Luteolin-7-O-β-d-glucuronide Ameliorates Cerebral Ischemic Injury: Involvement of RIP3/MLKL Signaling Pathway
Abstract
1. Introduction
2. Results
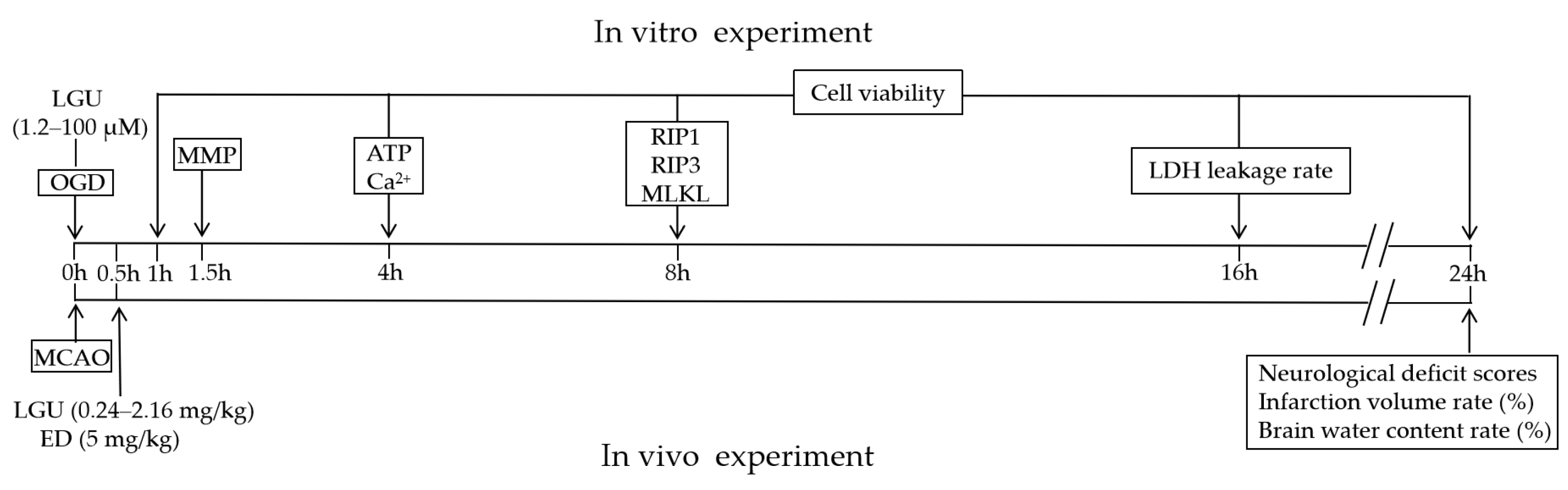
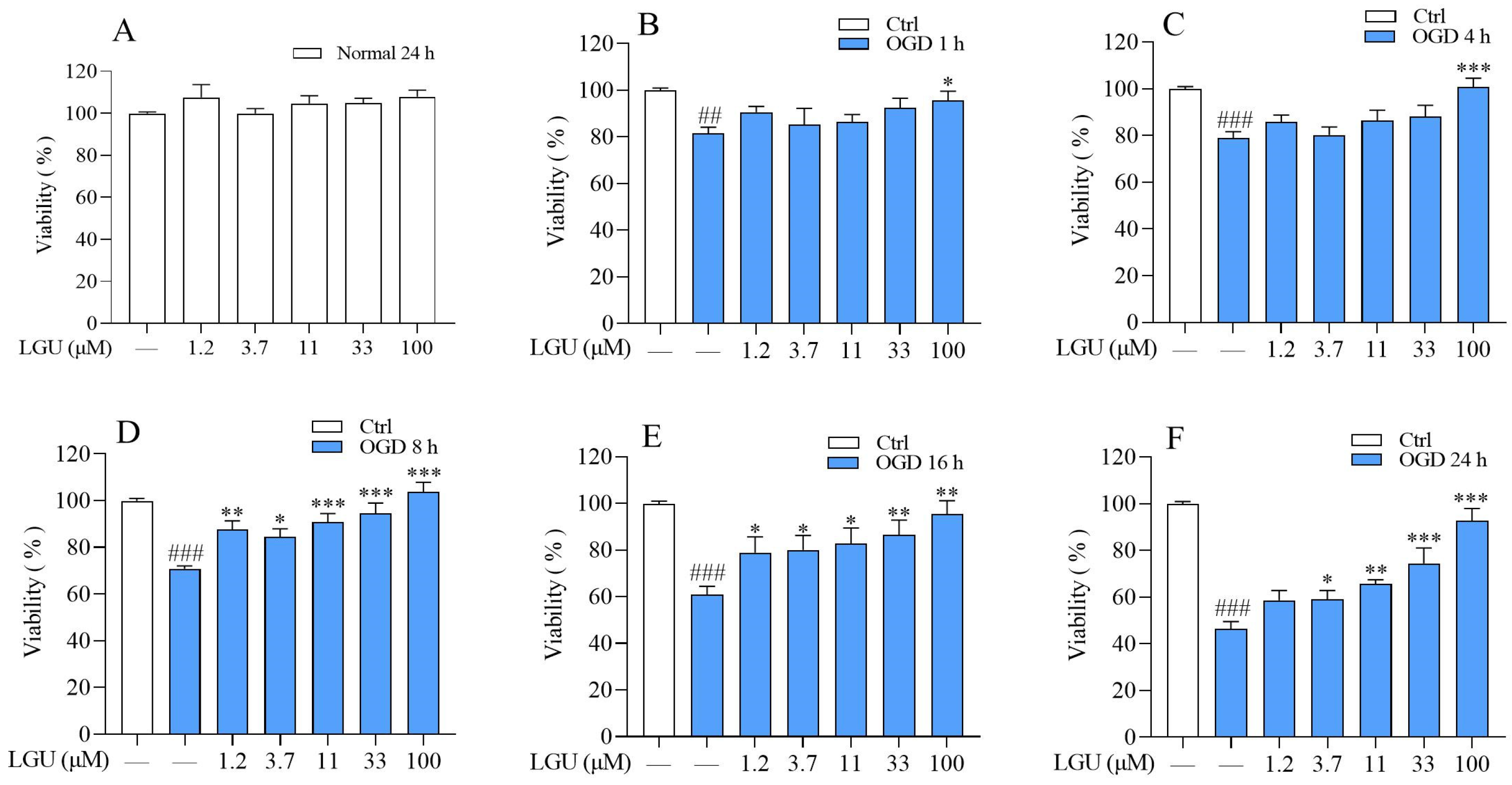
2.1. Protective Effect of LGU on the OGD-Induced Decrease in Cell Viability in Rat Primary Cortical Neurons
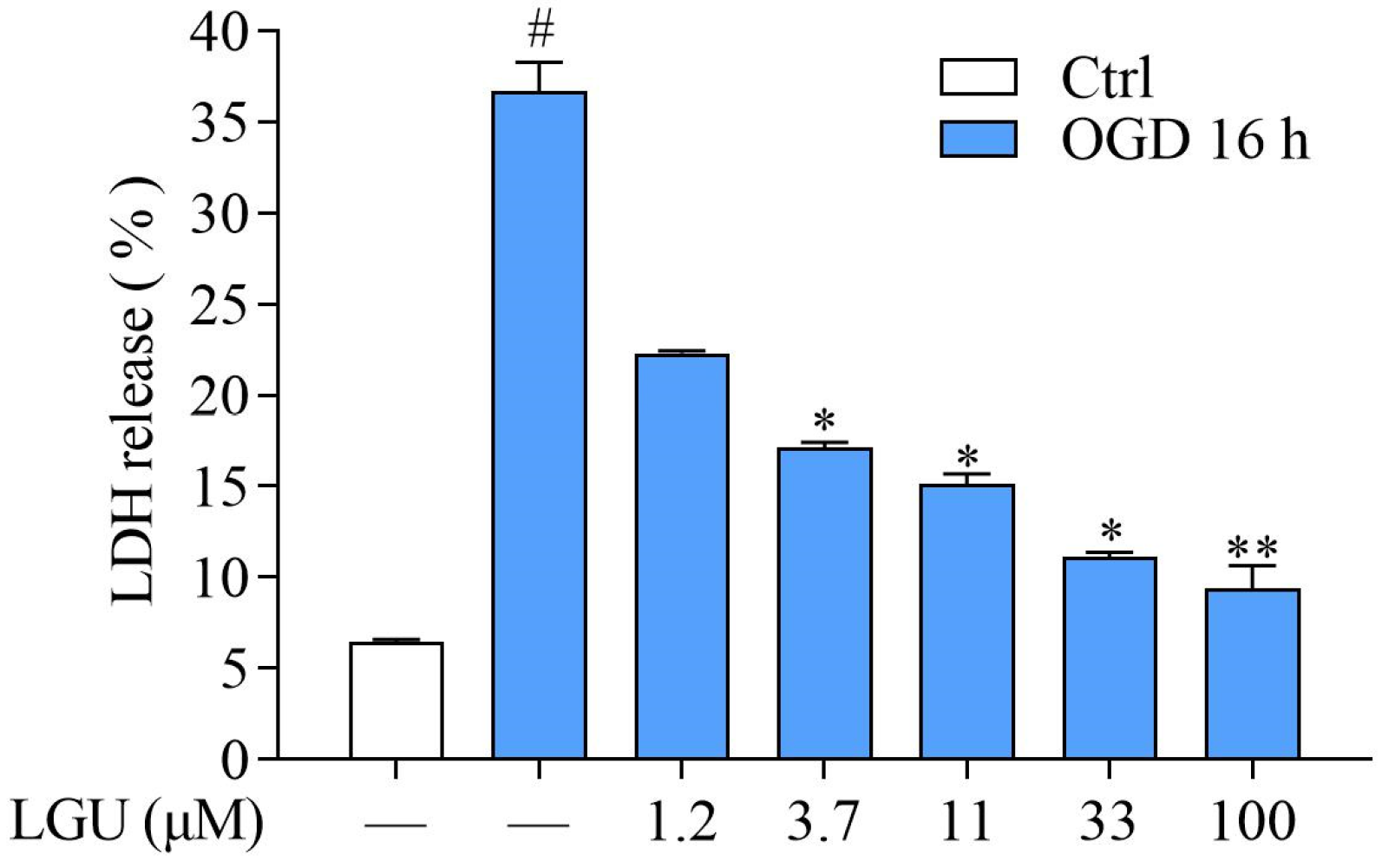
2.2. Protective Effect of LGU on OGD-Induced Neuronal Death in Rat Primary Cortical Neurons
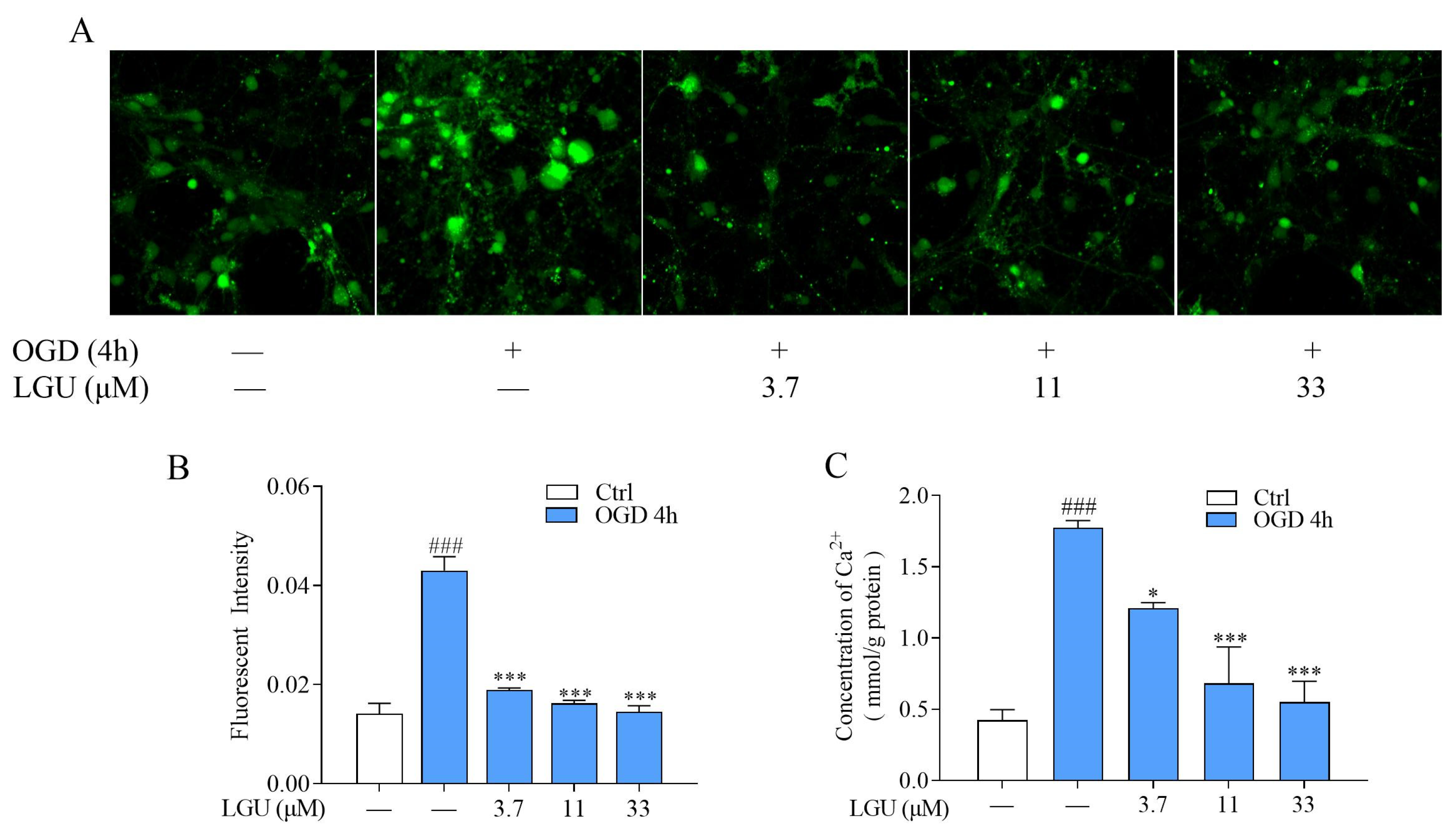
2.3. Inhibitory Effect of LGU on OGD-Induced Intracellular Ca2+ Overload in Rat Primary Cortical Neurons
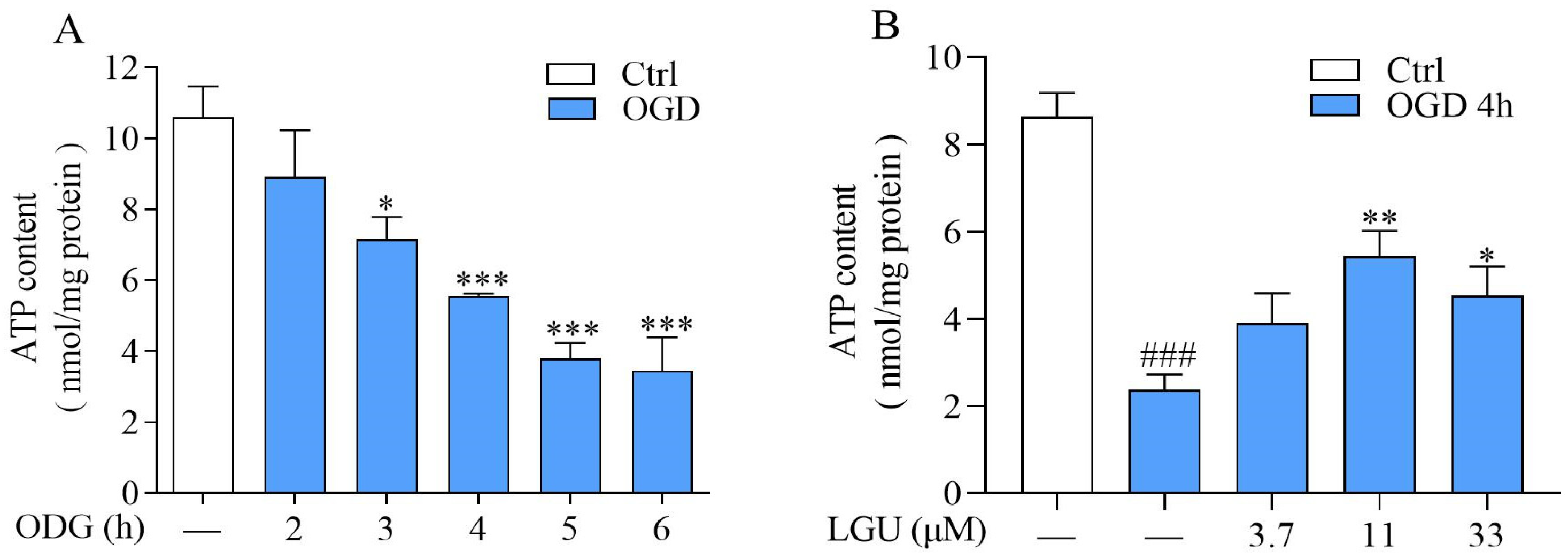
2.4. Improvement Effect of LGU on OGD-Induced ATP Depletion in Rat Primary Cortical Neurons
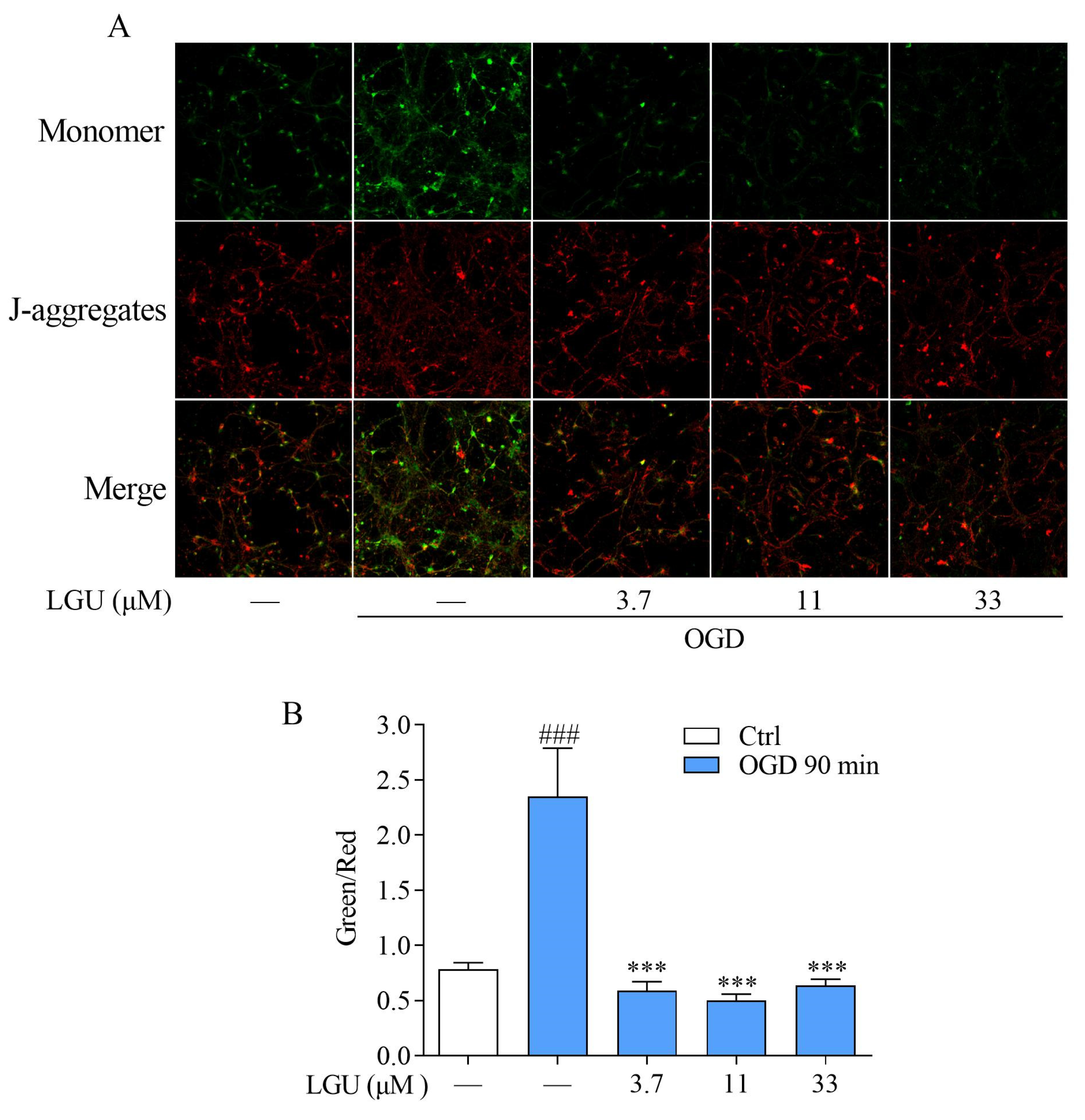
2.5. Improvement Effect of LGU on OGD-Induced Mitochondria Disfunction in Rat Primary Cortical Neurons
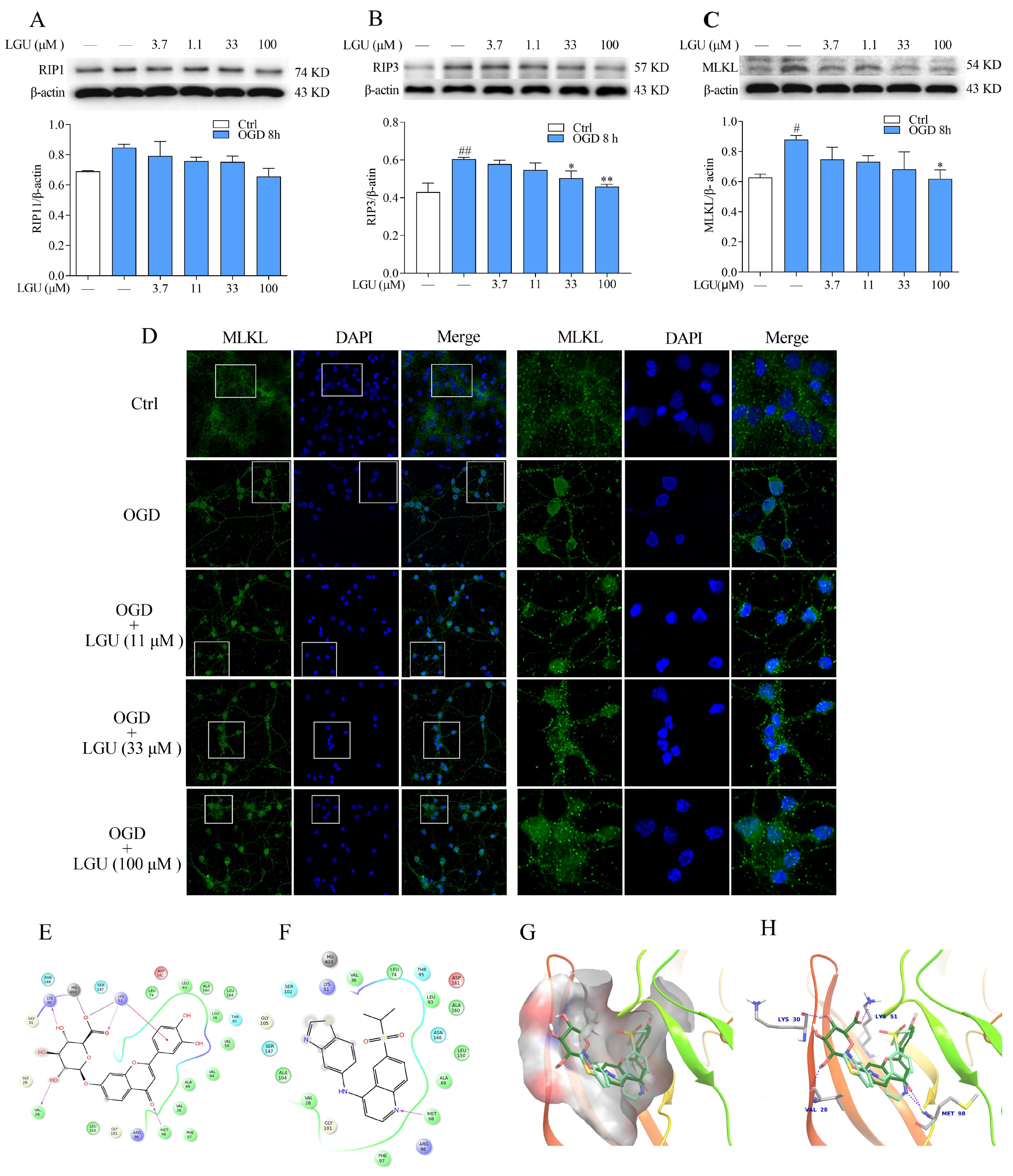
2.6. Improvement Effect of LGU on the OGD-Induced Activation of Necroptosis Signaling Pathway in Rat Primary Cortical Neurons
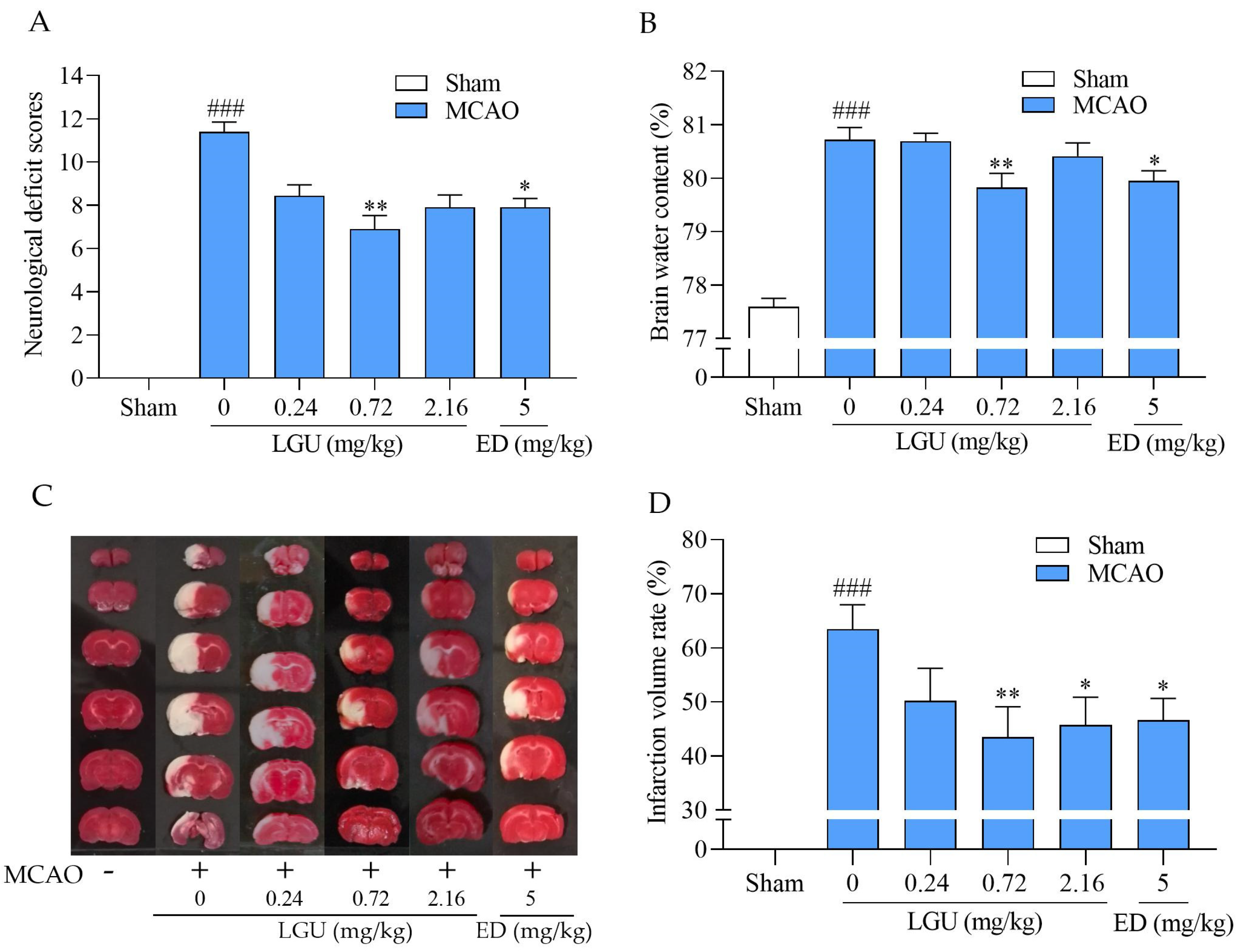
2.7. Protective Effect of LGU on Ischemic Brain Injury in MCAO Rats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Animal
4.3. Primary Culture of Cerebral Cortical Neurons
4.4. Oxygen–Glucose Deprivation (OGD) and Drug Treatment
4.5. Cell Viability Assay
4.6. Lactate Dehydrogenase (LDH) Assay
4.7. Detection of Intracellular Calcium ([Ca2+]i)
4.8. Detection of Intracellular ATP Level
4.9. Measurement of the Mitochondrial Membrane Potential (MMP)
4.10. Western Blot Detection
4.11. Immunofluorescence Staining
4.12. Molecular Docking
4.13. MCAO Model
4.14. Modified Neurological Severity Score (mNSS)
4.15. 2,3,5-Triphenyl-Tetrazolium (TTC) Staining
4.16. Brain Water Content
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Q.; Liu, J.; Mao, Z.; Tian, L.; Wang, N.; Wang, G.; Wang, Y.; Seto, S. Ligustilide attenuates ischemic stroke injury by promoting Drp1-mediated mitochondrial fission via activation of AMPK. Phytomedicine 2022, 95, 153884. [Google Scholar] [CrossRef]
- Hribljan, V.; Lisjak, D.; Petrović, D.J.; Mitrečić, D. Necroptosis is one of the modalities of cell death accompanying ischemic brain stroke: From pathogenesis to therapeutic possibilities. Croat. Med. J. 2019, 60, 121–126. [Google Scholar] [CrossRef]
- Kourti, M.; Liaropoulou, D.; Paschou, M.; Giagklisi, I.; Paschalidi, M.; Petani, E.; Papazafiri, P. Enhanced Ca(2+) Entry Sustains the Activation of Akt in Glucose Deprived SH-SY5Y Cells. Int. J. Mol. Sci. 2022, 23, 1386. [Google Scholar] [CrossRef]
- Mosconi, M.G.; Paciaroni, M. Treatments in Ischemic Stroke: Current and Future. Eur. Neurol. 2022, 85, 349–366. [Google Scholar] [CrossRef]
- Meschia, J.F.; Bushnell, C.; Boden-Albala, B.; Braun, L.T.; Bravata, D.M.; Chaturvedi, S.; Creager, M.A.; Eckel, R.H.; Elkind, M.S.; Fornage, M.; et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 3754–3832. [Google Scholar] [CrossRef]
- Dong, C.; Li, J.; Zhao, M.; Chen, L.; Zhai, X.; Song, L.; Zhao, J.; Sun, Q.; Wu, J.; Xie, X. Pharmacological Effect of Panax notoginseng Saponins on Cerebral Ischemia in Animal Models. Biomed. Res. Int. 2022, 2022, 4281483. [Google Scholar] [CrossRef]
- Yin, R.; Deng, X.X.; Han, F.; Song, Z.; Cheng, W.M.; Chen, X.H.; Bi, K.S. Determination of five components in Ixeris sonchifolia by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2007, 43, 1364–1369. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Zhang, J.; Sun, G. Microemulsion Electrokinetic Chromatography in Combination with Chemometric Methods to Evaluate the Holistic Quality Consistency and Predict the Antioxidant Activity of Ixeris sonchifolia (Bunge) Hance Injection. PLoS ONE 2016, 11, e0157601. [Google Scholar] [CrossRef]
- Lu, J.; Feng, X.; Sun, Q.; Lu, H.; Manabe, M.; Sugahara, K.; Ma, D.; Sagara, Y.; Kodama, H. Effect of six flavonoid compounds from Ixeris sonchifolia on stimulus-induced superoxide generation and tyrosyl phosphorylation in human neutrophils. Clin. Chim. Acta 2002, 316, 95–99. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Gan, F.F.; Shelar, S.B.; Ng, K.Y.; Chew, E.H. Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food Chem. Toxicol. 2013, 59, 272–280. [Google Scholar] [CrossRef]
- Yuan, Y.; Hu, Z.F.; Wang, F.L.; Wan, L.S.; Gong, Y.; Chen, J.C. Sesquiterpenoids from Ixeris sonchifolia and their neuroprotective activities. J. Asian Nat. Prod. Res. 2022, 24, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Liu, Z.; Lin, Z.; Shi, P.; Chen, B.; Li, S.; Li, G.; Huang, L.; Lin, X.; Yao, H. Potential mechanism of action of Ixeris sonchifolia extract injection against cardiovascular diseases revealed by combination of HPLC-Q-TOF-MS, virtual screening and systems pharmacology approach. RSC Adv. 2020, 10, 38497–38504. [Google Scholar] [CrossRef]
- Tang, M.; Tang, S.H.; Huang, J.Y.; Hattori, M.; Zhang, N.; Yang, B.; Wu, X.H.; Zhang, H.L.; Wang, Z.G. Three new sesquiterpenes from Ixeris sonchifolia. J. Asian Nat. Prod. Res. 2022, 25, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Li, K.W.; Liang, Y.Y.; Xie, S.M.; Niu, F.J.; Guo, L.Y.; Liu, Z.H.; Zhou, C.Z.; Wang, L.Z. Ixeris sonchifolia: A review of its traditional uses, chemical constituents, pharmacology and modern applications. Biomed. Pharmacother. 2020, 125, 109869. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Jee, H.J.; Kim, S.Y.; Hwang, S.H.; Pil, G.B.; Jung, Y.S. Luteolin-7-O-Glucuronide Improves Depression-like and Stress Coping Behaviors in Sleep Deprivation Stress Model by Activation of the BDNF Signaling. Nutrients 2022, 14, 3314. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Park, H.J.; Nugroho, A.; Kim, E.J.; Jung, H.A.; Choi, J.S. Quantification of major compounds from Ixeris dentata, Ixeris dentata Var. albiflora, and Ixeris sonchifolia and their comparative anti-inflammatory activity in lipopolysaccharide-stimulated RAW 264.7 cells. J. Med. Food 2015, 18, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.C.; Park, J.; Cho, S. Anti-Inflammatory and Anti-Oxidative Effects of luteolin-7-O-glucuronide in LPS-Stimulated Murine Macrophages through TAK1 Inhibition and Nrf2 Activation. Int. J. Mol. Sci. 2020, 21, 2007. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Hwang, S.H.; Suh, H.W.; Lim, S.S. Phytochemical Analysis of Agrimonia pilosa Ledeb, Its Antioxidant Activity and Aldose Reductase Inhibitory Potential. Int. J. Mol. Sci. 2017, 18, 379. [Google Scholar] [CrossRef] [PubMed]
- Orhan, F.; Gulluce, M.; Ozkan, H.; Alpsoy, L. Determination of the antigenotoxic potencies of some luteolin derivatives by using a eukaryotic cell system, Saccharomyces cerevisiae. Food Chem. 2013, 141, 366–372. [Google Scholar] [CrossRef]
- Crascì, L.; Basile, L.; Panico, A.; Puglia, C.; Bonina, F.P.; Basile, P.M.; Rizza, L.; Guccione, S. Correlating In Vitro Target-Oriented Screening and Docking: Inhibition of Matrix Metalloproteinases Activities by Flavonoids. Planta Med. 2017, 83, 901–911. [Google Scholar] [CrossRef]
- Datta, A.; Sarmah, D.; Mounica, L.; Kaur, H.; Kesharwani, R.; Verma, G.; Veeresh, P.; Kotian, V.; Kalia, K.; Borah, A.; et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl. Stroke Res. 2020, 11, 1185–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Chen, X.; Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 2022, 49, 15. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Liu, M.; Chen, M.; Luo, Y.; Wang, C.; Xu, T.; Jiang, Y.; Guo, Y.; Zhang, J.H. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol. Ther. 2020, 216, 107695. [Google Scholar] [CrossRef] [PubMed]
- Tasca, C.I.; Dal-Cim, T.; Cimarosti, H. In vitro oxygen-glucose deprivation to study ischemic cell death. Methods Mol. Biol. 2015, 1254, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Liu, N.; Yao, M.; Zhang, Y.; Yao, Z.; Feng, Y.; Liu, J.; Zhou, G. A Review of Neuroprotective Effects and Mechanisms of Ginsenosides From Panax Ginseng in Treating Ischemic Stroke. Front. Pharmacol. 2022, 13, 946752. [Google Scholar] [CrossRef]
- Piccirillo, S.; Magi, S.; Castaldo, P.; Preziuso, A.; Lariccia, V.; Amoroso, S. NCX and EAAT transporters in ischemia: At the crossroad between glutamate metabolism and cell survival. Cell Calcium 2020, 86, 102160. [Google Scholar] [CrossRef] [PubMed]
- Szydlowska, K.; Tymianski, M. Calcium, ischemia and excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar] [CrossRef]
- Wang, B.; Ma, L.; Liu, L.; Qin, J.; Li, T.; Bu, K.; Li, Z.; Lu, H.; Song, X.; Cao, Y.; et al. Receptor-Interacting Protein 3/Calmodulin-Dependent Kinase II/Proline-Rich Tyrosine Kinase 2 Pathway is Involved in Programmed Cell Death in a Mouse Model of Brain Ischaemic Stroke. Neuroscience 2022, 506, 14–28. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, F.; Duo, K.; Liu, Y.; Yu, J.; Wu, Q.; Cai, Z. The Role of Necroptosis in Cerebral Ischemic Stroke. Mol. Neurobiol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Li, X.; Zhang, H.; Wang, L.; Wu, X.; Zhang, H.; Luo, Y. Catalytically inactive RIP1 and RIP3 deficiency protect against acute ischemic stroke by inhibiting necroptosis and neuroinflammation. Cell Death Dis. 2020, 11, 565. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, M.; Wang, Y.; Wang, Q.; Wu, J. Cell Death Mechanisms in Cerebral Ischemia-Reperfusion Injury. Neurochem. Res. 2022, 47, 3525–3542. [Google Scholar] [CrossRef]
- Vieira, M.; Fernandes, J.; Carreto, L.; Anuncibay-Soto, B.; Santos, M.; Han, J.; Fernández-López, A.; Duarte, C.B.; Carvalho, A.L.; Santos, A.E. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous RIP3. Neurobiol. Dis. 2014, 68, 26–36. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Upton, J.W.; Long, A.B.; Livingston-Rosanoff, D.; Daley-Bauer, L.P.; Hakem, R.; Caspary, T.; Mocarski, E.S. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 2011, 471, 368–372. [Google Scholar] [CrossRef]
- Wu, Z.; Deshpande, T.; Henning, L.; Bedner, P.; Seifert, G.; Steinhäuser, C. Cell death of hippocampal CA1 astrocytes during early epileptogenesis. Epilepsia 2021, 62, 1569–1583. [Google Scholar] [CrossRef]
- Fujita, Y.; Yano, T.; Kanamori, H.; Nagahara, D.; Muranaka, A.; Kouzu, H.; Mochizuki, A.; Koyama, M.; Nagano, N.; Fujito, T.; et al. Enhanced nuclear localization of phosphorylated MLKL predicts adverse events in patients with dilated cardiomyopathy. ESC Heart Fail. 2022, 9, 3435–3451. [Google Scholar] [CrossRef]
- Yoon, S.; Bogdanov, K.; Kovalenko, A.; Wallach, D. Necroptosis is preceded by nuclear translocation of the signaling proteins that induce it. Cell Death Differ. 2016, 23, 253–260. [Google Scholar] [CrossRef]
- Maud, P.; Thavarak, O.; Cedrick, L.; Michele, B.; Vincent, B.; Olivier, P.; Regis, B. Evidence for the use of isoflurane as a replacement for chloral hydrate anesthesia in experimental stroke: An ethical issue. Biomed. Res. Int. 2014, 2014, 802539. [Google Scholar] [CrossRef]
- Keshavarz, S.; Nemati, M.; Saied Salehi, M.; Naseh, M. The impact of anesthetic drugs on hemodynamic parameters and neurological outcomes following temporal middle cerebral artery occlusion in rats. Neuroreport 2023, 34, 199–204. [Google Scholar] [CrossRef]
- Ward-Flanagan, R.; Dickson, C.T. Intravenous chloral hydrate anesthesia provides appropriate analgesia for surgical interventions in male Sprague-Dawley rats. PLoS ONE 2023, 18, e0286504. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; de Oliveira, M.A.; Martins, J.O.; Sannomiya, P.; de Cassia Tostes, R.; Nigro, D.; Carvalho, M.H.; Fortes, Z.B. Differential effects of chloral hydrate- and ketamine/xylazine-induced anesthesia by the s.c. route. Life Sci. 2006, 79, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Field, K.J.; White, W.J.; Lang, C.M. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab. Anim. 1993, 27, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.G.; Murphy, K.L.; Taylor, P.M.; Wolfensohn, S.E. Chloral hydrate is not acceptable for anesthesia or euthanasia of small animals. Anesthesiology 2009, 111, 209. [Google Scholar] [CrossRef]
- Silverman, J.; Muir, W.W., 3rd. A review of laboratory animal anesthesia with chloral hydrate and chloralose. Lab. Anim. Sci. 1993, 43, 210–216. [Google Scholar]
- Watanabe, K.; Tanaka, M.; Yuki, S.; Hirai, M.; Yamamoto, Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J. Clin. Biochem. Nutr. 2018, 62, 20–38. [Google Scholar] [CrossRef]
- Zhao, K.; Li, G.Z.; Nie, L.Y.; Ye, X.M.; Zhu, G.Y. Edaravone for Acute Ischemic Stroke: A Systematic Review and Meta-analysis. Clin. Ther. 2022, 44, e29–e38. [Google Scholar] [CrossRef]
- Wang, T.; Gu, J.; Wu, P.F.; Wang, F.; Xiong, Z.; Yang, Y.J.; Wu, W.N.; Dong, L.D.; Chen, J.G. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: Involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic. Biol. Med. 2009, 47, 229–240. [Google Scholar] [CrossRef]
- Tavakoli-Far, B.; Rahbar-Roshandel, N.; Rahimi-Moghaddam, P.; Mahmoudian, M. Neuroprotective effects of mebudipine and dibudipine on cerebral oxygen-glucose deprivation/reperfusion injury. Eur. J. Pharmacol. 2009, 610, 12–17. [Google Scholar] [CrossRef]
- Que, J.; Ye, M.; Zhang, Y.; Xu, W.; Li, H.; Xu, W.; Chu, K. Bryonolic Acid, a Triterpenoid, Protect Against N-methyl-d-Aspartate-Induced Neurotoxicity in PC12 Cells. Molecules 2016, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, X.; Chen, M.; Ge, Q.; Shen, Y.; Pan, S. Resveratrol protects PC12 cells against OGD/R-induced apoptosis via the mitochondrial-mediated signaling pathway. Acta Biochim. Biophys. Sin. 2016, 48, 342–353. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sanberg, P.R.; Li, Y.; Wang, L.; Lu, M.; Willing, A.E.; Sanchez-Ramos, J.; Chopp, M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001, 32, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, X.; Li, Q.; Li, Z.; Du, F. Anti-inflammation effects of picroside 2 in cerebral ischemic injury rats. Behav. Brain Funct. 2010, 6, 43. [Google Scholar] [CrossRef]
- Yu, L.; Duan, Y.; Zhao, Z.; He, W.; Xia, M.; Zhang, Q.; Cao, X. Hydroxysafflor Yellow A (HSYA) Improves Learning and Memory in Cerebral Ischemia Reperfusion-Injured Rats via Recovering Synaptic Plasticity in the Hippocampus. Front. Cell. Neurosci. 2018, 12, 371. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Lin, F.; Chen, Y.; Dou, Y.; Li, T.; Jin, X.; Song, J.; Wang, F. Luteolin-7-O-β-d-glucuronide Ameliorates Cerebral Ischemic Injury: Involvement of RIP3/MLKL Signaling Pathway. Molecules 2024, 29, 1665. https://doi.org/10.3390/molecules29071665
Fan X, Lin F, Chen Y, Dou Y, Li T, Jin X, Song J, Wang F. Luteolin-7-O-β-d-glucuronide Ameliorates Cerebral Ischemic Injury: Involvement of RIP3/MLKL Signaling Pathway. Molecules. 2024; 29(7):1665. https://doi.org/10.3390/molecules29071665
Chicago/Turabian StyleFan, Xing, Fang Lin, Yu Chen, Yuling Dou, Ting Li, Xinxin Jin, Jintao Song, and Fang Wang. 2024. "Luteolin-7-O-β-d-glucuronide Ameliorates Cerebral Ischemic Injury: Involvement of RIP3/MLKL Signaling Pathway" Molecules 29, no. 7: 1665. https://doi.org/10.3390/molecules29071665
APA StyleFan, X., Lin, F., Chen, Y., Dou, Y., Li, T., Jin, X., Song, J., & Wang, F. (2024). Luteolin-7-O-β-d-glucuronide Ameliorates Cerebral Ischemic Injury: Involvement of RIP3/MLKL Signaling Pathway. Molecules, 29(7), 1665. https://doi.org/10.3390/molecules29071665





