Supercritical CO2 Extraction of Fatty Acids, Phytosterols, and Volatiles from Myrtle (Myrtus communis L.) Fruit
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield
2.2. Fatty Acids, Phytosterols, and Volatiles in Myrtle Berries
2.2.1. Fatty Acid Composition
2.2.2. Phytosterols Composition
2.2.3. Volatile Composition
2.3. Influence of SFE Conditions
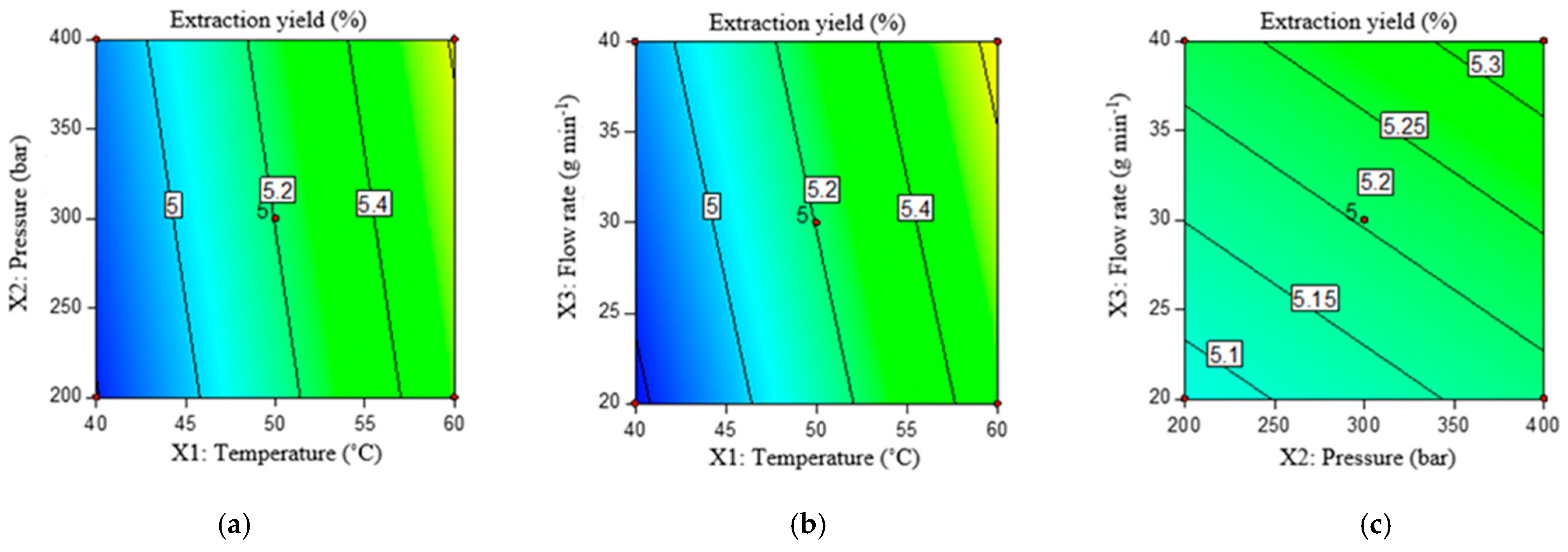
2.3.1. Extraction Yield
2.3.2. Polyunsaturated Fatty Acid Yield
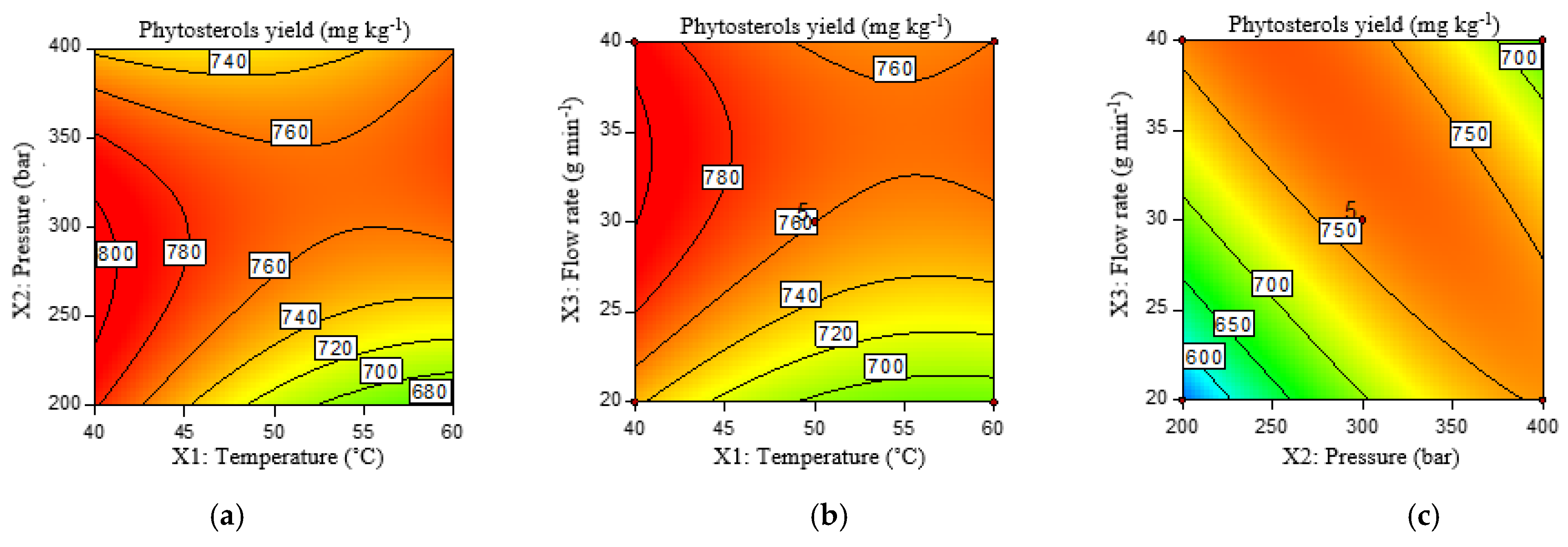
2.3.3. Phytosterols Yield
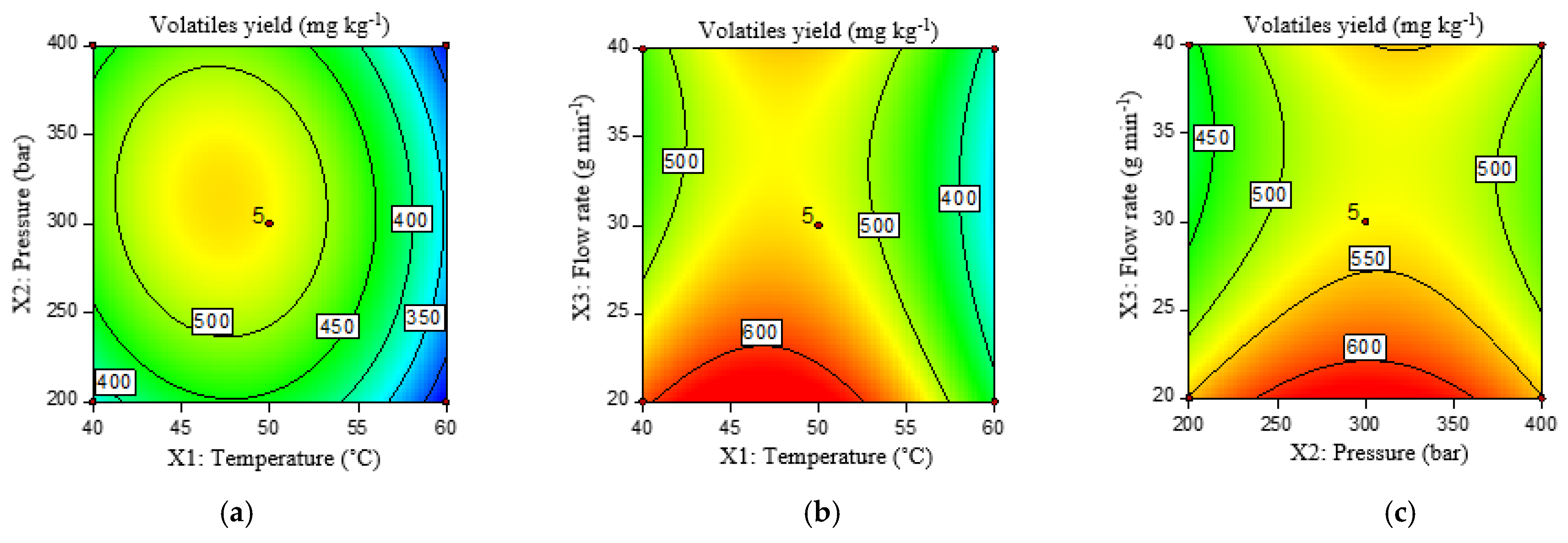
2.3.4. Volatiles Yield
2.4. Results of the Optimization of SFE Conditions
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Supercritical Fluid Extraction
3.4. Determination of Fatty Acids
3.5. Determination of Sterols
3.6. Determination of Volatiles
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sumbul, S.; Aftab Ahmad, M.; Asif, M.; Akhtar, M. Myrtus communis Linn.—A review. Indian J. Nat. Prod. Resour. 2011, 2, 395–402. [Google Scholar]
- Giampieri, F.; Cianciosi, D.; Forbes-hernández, T.Y. Myrtle (Myrtus communis L.) berries, seeds, leaves, and essential oils: New undiscovered sources of natural compounds with promising health benefits. Food Front. 2020, 1, 276–295. [Google Scholar] [CrossRef]
- Hussain, A.Y.; Hussein, H.J.; Al-Rubaye, A.F. Antifungal Efficacy of the crude Flavonoid, Terpenoid, and Alkaloid Extracted from Myrtus communis L. against Aspergillus species isolated from Stored Medicinal plants seeds in the Iraqi Markets Second International Virtual Conference of Biotechnology. J. Biotechnol. Res. Cent. 2021, 15, 73–80. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef]
- Correddu, F.; Maldini, M.; Addis, R.; Petretto, G.L.; Palomba, M.; Battacone, G.; Pulina, G.; Nudda, A.; Pintore, G. Myrtus communis Liquor Byproduct as a Source of Bioactive Compounds. Foods 2019, 8, 237. [Google Scholar] [CrossRef]
- Şan, B.; Yildirim, A.N.; Polat, M.; Yildirim, F. Chemical Compositions of Myrtle (Myrtus communis L.) Genotypes Having Bluish-Black and Yellowish-White Fruits. Erwerbs-Obstbau 2015, 57, 203–210. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Singh, R.K. Supercritical fluids and the food industry. Compr. Rev. Food Sci. Food Saf. 2002, 1, 33–44. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical fluid extraction of seed oils—A short review of current trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- De Giorgi, M.R.; Cadoni, E.; Medda, E.; Alessi, P.; Cortesi, A. Supercritical CO2 Extraction of Essential Oil from Myrtus communis Berries. In Proceedings of the Fifth Italian Conference on Supercritical Fluids and Their Applications, Trieste, Italy, 13–16 June 1999; AIDIC-Università di Padova: Padova, Italy, 1999. [Google Scholar]
- Pereira, P.; Cebola, M.J.; Oliveira, M.C.; Bernardo-Gil, M.G. Supercritical fluid extraction vs. conventional extraction of myrtle leaves and berries: Comparison of antioxidant activity and identification of bioactive compounds. J. Supercrit. Fluids 2016, 113, 1–9. [Google Scholar] [CrossRef]
- Batchu, S.N.; Chaudhary, K.; Zlobine, I.; Pawa, J.; Seubert, J.M. Handbook of Lipids in Human Function: Fatty Acids. In Handbook of Lipids in Human Function: Fatty Acids; Watson, R.R., De Meester, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–809. ISBN 9781630670368. [Google Scholar]
- Anez-Bustillos, L.; Dao, D.T.; Fell, G.L.; Baker, M.A.; Gura, K.M.; Bistrian, B.R.; Puder, M. Redefining essential fatty acids in the era of novel intravenous lipid emulsions. Clin. Nutr. 2018, 37, 784–789. [Google Scholar] [CrossRef]
- Aydın, C.; Ozcan, M.M. Determination of nutritional and physical properties of myrtle (Myrtus communis L.) fruits growing wild in Turkey. J. Food Eng. 2007, 79, 453–458. [Google Scholar] [CrossRef]
- Wannes, W.A.; Marzouk, B. Characterization of myrtle seed (Myrtus communis var. baetica) as a source of lipids, phenolics, and antioxidant activities. J. Food Drug Anal. 2016, 24, 316–323. [Google Scholar] [CrossRef]
- Wannes, W.A.; Mhamdi, B.; Marzouk, B. Variations in essential oil and fatty acid composition during Myrtus communis var. italica fruit maturation. Food Chem. 2009, 112, 621–626. [Google Scholar] [CrossRef]
- Bai, G.; Ma, C.; Chen, X. Phytosterols in edible oil: Distribution, analysis and variation during processing. Grain Oil Sci. Technol. 2021, 4, 33–44. [Google Scholar] [CrossRef]
- Nattagh-Eshtiva, E.; Barghchi, H.; Pahlavani, N.; Ghavami; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phyther. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, X.; Xu, J.; Li, C.; Yu, Y.; Wang, W.; Zhu, L. The Protective Effect of Dietary Phytosterols on Cancer Risk: A Systematic Meta-Analysis. J. Oncol. 2019, 2019, 7479518. [Google Scholar] [CrossRef]
- Bowles, E.J. Plants and Essential Oils. In The Chemistry of Aromatherapeutic Oils; Allen & Unwin: Crows Nest, Australia, 2003; pp. 21–41. ISBN 174114051X. [Google Scholar]
- Hennia, A.; Nemmiche, S.; Guerreiro, A.; Faleiro, M.L.; Antunes, M.D.; Aazza, S.; Miguel, M.G. Antioxidant and Antiproliferative Activities of Myrtus communis L. Essential Oils from Different Algerian Regions. J. Essent. Oil-Bearing Plants 2019, 22, 1488–1499. [Google Scholar] [CrossRef]
- Jerkovic, I.; Radonic, A.; Borcic, I. Comparative study of leaf, fruit and flower essential oils of croatian Myrtus communis (L.) during a one-year vegetative cycle. J. Essent. Oil Res. 2002, 14, 266–270. [Google Scholar] [CrossRef]
- Wannes, W.A.; Mhamdi, B.; Sriti, J.; Marzouk, B. Glycerolipid and fatty acid distribution in pericarp, seed and whole fruit oils of Myrtus communis var. italica. Ind. Crops Prod. J. 2010, 31, 77–83. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al Juhaimi, F.; Ahmed, I.A.M.; Babiker, E.E.; Ghafoor, K. Antioxidant activity, fatty acid composition, phenolic compounds and mineral contents of stem, leave and fruits of two morphs of wild myrtle plants. J. Food Meas. Charact. 2020, 14, 1376–1382. [Google Scholar] [CrossRef]
- Serce, S.; Ercisli, S.; Sengul, M.; Gunduz, K.; Orhan, E. Antioxidant activities and fatty acid composition of wild grown myrtle (Myrtus communis L.) fruits. Pharmacogn. Mag. 2010, 6, 9–13. [Google Scholar] [CrossRef]
- Cakir, A. Essential oil and fatty acid composition of the fruits of Hippophae rhamnoides L. (Sea Buckthorn) and Myrtus communis L. from Turkey. Biochem. Syst. Ecol. 2004, 32, 809–816. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, S.; Zhang, F.; Huang, M.; Xie, J. Characterization and authentication of olive, camellia and other vegetable oils by combination of chromatographic and chemometric techniques: Role of fatty acids, tocopherols, sterols and squalene. Eur. Food Res. Technol. 2021, 247, 411–426. [Google Scholar] [CrossRef]
- Vecka, M.; Staňková, B.; Kutová, S.; Tomášová, P.; Tvrzická, E.; Žák, A. Comprehensive sterol and fatty acid analysis in nineteen nuts, seeds, and kernel. SN Appl. Sci. 2019, 1, 1531. [Google Scholar] [CrossRef]
- Moghadasian, M.H. Pharmacological properties of plant sterols In vivo and in vitro observations. Life Sci. 2000, 67, 605–615. [Google Scholar] [CrossRef]
- Balbino, S.; Repaji, M.; Medved, A.M.; Tonkovi, P. Characterization of lipid fraction of Apiaceae family seed spices: Impact of species and extraction method. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100326. [Google Scholar] [CrossRef]
- Yang, B.; Karlsson, R.M.; Oksman, P.H.; Kallio, H.P.; Hippophae, S. Phytosterols in Sea Buckthorn (Hippophae rhamnoides L.) Berries: Identification and Effects of Different Origins and Harvesting Times. J. Agric. Food Chem. 2001, 49, 5620–5629. [Google Scholar] [CrossRef]
- Bradesi, P.; Tomi, F.; Casanova, J.; Costa, J.; Bemardini, A.F. Chemical composition of myrtle leaf essential oil from Corsica (France). J. Essent. Oil Res. 1997, 9, 283–288. [Google Scholar] [CrossRef]
- Pereira, P.C.; Cebola, M.J.; Bernardo-Gil, M.G. Evolution of the yields and composition of essential oil from Portuguese myrtle (Myrtus comunis L.) through the vegetative cycle. Molecules 2009, 14, 3094–3105. [Google Scholar] [CrossRef]
- Ghasemi, E.; Raofie, F.; Najafi, N.M. Application of response surface methodology and central composite design for the optimisation of supercritical fluid extraction of essential oils from Myrtus communis L. leaves. Food Chem. 2011, 126, 1449–1453. [Google Scholar] [CrossRef]
- Usai, M.; Marchetti, M.; Culeddu, N.; Mulas, M. Chemical Composition of Myrtle (Myrtus communis L.) Berries Essential Oils as Observed in a Collection of Genotypes. Molecules 2018, 23, 2502. [Google Scholar] [CrossRef]
- Dai, Q.; Yang, Y.; Chen, K.; Cheng, Z.; Ni, Y.; Li, J. Optimization of Supercritical CO2 Operative Parameters to Simultaneously Increase the Extraction Yield of Oil and Pentacyclic Triterpenes from Artichoke Leaves and Stalks by Response Surface Methodology and Ridge Analysis. Eur. J. Lipid Sci. Technol. 2019, 121, 1–11. [Google Scholar] [CrossRef]
- Gustinelli, G.; Eliasson, L.; Svelander, C.; Alminger, M.; Ahrné, L. Supercritical CO2 extraction of bilberry (Vaccinium myrtillus L.) seed oil: Fatty acid composition and antioxidant activity. J. Supercrit. Fluids 2018, 135, 91–97. [Google Scholar] [CrossRef]
- Hamid, I.A.A.; Ismail, N.; Rahman, N.A. Supercritical Carbon Dioxide Extraction of Selected Herbal Leaves: An Overview. Mater. Sci. Eng. 2018, 358, 2–7. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Ghazanib, S.M.; Corazzaa, M.L.; Marangonib, A.G.; Ribania, R.H. Assessment of subcritical propane, supercritical CO2 and Soxhlet extraction of oil from sapucaia (Lecythis pisonis) nuts. J. Supercrit. Fluids 2018, 133, 122–132. [Google Scholar] [CrossRef]
- Jokić, S.; Sudar, R.; Svilović, S.; Vidović, S.; Bilić, M.; Velić, D.; Jurković, V. Fatty Acid Composition of Oil Obtained from Soybeans. Czech J. Food Sci. 2013, 31, 116–125. [Google Scholar] [CrossRef]
- Teslić, N.; Bojanić, N.; Čolović, D.; Fišteš, A.; Rakić, D.; Solarov, M.B.; Zeković, Z.; Pavlić, B. Conventional versus novel extraction techniques for wheat germ oil recovery: Multi-response optimization of supercritical fluid extraction. Sep. Sci. Technol. 2021, 56, 1546–1561. [Google Scholar] [CrossRef]
- Maheshwari, P.; Nikolov, Z.L.; White, T.M.; Hartel, R. Solubility of fatty acids in supercritical carbon dioxide. J. Am. Oil Chem. Soc. 1992, 69, 1069–1076. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Herrero, M.; Castro-Puyana, M.; Ibanez, E. Supercritical Fluid Extraction. In Natural Product Extraction Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; The Royal Society of Chemistry: London, UK, 2013; pp. P001–P004. ISBN 9781849737579. [Google Scholar]
- Lu, T.; Gaspar, F.; Marriott, R.; Mellor, S.; Watkinson, C.; Al-duri, B.; Seville, J.; Santos, R.; Limited, B.; Lane, H.P.; et al. Extraction of borage seed oil by compressed CO2: Effect of extraction parameters and modelling. J. Supercrit. Fluids 2007, 41, 68–73. [Google Scholar] [CrossRef]
- Machmudah, S.; Kawahito, Y.; Sasaki, M.; Goto, M. Process optimization and extraction rate analysis of carotenoids extraction from rosehip fruit using supercritical CO2. J. Supercrit. Fluids 2008, 44, 308–314. [Google Scholar] [CrossRef]
- Zuknik, M.H.; Norulaini, N.A.N.; Omar, A.K.M. Supercritical carbon dioxide extraction of lycopene: A review. J. Food Eng. 2012, 112, 253–262. [Google Scholar] [CrossRef]
- Grzegorz, D.; Sylwester, C.; Iwona, K. Fractionation of sterols, tocols and squalene in fl axseed oils under the impact of variable conditions of supercritical CO2 extraction. J. Food Compos. Anal. 2019, 83, 103261. [Google Scholar] [CrossRef]
- Uddin, S.; Sarker, I.; Ferdosh, S.; Akanda, H.; Easmin, S.; Bt, H.; Bin, K. Phytosterols and their extraction from various plant matrices using supercritical carbon dioxide: A review. J. Sci. Food Agric. 2015, 95, 1385–1394. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical fluid extraction and characterisation of Moringa oleifera leaves oil. Sep. Purif. Technol. 2013, 118, 497–502. [Google Scholar] [CrossRef]
- Grosso, C.; Ferraro, V.; Figueiredo, A.C.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M. Supercritical carbon dioxide extraction of volatile oil from Italian coriander seeds. Food Chem. 2008, 111, 197–203. [Google Scholar] [CrossRef]
- Maitusong, J.; Aimila, A.; Zhang, J.; Mahinur, B.; Maiwulanjiang, M.; Akber, H. Process optimization for the supercritical carbon dioxide extraction of Foeniculum vulgare Mill. seeds aromatic extract with respect to yield and trans-anethole contents using Box-Behnken design. Flavour Fragr. J. 2020, 280–291. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction—A review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical carbon dioxide applications in food processing. Food Eng. Rev. 2021, 1–22. [Google Scholar] [CrossRef]
- Sajfrtová, M.; Lickova, I.; Wimmerová, M.; Sovová, H.; Wimmer, Z. B-Sitosterol: Supercritical Carbon Dioxide Extraction from Sea Buckthorn (Hippophae rhamnoides L.) Seeds. Int. J. Mol. Sci. 2010, 11, 1842–1850. [Google Scholar] [CrossRef]
- Pereira, P.; Bernardo-Gil, M.G.; Cebola, M.J.; Mauricio, E.; Romano, A. Supercritical fluid extracts with antioxidant and antimicrobial activities from myrtle (Myrtus communis L.) leaves. Response surface optimization. J. Supercrit. Fluids 2013, 83, 57–64. [Google Scholar] [CrossRef]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017.
- ISO 12966-4:2015; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 4: Determination by Capillary Gas Chromatography. ISO: Geneva, Switzerland, 2015.
- ISO 12228-1:2014; Determination of Individual and Total Sterols Contents—Gas Chromatographic Method—Part 1: Animal and Vegetable Fats and Oils. ISO: Geneva, Switzerland, 2014.

| Exp. | X1: T (°C) | X2: p (bar) | X3: Q (g min−1) | Yield |
|---|---|---|---|---|
| 1 | 50 | 300 | 30 | 5.26 |
| 2 | 60 | 300 | 20 | 5.33 |
| 3 | 60 | 200 | 30 | 5.49 |
| 4 | 60 | 300 | 40 | 5.92 |
| 5 | 50 | 300 | 30 | 5.18 |
| 6 | 50 | 200 | 20 | 5.10 |
| 7 | 60 | 400 | 30 | 5.59 |
| 8 | 50 | 300 | 30 | 5.03 |
| 9 | 50 | 400 | 20 | 5.37 |
| 10 | 40 | 400 | 30 | 4.95 |
| 11 | 50 | 300 | 30 | 5.27 |
| 12 | 50 | 400 | 40 | 5.05 |
| 13 | 40 | 200 | 30 | 4.75 |
| 14 | 50 | 300 | 30 | 5.19 |
| 15 | 50 | 200 | 40 | 5.20 |
| 16 | 40 | 300 | 20 | 4.77 |
| 17 | 40 | 300 | 40 | 5.01 |
| Fatty Acids (%) | Phytosterols (mg kg−1) | Volatiles (mg kg−1) | |||
|---|---|---|---|---|---|
| C11:0 | 0.6 ± 0.5 | Campesterol | 238 ± 69 | α-Pinene | 1280 ± 436 |
| C15:0 | 0.1 ± 0.1 | 24-Methylenecholesterol | 88 ± 89 | β-Pinene | 2 ± 3 |
| C16:0 | 8.5 ± 0.2 | Stigmasterol | 76 ± 6 | Myrcene | 1 ± 2 |
| C17:0 | 0.2 ± 0.1 | β-Sitosterol | 12,465 ± 1308 | α-Phellandrene | 35 ± 21 |
| C18:0 | 3.1 ± 0.8 | Δ5-Avenasterol | 590 ± 71 | 3-Carene | 42 ± 23 |
| C20:0 | 0.5 ± 0.0 | Δ7-Sitosterol | 166 ± 29 | Limonene | 238 ± 58 |
| C22:0 | 0.1 ± 0.2 | Citrostadienol | 90 ± 58 | 1,8-Cineole | 2183 ± 487 |
| C23:0 | 0.8 ± 0.7 | Total content in extract | 13,937 ± 149 | Linalool | 659 ± 530 |
| C24:0 | 0.1 ± 0.1 | Yield per fruit DW | 723 ± 63 | α-Terpineol | 105 ± 39 |
| C18:1c | 7.3 ± 0.3 | Myrtenol | 23 ± 19 | ||
| C20:1 | 0.2 ± 0.1 | Carvone | 77 ± 20 | ||
| C18:2t | 0.3 ± 0.1 | Geraniol | 78 ± 26 | ||
| C18:2c | 76.8 ± 1.6 | Myrtenyl acetate | 4297 ± 917 | ||
| C18:3n3 | 0.4 ± 0.1 | Total content in extract | 9020 ± 2219 | ||
| C20:5n3 | 0.2 ± 0.1 | Yield per fruit DW | 466 ± 102 | ||
| Σ SFA | 14.0 ± 1.5 | ||||
| Σ MUFA | 7.8 ± 0.2 | ||||
| Σ PUFA | 77.9 ± 1.4 | ||||
| Source of Variation | Extraction Yield | PUFA Content | Phytosterols Yield | Volatiles Yield | ||||
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (mg kg−1) | (mg kg−1) | |||||
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Model | 17.22 | <0.001 * | 6.16 | 0.008 * | 5.03 | 0.024 * | 5.48 | 0.018 * |
| Linear | ||||||||
| X1 | 48.40 | <0.001 * | 16.19 | 0.001 * | 2.78 | 0.124 | 11.12 | 0.013 * |
| X2 | 1.05 | 0.324 | 0.01 | 0.908 | 5.31 | 0.063 | 0.64 | 0.449 |
| X3 | 2.22 | 0.160 | 2.27 | 0.156 | 5.83 | 0.047 * | 4.20 | 0.080 |
| Quadratic | ||||||||
| X12 | - | - | - | - | 0.99 | 0.333 | 20.32 | 0.003 * |
| X22 | - | - | - | - | 7.35 | 0.036 * | 7.98 | 0.026 * |
| X32 | - | - | - | - | 3.08 | 0.105 | 4.11 | 0.082 |
| Interaction | ||||||||
| X1X2 | - | - | - | - | 2.71 | 0.121 | 0.20 | 0.666 |
| X1X3 | - | - | - | - | 0.13 | 0.735 | 0.45 | 0.522 |
| X2X3 | - | - | - | - | 16.96 | 0.005 * | 0.35 | 0.574 |
| Lack of fit | 2.84 | 0.164 | 4.06 | 0.095 | 3.25 | 0.164 | 0.49 | 0.710 |
| R2 | 0.799 | 0.587 | 0.866 | 0.876 | ||||
| Factor | Extraction Yield (%) | PUFA Content (%) | Phytosterol Yield (mg kg−1) | Volatile Yield (mg kg−1) |
|---|---|---|---|---|
| Optimal value | 5.69 | 79.34 | 793.48 | 629.85 |
| Temperature (°C) | 60.0 | 40.2 | 40.5 | 49.5 |
| Pressure (bar) | 400.0 | 235.7 | 225.0 | 298.0 |
| Flow rate (g min−1) | 40.0 | 31.1 | 32.5 | 20.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvitković, D.; Škarica, I.; Dragović-Uzelac, V.; Balbino, S. Supercritical CO2 Extraction of Fatty Acids, Phytosterols, and Volatiles from Myrtle (Myrtus communis L.) Fruit. Molecules 2024, 29, 1755. https://doi.org/10.3390/molecules29081755
Cvitković D, Škarica I, Dragović-Uzelac V, Balbino S. Supercritical CO2 Extraction of Fatty Acids, Phytosterols, and Volatiles from Myrtle (Myrtus communis L.) Fruit. Molecules. 2024; 29(8):1755. https://doi.org/10.3390/molecules29081755
Chicago/Turabian StyleCvitković, Daniela, Iva Škarica, Verica Dragović-Uzelac, and Sandra Balbino. 2024. "Supercritical CO2 Extraction of Fatty Acids, Phytosterols, and Volatiles from Myrtle (Myrtus communis L.) Fruit" Molecules 29, no. 8: 1755. https://doi.org/10.3390/molecules29081755
APA StyleCvitković, D., Škarica, I., Dragović-Uzelac, V., & Balbino, S. (2024). Supercritical CO2 Extraction of Fatty Acids, Phytosterols, and Volatiles from Myrtle (Myrtus communis L.) Fruit. Molecules, 29(8), 1755. https://doi.org/10.3390/molecules29081755









