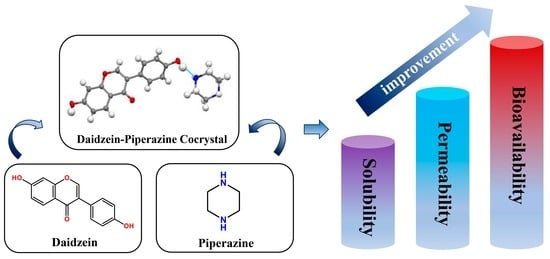
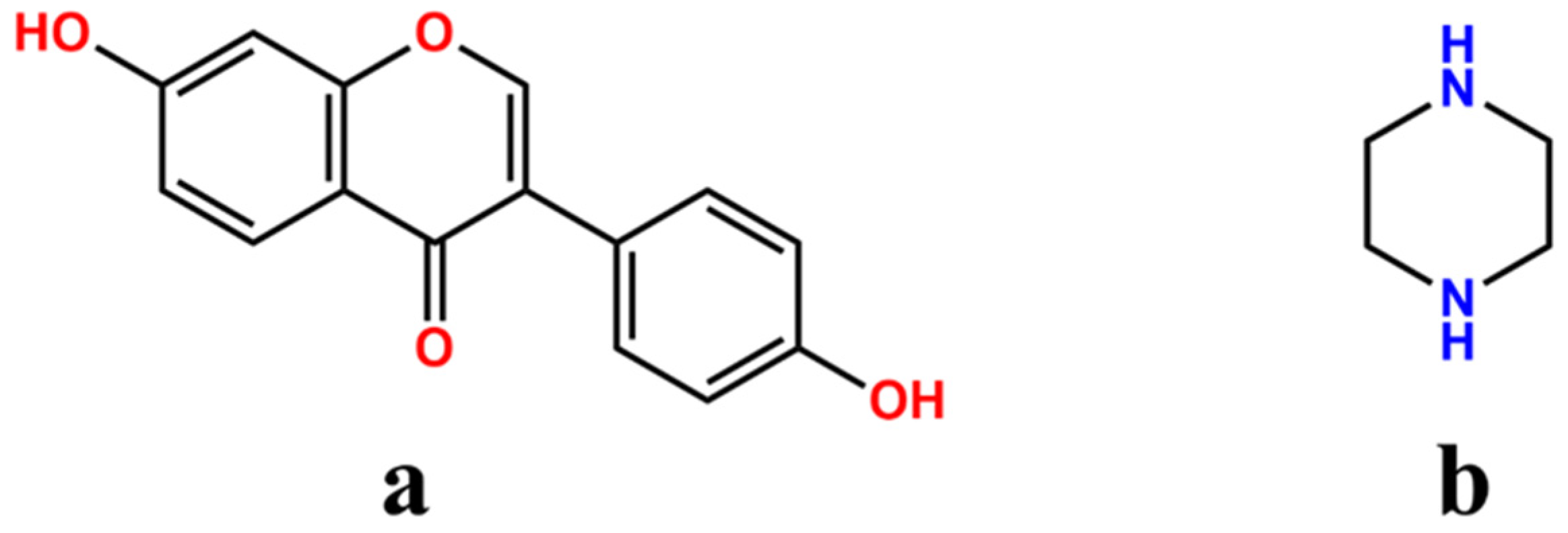
A Novel Cocrystal of Daidzein with Piperazine to Optimize the Solubility, Permeability and Bioavailability of Daidzein
Abstract
1. Introduction
2. Results and Discussion
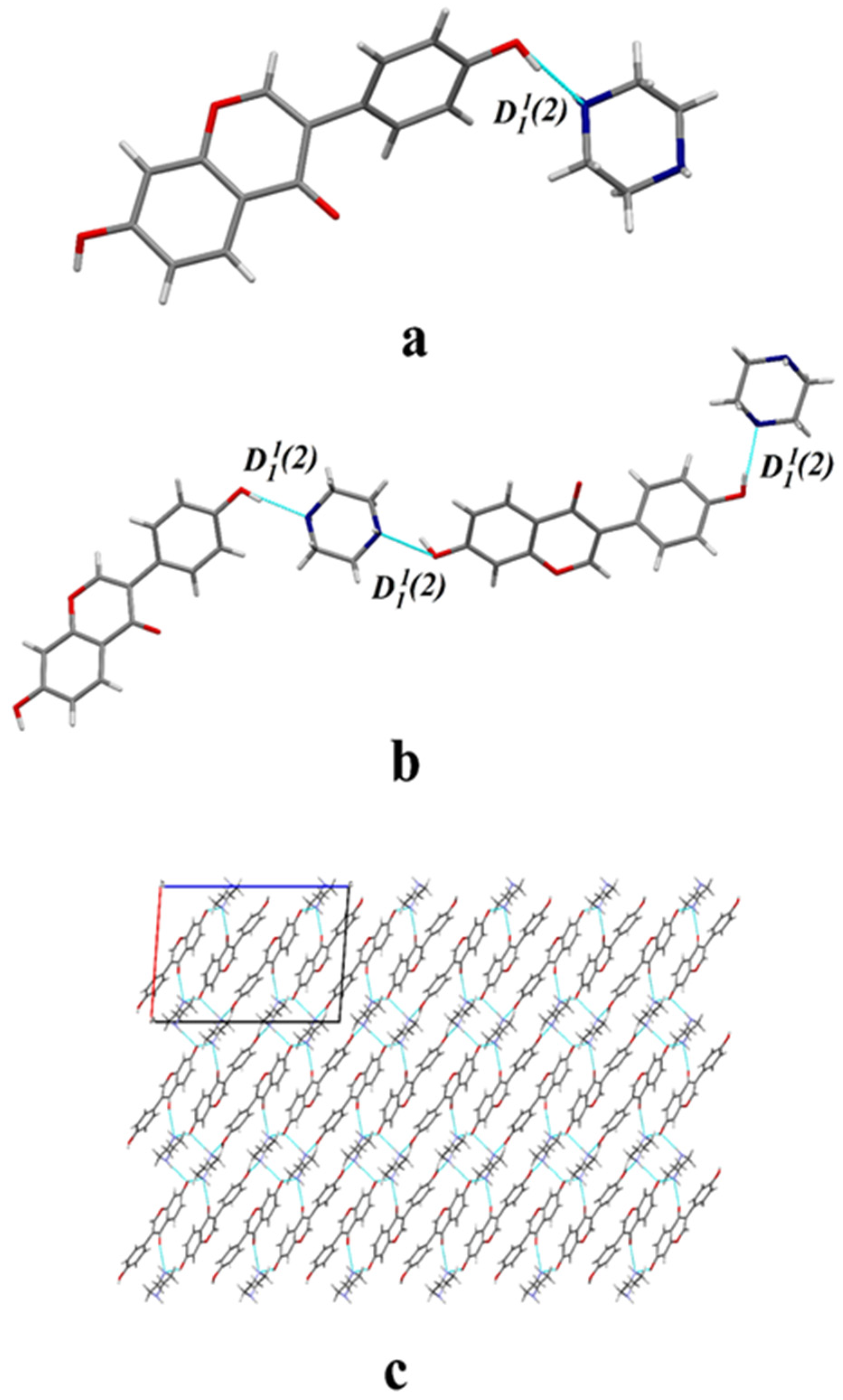
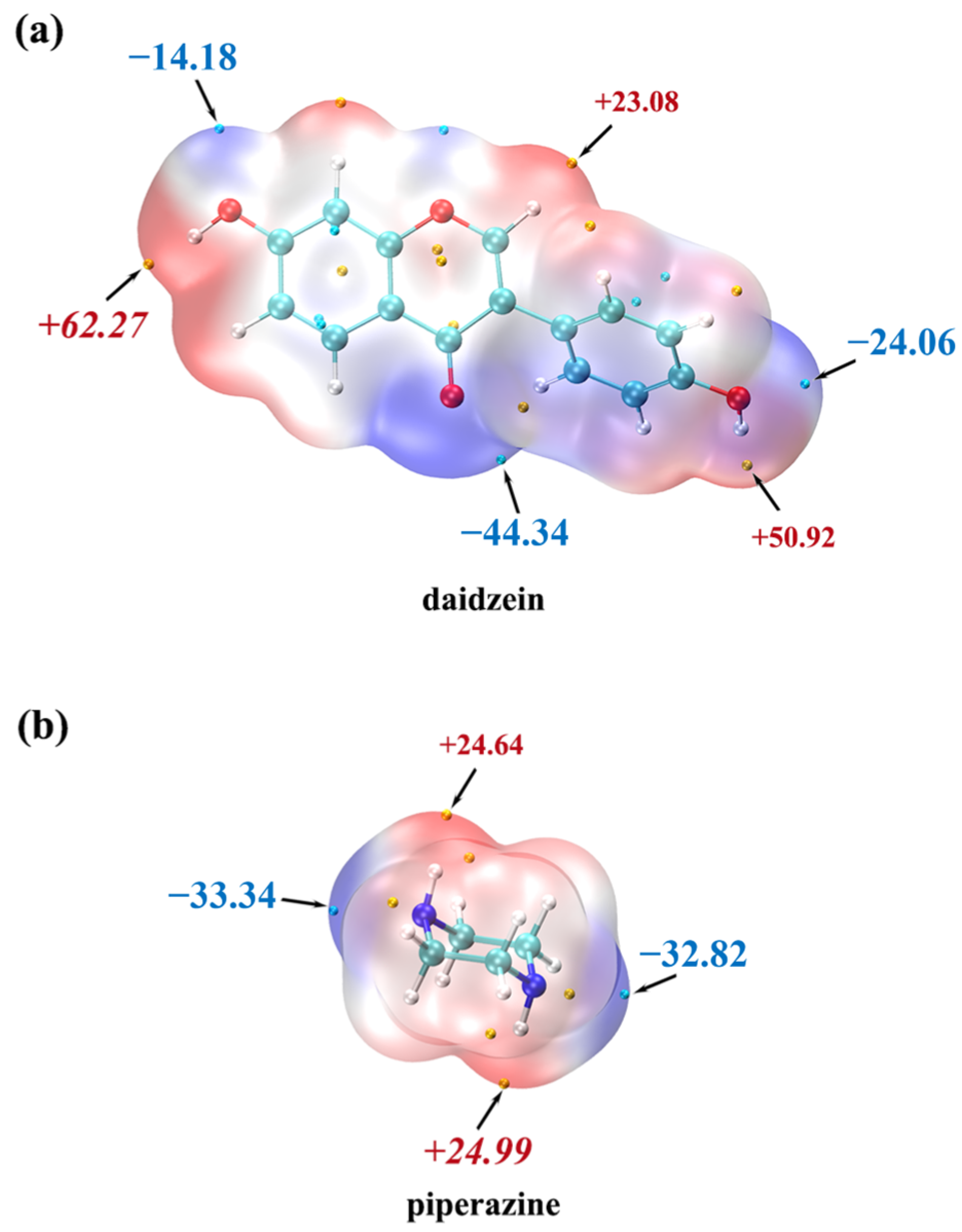
2.1. Crystal Structure Analysis
2.2. PXRD Analysis
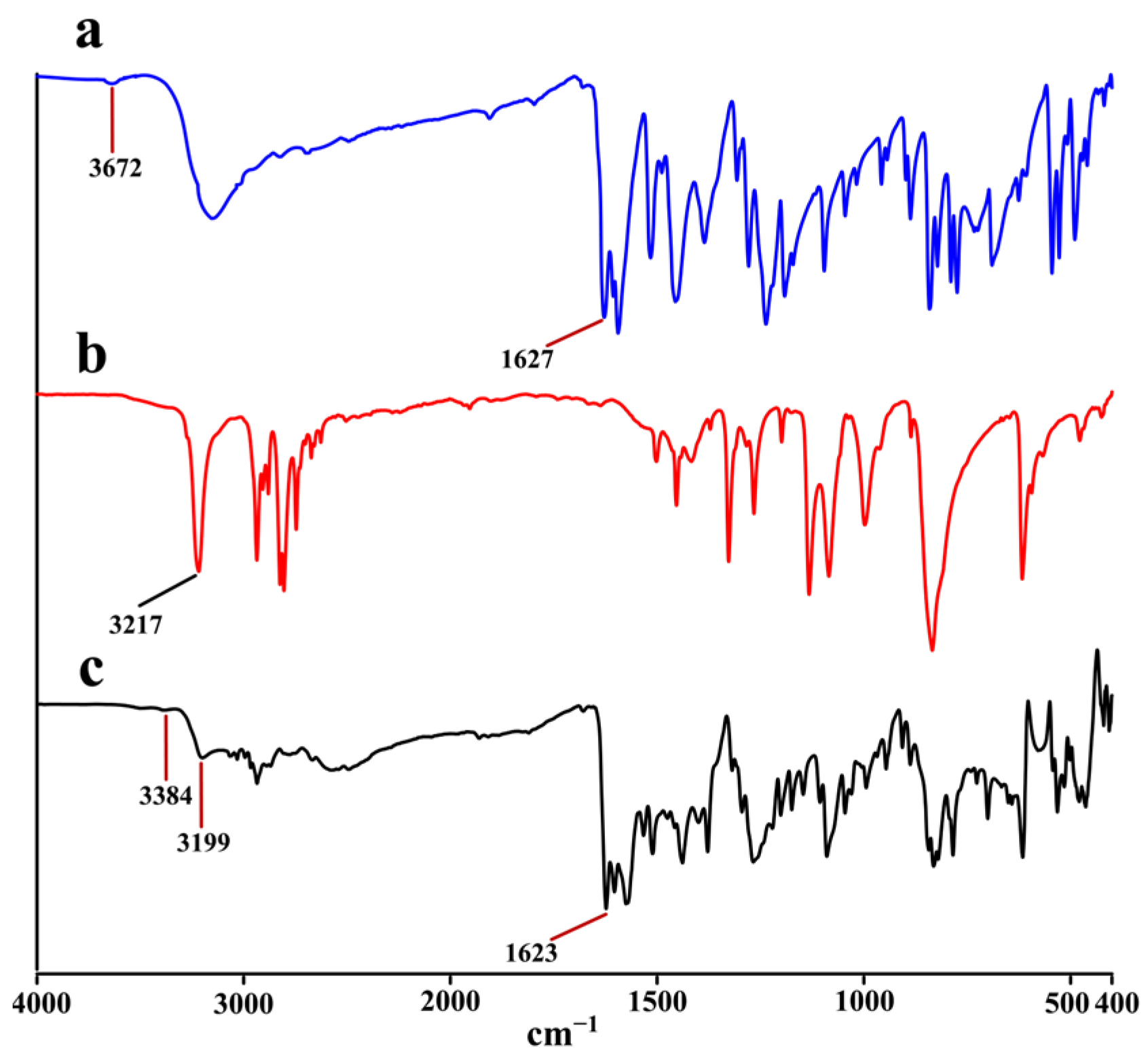
2.3. IR Analysis
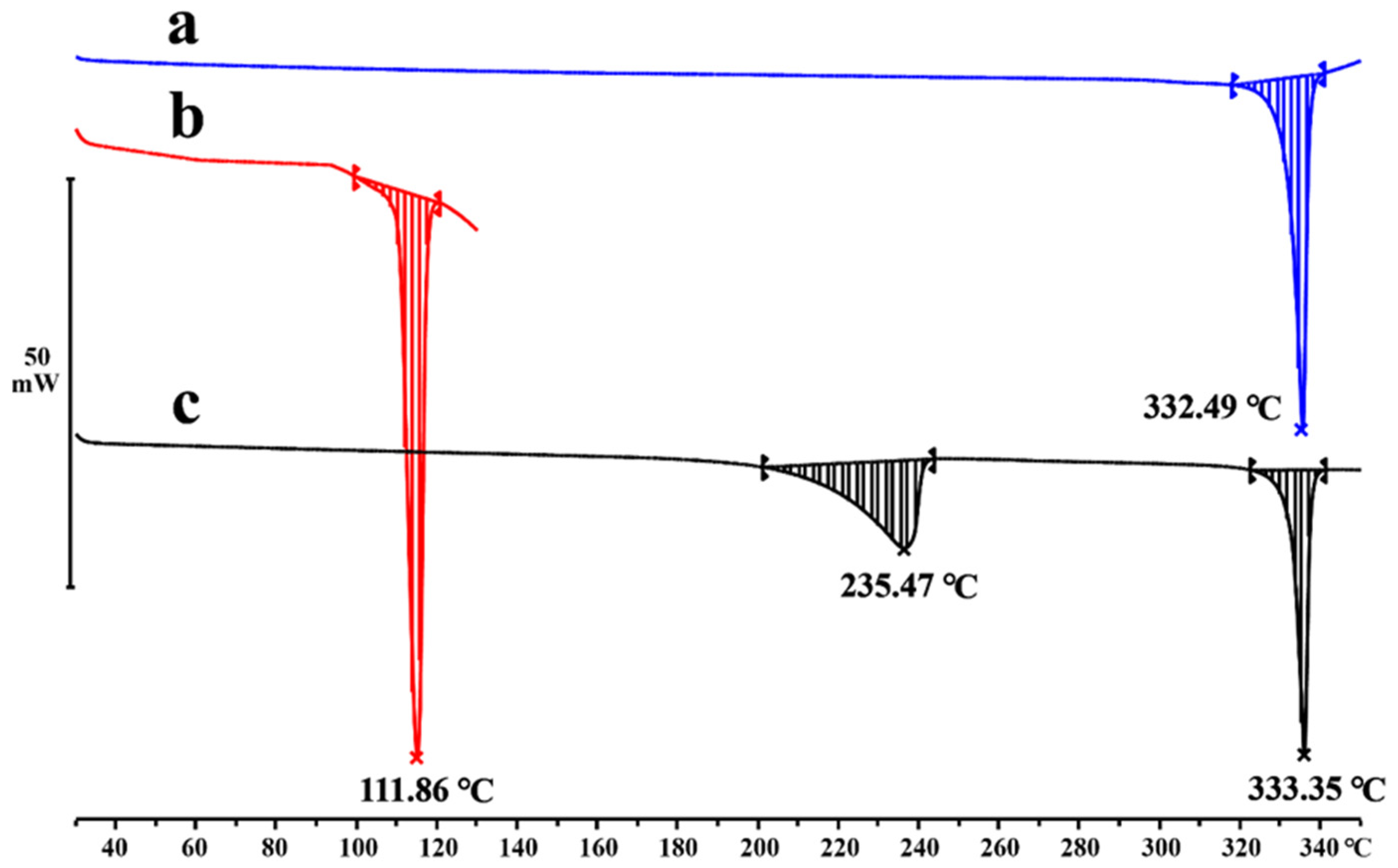
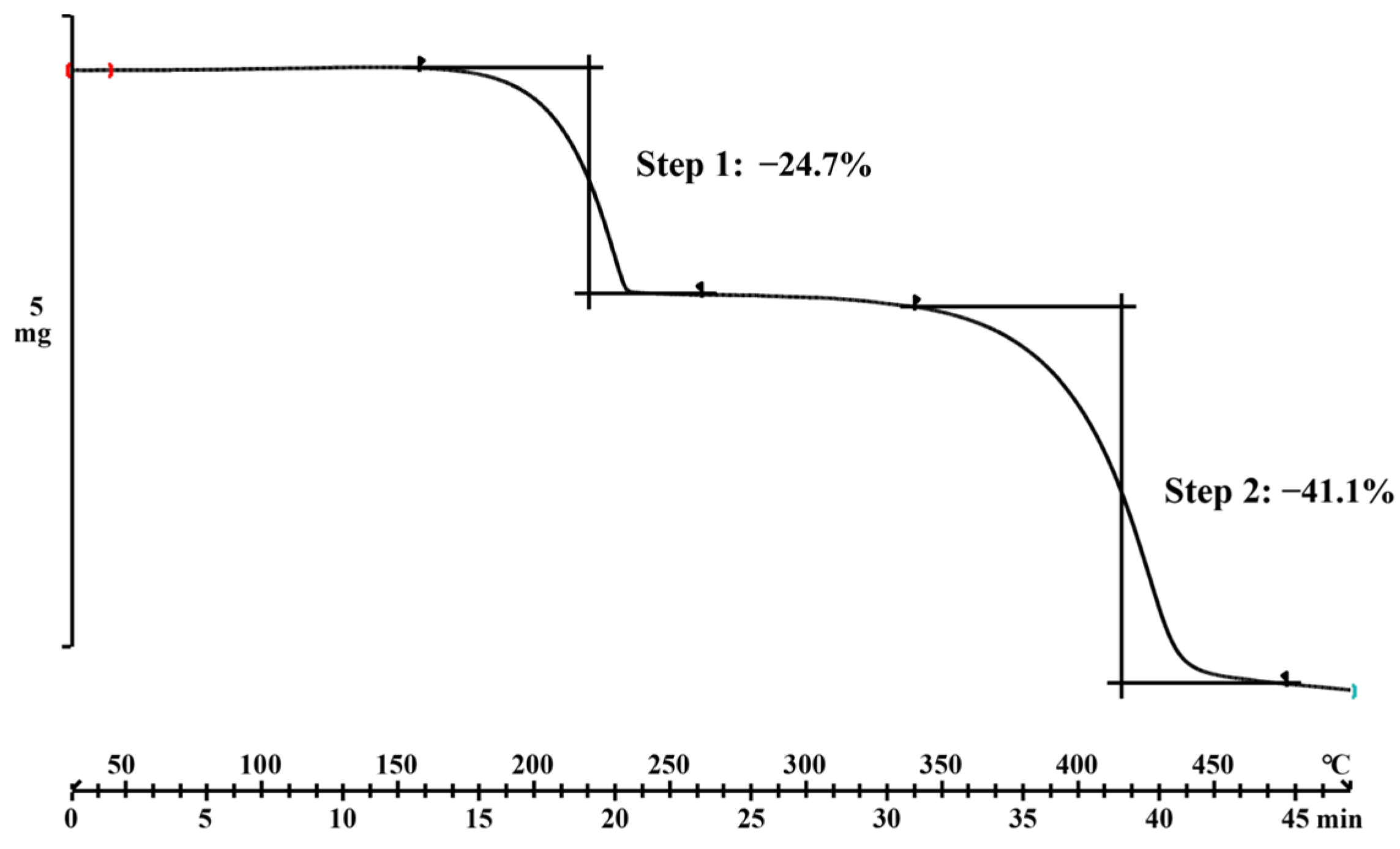
2.4. Thermal Analysis
2.5. Stability Study
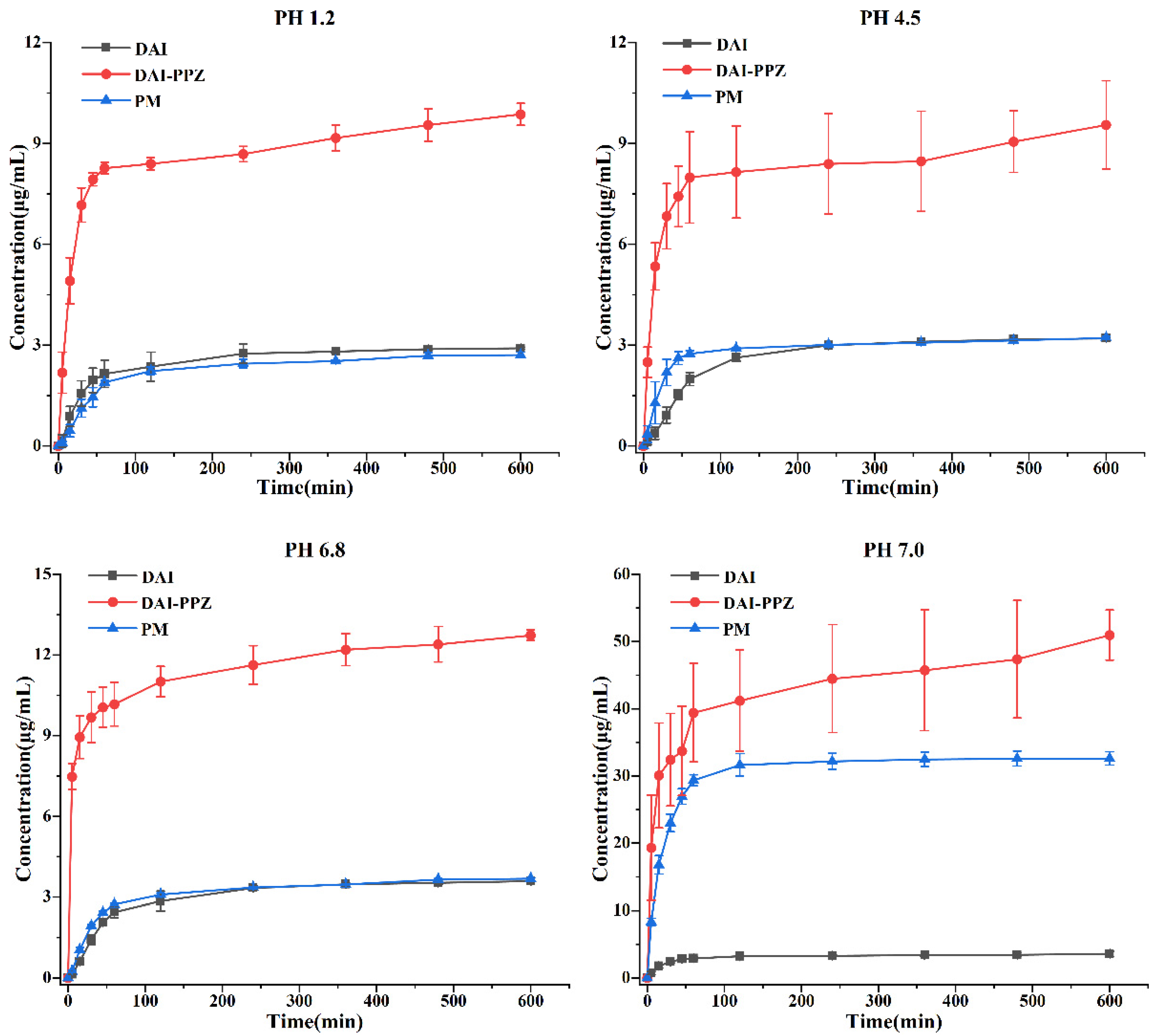
2.6. Solubility and Powder Dissolution In Vitro
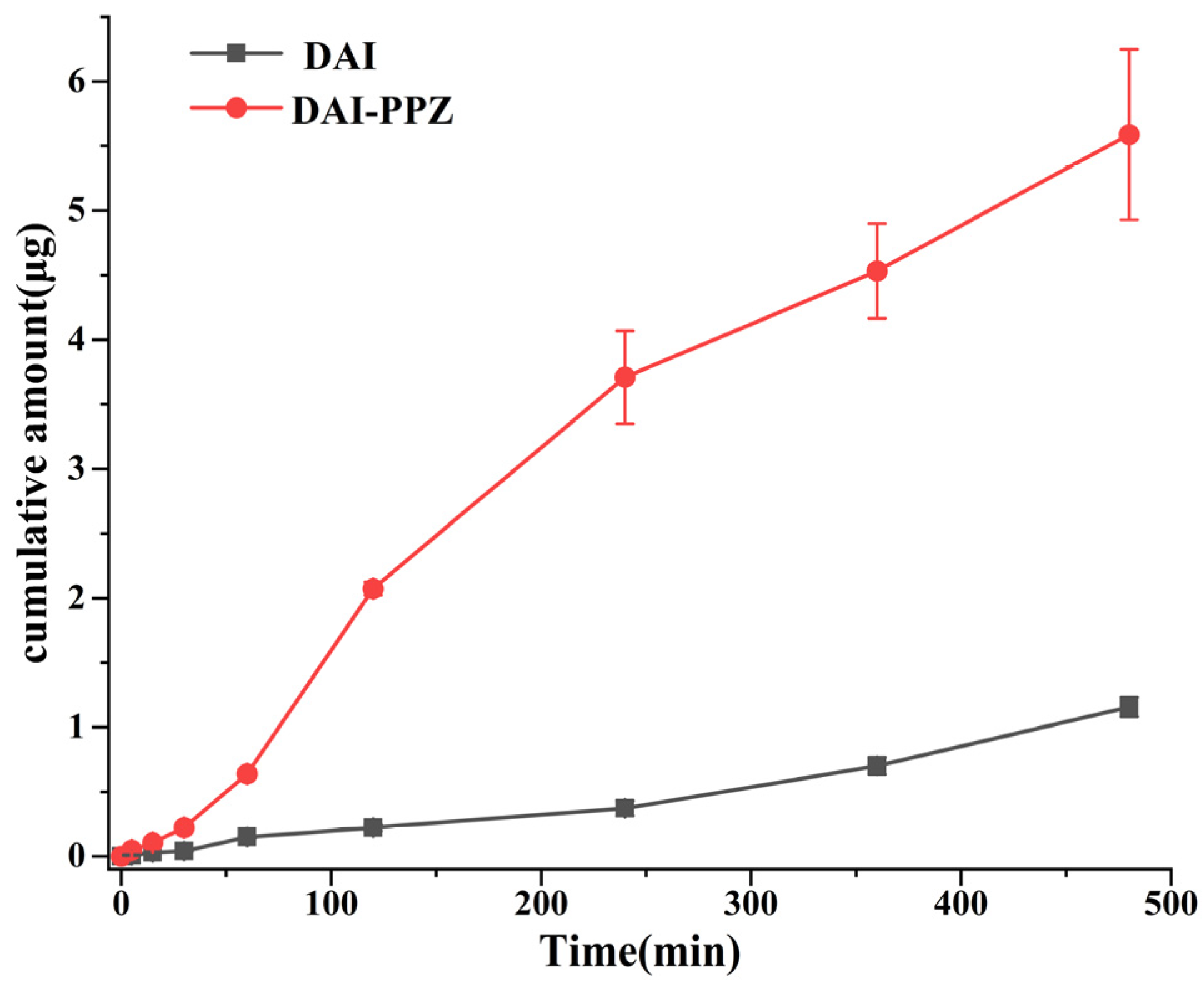
2.7. Permeability Study
2.8. Pharmacokinetics In Vivo
3. Materials and Methods
3.1. Materials
3.2. Preparation of DAI-PPZ Cocrystal
3.3. Preparation of Single Crystal
3.4. SCXRD Analysis
3.5. MEPS Calculations
3.6. PXRD Analysis
3.7. IR Analysis
3.8. Thermal Analysis
3.9. Stability Evaluation
3.10. Solubility Determination and PH Measurement
3.11. Powder Dissolution In Vitro
3.12. Flux Measurements
3.13. Pharmacokinetic Study In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laurenz, R.; Tumbalam, P.; Naeve, S.; Thelen, K.D. Determination of isoflavone (genistein and daidzein) concentration of soybean seed as affected by environment and management inputs. J. Sci. Food Agric. 2017, 97, 3342–3347. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Grewal, S.; Sharma, N.; Behl, T.; Gupta, S.; Anwer, M.K.; Vargas-De-La-Cruz, C.; Mohan, S.; Bungau, S.G.; Bumbu, A. Unveiling the Pharmacological and Nanotechnological Facets of Daidzein: Present State-of-the-Art and Future Perspectives. Molecules 2023, 28, 1765–1785. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-H.; Ju, J.-W.; Park, M.J.; Han, J.S. Daidzein inhibits carbohydrate digestive enzymes in vitro and alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2013, 712, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Laddha, A.P.; Kulkarni, Y.A. Daidzein mitigates myocardial injury in streptozotocin-induced diabetes in rats. Life Sci. 2021, 284, 119664. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Caradonna, F.; Giavaresi, G. Flavonoids in Bone Erosive Diseases: Perspectives in Osteoporosis Treatment. Trends Endocrin. Metab. 2021, 32, 76–94. [Google Scholar] [CrossRef]
- Laddha, A.P.; Murugesan, S.; Kulkarni, Y.A. In-vivo and in-silico toxicity studies of daidzein: An isoflavone from soy. Drug Chem. Toxicol. 2020, 45, 1408–1416. [Google Scholar] [CrossRef]
- Kulling, S.E.; Honig, D.M.; Metzler, M. Oxidative metabolism of the soy isoflavonesdaidzein and genistein in humans in vitro and in vivo. J. Agric. Food Chem. 2001, 49, 3024–3033. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Lehmann, L.; Metzler, M. Oxidative metabolism and genotoxic potential of major isoflavone phytoestrogens. J. Chromatogr. B 2002, 777, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Stancanelli, R.; Mazzaglia, A.; Tommasini, S.; Calabrò, M.L.; Villari, V.; Guardo, M.; Ficarra, P.; Ficarra, R. The enhancement of isoflavones water solubility by complexation with modified cyclodextrins: A spectroscopic investigation with implications in the pharmaceutical analysis. J. Pharm. Biomed. 2007, 44, 980–984. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Wang, J.; Liu, H.; Chen, Y. Preparation and Pharmacokinetic Study of Daidzein Long-Circulating Liposomes. Nanoscale Res. Lett. 2019, 14, 321–330. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, X.; Li, J.; Shen, Q. The comparison of different daidzein-PLGA nanoparticles in increasing its oral bioavailability. Int. J. Nanomed. 2012, 7, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-B.; Chen, D.-W.; Xie, L.-P.; Zhang, R.-Q. Optimized preparation of daidzein-loaded chitosan microspheres and in vivo evaluation after intramuscular injection in rats. Int. J. Pharm. 2007, 338, 142–151. [Google Scholar] [CrossRef]
- Shen, Q.; Li, X.; Yuan, D.; Jia, W. Enhanced oral bioavailability of daidzein by selfmicroemulsifying drug delivery system. Chem. Pharm. Bull. 2010, 58, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, Y.; Chadha, K.; Chadha, R.; Karan, M. Daidzein cocrystals: An opportunity to improve its biopharmaceutical parameters. Heliyon 2019, 5, e02669. [Google Scholar] [CrossRef] [PubMed]
- Panzade, P.S.; Shendarkar, G.R. Pharmaceutical cocrystal: A game changing approach for the administration of old drugs in new crystalline form. Drug Dev. Ind. Pharm. 2020, 46, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Bolla, G.; Nangia, A. Pharmaceutical cocrystals: Walking the talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Emami, S.; Siahi-Shadbad, M.; Adibkia, K.; Barzegar-Jalali, M. Recent advances in improving oral drug bioavailability by cocrystals. Bioimpacts 2018, 8, 305–320. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, Y.; Yu, M.; Yang, S.; Lu, Y.; Du, G. Recent Advances on the Biological Study of Pharmaceutical Cocrystals. AAPS PharmSciTech 2022, 23, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Ghosh, A. Progress on cocrystallization of poorly soluble NME’s in the last decade. CrystEngComm 2020, 22, 6958–6974. [Google Scholar] [CrossRef]
- Sinha, A.S.; Maguire, A.R.; Lawrence, S.E. Cocrystallization of nutraceuticals. Cryst. Growth Des. 2015, 15, 984–1009. [Google Scholar] [CrossRef]
- Wang, J.-R.; Wang, X.; Yang, Y.; Chen, X.; Mei, X. Solid-state characterization of 17β-estradiol co-crystals presenting improved dissolution and bioavailability. CrystEngComm 2016, 18, 3498–3505. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; An, Q.; Gong, L.; Yang, S.; Zhang, B.; Su, B.; Yang, D.; Zhang, L.; Lu, Y.; et al. Optimized solubility and bioavailability of genistein based on cocrystal engineering. Nat. Product. Bioprospecting 2023, 13, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, R.; Wu, L.; Zhang, X.; Guan, S.; Zhang, L.; Ning, L.; Li, S. Azilsartan piperazine salt solvate and monohydrate: Preparation, crystal structure, enhanced solubility and oral bioavailability. New J. Chem. 2020, 44, 852–860. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, X.-L.; Jia, J.-L.; Zhao, X.-H.; Li, Z.-W.; Lu, T.-B.; Chen, J.-M. Crystal Structures, Stability, and Solubility Evaluation of Two Polymorphs of a 2:1 Melatonin–Piperazine Cocrystal. Cryst. Growth Des. 2019, 20, 1079–1087. [Google Scholar] [CrossRef]
- Bolus, L.; Wang, K.; Pask, C.; Lai, X.; Li, M. Cocrystallisation of Daidzein with pyridine-derived molecules: Screening, structure determination and characterisation. J. Mol. Struct. 2020, 1222, 128893. [Google Scholar] [CrossRef]
- Huang, S.; Xue, Q.; Xu, J.; Ruan, S.; Cai, T. Simultaneously Improving the Physicochemical Properties, Dissolution Performance, and Bioavailability of Apigenin and Daidzein by Co-Crystallization with Theophylline. J. Pharm. Sci. 2019, 108, 2982–2993. [Google Scholar] [CrossRef]
- Wang, H.; Yang, D.; Zhang, W.; Song, J.; Gong, N.; Yu, M.; Yang, S.; Zhang, B.; Liu, Q.; Du, G. An innovative rhein-matrine cocrystal: Synthesis, characterization, formation mechanism and pharmacokinetic study. Chin. Chem. Lett. 2023, 34, 107258. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Song, J.; Wang, C.; Li, J.; Liu, L.; He, X.; Zhao, X.; Sun, C.C. Drug–drug cocrystallization simultaneously improves pharmaceutical properties of genistein and ligustrazine. Cryst. Growth Des. 2021, 21, 3461–3468. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, Y.; Wang, W.; An, Q.; Li, S.; Zhang, B.; Zhou, J.; Hu, K.; Zhang, L.; Yang, D.; et al. Characterization, Analysis, and Theoretical Calculation Studies of Solvates and Cocrystals of Betulin: An Exploration of the Boundary between Solvates and Cocrystals. Cryst. Growth Des. 2023, 23, 8694–8706. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Manin, A.N.; Manin, N.G.; Kuzmina, L.G.; Churakov, A.V.; Perlovich, G.L. Pharmaceutical cocrystals of diflunisal and diclofenac with theophylline. Mol. Pharm. 2014, 11, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Piskula, M.K.; Yamakoshi, J.; Iwai, Y. Daidzein and genistein but not their glucosides are absorbed from the rat stomach. FEBS Lett. 1999, 447, 287–291. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Zhang, C.; Yang, D.; Lu, Y.; Zhou, Z. The enhanced pH-dependent solubility behavior of three novel lamotrigine-acid salts. J. Mol. Liq. 2023, 382, 121929. [Google Scholar] [CrossRef]
- Roy, P.; Chakraborty, S.; Pandey, N.; Kumari, N.; Chougule, S.; Chatterjee, A.; Chatterjee, K.; Mandal, P.; Gorain, B.; Dhotre, A.V.; et al. Study on Sulfamethoxazole–Piperazine Salt: A Mechanistic Insight into Simultaneous Improvement of Physicochemical Properties. Mol. Pharm. 2023, 20, 5226–5239. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Devi, V.K.; Clara, D.; Malviya, N.; Ganguly, S.; Desiraju, G.R. Cocrystals of hydrochlorothiazide: Solubility and diffusion/permeability enhancements through drug–coformer interactions. Mol. Pharm. 2015, 12, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.O.; Volkova, T.V.; Churakov, A.V.; Proshin, A.N.; Terekhova, I.V.; Perlovich, G.L. Cocrystal formation, crystal structure, solubility and permeability studies for novel 1,2,4-thiadiazole derivative as a potent neuroprotector. Eur. J. Pharm. Sci. 2017, 109, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.L.; Voronin, A.P.; Gao, W.; Perlovich, G.L.; Lu, T.B.; Chen, J.M. Intermolecular interactions and permeability of 5-fluorouracil cocrystals with a series of isomeric hydroxybenzoic acids: A combined theoretical and experimental study. CrystEngComm 2019, 21, 5095–5105. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131, 1362S–1375S. [Google Scholar] [CrossRef]
- Yang, D.; Wang, H.; Liu, Q.; Yuan, P.; Chen, T.; Zhang, L.; Yang, S.; Zhou, Z.; Lu, Y.; Du, G. Structural landscape on a series of rhein: Berberine cocrystal salt solvates: The formation, dissolution elucidation from experimental and theoretical investigations. Chin. Chem. Lett. 2022, 33, 3207–3211. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Daidzein–Piperazine Cocrystal |
|---|---|
| Empirical formula | C19H20N2O4 |
| Formula weight | 340.37 |
| Crystal size/mm | 0.22 × 0.18 × 0.12 |
| Description | block |
| Crystal system | Monoclinic |
| Space group | P21/C |
| a (Å) | 12.162(1) |
| b (Å) | 6.385(1) |
| c (Å) | 21.224(1) |
| β (◦) | 95.02(1) |
| Volume (Å3) | 1641.9(1) |
| Z | 4 |
| Completeness | 99.7% |
| Density (g·cm−3) | 1.377 |
| Reflections with I > 2σ (I) | 2792 |
| Rindexs (I > 2σI) | R1 = 0.0556, wR2 = 0.1578 |
| Goodness of fit on F2 | 1.035 |
| CCDC deposition number | 2313133 |
| D–H⋯A | d(D⋯A) (Å) | ∠(DHA) (deg) | Symmetry Code |
|---|---|---|---|
| O4−H4⋯N2P | 2.738 | 146.91 | - |
| O3−H3⋯N1P | 2.557 | 131.51 | [x + 1, −y + 1/2, z − 1/2] |
| N1P−H1P⋯O2 | 2.919 | 127.93 | [−x, −y + 1, −z + 1] |
| N2P−H2P⋯O3 | 3.092 | 175.17 | [−x + 1, −y + 1, −z + 1] |
| Compound | PH 1.2 | PH 4.5 | PH 6.8 | PH 7.0 |
|---|---|---|---|---|
| DAI | 1.31 ± 0.06 | 4.72 ± 0.01 | 6.80 ± 0.04 | 6.62 ± 0.57 |
| DAI-PPZ | 1.80 ± 0.35 | 4.86 ± 0.01 | 7.23 ± 0.05 | 9.67 ± 0.21 |
| PM | 1.88 ± 0.01 | 4.82 ± 0.02 | 7.18 ± 0.07 | 9.70 ± 0.17 |
| Parameter | Daidzein | Daidzein-Piperazine |
|---|---|---|
| AUC(0–t) (µg/L·h) | 798.15 ± 398.10 | 2600.55 ± 701.41 ** |
| AUC(0–∞) (µg/L·h) | 895.53 ± 388.94 | 2824.12 ± 764.66 ** |
| MRT(0–t) (h) | 7.89 ± 1.04 | 9.79 ± 1.14 * |
| MRT(0–∞) (h) | 12.29 ± 5.50 | 11.61 ± 3.04 |
| t1/2z (h) | 8.58 ± 6.16 | 4.76 ± 2.18 |
| Tmax (h) | 3.75 ± 2.89 | 8.17 ± 2.71 * |
| CLz/F (L/h/kg) | 128.09 ± 47.35 | 50.57 ± 14.12 ** |
| Cmax (µg/L) | 131.50 ± 74.29 | 268.47 ± 45.73 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, S.; Li, Q.; Wang, W.; Liu, M.; Yang, S.; Zhang, L.; Yang, D.; Du, G.; Lu, Y. A Novel Cocrystal of Daidzein with Piperazine to Optimize the Solubility, Permeability and Bioavailability of Daidzein. Molecules 2024, 29, 1710. https://doi.org/10.3390/molecules29081710
Wang Z, Li S, Li Q, Wang W, Liu M, Yang S, Zhang L, Yang D, Du G, Lu Y. A Novel Cocrystal of Daidzein with Piperazine to Optimize the Solubility, Permeability and Bioavailability of Daidzein. Molecules. 2024; 29(8):1710. https://doi.org/10.3390/molecules29081710
Chicago/Turabian StyleWang, Zhipeng, Shuang Li, Qi Li, Wenwen Wang, Meiru Liu, Shiying Yang, Li Zhang, Dezhi Yang, Guanhua Du, and Yang Lu. 2024. "A Novel Cocrystal of Daidzein with Piperazine to Optimize the Solubility, Permeability and Bioavailability of Daidzein" Molecules 29, no. 8: 1710. https://doi.org/10.3390/molecules29081710
APA StyleWang, Z., Li, S., Li, Q., Wang, W., Liu, M., Yang, S., Zhang, L., Yang, D., Du, G., & Lu, Y. (2024). A Novel Cocrystal of Daidzein with Piperazine to Optimize the Solubility, Permeability and Bioavailability of Daidzein. Molecules, 29(8), 1710. https://doi.org/10.3390/molecules29081710