Temperature-Controlled Divergent Synthesis of Pyrazoles and 1-Tosyl-1H-pyrazoles under Transition-Metal-Catalyst- and Oxidant-Free Conditions
Abstract
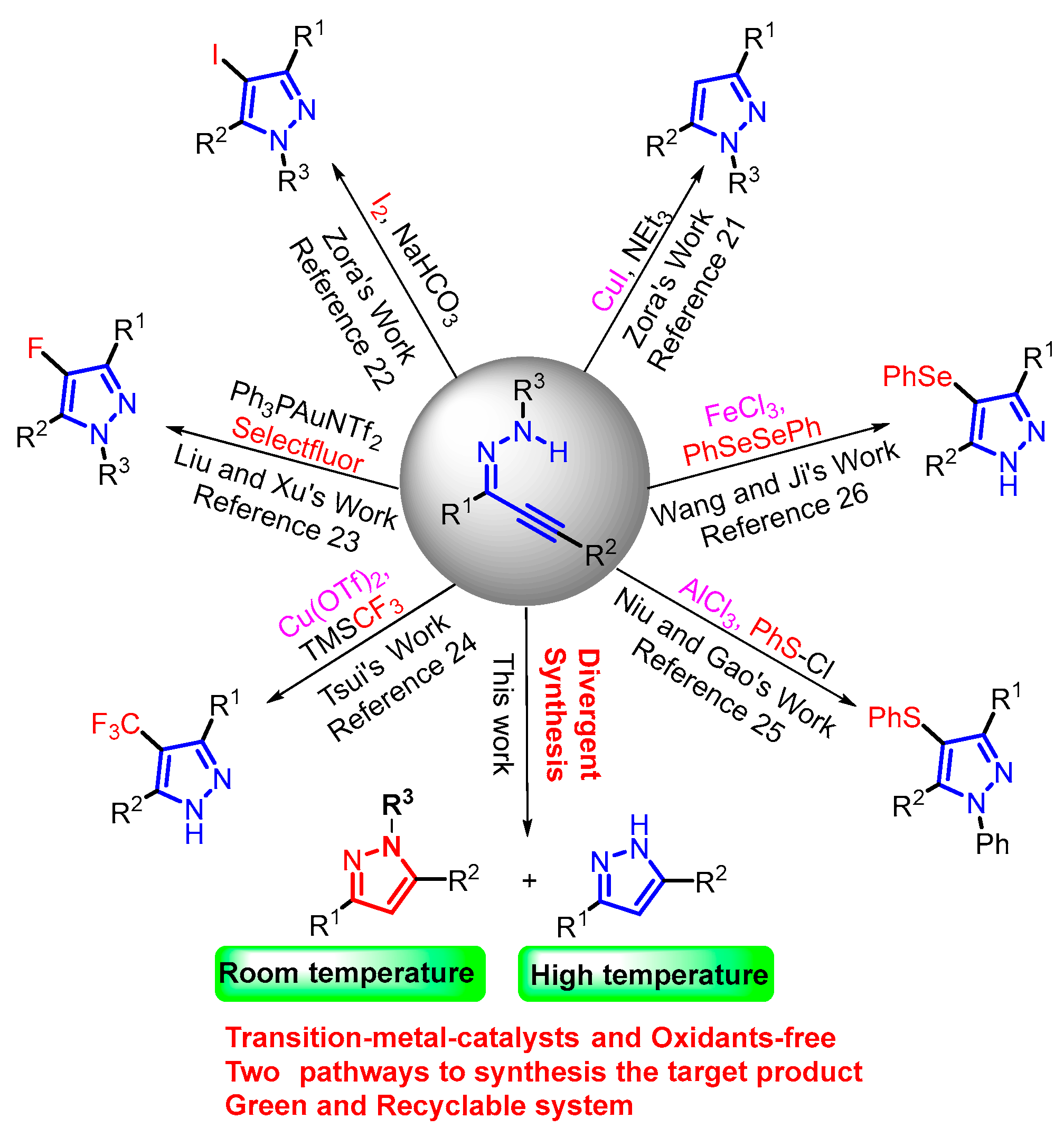
1. Introduction
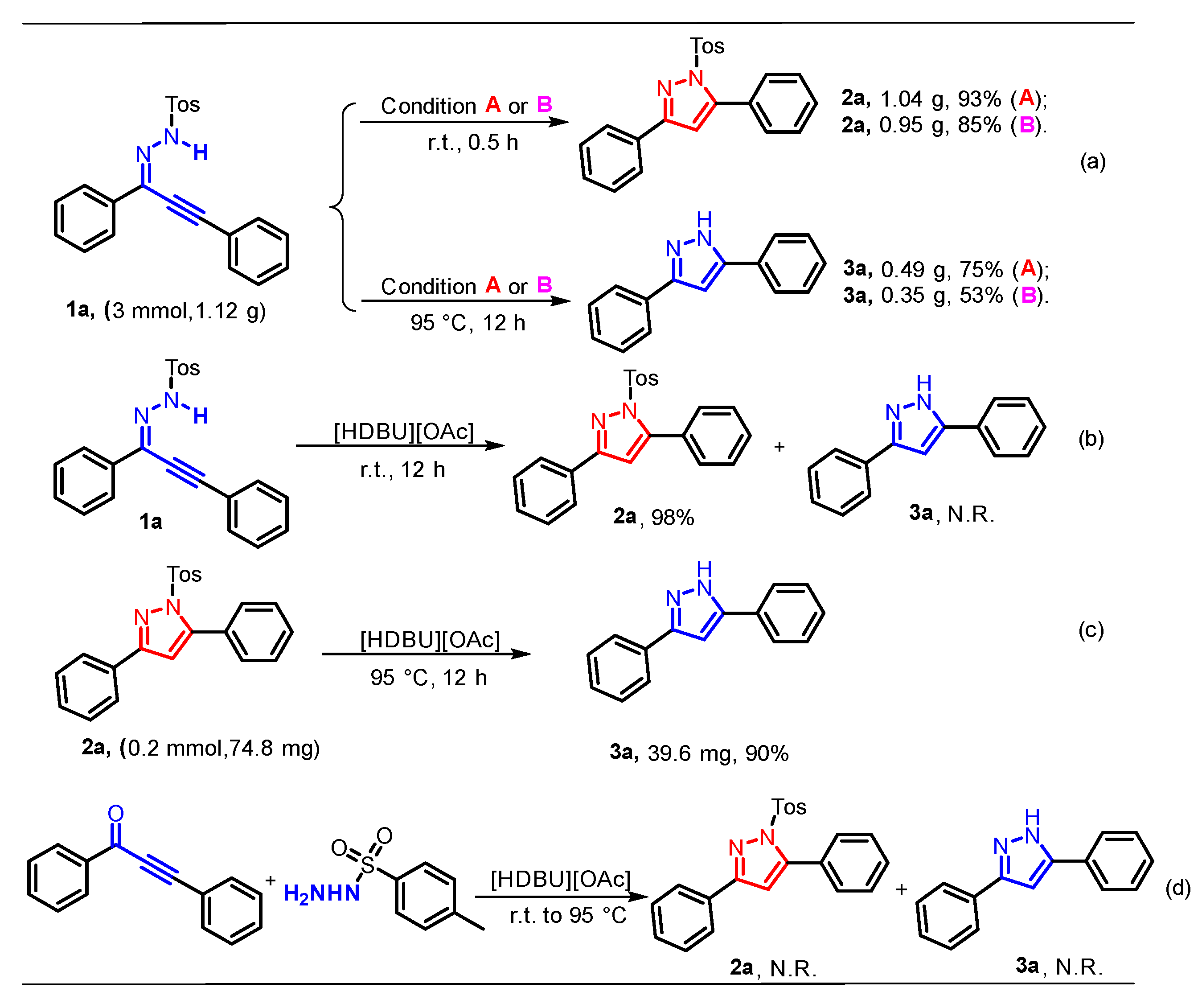
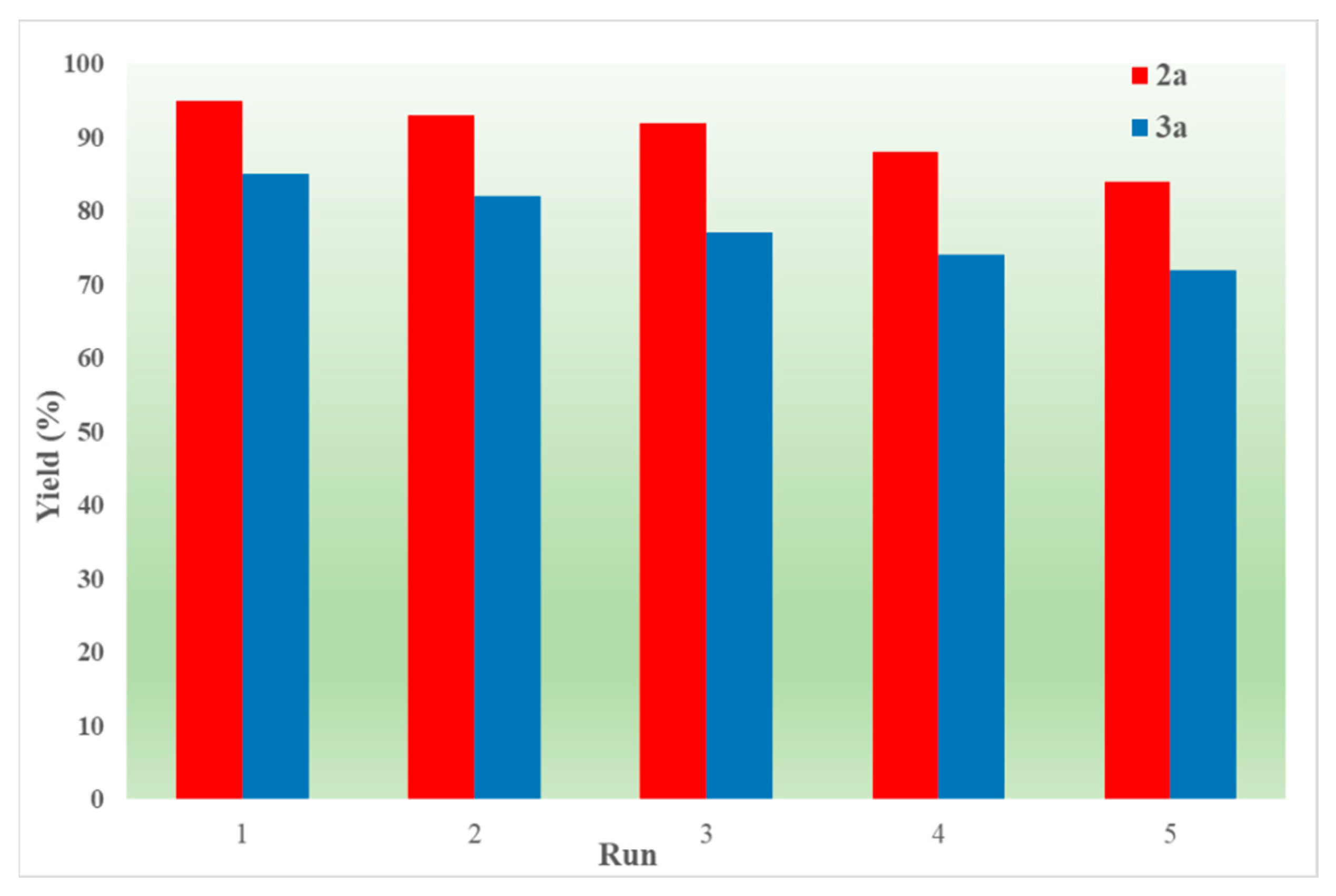
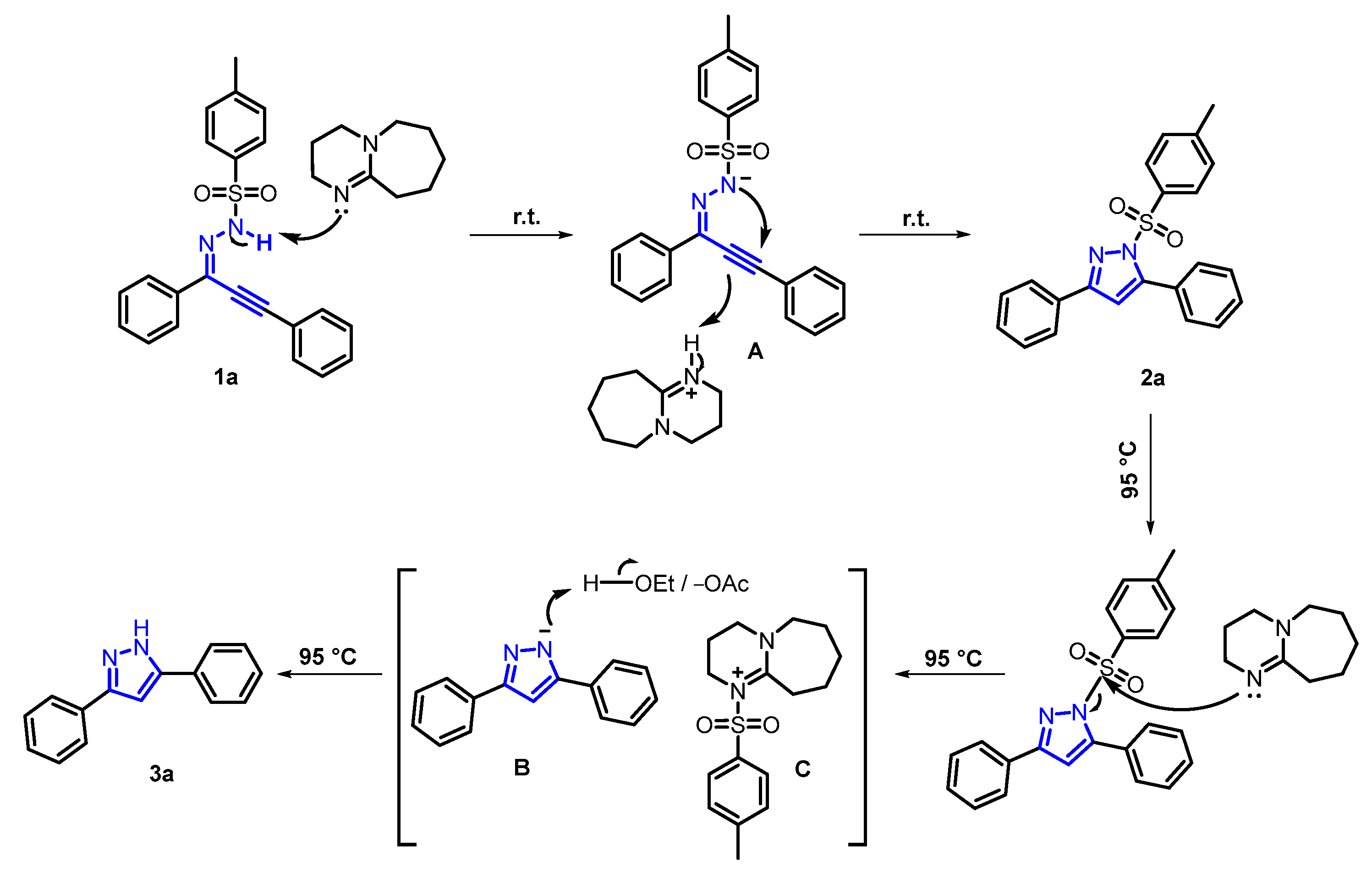
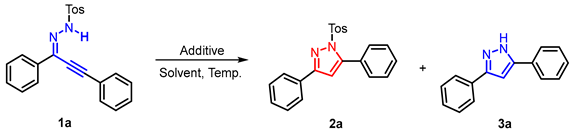
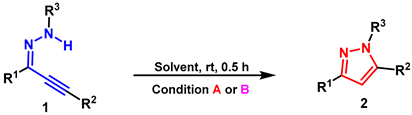
2. Results and Discussion
3. Experimental Section
Preparation of the Starting Materials
- General procedure for synthesis of 2a: Reaction conditions A: A mixture of the 1a (0.2 mmol), [HDBU][OAc] (2.0 mL), stirred at r.t., under air, 0.5 h. Condition B: 1a (0.2 mmol), DBU (1.0 equiv.), EtOH (2.0 mL), stirred at r.t., under air, 0.5 h. The product 2a was purified by silica gel column flash chromatography using PE/AcOEt as an eluent.
- Procedure for Gram-scale synthesis of 2a: Reaction conditions A: A mixture of the 1a (3 mmol), [HDBU][OAc] (30.0 mL), r.t., under air, 0.5 h. Conditions B: A mixture of the 1a (3 mmol), DBU (1.0 equiv.), EtOH (30.0 mL), r.t., under air, 0.5 h. The product 2a was purified by silica gel column flash chromatography using PE/AcOEt as an eluent.
- Procedure for Gram-scale synthesis of 3a: Reaction conditions A: A mixture of the 1a (3 mmol), [HDBU][OAc] (30.0 mL), 95 °C, under air, 12 h. Conditions B: A mixture of the 1a (3 mmol), DBU (1.0 equiv.), EtOH (30.0 mL), 95 °C, under air, 12 h. The product 3a was purified by silica gel column flash chromatography using PE/AcOEt as an eluent.
- 3,5-diphenyl-1-tosyl-1H-pyrazole (2a): White solid, (A: 95%, 71.1 mg; B: 93%, 69.6 mg); 1H NMR (400 MHz, CDCl3) δ 7.87 (dd, J = 8.0, 1.4 Hz, 2H), 7.65 (d, J = 8.4 Hz, 2H), 7.53–7.39 (m, 8H), 7.22 (d, J = 8.2 Hz, 2H), 6.63 (s, 1H), 2.38 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 155.19, 149.47, 145.33, 134.90, 131.38, 130.02, 129.66, 129.63, 129.48, 129.32, 128.72, 128.05, 127.83, 126.48, 109.52, 21.68. HRMS (ESI): Calculated for C22H19N2O2S: [M+H]+ 375.1162, Found 375.1165.
- 5-phenyl-3-(p-tolyl)-1-tosyl-1H-pyrazole (2b): White solid, (A: 98%, 76.0 mg; B: 95%, 73.7 mg); 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 8.1 Hz, 2H), 7.63 (d, J = 8.4 Hz, 2H), 7.50–7.43 (m, 5H), 7.23–7.19 (m, 4H), 6.59 (s, 1H), 2.38 (s, 3H), 2.37 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 155.34, 149.48, 145.22, 139.36, 134.93, 130.00, 129.70, 129.60, 129.42, 129.40, 128.56, 128.02, 127.80, 126.38, 109.53, 21.67, 21.40. HRMS (ESI): Calculated for C23H21N2O2S: [M+H]+ 389.1318, Found 389.1310.
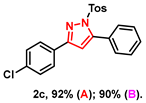
- 3-(4-chlorophenyl)-5-phenyl-1-tosyl-1H-pyrazole (2c): White solid, (A: 92%, 75.1 mg; B: 90%, 73.4 mg) 1H NMR (400 MHz, CDCl3) δ 7.82–7.75 (m, 2H), 7.63 (d, J = 8.4 Hz, 2H), 7.52–7.42 (m, 5H), 7.41–7.34 (m, 2H), 7.22 (d, J = 8.1 Hz, 2H), 6.58 (s, 1H), 2.38 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 153.94, 149.52, 145.47, 135.20, 134.79, 130.00, 129.92, 129.70, 129.56, 129.42, 128.93, 128.07, 127.85, 127.72, 109.25, 21.70. HRMS (ESI): Calculated for C22H18ClN2O2S: [M+H]+ 409.0772, Found 409.0775.
- 3-(4-bromophenyl)-5-phenyl-1-tosyl-1H-pyrazole (2d): White solid, (A: 93%, 84.1 mg; B: 89%, 80.4 mg); 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 8.5 Hz, 2H), 7.62 (d, J = 8.3 Hz, 2H), 7.53 (d, J = 8.5 Hz, 2H), 7.47 (d, J = 3.3 Hz, 1H), 7.46–7.41 (m, 4H), 7.21 (d, J = 8.3 Hz, 2H), 6.57 (s, 1H), 2.37 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 153.99, 149.53, 145.51, 134.72, 131.88, 130.34, 130.00, 129.72, 129.58, 129.37, 128.06, 127.98, 127.86, 123.48, 109.25, 21.72. HRMS (ESI): Calculated for C22H18BrN2O2S: [M+H]+ 453.0267, Found 453.0260.
- 5-phenyl-3-(m-tolyl)-1-tosyl-1H-pyrazole (2e): White solid, (A: 95%, 73.7 mg; B: 91%, 70.6 mg); 1H NMR (500 MHz, CDCl3) δ 7.71 (s, 1H), 7.61 (d, J = 8.2 Hz, 3H), 7.49–7.44 (m, 5H), 7.29 (t, J = 7.6 Hz, 1H), 7.19 (d, J = 8.2 Hz, 3H), 6.60 (s, 1H), 2.39 (s, 3H), 2.35 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 155.41, 149.44, 145.28, 138.44, 134.88, 131.23, 130.13, 130.03, 129.64, 129.46, 128.60, 127.99, 127.81, 127.08, 123.64, 109.72, 21.69, 21.44. HRMS (ESI): Calculated for C23H21N2O2S: [M+H]+ 389.1318, Found 389.1317.
- 3-(3-methoxyphenyl)-5-phenyl-1-tosyl-1H-pyrazole (2f): White solid, (A: 97%, 78.3 mg; B: 93%, 75.1 mg); 1H NMR (400 MHz, CDCl3) δ 7.63 (d, J = 8.4 Hz, 2H), 7.51–7.43 (m, 5H), 7.41 (t, J = 4.9 Hz, 2H), 7.32 (t, J = 7.9 Hz, 1H), 7.21 (d, J = 8.2 Hz, 2H), 6.96–6.89 (m, 1H), 6.60 (s, 1H), 3.86 (s, 3H), 2.37 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 159.89, 155.08, 149.44, 145.35, 134.83, 132.71, 130.01, 129.75, 129.66, 129.58, 129.48, 128.05, 127.82, 119.03, 115.17, 111.65, 109.70, 55.44, 21.70. HRMS (ESI): Calculated for C23H21N2O3S: [M+H]+ 405.1267, Found 405.1265.
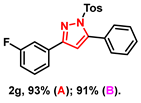
- 3-(3-fluorophenyl)-5-phenyl-1-tosyl-1H-pyrazole (2g): White solid, (A: 93%, 72.9 mg; B: 91%, 71.3 mg); 1H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 8.4 Hz, 2H), 7.62–7.55 (m, 2H), 7.52–7.43 (m, 5H), 7.40–7.35 (m, 1H), 7.23 (d, J = 8.2 Hz, 2H), 7.09–7.04 (m, 1H), 6.59 (s, 1H), 2.38 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 164.26 (d, J = 245.0 Hz), 153.85, 149.45, 145.50, 134.75, 133.63 (d, J = 8.0 Hz), 130.31 (d, J = 8.0 Hz), 129.99, 129.70, 129.56, 129.38, 128.10, 127.84, 122.11 (d, J = 2.0 Hz), 116.21 (d, J = 21.0 Hz), 113.43 (d, J = 21.0 Hz), 109.31, 21.69. HRMS (ESI): Calculated for C22H18FN2O2S: [M+H]+ 393.1068, Found 393.1066.
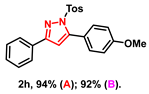
- 5-(4-methoxyphenyl)-3-phenyl-1-tosyl-1H-pyrazole (2h): White solid, (A: 94%, 76.0 mg; B: 92%, 74.3 mg); 1H NMR (400 MHz, CDCl3) δ 7.89–7.82 (m, 2H), 7.62 (d, J = 8.4 Hz, 2H), 7.45–7.35 (m, 5H), 7.20 (d, J = 8.2 Hz, 2H), 7.02–6.92 (m, 2H), 6.57 (s, 1H), 3.88 (s, 3H), 2.36 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 160.56, 155.27, 149.56, 145.26, 134.88, 131.43, 131.38, 129.63, 129.29, 128.70, 127.99, 126.47, 121.72, 113.29, 109.29, 55.38, 21.69. HRMS (ESI): Calculated for C23H21N2O3S: [M+H]+ 405.1267, Found 405.1260.
- 5-(3-fluorophenyl)-3-phenyl-1-tosyl-1H-pyrazole (2i): White solid, (A: 91%, 71.3 mg; B: 88%, 69.0 mg); 1H NMR (500 MHz, CDCl3) δ 7.77–7.75 (m, 2H), 7.58 (d, J = 8.4 Hz, 2H), 7.37–7.29 (m, 4H), 7.19–7.14 (m, 3H), 7.13–7.06 (m, 2H), 6.55 (s, 1H), 2.30 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 161.89 (d, J = 245.0 Hz), 154.15, 146.80 (d, J = 2.5 Hz), 144.50, 133.63, 130.45 (d, J = 7.5 Hz), 130.09, 128.69, 128.41, 128.36 (d, J = 2.5 Hz), 127.69, 126.99, 125.39, 124.88, 124.86, 116.09 (d, J = 22.5 Hz), 115.47 (d, J = 20.0 Hz), 108.69, 20.64. HRMS (ESI): Calculated for C22H18FN2O2S: [M+H]+ 393.1068, Found 393.1064.
- 5-(3,4-dichlorophenyl)-3-phenyl-1-tosyl-1H-pyrazole (2j): White solid, (A: 92%, 81.3 mg; B: 87%, 76.9 mg); 1H NMR (500 MHz, CDCl3) δ 7.76–7.74 (m, 2H), 7.59 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 8.3 Hz, 1H), 7.41 (d, J = 2.0 Hz, 1H), 7.37–7.31 (m, 3H), 7.27 (dd, J = 8.3, 2.0 Hz, 1H), 7.20–7.16 (m, 2H), 6.55 (s, 1H), 2.32 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 154.22, 145.55, 144.70, 133.52, 132.86, 131.10, 130.40, 129.93, 128.88, 128.79, 128.46, 128.39, 127.72, 127.01, 125.39, 108.81, 20.68. HRMS (ESI): Calculated for C22H17Cl2N2O2S: [M+H]+ 443.0382, Found 443.0381.
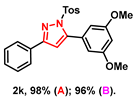
- 5-(3,5-dimethoxyphenyl)-3-phenyl-1-tosyl-1H-pyrazole (2k): White solid, (A: 98%, 85.0 mg; B: 96%, 83.3 mg); 1H NMR (400 MHz, DMSO-d6) δ 7.82 (d, J = 7.0 Hz, 2H), 7.59 (d, J = 8.1 Hz, 2H), 7.46–7.32 (m, 5H), 7.11 (s, 1H), 6.61–6.57 (m, 3H), 3.75 (s, 6H), 2.29 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 160.50, 159.78, 154.74, 149.12, 145.92, 134.01, 130.86, 130.72, 130.17, 129.64, 128.99, 127.54, 126.16, 110.14, 108.06, 101.35, 55.45, 21.16. HRMS (ESI): Calculated for C24H23N2O4S: [M+H]+ 435.1373, Found 435.1377.
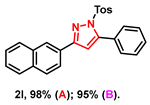
- 3-(naphthalen-2-yl)-5-phenyl-1-tosyl-1H-pyrazole (2l): White solid, (A: 98%, 83.1 mg; B: 95%, 80.5 mg); 1H NMR (500 MHz, CDCl3) δ 8.30 (s, 1H), 8.04 (dd, J = 8.6, 1.5 Hz, 1H), 7.89 (d, J = 8.6 Hz, 2H), 7.85 (dd, J = 6.6, 2.7 Hz, 1H), 7.67 (d, J = 8.4 Hz, 2H), 7.54–7.46 (m, 7H), 7.22 (d, J = 8.3 Hz, 2H), 6.76 (s, 1H), 2.36 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 154.14, 148.51, 144.29, 133.77, 132.68, 132.18, 128.97, 128.60, 128.52, 128.45, 127.67, 127.39, 127.34, 126.96, 126.77, 126.73, 125.58, 125.43, 124.75, 122.98, 108.71, 20.61. HRMS (ESI): Calculated for C26H21N2O2S: [M+H]+ 425.1318, Found 425.1311.
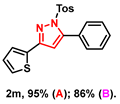
- 5-phenyl-3-(thiophen-2-yl)-1-tosyl-1H-pyrazole (2m): White solid, (A: 95%, 71.0 mg; B: 86%, 64.3 mg); 1H NMR (400 MHz, DMSO-d6) δ 7.61–7.58 (m, 2H), 7.52–7.46 (m, 7H), 7.36 (d, J = 7.3 Hz, 2H), 7.11 (t, J = 4.9 Hz, 1H), 7.04 (s, 1H), 2.31 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 150.76, 149.67, 145.92, 133.83, 133.35, 130.17, 129.77, 128.95, 128.04, 127.95, 127.39, 110.16, 21.14. HRMS (ESI): Calculated for C22H19N2O2S: [M+H]+ 375.1089, Found 375.1082.
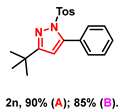
- 3-(tert-butyl)-5-phenyl-1-tosyl-1H-pyrazole (2n): White solid, (A: 90%, 63.7 mg; B: 85%, 60.2 mg); 1H NMR (500 MHz, CDCl3) δ 7.55 (d, J = 8.3 Hz, 2H), 7.44–7.40 (m, 5H), 7.19 (d, J = 8.2 Hz, 2H), 6.19 (s, 1H), 2.38 (s, 3H), 1.26 (s, 9H). 13C NMR (125 MHz, CDCl3) δ 166.01, 148.46, 143.78, 133.74, 128.92, 128.77, 128.23, 128.15, 126.82, 126.65, 108.86, 31.60, 28.73, 20.60. HRMS (ESI): Calculated for C20H23N2O2S: [M+H]+ 355.1475, Found 355.1479.
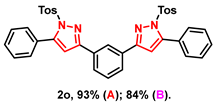
- 1,3-bis(5-phenyl-1-tosyl-1H-pyrazol-3-yl)benzene (2o): White solid, (A: 93%, 124.6 mg; B: 84%, 112.5 mg); 1H NMR (400 MHz, CDCl3) δ 8.24 (s, 1H), 7.89 (d, J = 7.6 Hz, 2H), 7.62 (d, J = 8.1 Hz, 4H), 7.49–7.46 (m, 11H), 7.21 (d, J = 8.1 Hz, 4H), 6.69 (s, 2H), 2.37 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 154.77, 149.55, 145.44, 134.77, 131.92, 130.04, 129.70, 129.54, 129.48, 129.15, 128.03, 127.85, 127.34, 124.49, 109.78, 21.71. HRMS (ESI): Calculated for C38H31N4O4S2: [M+H]+ 671.1781, Found 671.1788.
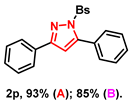
- 3,5-diphenyl-1-(phenylsulfonyl)-1H-pyrazole (2p): White solid, (A: 93%, 66.9 mg; B: 85%, 61.2 mg); 1H NMR (400 MHz, CDCl3) δ 7.86 (dd, J = 7.9, 1.4 Hz, 2H), 7.77–7.73 (m, 2H), 7.58–7.55 (m, 1H), 7.51–7.48 (m, 1H), 7.57–7.45 (m, 5H), 7.43–7.39 (m, 4H), 6.63 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 155.35, 149.56, 137.80, 134.12, 131.25, 130.00, 129.53, 129.47, 129.38, 129.01, 128.72, 127.97, 127.85, 126.47, 109.63. HRMS (ESI): Calculated for C21H17N2O2S: [M+H]+ 361.1005, Found 361.1008.
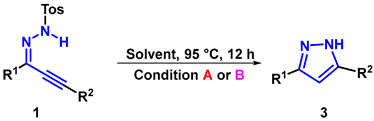
- General procedure for synthesis of 3a: Reaction conditions A: A mixture of the 1a (0.2 mmol), [HDBU][OAc] (2.0 mL), stirred at 95 °C, under air, 12 h. Reaction conditions A for 3a: A mixture of the 1a (0.2 mmol), DBU (1.0 equiv.), EtOH (2.0 mL), stirred at 95 °C, under air, 12 h. The product 3a was purified by silica gel column flash chromatography using PE/AcOEt as an eluent.
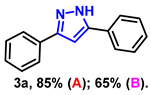
- 3,5-diphenyl-1H-pyrazole (3a): White solid, (A: 85%, 37.4 mg; B: 65%, 28.6 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.33 (s, 1H), 7.81 (d, J = 7.4 Hz, 2H), 7.74 (d, J = 7.5 Hz, 2H), 7.41–7.33 (m, 4H), 7.29–7.21 (m, 2H), 7.12 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 151.34, 143.39, 133.69, 129.36, 129.06, 128.67, 128.17, 127.49, 125.14, 99.66. HRMS (ESI): Calculated for C15H13N2: [M+H]+ 221.1073, Found 221.1079.
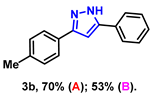
- 5-phenyl-3-(p-tolyl)-1H-pyrazole (3b): White solid, (A: 70%, 32.7 mg; B: 53%, 24.8 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.24 (s, 1H), 7.79 (d, J = 7.5 Hz, 2H), 7.68 (d, J = 7.9 Hz, 2H), 7.40 (t, J = 7.6 Hz, 2H), 7.28 (t, J = 7.3 Hz, 1H), 7.21 (d, J = 7.9 Hz, 2H), 7.08 (s, 1H), 2.28 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 137.12, 129.40, 128.91, 128.82, 127.71, 125.10, 125.05, 99.29, 20.86. HRMS (ESI): Calculated for C16H15N2: [M+H]+ 235.1230, Found 235.1233.
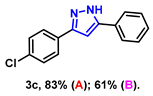
- 3-(4-chlorophenyl)-5-phenyl-1H-pyrazole (3c): White solid, (A: 83%, 42.1 mg; B: 61%, 30.9 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.44 (s, 1H), 7.89–7.78 (m, 4H), 7.65–7.62 (m, 2H), 7.49–7.47 (m, 2H), 7.35 (s, 1H), 7.22 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 131.64, 128.99, 127.09, 125.12, 99.86. HRMS (ESI): Calculated for C15H12ClN2: [M+H]+ 255.0684, Found 255.0689.
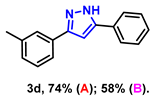
- 5-phenyl-3-(m-tolyl)-1H-pyrazole (3d): White solid, (A: 74%, 34.6 mg; B: 58%, 27.1 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.25 (s, 1H), 7.71 (d, J = 7.1 Hz, 2H), 7.55 (s, 1H), 7.50 (d, J = 7.1 Hz, 1H), 7.29 (t, J = 7.5 Hz, 2H), 7.17 (t, J = 7.3 Hz, 2H), 7.03 (s, 1H), 6.98 (d, J = 7.3 Hz, 1H), 2.20 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 138.02, 128.91, 127.84, 125.79, 125.18, 122.38, 99.67, 21.21. HRMS (ESI): Calculated for C16H15N2: [M+H]+ 235.1230, Found 235.1234.
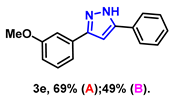
- 3-(3-methoxyphenyl)-5-phenyl-1H-pyrazole (3e): White solid, (A: 69%, 34.5 mg; B: 49%, 24.5 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.30 (s, 1H), 7.77 (d, J = 7.4 Hz, 2H), 7.39–7.35 (m, 4H), 7.30–7.23 (m, 2H), 7.14 (s, 1H), 6.83 (d, J = 7.6 Hz, 1H), 3.74 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 159.70, 129.96, 128.84, 127.79, 125.14, 117.56, 113.44, 110.53, 99.91, 55.17. HRMS (ESI): Calculated for C16H15N2O: [M+H]+ 251.1179, Found 251.1171.
- 3-(3-chlorophenyl)-5-phenyl-1H-pyrazole (3f): White solid, (A: 80%, 40.6 mg; B: 70%, 35.5 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.33 (s, 1H), 7.78–7.35 (m, 4H), 7.40 (d, J = 8.3 Hz, 2H), 7.35 (t, J = 7.6 Hz, 2H), 7.24 (t, J = 7.4 Hz, 1H), 7.10 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 132.23, 129.40, 128.90, 128.85, 127.97, 126.82, 125.16, 125.07, 99.90. HRMS (ESI): Calculated for C15H12ClN2: [M+H]+ 255.0684, Found 255.0679.
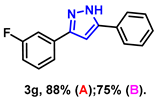
- 3-(3-fluorophenyl)-5-phenyl-1H-pyrazole (3g): White solid, (A: 88%, 41.8 mg; B: 75%, 35.7 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.38 (s, 1H), 7.74 (d, J = 7.2 Hz, 2H), 7.62–7.57 (m, 2H), 7.41–7.34 (m, 3H), 7.24 (t, J = 7.3 Hz, 1H), 7.17 (s, 1H), 7.06 (t, J = 7.7 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 163.87 (d, J = 240.0 Hz), 130.84, 128.93, 128.02, 125.17, 121.21 (d, J = 3.0 Hz), 114.49 (d, J = 20.0 Hz), 111.82 (d, J = 23.0 Hz), 100.24. HRMS (ESI): Calculated for C15H12FN2: [M+H]+ 239.0979, Found 239.0977.
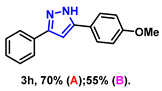
- 5-(4-methoxyphenyl)-3-phenyl-1H-pyrazole (3h): White solid, (A: 70%, 35.0 mg; B: 55%, 27.5 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.16 (s, 1H), 7.81–7.60 (m, 4H), 7.32 (t, J = 7.3 Hz, 2H), 7.20 (t, J = 7.0 Hz, 1H), 6.95 (s, 1H), 6.90 (d, J = 8.1 Hz, 2H), 3.66 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 159.06, 151.20, 143.36, 133.72, 128.81, 127.65, 126.52, 125.13, 114.28, 98.87, 55.16. HRMS (ESI): Calculated for C16H15N2O: [M+H]+ 251.1179, Found 251.1173.
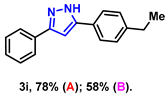
- 5-(4-ethylphenyl)-3-phenyl-1H-pyrazole (3i): White solid, (A: 78%, 38.6 mg; B: 58%, 28.7 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.22 (s, 1H), 7.71 (d, J = 7.1 Hz, 2H), 7.60 (d, J = 7.5 Hz, 2H), 7.28 (t, J = 7.5 Hz, 2H), 7.16 (t, J = 7.3 Hz, 1H), 7.10 (d, J = 7.8 Hz, 2H), 6.97 (s, 1H), 2.43 (q, J = 7.6 Hz, 2H), 1.01 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 143.53, 128.88, 128.30, 127.77, 125.21, 125.18, 99.35, 28.07, 15.63. HRMS (ESI): Calculated for C17H17N2: [M+H]+ 249.1386, Found 249.1388.
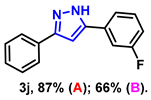
- 5-(3-fluorophenyl)-3-phenyl-1H-pyrazole (3j): White solid, (A: 87%, 41.4 mg; B: 66%, 31.4 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.41 (s, 1H), 7.85–7.55 (m, 4H), 7.39–7.30 (m, 3H), 7.23 (d, J = 24.2 Hz, 2H), 7.07 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 163.96, (d, J = 242.0 Hz), 130.92 (d, J = 3.0 Hz), 128.99, 128.06, 125.25, 121.26, 114.36 (d, J = 21.0 Hz), 111.91 (d, J = 23.0 Hz), 100.27. HRMS (ESI): Calculated for C15H12FN2: [M+H]+ 239.0979, Found 239.0982.
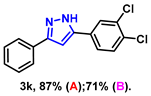
- 5-(3,4-dichlorophenyl)-3-phenyl-1H-pyrazole (3k): White solid, (A: 87%, 50.1 mg; B: 71%, 40.8 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.23 (s, 1H), 7.73 (d, J = 7.6 Hz, 2H), 7.39–7.35 (m, 4H), 7.26 (t, J = 7.3 Hz, 1H), 7.04–7.02 (m, 1H), 6.96 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 128.95, 128.12, 127.77, 125.20, 123.95, 99.57. HRMS (ESI): Calculated for C15H12Cl2N2: [M+H]+ 289.0298, Found 289.0295.
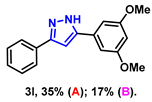
- 5-(3,5-dimethoxyphenyl)-3-phenyl-1H-pyrazole (3l): White solid, (A: 35%, 19.6 mg; B: 17%, 9.5 mg); 1H NMR (400 MHz, DMSO-d6) δ 7.90–7.87 (m, 2H), 7.48–7.44 (m, 2H), 7.36–7.32 (m, 1H), 7.26 (s, 1H), 7.09 (d, J = 2.3 Hz, 2H), 6.51 (t, J = 2.2 Hz, 1H), 3.83 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 160.89, 147.40, 133.27, 131.75, 128.82, 127.76, 125.15, 103.27, 100.08, 99.80, 55.32. HRMS (ESI): Calculated for C17H17N2O2: [M+H]+ 281.1285, Found 281.1280.
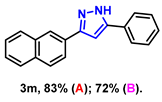
- 3-(naphthalen-2-yl)-5-phenyl-1H-pyrazole (3m): White solid, (A: 83%, 44.8 mg; B: 72%, 38.8 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.43 (s, 1H), 8.32 (s, 1H), 8.08–7.66 (m, 6H), 7.53–7.35 (m, 4H), 7.29 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 133.22, 132.56, 128.98, 128.42, 128.03, 127.77, 126.67, 126.12, 125.18, 123.72, 123.50, 100.11. HRMS (ESI): Calculated for C19H15N2: [M+H]+ 271.1230, Found 271.1239.
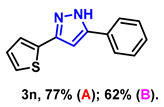
- 5-phenyl-3-(thiophen-2-yl)-1H-pyrazole (3n): White solid, (A: 77%, 34.8 mg; B: 62%, 28.0 mg); 1H NMR (400 MHz, DMSO-d6) δ 13.43 (s, 1H), 7.90 (d, J = 7.5 Hz, 2H), 7.56–7.53 (m, 4H), 7.43 (t, J = 7.3 Hz, 1H), 7.25–7.17 (m, 1H), 7.14 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 129.03, 128.20, 127.84, 125.23, 124.00, 99.61. HRMS (ESI): Calculated for C13H11N2S: [M+H]+ 227.0637, Found 227.0630.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lan, R.X.; Liu, Q.; Fan, P.S.; Lin, S.; Fernando, S.R.; McCallion, D.; Pertwee, R.; Makriyannis, A. Structure−activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J. Med. Chem. 1999, 42, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Daidone, G.; Maggio, B.; Plescia, S.; Raffa, D.; Musiu, C.; Milia, C.; Perra, G.; Marongiu, M.E. Antimicrobial and antineoplastic activities of new 4-diazopyrazole derivatives. Eur. J. Med. Chem. 1998, 33, 375–382. [Google Scholar] [CrossRef]
- Haque, T.S.; Tadesse, S.; Marcinkeviciene, J.; Rogers, M.J.; Sizemore, C.; Kopcho, L.M.; Amsler, K.; Ecret, L.D.; Zhan, D.L.; Hobbs, F.; et al. Parallel Synthesis of Potent, Pyrazole-Based Inhibitors of Helicobacter pylori Dihydroorotate Dehydrogenase. J. Med. Chem. 2002, 45, 4669–4678. [Google Scholar] [CrossRef] [PubMed]
- Castagnolo, D.; Manetti, F.; Radi, M.; Bechi, B.; Pagano, M.; Logu, A.; Meleddu, R.; Saddi, M.; Botta, M. Synthesis, biological evaluation, and SAR study of novel pyrazole analogues as inhibitors of Mycobacterium tuberculosis: Part 2. Synthesis of rigid pyrazolones. Bioorg. Med. Chem. 2009, 17, 5716–5721. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; Bonner, K.; Jones, E.A.; Emms, F.; Leeson, P.D.; Marwood, R.; Patel, S.; Patel, S.; Rowley, M.; Thomas, S.; et al. 4-N-linked-heterocyclic piperidine derivatives with high affinity and selectivity for human dopamine D4 receptors. Bioorg. Med. Chem. 1999, 9, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; Ashour, H.M.A.; Guemei, A.A. Novel Pyrazole Derivatives as Potential Promising Anti-inflammatory Antimicrobial Agents. Arch. Pharm. Chem. Life Sci. 2005, 338, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Almansa, C.; Gómez, L.A.; Cavalcanti, F.L.; Arriba, A.F.; García-Rafanell, J.; Forn, J. Synthesis and Structure−Activity Relationship of a New Series of Potent AT1 Selective Angiotensin II Receptor Antagonists: 5-(Biphenyl-4-yl-methyl)pyrazoles. J. Med. Chem. 1997, 40, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, R.; Ligresti, A.; La Regina, G.; Piscitelli, F.; Gatti, V.; Brizzi, A.; Pasquini, S.; Lavecchia, A.; Allarà, M.; Fantini, N.M.; et al. Synthesis, cannabinoid receptor affinity, molecular modeling studies and in vivo pharmacological evaluation of new substituted 1-aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. 2. Effect of the 3-carboxamide substituent on the affinity and selectivity profile. Bioorg. Med. Chem. 2009, 17, 5549–5564. [Google Scholar]
- Kees, K.L.; Fitzgerald, J.J.; Steiner, K.E.; Mattes, J.F.; Mihan, B.; Tosi, T.; Mondoro, D.; McCaleb, M.L. New potent antihyperglycemic agents in db/db mice: Synthesis and structure-activity relationship studies of (4-substituted benzyl) (trifluoromethyl)pyrazoles and -pyrazolones. J. Med. Chem. 1996, 39, 3920–3928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Wu, C.Y.; Zhang, N.N.; Fan, R.; Ye, Y.; Xu, J. Recent advances in the development of pyrazole derivatives as anticancer agents. Int. J. Mol. Sci. 2023, 24, 12724. [Google Scholar] [CrossRef] [PubMed]
- Grotjahn, D.B.; Van, S.; Combs, D.; Lev, D.A.; Schneider, C.; Rideout, M.; Meyer, C.; Hernandez, G.; Mejorado, L. New flexible synthesis of pyrazoles with different, functionalized substituents at C3 and C5. J. Org. Chem. 2002, 67, 9200–9209. [Google Scholar] [CrossRef] [PubMed]
- Dastrup, D.M.; Yap, A.H.; Weinreb, S.M.; Henryb, J.R.; Lechleiter, A.J. Synthesis of β-tosylethylhydrazine and its use in preparation of N-protected pyrazoles and 5-aminopyrazoles. Tetrahedron 2004, 60, 901–906. [Google Scholar] [CrossRef]
- Smith, C.D.; Tchabanenko, K.; Adlington, R.M.; Baldwin, J.E. Synthesis of linked heterocycles via use of bis-acetylenic compounds. Tetrahedron Lett. 2006, 47, 3209–3212. [Google Scholar] [CrossRef]
- Liu, H.L.; Jiang, H.F.; Zhang, M.; Yao, W.J.; Zhu, Q.H.; Tang, Z. One-pot three-component synthesis of pyrazoles through a tandem coupling-cyclocondensation sequence. Tetrahedron Lett. 2008, 49, 3805–3809. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.-Y.; Ning, J.; Jiang, X.; Deng, J.; Deng, Y.; Xu, R.; He, W.-M. Electrochemical multicomponent synthesis of 4-selanylpyrazoles under catalyst-and chemical-oxidant-free conditions. Green Chem. 2021, 23, 3950–3954. [Google Scholar] [CrossRef]
- Bamoniri, A.; Yaghmaeiyan, N.; Khaje, S. Synthesis and characterization of highly substituted pyrazoles using silicaphosphoric acid nanoparticles as a recoverable heterogeneous solid acid catalyst. Indian J. Chem. Technol. 2023, 30, 476–482. [Google Scholar]
- Unoh, Y.; Hirano, K.; Miura, M. Metal-Free Electrophilic phosphination/cyclization of alkynes. J. Am. Chem. Soc. 2017, 139, 6106–6109. [Google Scholar] [CrossRef] [PubMed]
- Slivka, M.; Onysko, M. The use of electrophilic cyclization for the preparation of condensed heterocycles. Synthesis 2021, 53, 3497–3512. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.L.; Tan, P.P.; Gu, X.W.; Sun, W.J.; Liu, C.; Chen, J.C.; Li, J.Z.; Sun, K. K2S2O8/I2-Promoted Electrophilic Selenylative Cyclization To Access Seleno-Benzo[b]azepines. Org. Lett. 2022, 24, 2288–2293. [Google Scholar] [CrossRef] [PubMed]
- Muzalevskiy, V.M.; Rulev, A.Y.; Romanov, A.R.; Kondrashov, E.V.; Ushakov, I.A.; Chertkov, A.V.; Nenajdenko, G. Selective, Metal-Free Approach to 3- or 5-CF3-Pyrazoles: Solvent Switchable Reaction of CF3-Ynones with Hydrazines. J. Org. Chem. 2017, 82, 7200–7214. [Google Scholar] [CrossRef] [PubMed]
- Zora, M.; Kivrak, A. Synthesis of pyrazoles via CuI-mediated electrophilic cyclizations of α, β-alkynic hydrazones. J. Org. Chem. 2011, 76, 9379–9390. [Google Scholar] [CrossRef]
- Zora, M.; Kivrak, A.; Yazici, C. Synthesis of pyrazoles via electrophilic cyclization. J. Org. Chem. 2011, 76, 6726–6742. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.Q.; Liu, Y.K.; Zhu, J.; Jiang, B.; Xu, Z.Y. A novel synthesis of fluorinated pyrazoles via gold (I)-catalyzed tandem aminofluorination of alkynes in the presence of selectfluor. Org. Lett. 2011, 13, 4220–4223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.D.; He, L.S.; Li, K.K.; Tsui, G.C. Copper-Mediated Domino Cyclization/Trifluoromethylation/Deprotection with TMSCF3: Synthesis of 4-(Trifluoromethyl)pyrazoles. Org. Lett. 2017, 19, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Shang, Y.-Z.; Cheng, Y.-F.; Tian, J.; Niu, Y.L.; Gao, W.-C. Synthesis of 4-chalcogenyl pyrazoles via electrophilic chalcogenation/cyclization of α,β-alkynic hydrazones. Org. Biomol. Chem. 2020, 18, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.-F.; Li, F.-H.; Li, J.; Wang, S.-Y.; Ji, S.-J. Iron(III) chloride-promoted cyclization of α,β-alkynic tosylhydrazones with diselenides: Synthesis of 4-(arylselanyl)-1H-pyrazoles. Org. Biomol. Chem. 2020, 18, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhu, Q.; Lin, Y.-W.; He, W.-M. The concept of dual roles design in clean organic preparation. Chin. Chem. Lett. 2019, 30, 2132–2138. [Google Scholar] [CrossRef]
- Lu, L.-H.; Wang, Z.; Xia, W.; Cheng, P.; Zhang, B.; Cao, Z.; He, W.-M. Sustainable routes for quantitative green selenocyanation of activated alkynes. Chin. Chem. Lett. 2019, 30, 1237–1240. [Google Scholar] [CrossRef]
- Zheng, H.; Cao, X.-T.; Du, K.; Xu, J.; Zhang, P.F. A highly efficient way to capture CX2 (O, S) mildly in reusable ReILs at atmospheric pressure. Green Chem. 2014, 16, 3142–3148. [Google Scholar] [CrossRef]
- Taheri, A.; Lai, B.; Cheng, C.; Gu, Y. Brønsted acid ionic liquid-catalyzed reductive Friedel–Crafts alkylation of indoles and cyclic ketones without using an external reductant. Green Chem. 2015, 17, 812–816. [Google Scholar] [CrossRef]
- Liu, F.S.; Ping, R.; Gu, Y.Q.; Zhao, P.H.; Liu, B.; Gao, J.; Liu, M.S. Efficient one pot capture and conversion of CO2 into quinazoline-2,4(1H, 3H)-diones using triazolium-based ionic liquids. ACS Sustain. Chem. Eng. 2020, 8, 2910–2918. [Google Scholar] [CrossRef]

 | |||
|---|---|---|---|
| Entry | Solvent | Yield of 2a [%] b | Yield of 3a [%] b |
| 1 | CH3CN | N.R. | N.R. |
| 2 | DMSO | N.R. | N.R. |
| 3 | THF | N.R. | N.R. |
| 4 | EtOH | N.R. (95) d | N.R. (65) e |
| 5 | [HDBU][OAc] | 98 | 85 c |
| 6 | [HDBU][NHS] | 95 | 78 c |
| 7 | [HTMG][NHS] | 78 | 63 c |
| 8 | [HDBU][OAc][NHS] | 86 | 68 c |
| 9 | [HTMG][HDBU][OAc][NHS] | 80 | 58 c |
 | |||
 |  |  |  |
 |  |  |  |
 |  |  |  |
 |  |  | |
 |  | ||
 | |||
 |  |  |  |
 |  |  |  |
 |  |  |  |
 |  |  | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Xu, W.; Xia, C.; Cao, X. Temperature-Controlled Divergent Synthesis of Pyrazoles and 1-Tosyl-1H-pyrazoles under Transition-Metal-Catalyst- and Oxidant-Free Conditions. Molecules 2024, 29, 1706. https://doi.org/10.3390/molecules29081706
Wang K, Xu W, Xia C, Cao X. Temperature-Controlled Divergent Synthesis of Pyrazoles and 1-Tosyl-1H-pyrazoles under Transition-Metal-Catalyst- and Oxidant-Free Conditions. Molecules. 2024; 29(8):1706. https://doi.org/10.3390/molecules29081706
Chicago/Turabian StyleWang, Kai, Wenjing Xu, Chengcai Xia, and Xianting Cao. 2024. "Temperature-Controlled Divergent Synthesis of Pyrazoles and 1-Tosyl-1H-pyrazoles under Transition-Metal-Catalyst- and Oxidant-Free Conditions" Molecules 29, no. 8: 1706. https://doi.org/10.3390/molecules29081706
APA StyleWang, K., Xu, W., Xia, C., & Cao, X. (2024). Temperature-Controlled Divergent Synthesis of Pyrazoles and 1-Tosyl-1H-pyrazoles under Transition-Metal-Catalyst- and Oxidant-Free Conditions. Molecules, 29(8), 1706. https://doi.org/10.3390/molecules29081706






