, 3264–3268. [Google Scholar] [CrossRef]
- Pramanik, M.M.D.; Yuan, F.; Yan, D.-M.; Xiao, W.-J.; Chen, J.-R. Visible-Light-Driven Radical Multicomponent Reaction of 2-Vinylanilines, Sulfonyl Chlorides, and Sulfur Ylides for Synthesis of Indolines. Org. Lett. 2020, 22, 2639–2644. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Daniliuc, C.G.; Alasmary, F.A.; Studer, A. Direct Access to α-Aminosilanes Enabled by Visible-Light-Mediated Multicomponent Radical Cross-Coupling. Angew. Chem. Int. Ed. 2021, 60, 23335–23341. [Google Scholar] [CrossRef]
- Deneny, P.J.; Kumar, R.; Gaunt, M.J. Visible light-mediated radical fluoromethylation via halogen atom transfer activation of fluoroiodomethane. Chem. Sci. 2021, 12, 12812–12818. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Guo, L.; Qi, D.; Gao, F.; Yang, C.; Xia, W. Visible-Light-Induced Multicomponent Synthesis of γ-Amino Esters with Diazo Compounds. Org. Lett. 2021, 23, 6278–6282. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Afonso, M.J.; Sookezian, A.; Badir, S.O.; Khatib, M.E.; Molander, G.A. Photoinduced 1,2-dicarbofunctionalization of alkenes with organotrifluoroborate nucleophiles via radical/polar crossover. Chem. Sci. 2021, 12, 9189–9195. [Google Scholar] [CrossRef]
- Shen, J.; Xu, J.; He, L.; Ouyang, Y.; Huang, L.; Li, W.; Zhu, Q.; Zhang, P. Photoinduced Rapid Multicomponent Cascade Reaction of Aryldiazonium Salts with Unactivated Alkenes and TMSN3. Org. Lett. 2021, 23, 1204–1208. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Liu, H.; Li, X.; Wang, B. tert-Butyl nitrite triggered radical cascade reaction for synthesizing isoxazoles by a one-pot multicomponent strategy. Chem. Commun. 2022, 58, 9152–9155. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Zeng, L.-H.; Li, S.; Chen, Z.; Wu, J. Accessing chiral sulfones bearing quaternary carbon stereocenters via photoinduced radical sulfur dioxide insertion and Truce–Smiles rearrangement. Nat. Commun. 2022, 13, 7081. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Geng, X.; Tao, P.; Shen, Y.; Su, Z.; Zheng, K. Modular Construction of Unnatural α-Tertiary Amino Acid Derivatives by Multicomponent Radical Cross-Couplings. Angew. Chem. Int. Ed. 2022, 61, e202210755. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.-T.; Chen, H.-L.; Wei, D.; Wei, B.-Y.; Li, Z.-H.; Zhang, J.-W.; Yu, W.; Han, B. Regioselective Fluoroalkylphosphorylation of Unactivated Alkenes by Radical-Mediated Alkoxyphosphine Rearrangement. Angew. Chem. Int. Ed. 2022, 61, e202203398. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; You, E.; Kim, J.; Hong, S. Site-Selective Pyridylic C−H Functionalization by Photocatalytic Radical Cascades. Angew. Chem. Int. Ed. 2022, 61, e202204217. [Google Scholar] [CrossRef]
- Burykina, J.V.; Kobelev, A.D.; Shlapakov, N.S.; Kostyukovich, A.Y.; Fakhrutdinov, A.N.; König, B.; Ananikov, V.P. Intermolecular Photocatalytic Chemo-, Stereo- and Regioselective Thiol–Yne–Ene Coupling Reaction. Angew. Chem. Int. Ed. 2022, 61, e202116888. [Google Scholar] [CrossRef]
- Su, Y.-L.; Liu, G.-X.; Angelis, L.D.; He, R.; Al-Sayyed, A.; Schanze, K.S.; Hu, W.-H.; Qiu, H.; Doyle, M.P. Radical Cascade Multicomponent Minisci Reactions with Diazo Compounds. ACS Catal. 2022, 12, 1357–1363. [Google Scholar] [CrossRef]
- Yang, N.; Mao, C.; Zhang, H.; Wang, P.; Li, S.; Xie, L.; Liao, S. FSO2 Radical-Initiated Tandem Addition Reaction of Two Different Olefins: A Facile Access to Multifunctional Aliphatic Sulfonyl Fluorides. Org. Lett. 2023, 25, 4478–4482. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, Y.-T.; Wang, Y.-X.; Wang, H.-Y.; Hao, W.-J.; Jiang, B. Multicomponent Annulative SO2 Insertion of Heteroatom-Linked 1,7-Diynes for Accessing Tricyclic Sulfones. Adv. Synth. Catal. 2023, 365, 1693–1698. [Google Scholar] [CrossRef]
- Venditto, N.J.; Boerth, J.A. Photoredox-Catalyzed Multicomponent Synthesis of Functionalized γ-Amino Butyric Acids via Reductive Radical Polar Crossover. Org. Lett. 2023, 25, 3429–3434. [Google Scholar] [CrossRef]
- Huang, W.; Keess, S.; Molander, G.A. A General and Practical Route to Functionalized Bicyclo[1.1.1]Pentane-Heteroaryls Enabled by Photocatalytic Multicomponent Heteroarylation of [1.1.1]Propellane. Angew. Chem. Int. Ed. 2023, 62, e202302223. [Google Scholar] [CrossRef]
- Sun, B.; Tang, X.-L.; Zhuang, X.; Ling, L.; Huang, P.; Wang, J.; Jin, C. Visible-Light-Driven Multicomponent Radical Cascade Versatile Alkylation of Quinoxalinones Enabled by Electron Donor Acceptor Complex in Water. Adv. Synth. Catal. 2023, 365, 1020–1026. [Google Scholar] [CrossRef]
- Zhang, K.-Y.; Long, F.; Peng, C.-C.; Liu, J.-H.; Hu, Y.-C.; Wu, L.-J. Multicomponent Sulfonylation of Alkenes to Access β-Substituted Arylsulfones. J. Org. Chem. 2023, 88, 3772–3780. [Google Scholar] [CrossRef]
- Sookezian, A.; Molander, G.A. Photoinduced Vicinal 1,2-Difunctionalization of Olefins for the Synthesis of Alkyl Sulfonamides. Org. Lett. 2023, 25, 1014–1019. [Google Scholar] [CrossRef]
- Bhat, V.S.; Lee, A. Three-Component Synthesis of 3-(Arylsulfonyl)benzothiophenes Using Acetic Acid as a Quencher for Methyl Radical-Mediated Side Reactions. Adv. Synth. Catal. 2023, 365, 1514–1520. [Google Scholar] [CrossRef]
- Cukalovic, A.; Monbaliu, J.-C.M.R.; Stevens, C. Microreactor Technology as an Efficient Tool for Multicomponent Reactions. Synth. Heterocycles Via Multicomponent React. I 2010, 23, 161–198. [Google Scholar]
- Zhang, W.; Yi, W.-B. Pot, Atom, and Step Economy (PASE) Synthesis; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Magnus Rueping, M.; Vila, C.; Bootwicha, T. Continuous Flow Organocatalytic C−H Functionalization and Cross-Dehydrogenative Coupling Reactions: Visible Light Organophotocatalysis for Multicomponent Reactions and C−C, C−P Bond Formations. ACS Catal. 2013, 3, 1676–1680. [Google Scholar] [CrossRef]
- Maya Shankar Singh, M.S.; Chowdhury, S. Recent developments in solvent-free multicomponent reactions: A perfect synergy for eco-compatible organic synthesis. RSC Adv. 2012, 2, 4547–4592. [Google Scholar] [CrossRef]
- Verma, C.; Haque, J.; Quraishi, M.A.; Ebenso, E.E. Aqueous phase environmental friendly organic corrosion inhibitors derived from one step multicomponent reactions: A review. J. Mol. Liq. 2019, 275, 18–40. [Google Scholar] [CrossRef]
- Zhang, X.; Smith, R.T.; Le, C.; McCarver, S.J.; Shireman, B.T.; Carruthers, N.I.; MacMillan, D.W.C. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 2020, 580, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Campo, J.; García-Valverde, M.; Marcaccini, S.; Rojoa, M.J.; Torroba, T. Synthesis of indole derivatives via isocyanides. Org. Biomol. Chem. 2006, 4, 757–765. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, T.; Zhao, X.; Liu, P.; Sun, P. Electrochemical Difunctionalization of Alkenes by a Four-Component Reaction Cascade Mumm Rearrangement: Rapid Access to Functionalized Imides. Angew. Chem. Int. Ed. 2020, 59, 3465–3469. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, A.; Yamamoto, Y. Multicomponent Reactions between Heteroatom Compounds and Unsaturated Compounds in Radical Reactions. Molecules 2023, 28, 6356. https://doi.org/10.3390/molecules28176356
Ogawa A, Yamamoto Y. Multicomponent Reactions between Heteroatom Compounds and Unsaturated Compounds in Radical Reactions. Molecules. 2023; 28(17):6356. https://doi.org/10.3390/molecules28176356
Chicago/Turabian StyleOgawa, Akiya, and Yuki Yamamoto. 2023. "Multicomponent Reactions between Heteroatom Compounds and Unsaturated Compounds in Radical Reactions" Molecules 28, no. 17: 6356. https://doi.org/10.3390/molecules28176356
APA StyleOgawa, A., & Yamamoto, Y. (2023). Multicomponent Reactions between Heteroatom Compounds and Unsaturated Compounds in Radical Reactions. Molecules, 28(17), 6356. https://doi.org/10.3390/molecules28176356




