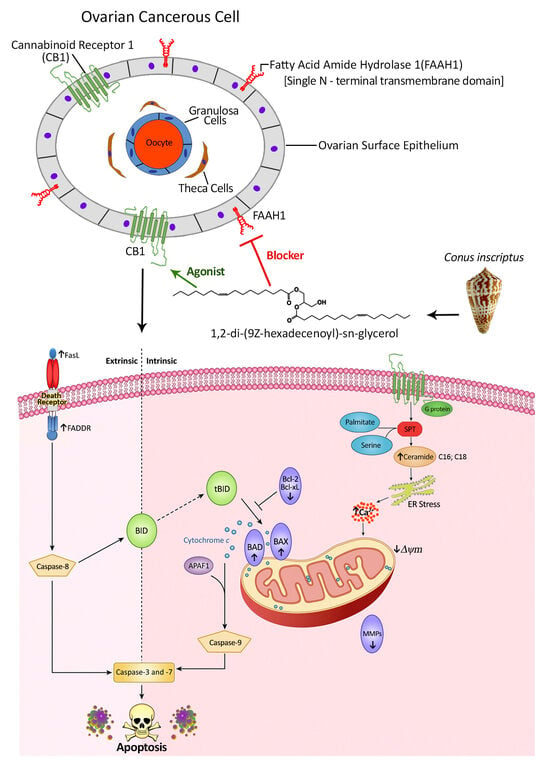
A Novel Non-Psychoactive Fatty Acid from a Marine Snail, Conus inscriptus, Signals Cannabinoid Receptor 1 (CB1) to Accumulate Apoptotic C16:0 and C18:0 Ceramides in Teratocarcinoma Cell Line PA1
Abstract
1. Introduction
2. Results
2.1. Background Information on the Structural Details of the Compound and Preliminary Bioactivity Testing
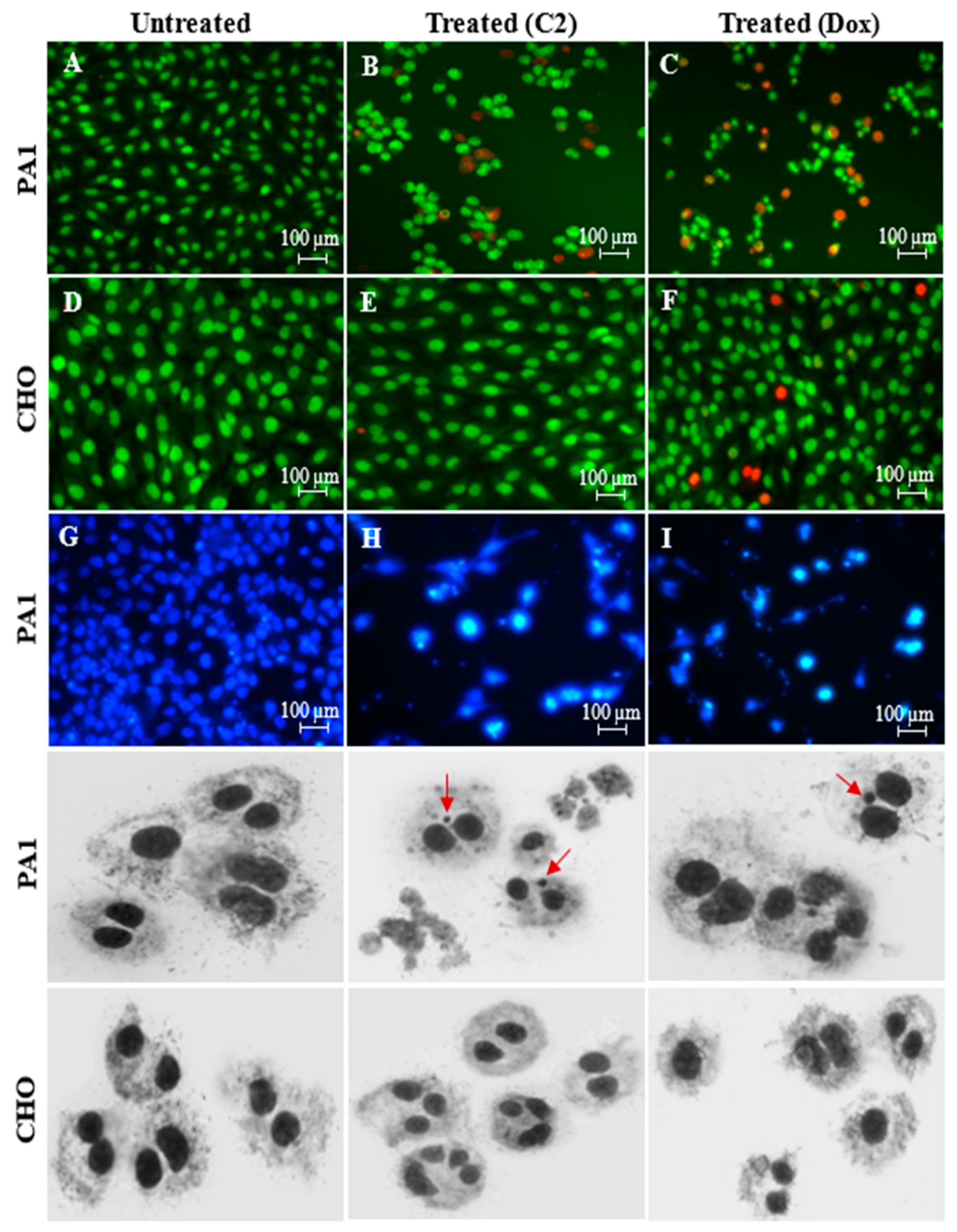
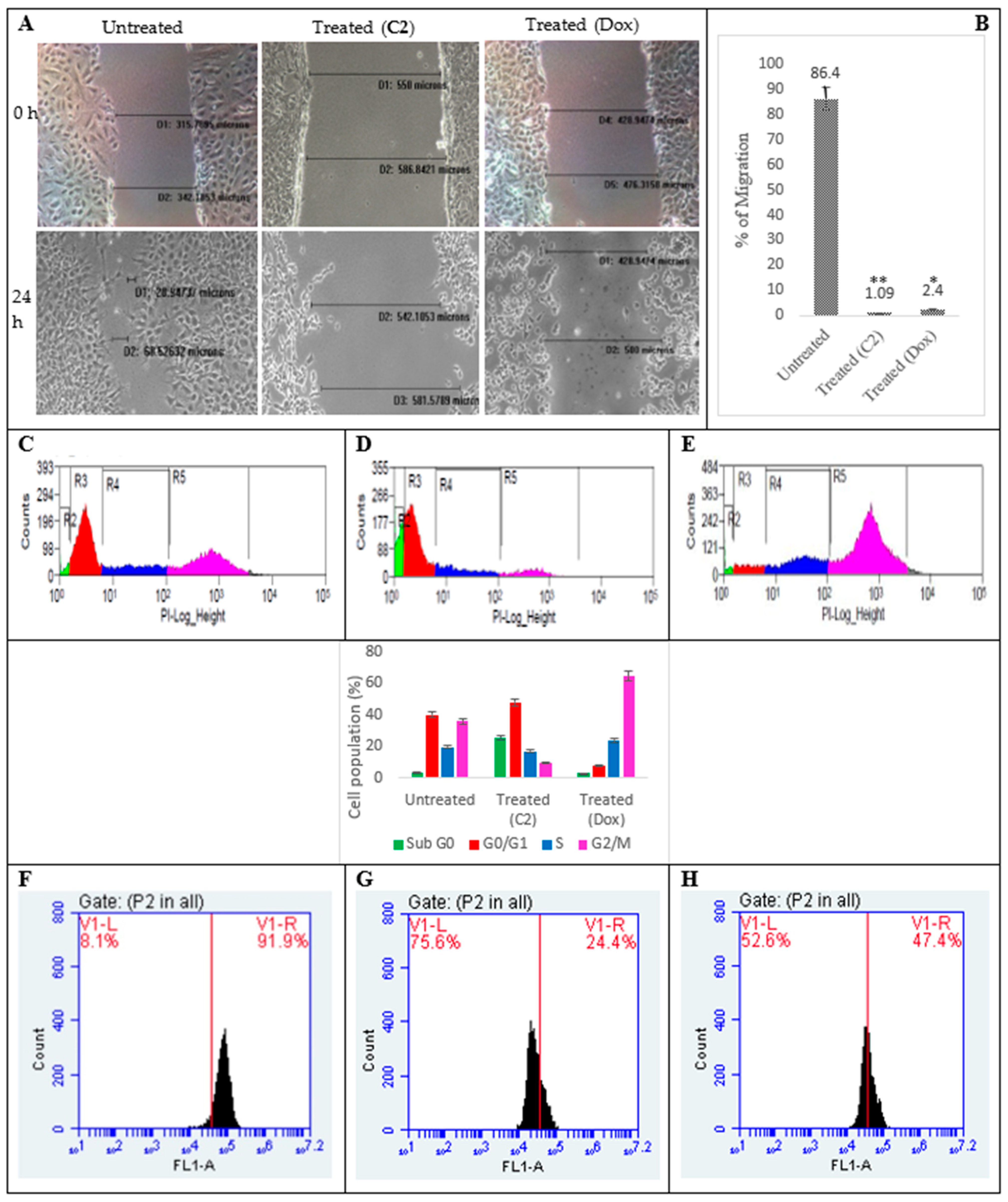
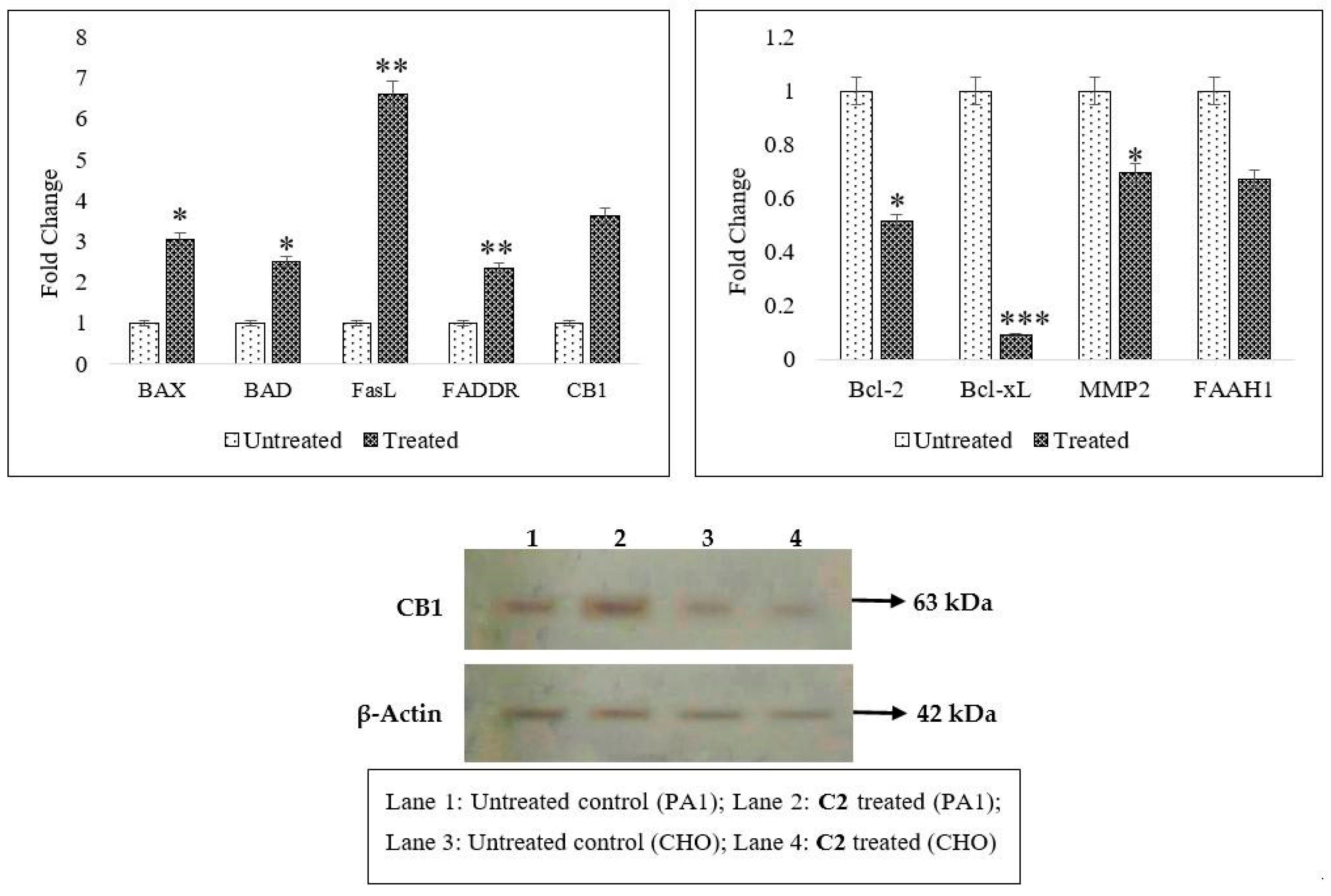
2.2. Effects of C2 on Cell Migration, Cell Cycle Arrest, Mitochondrial Membrane Potential (MMP) Disruption and Gene/Protein Expression in PA1 Cells
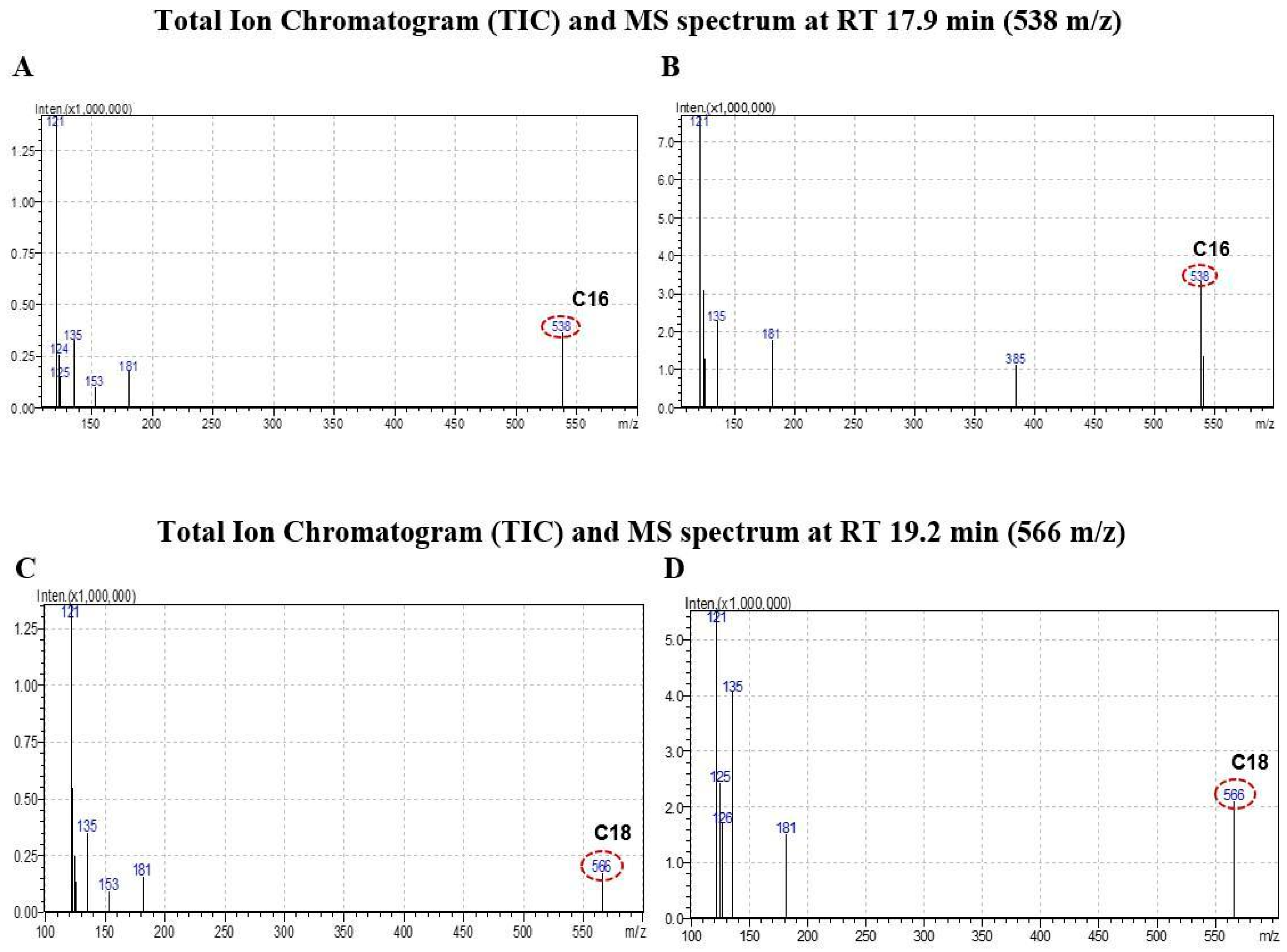
2.3. CB1 Overexpression in PA1 Cells and Acute Generation of Intracellular Ceramide (Especially C16:0 and C18:0) Pools Are Attributed to C2 Treatment
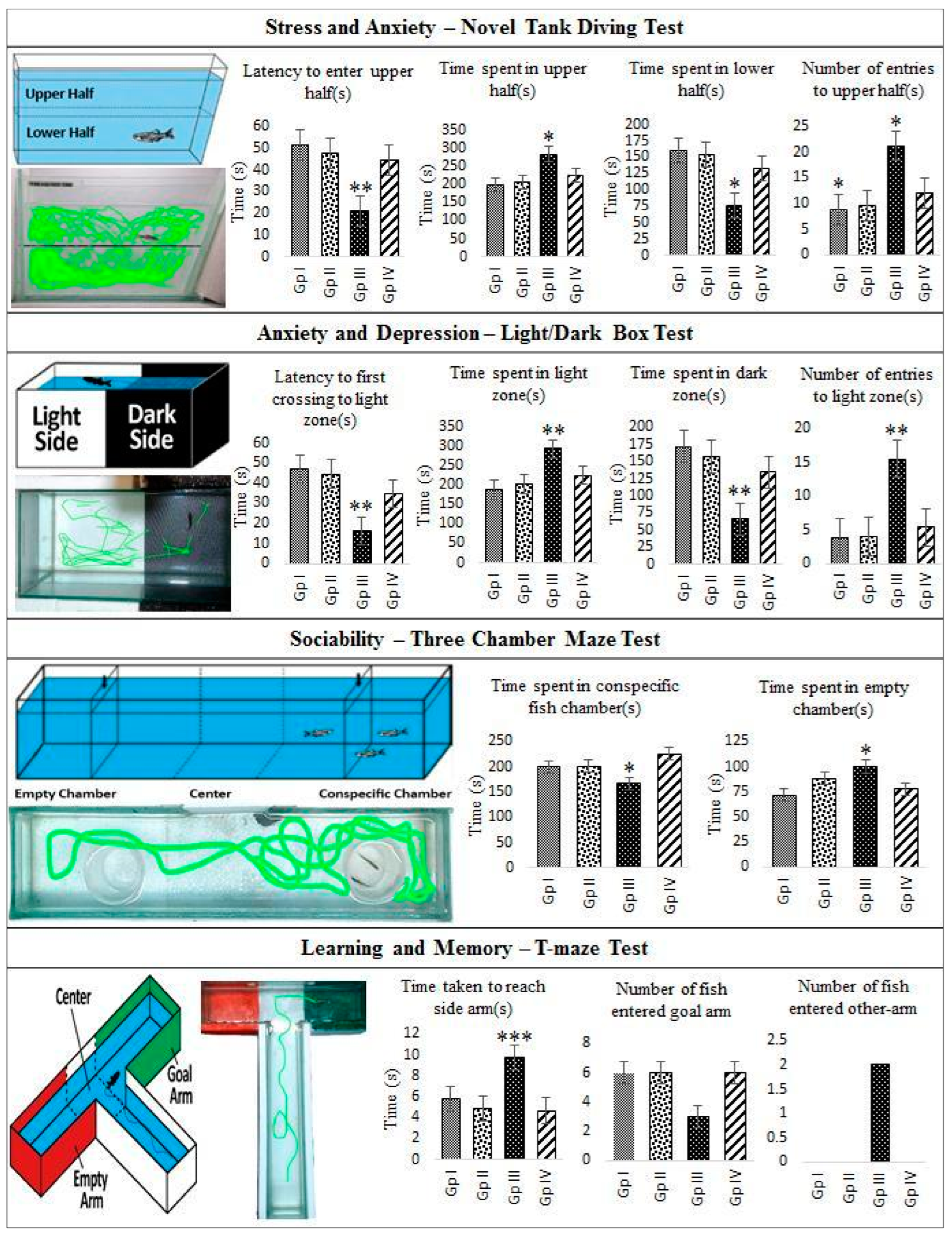
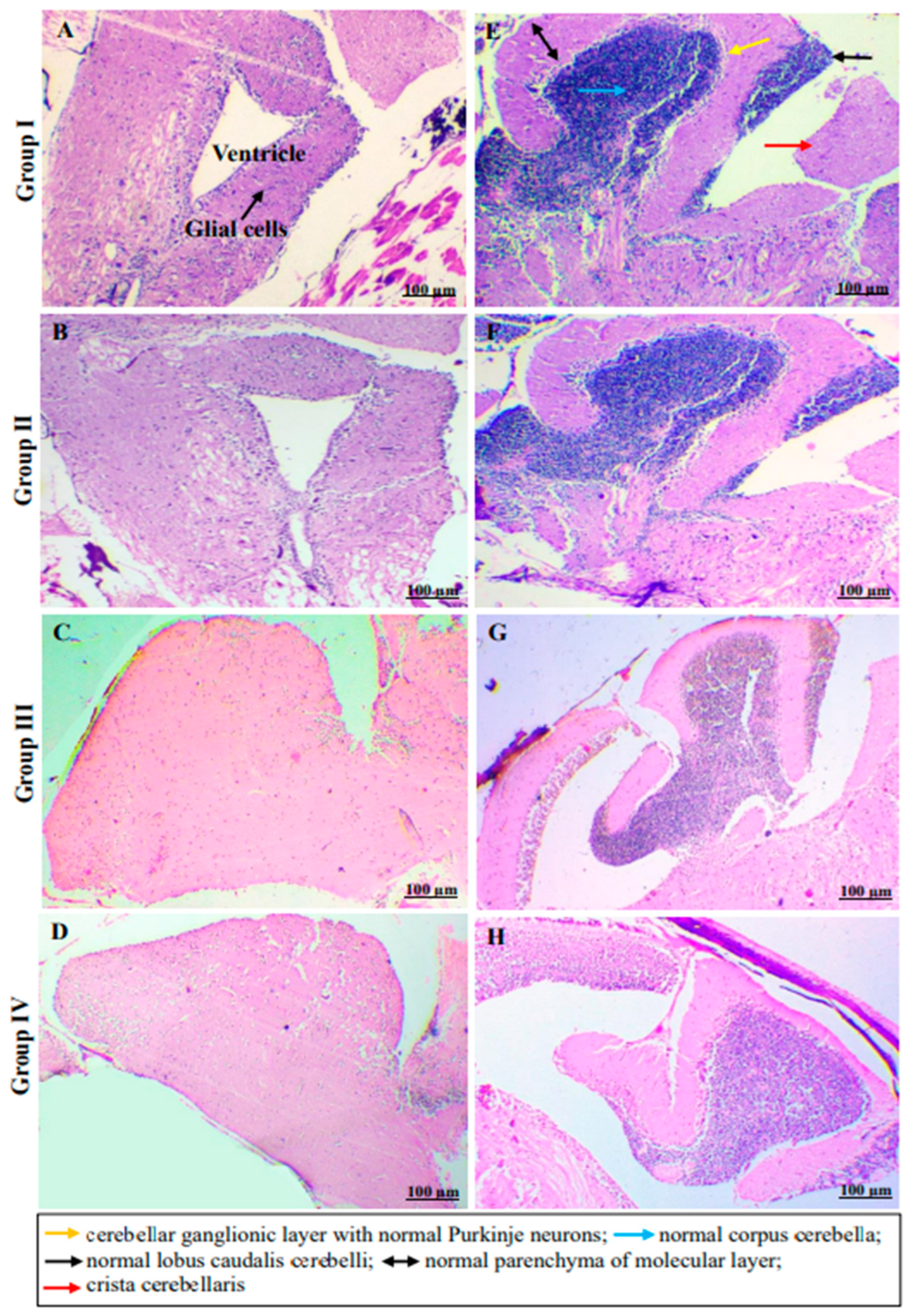
2.4. In Vivo Monitoring of Neurotoxic Effects, Neurobehavioral Endpoints and Histopathological Examination of Brain Tissues of Freshwater Fish, Danio Rerio, Exposed to C2
3. Materials and Methods
3.1. Materials
3.2. Cell Culture, Maintenance and Assessment of Cell Viability by MTT Assay
3.3. Assessment of PA1 Cell Death Using Nuclear Stains: Dual-Stained Acridine Orange–Propidium Iodide (AO/PI) and 4′,6-Diamidino-2-Phenylindole (DAPI) and Genotoxicity Analysis by Cytokinesis-Block Micronucleus (CBMN) Assay
3.4. Inhibition of Cellular Migration (Scratch Assay), Cell Cycle, Mitochondrial Membrane Potential (∆ψmit) and C2-Induced Changes in Gene and Protein Profiling
3.5. Analysis of Ceramide Accumulation in Response to C2 Treatment Using Liquid Chromatography–Mass Spectrometry (LC-MS)
3.6. Effect of C2 on the Neurobehavioral Properties in Adult Zebrafish, Danio Rerio, Models
3.6.1. Housing and Acclimatization
3.6.2. Acute Toxicity Test
3.6.3. Stress and Anxiety—Novel Tank Diving Test
3.6.4. Anxiety and Depression—Light/Dark Test
3.6.5. Sociability—Three Chamber Maze Test
3.6.6. Learning and Memory—T-Maze Test
3.6.7. Histopathological Examination of Brain Tissues
3.7. Statistical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannun, Y.A. Functions of Ceramide in Coordinating Cellular Responses to Stress. Science 1996, 274, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Westwick, J.; Bielawska, A.; Dbaibo, G.; Hannun, Y.A.; Brenner, D.A. Ceramide Activates the Stress-Activated Protein Kinases. J. Biol. Chem. 1995, 270, 22689–22692. [Google Scholar] [CrossRef] [PubMed]
- Latinis, K.M.; Koretzky, G.A. Fas Ligation Induces Apoptosis and Jun Kinase Activation Independently of CD45 and Lck in Human T Cells. Blood 1996, 87, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Jayadev, S.; Liu, B.; Bielawska, A.; Lee, J.Y.; Nazaire, F.; Pushkareva, M.Y.; Obeid, L.M.; Hannun, Y.A. Role for Ceramide in Cell Cycle Arrest. J. Biol. Chem. 1995, 270, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Dbaibo, G. Regulation of the Stress Response by Ceramide. Biochem. Soc. 1997, 25, 557–561. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N. The Endocannabinoid Anandamide Induces Apoptosis of Rat Decidual Cells through a Mechanism Involving Ceramide Synthesis and P38 MAPK Activation. Apoptosis 2013, 18, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Ramer, R.; Schwarz, R.; Hinz, B. Modulation of the Endocannabinoid System as a Potential Anticancer Strategy. Front Pharmacol. 2019, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.; Henderson, K.; Danell, A.S.; Van Dross, R. Arachidonoyl-ethanolamide Activates Endoplasmic Reticulum Stress-apoptosis in Tumorigenic Keratinocytes: Role of Cyclooxygenase-2 and Novel J-series Prostamides. Mol. Carcinog. 2015, 55, 117–130. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C.; Perry, E.; MacDougall, L.; Ammerman, Y.; Cooper, T.B.; Wu, Y.T.; Braley, G.; Gueorguieva, R.; Krystal, J.H. The Psychotomimetic Effects of Intravenous Delta-9-Tetrahydrocannabinol in Healthy Individuals: Implications for Psychosis. Neuropsychopharmacol 2004, 29, 1558–1572. [Google Scholar] [CrossRef]
- Lee, P.J.; Woo, S.J.; Jee, J.-G.; Sung, S.H. Bisdemethoxycurcumin Induces Apoptosis in Activated Hepatic Stellate Cells via Cannabinoid Receptor 2. Molecules 2015, 20, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Paul, V.J.; Luesch, H. Marine Cyanobacterial Fatty Acid Amides Acting on Cannabinoid Receptors. ChemBioChem 2012, 13, 2676–2681. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jutooru, I.; Lei, P.; Kim, K.; Lee, S.; Brents, L.K.; Prather, P.L.; Safe, S. Betulinic Acid Targets YY1 and ErbB2 through Cannabinoid Receptor-Dependent Disruption of MicroRNA-27a:ZBTB10 in Breast Cancer. Mol. Cancer Ther. 2012, 11, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as Potential Anticancer Drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.B.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a Non-Psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells through a Multitarget Effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive Effect of the Non-Psychotropic Phytocannabinoid Cannabidiol on Experimental Colon Cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef]
- Christina, V.S.; Raman, L.S.; Sivamurugan, V.; Kumar, T.D.; Panchatcharam, T.S.; Narasimhan, G.; Doss, G.C.P.; Krishnan, M.E.G. 3-Hydroxypropane-1,2-Diyl Dipalmitoleate—A Natural Compound with Dual Roles (CB1 Agonist/FAAH1 Blocker) in Inhibiting Ovarian Cancer Cell Line. Pharmaceuticals 2021, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Huang, H.; Tong, T.; Yau, L.-F.; Chen, C.-Y.; Mi, J.; Wang, J.; Jiang, Z. LC-MS Based Sphingolipidomic Study on A2780 Human Ovarian Cancer Cell Line and Its Taxol-Resistant Strain. Sci. Rep. 2016, 6, 34684. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.; Canavello, P.R.; Elegante, M.; Elkhayat, S.; Bartels, B.; Tien, A.K.; Tien, D.; et al. Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Fontana, B.D.; Duarte, T.; Müller, T.E.; Canzian, J.; Ziani, P.R.; Mezzomo, N.J.; Parker, M.O.; Rosemberg, D.B. Concomitant Taurine Exposure Counteracts Ethanol-Induced Changes in Locomotor and Anxiety-like Responses in Zebrafish. Psychopharmacol 2019, 237, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Surenderan, S.; Murkunde, Y. Elucidation of Acute and Neurobehavioral Toxicity of E-Waste Extracts with Special Reference to Cognitive Impairment, Anxiety and Stress Response Using Zebrafish. Toxicol. Environ. Health Sci. 2019, 11, 27–35. [Google Scholar] [CrossRef]
- Gould, G.G. Modified Associative Learning T-Maze Test for Zebrafish (Danio Rerio) and Other Small Teleost Fish. In Zebrafish Neurobehavioral Protocols; Humana Press: Totowa, NJ, USA, 2010; pp. 61–73. [Google Scholar]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Karakasis, K.; Rottapel, R.; Oza, A.M. Advances in Ovarian Cancer, from Biology to Treatment. Nat. Cancer 2021, 2, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.A.; Nemeth, E.; Tran, H.; Shvartsman, H.S.; Cass, I.; Narod, S.A.; Karlan, B.Y. BRCA1 Promoter Region Hypermethylation in Ovarian Carcinoma: A Population-Based Study. PubMed 2000, 60, 5329–5333. [Google Scholar]
- Widschwendter, M.; Jiang, G.; Woods, C.B.; Müller, H.M.; Fiegl, H.; Goebel, G.; Marth, C.; Müller-Holzner, E.; Zeimet, A.G.; Laird, P.W.; et al. DNA Hypomethylation and Ovarian Cancer Biology. Cancer Res. 2004, 64, 4472–4480. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, I.I.; Battagli, C.; Esteller, M.; Herman, J.G.; Dulaimi, E.; Edelson, M.I.; Bergman, C.; Ehya, H.; Eisenberg, B.L.; Cairns, P. Tumor Cell-Specific BRCA1 and RASSF1A Hypermethylation in Serum, Plasma, and Peritoneal Fluid from Ovarian Cancer Patients. Cancer Res. 2004, 64, 6476–6481. [Google Scholar] [CrossRef] [PubMed]
- Helzlsouer, K.J. Prospective Study of Serum CA-125 Levels as Markers of Ovarian Cancer. JAMA 1993, 269, 1123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Loganath, A.; Guo, C.; Narasimhan, K.; Koh, S.; Zhang, H.; Biswas, A.; Choolani, M. Circulating Haptoglobin Is an Independent Prognostic Factor in the Sera of Patients with Epithelial Ovarian Cancer. Neoplasia 2007, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.; Brown, L.; Davidson, A.C.; Konje, J.C. Localisation and Function of the Endocannabinoid System in the Human Ovary. PLoS ONE 2009, 4, e4579. [Google Scholar] [CrossRef]
- Fowler, C.J. Monoacylglycerol Lipase – a Target for Drug Development? Br. J. Pharmacol. 2012, 166, 1568–1585. [Google Scholar] [CrossRef]
- Ravi, J.; Sneh, A.; Shilo, K.; Nasser, M.W.; Ganju, R.K. FAAH Inhibition Enhances Anandamide Mediated Anti-Tumorigenic Effects in Non-Small Cell Lung Cancer by Downregulating the EGF/EGFR Pathway. Oncotarget 2014, 5, 2475–2486. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Murase, R.; Limbad, C.; Singer, E.A.; Allison, J.; Adrados, I.; Kawamura, R.; Pakdel, A.; Fukuyo, Y.; Nguyen, D.; et al. ID-1 Is a Key Transcriptional Regulator of Glioblastoma Aggressiveness and a Novel Therapeutic Target. Cancer Res. 2013, 73, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Ayakannu, T.; Taylor, A.H.; Willets, J.M.; Konje, J.C. The Evolving Role of the Endocannabinoid System in Gynaecological Cancer. Hum. Reprod. Update 2015, 21, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Messalli, E.; Grauso, F.; Luise, R.; Angelini, A.; Rossiello, R. Cannabinoid Receptor Type 1 Immunoreactivity and Disease Severity in Human Epithelial Ovarian Tumors. Am. J. Obstet. Gynecol. 2014, 211, 234.e1–234.e6. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.A.; Yang, J.; Trauger, S.A.; Nakayama, H.; Huang, L.; Strunk, D.; Moses, M.A.; Klagsbrun, M.; Bischoff, J.; Graier, W.F. TheGPR55 Agonist, L-α-lysophosphatidylinositol, Mediates Ovarian Carcinoma Cell-induced Angiogenesis. Br. J. Pharmacol. 2015, 172, 4107–4118. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Z.; Shen, K.; Sui, W. ∆9-tetrahydrocannabinol Inhibits Epithelial-mesenchymal Transition and Metastasis by Targeting Matrix Metalloproteinase-9 in Endometrial Cancer. Oncol Lett. 2018, 15, 8527–8535. [Google Scholar] [CrossRef] [PubMed]
- Petra, L.; Schicho, R. Cannabinoids in Gynecological Diseases. Med. Cannabis Cannabinoids 2019, 2, 14–21. [Google Scholar]
- Oza, A.M.; Del Pilar Estevez-Diz, M.; Grischke, E.; Hall, M.; Marmé, F.; Provencher, D.; Uyar, D.; Weberpals, J.I.; Wenham, R.M.; Laing, N.; et al. A Biomarker-Enriched, Randomized Phase II Trial of Adavosertib (AZD1775) Plus Paclitaxel and Carboplatin for Women with Platinum-Sensitive TP53-Mutant Ovarian Cancer. Clin. Cancer Res. 2020, 26, 4767–4776. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial Ovarian Cancer: Evolution of Management in the Era of Precision Medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Bagavandoss, P.; Grimshaw, S. Temporal and Spatial Distribution of the Cannabinoid Receptors (CB1, CB2) and Fatty Acid Amide Hydroxylase in the Rat Ovary. Anat. Rec. 2010, 293, 1425–1432. [Google Scholar] [CrossRef]
- Bifulco, M.; Laezza, C.; Portella, G.; Vitale, M.; Orlando, P.; De Petrocellis, L.; Di Marzo, V. Control by the Endogenous Cannabinoid System of Ras Oncogene-dependent Tumor Growth. FASEB J. 2001, 15, 1–17. [Google Scholar] [CrossRef]
- McAllister, S.D.; Chan, C.M.; Taft, R.J.; Luu, T.; Abood, M.E.; Moore, D.H.; Aldape, K.; Yount, G. Cannabinoids Selectively Inhibit Proliferation and Induce Death of Cultured Human Glioblastoma Multiforme Cells. J. Neuro-Onco. 2005, 74, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, C.; Sánchez, C.; Daza, A.; Galve-Roperh, I.; Guzmán, M. The Stimulation of Ketogenesis by Cannabinoids in Cultured Astrocytes Defines Carnitine Palmitoyltransferase I as a New Ceramide-Activated Enzyme. J. Neurochem. 1999, 72, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; Izquierdo, M.L.; Guzmán, M. Anti-Tumoral Action of Cannabinoids: Involvement of Sustained Ceramide Accumulation and Extracellular Signal-Regulated Kinase Activation. Nature Med. 2000, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. The Ceramide-Centric Universe of Lipid-Mediated Cell Regulation: Stress Encounters of the Lipid Kind. J. Biol. Chem. 2002, 277, 25847–25850. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.K. Serine Palmitoyltransferase: Role in Apoptotic de Novo Ceramide Synthesis and Other Stress Responses. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2002, 1585, 146–152. [Google Scholar] [CrossRef]
- Blaess, M.; Le, H.; Claus, R.A.; Kohl, M.; Deigner, H.P. Stereospecific Induction of Apoptosis in Tumor Cells via Endogenous C16-Ceramide and Distinct Transcripts. Cell Death Discov. 2015, 1, 15013. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cao, K.; Tang, Y.; Liu, J.; Li, J.; Chen, J.; Wang, S.; Chen, Z.; Zhou, J. C16:0 Ceramide Effect on Melanoma Malignant Behavior and Glycolysis Depends on Its Intracellular or Exogenous Location. Am. J. Transl. Res. 2020, 12, 1123–1135. [Google Scholar]
- Han, X.; Holtzman, D.M.; McKeel, D.W.; Kelley, J.; Morris, J.C. Substantial Sulfatide Deficiency and Ceramide Elevation in Very Early Alzheimer’s Disease: Potential Role in Disease Pathogenesis. J. Neurochem. 2002, 82, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Tepper, A.D.; Cock, J.G.R.B.-D.; De Vries, E.; Borst, J.; Van Blitterswijk, W.J. CD95/Fas-Induced Ceramide Formation Proceeds with Slow Kinetics and Is Not Blocked by Caspase-3/CPP32 Inhibition. J. Biol. Chem. 1997, 272, 24308–24312. [Google Scholar] [CrossRef] [PubMed]
- Gamen, S.; Marzo, I.; Anel, A.; Piñeiro, A.; Naval, J. CPP32 Inhibition Prevents Fas-Induced Ceramide Generation and Apoptosis in Human Cells. FEBS Lett. 1996, 390, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Pru, J.K.; Hendry, I.R.; Davis, J.S.; Rueda, B.R. Soluble FAS Ligand Activates the Sphingomyelin Pathway and Induces Apoptosis in Luteal Steroidogenic Cells Independently of Stress-Activated P38MAPK. Endocrinology 2002, 143, 4350–4357. [Google Scholar] [CrossRef] [PubMed]
- Cremesti, A.; Pâris, F.; Grassmé, H.; Holler, N.; Tschopp, J.; Fuks, Z.; Gulbins, E.; Kolesnick, R. Ceramide Enables FAS to Cap and Kill. J. Biol. Chem. 2001, 276, 23954–23961. [Google Scholar] [CrossRef]
- Bielawska, A.; Greenberg, M.S.; Perry, D.K.; Jayadev, S.; Shayman, J.A.; McKay, C.; Hannun, Y.A. (1S,2R)-D-Erythro-2-(N-Myristoylamino)-1-Phenyl-1-Propanol as an Inhibitor of Ceramidase. J. Biol. Chem. 1996, 271, 12646–12654. [Google Scholar] [CrossRef] [PubMed]
- Del Pulgar, T.G.; Velasco, G.; Guzmán, M. The CB1 Cannabinoid Receptor Is Coupled to the Activation of Protein Kinase B/Akt. Biochem. J. 2000, 347, 369–373. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular Characterization of an Enzyme That Degrades Neuromodulatory Fatty-Acid Amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Wei, B.; Mikkelsen, T.S.; McKinney, M.K.; Lander, E.S.; Cravatt, B.F. A Second Fatty Acid Amide Hydrolase with Variable Distribution among Placental Mammals. J. Biol. Chem. 2006, 281, 36569–36578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayaraghavan, C.S.; Raman, L.S.; Surenderan, S.; Kaur, H.; Chinambedu, M.D.; Thyagarajan, S.P.; Gnanambal Krishnan, M.E. A Novel Non-Psychoactive Fatty Acid from a Marine Snail, Conus inscriptus, Signals Cannabinoid Receptor 1 (CB1) to Accumulate Apoptotic C16:0 and C18:0 Ceramides in Teratocarcinoma Cell Line PA1. Molecules 2024, 29, 1737. https://doi.org/10.3390/molecules29081737
Vijayaraghavan CS, Raman LS, Surenderan S, Kaur H, Chinambedu MD, Thyagarajan SP, Gnanambal Krishnan ME. A Novel Non-Psychoactive Fatty Acid from a Marine Snail, Conus inscriptus, Signals Cannabinoid Receptor 1 (CB1) to Accumulate Apoptotic C16:0 and C18:0 Ceramides in Teratocarcinoma Cell Line PA1. Molecules. 2024; 29(8):1737. https://doi.org/10.3390/molecules29081737
Chicago/Turabian StyleVijayaraghavan, Christina Sathyanathan, Lakshmi Sundaram Raman, Shanmugapriya Surenderan, Harpreet Kaur, Mohanapriya Dandapani Chinambedu, Sadras Panchatcharam Thyagarajan, and Mary Elizabeth Gnanambal Krishnan. 2024. "A Novel Non-Psychoactive Fatty Acid from a Marine Snail, Conus inscriptus, Signals Cannabinoid Receptor 1 (CB1) to Accumulate Apoptotic C16:0 and C18:0 Ceramides in Teratocarcinoma Cell Line PA1" Molecules 29, no. 8: 1737. https://doi.org/10.3390/molecules29081737
APA StyleVijayaraghavan, C. S., Raman, L. S., Surenderan, S., Kaur, H., Chinambedu, M. D., Thyagarajan, S. P., & Gnanambal Krishnan, M. E. (2024). A Novel Non-Psychoactive Fatty Acid from a Marine Snail, Conus inscriptus, Signals Cannabinoid Receptor 1 (CB1) to Accumulate Apoptotic C16:0 and C18:0 Ceramides in Teratocarcinoma Cell Line PA1. Molecules, 29(8), 1737. https://doi.org/10.3390/molecules29081737