Characteristics of Dialdehyde Cellulose Nanofibrils Derived from Cotton Linter Fibers and Wood Fibers
Abstract
1. Introduction
2. Results and Discussion
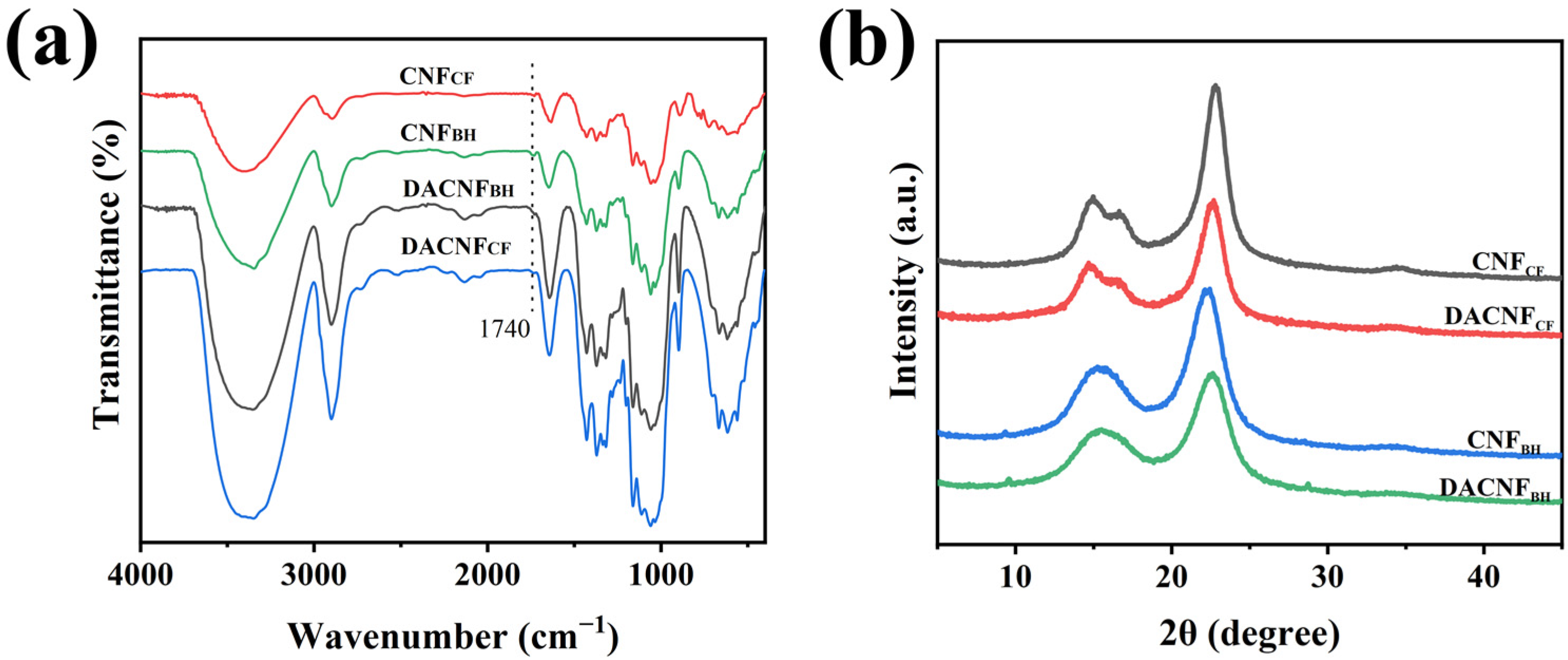
2.1. Chemical and Physical Properties of DACNFs
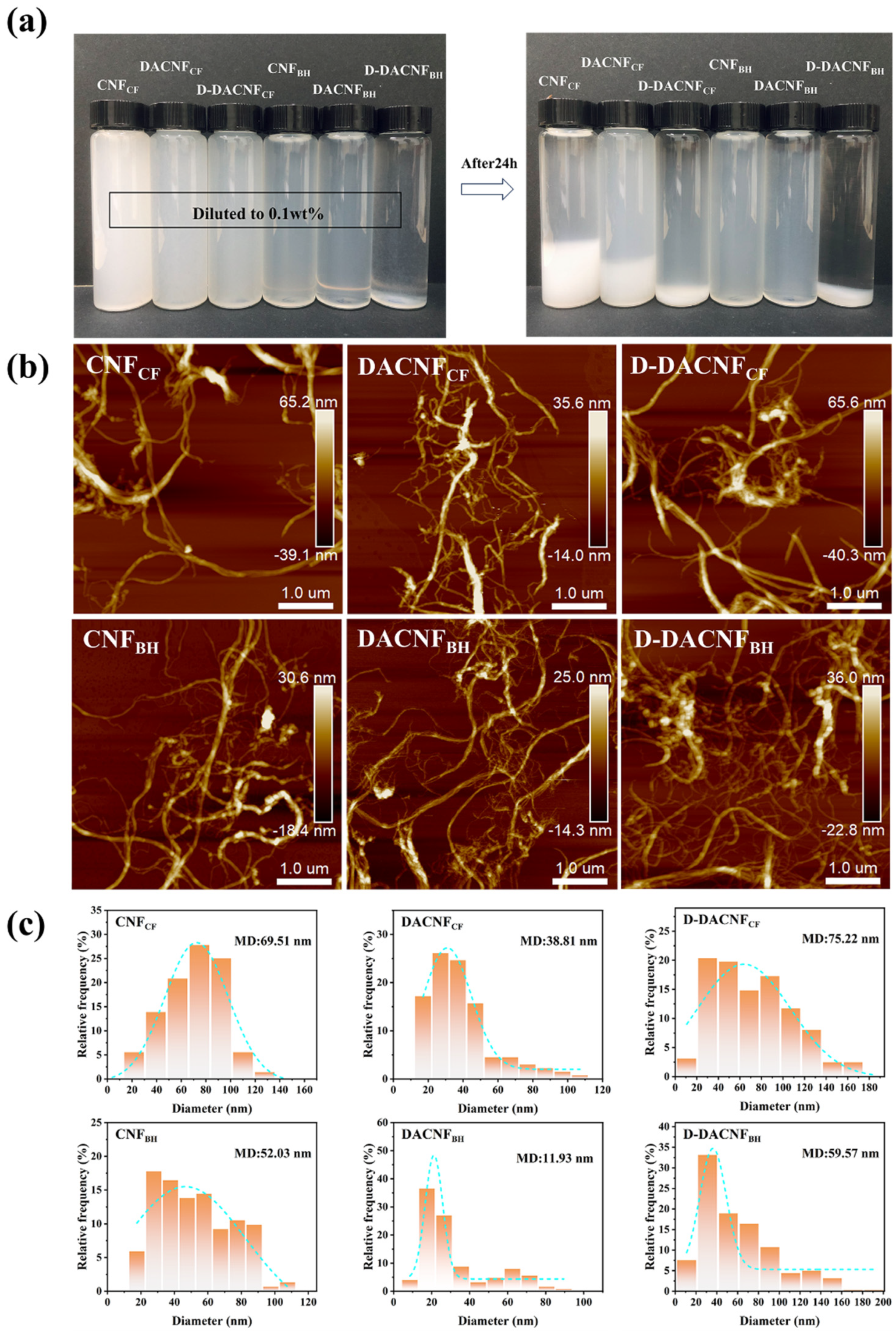
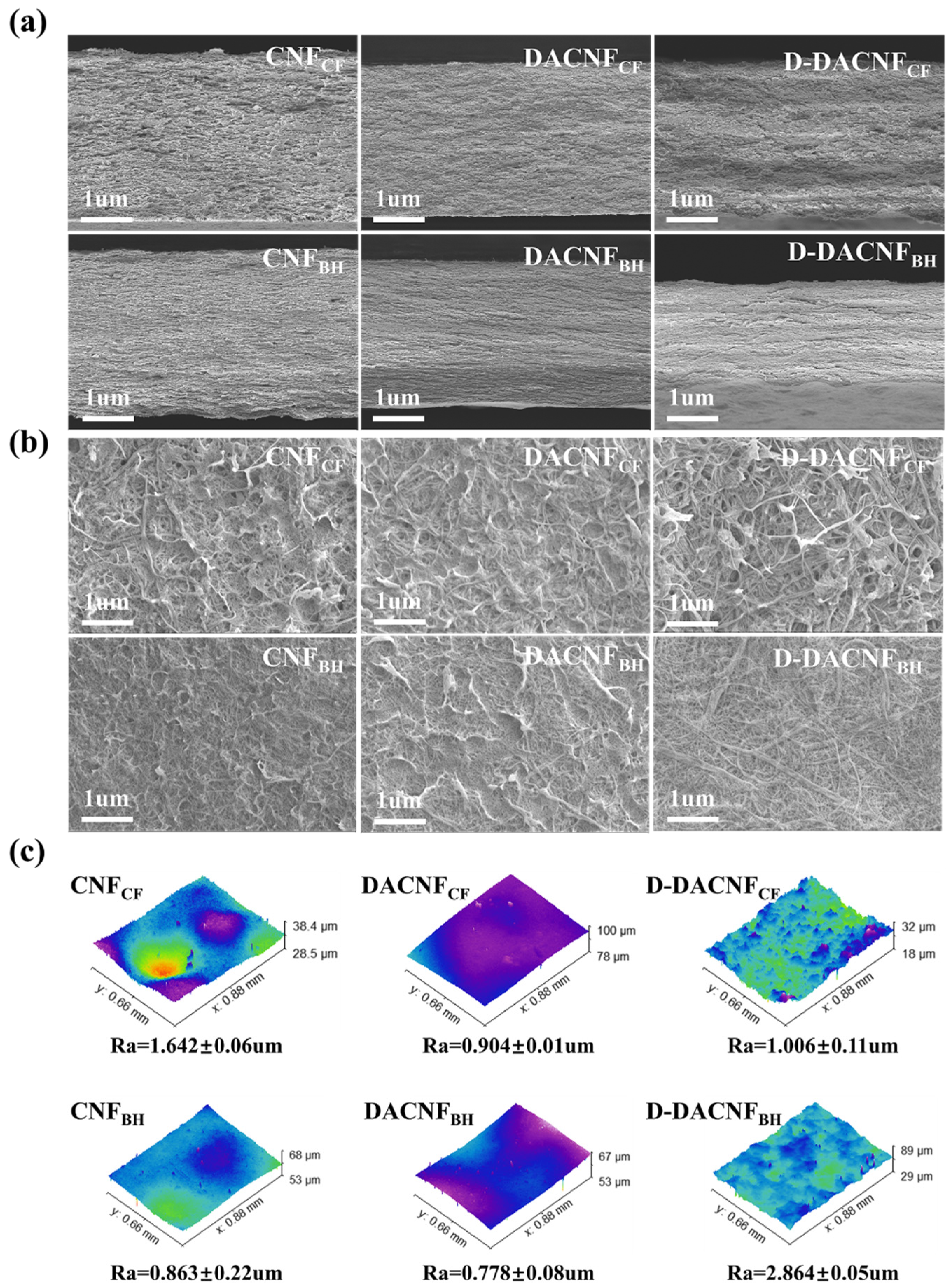
2.2. Morphological Structure of CNFs, DACNFs, and Redispersed DACNFs
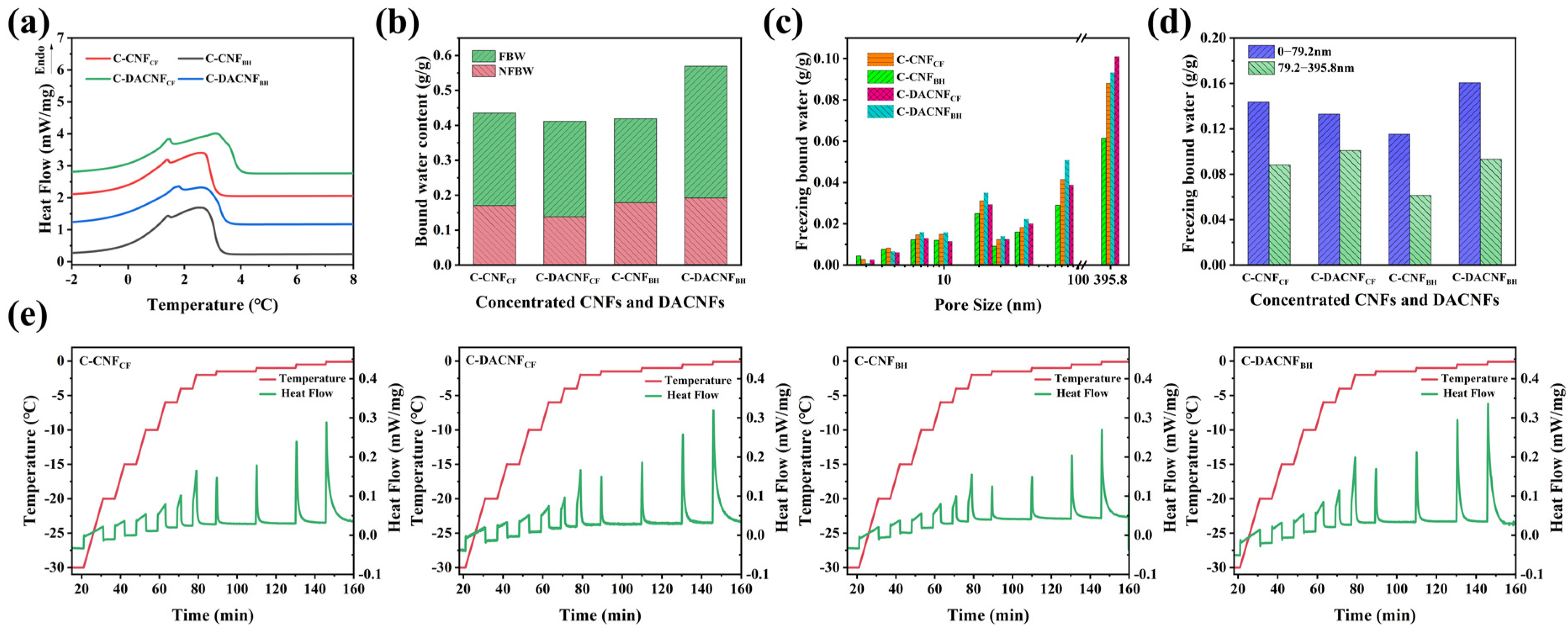
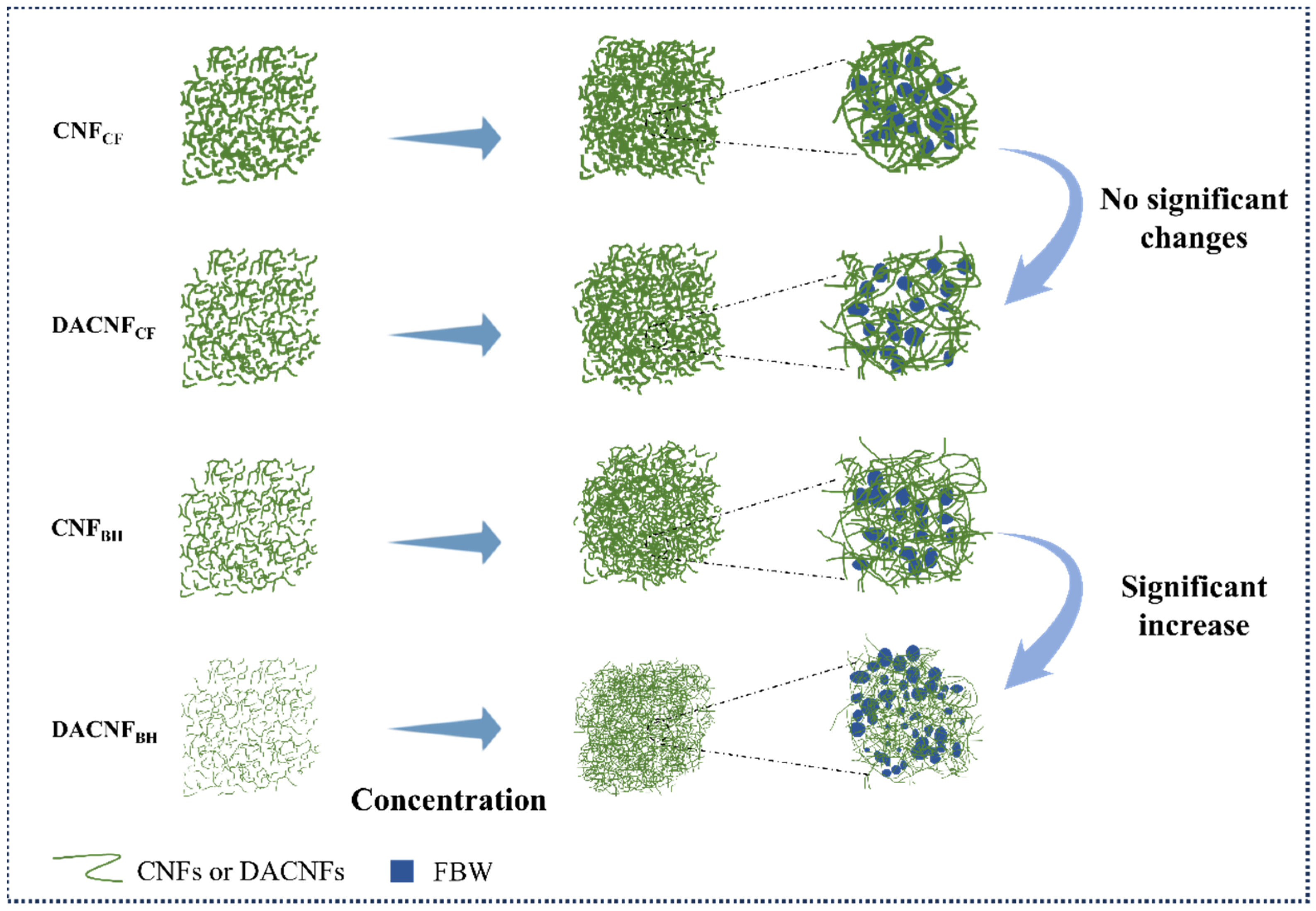
2.3. Bound Water and Pore Size Distribution of Concentrated CNFs and DACNFs
2.4. Properties of Films
2.4.1. Microstructure of Films
2.4.2. Mechanical Properties of Films
2.4.3. Optical Properties of Films
2.4.4. Swelling Capacity and Hydrophobicity of Films
2.4.5. Barrier Performance of Films
3. Experimental
3.1. Materials
3.2. Preparation of Cellulose Nanofibrils (CNFs)
3.3. Periodate Oxidation of CNFs
3.4. Concentration and Redispersion of CNFs and DACNFs
3.5. Preparation of Films
3.6. Characterization of CNFs and DACNFs
3.6.1. Determination of Aldehyde Content
3.6.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.6.3. Atomic Force Microscopy (AFM)
3.6.4. X-ray Diffraction (XRD)
3.6.5. Specific Surface Area (SSA)
3.6.6. Water Retention Value (WRV)
3.6.7. Zeta Potential
3.6.8. Surface Charge Measurements
3.6.9. Settling Performance
3.6.10. Bound Water of CNFs and DACNFs
3.6.11. Pore Detection of Concentrated CNFs and DACNFs
3.6.12. Field Emission Scanning Electron Microscopy (FESEM)
3.6.13. Surface Roughness of Films
3.6.14. Mechanical Properties of Films
3.6.15. UV Transmittance of Films
3.6.16. Water Absorption Properties of Films
3.6.17. Static Water Contact Angle (WCA) of the Films
3.6.18. Water Vapor Permeability and Oxygen Permeability of Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jechan, L.; Soosan, K.; Siming, Y.; Young-Kwon, P. Bioenergy generation from thermochemical conversion of lignocellulosic biomass-based integrated renewable energy systems. Renew. Sustain. Energy Rev. 2023, 178, 113240. [Google Scholar] [CrossRef]
- Wang, S.; Song, T.; Qi, H.; Xiang, Z. Exceeding High Concentration Limits of Aqueous Dispersion of Carbon Nanotubes Assisted by Nanoscale Xylan Hydrate Crystals. Chem. Eng. J. 2021, 419, 129602. [Google Scholar] [CrossRef]
- Sixta, H.; Potthast, A.; Krotschek, A.W. Chemical Pulping Processe: Sections 4.1–4.2.5. In Handbook of Pulp; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 109–229. [Google Scholar]
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 2019, 27, 575–593. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef] [PubMed]
- Sixta, H.; Süss, H.-U.; Potthast, A.; Schwanninger, M.; Krotscheck, A.W. Pulp Bleaching: Sections 7.1–7.3.5. In Handbook of Pulp; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 609–708. [Google Scholar]
- Gümüskaya, E.; Usta, M.; Kirci, H. The effects of various pulping conditions on crystalline structure of cellulose in cotton linters. Polym. Degrad. Stab. 2003, 81, 559–564. [Google Scholar] [CrossRef]
- Zhang, S.; Han, Y.; Wang, G.; Feng, L.; Lei, Y.; Wang, Z.; Xiong, S.; Yang, B.; Du, W.; Zhi, X.; et al. Long-term assessments of cotton fiber quality in response to plant population density: Reconciling fiber quality and its temporal stability. Ind. Crops Prod. 2023, 198, 116741. [Google Scholar] [CrossRef]
- Maiti, S.; Jayaramudu, J.; Das, K.; Reddy, S.M.; Sadiku, R.; Ray, S.S.; Liu, D. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr. Polym. 2013, 98, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, F.; Jafari, S.M.; Taheri, R.A. Optimization of homogenization-sonication technique for the production of cellulose nanocrystals from cotton linter. Int. J. Biol. Macromol. 2019, 137, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Kuga, S. Reactive interaction of aromatic amines with dialdehyde cellulose gel. Cellulose 2000, 7, 287–297. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Q.; Liu, Z.; Ni, Y. Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 2015, 22, 1135–1146. [Google Scholar] [CrossRef]
- Kim, U.-J.; Kimura, S.; Wada, M. Highly enhanced adsorption of Congo red onto dialdehyde cellulose-crosslinked cellulose-chitosan foam. Carbohydr. Polym. 2019, 214, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Silva Gomes, A.; Vitória Guimarães Leal, M.; Roefero Tolosa, G.; Camargo Cabrera, F.; Dognani, G.; Eloízo Job, A. Cationic dialdehyde cellulose microfibers for efficient removal of eriochrome black T from aqueous solution. Bioresour. Technol. 2023, 380, 129096. [Google Scholar] [CrossRef]
- Yao, M.; Wang, Z.; Liu, Y.; Yang, G.; Chen, J. Preparation of dialdehyde cellulose graftead graphene oxide composite and its adsorption behavior for heavy metals from aqueous solution. Carbohydr. Polym. 2019, 212, 345–351. [Google Scholar] [CrossRef]
- Lei, Z.; Gao, W.; Zeng, J.; Wang, B.; Xu, J. The mechanism of Cu (II) adsorption onto 2,3-dialdehyde nano-fibrillated celluloses. Carbohydr. Polym. 2019, 230, 115631. [Google Scholar] [CrossRef]
- Koprivica, S.; Siller, M.; Hosoya, T.; Roggenstein, W.; Rosenau, T.; Potthast, A. Regeneration of Aqueous Periodate Solutions by Ozone Treatment: A Sustainable Approach for Dialdehyde Cellulose Production. ChemSusChem 2016, 9, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A. Emerging Nanocellulose Technologies: Recent Developments. Adv. Mater. 2020, 33, 2000630. [Google Scholar] [CrossRef]
- Liu, H.; Tu, Q.; Huang, L.; Gao, W.; Zeng, J.; Wang, B.; Xu, J.; Wang, Z. Characteristics of concentrated lignocellulosic nanofibril suspensions. Cellulose 2021, 29, 147–158. [Google Scholar] [CrossRef]
- Yuko, O.; Miyuki, T.; Akira, I. Characterization of solid-state structures, molar masses, and microfibril structures of cellulose in never-dried cotton fibers and ramie bast fibers. Cellulose 2022, 29, 9105–9119. [Google Scholar] [CrossRef]
- Hefang, L.; Qiyuan, T.; Luyao, H.; Wenhua, G.; Jinsong, Z.; Bin, W.; Jinpeng, L.; Jun, X. Characteristics of concentrated cellulose nanofibrils measured by differential scanning calorimetry. Cellulose 2023, 30, 5019–5031. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Z.; Peng, Y.; Han, B.; Li, Z.; Li, X.; Liu, W. Preparation, characterization and feasibility study of dialdehyde carboxymethyl cellulose as a novel crosslinking reagent. Carbohydr. Polym. 2015, 137, 632–641. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Reiner, R.S.; Ralph, S.A.; Catchmark, J.; Chi, K.; Foster, E.J.; Hunt, C.G.; Baez, C.; Ibach, R.E.; Hirth, K.C. Characterization of the supramolecular structures of cellulose nanocrystals of different origins. Cellulose 2021, 28, 1369–1385. [Google Scholar] [CrossRef]
- Khandoker Samaher, S.; Nitesh Kumar, K.; Ashiqur, R.M.; Hasan, J.; Youssef, H.; Stephen, J.E.; Alfred, D.F.; Lokendra, P.; Lucian, A.L. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
- Duchemin, B. Size, shape, orientation and crystallinity of cellulose Iβ by X-ray powder diffraction using a free spreadsheet program. Cellulose 2017, 24, 2727–2741. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; Chen, H.; Hu, J.; Cui, L. Preparation of different morphologies cellulose nanocrystals from waste cotton fibers and its effect on PLLA/PDLA composites films. Compos. Part B Eng. 2021, 217, 108934. [Google Scholar] [CrossRef]
- Errokh, A.; Magnin, A.; Putaux, J.-L.; Boufi, S. Morphology of the nanocellulose produced by periodate oxidation and reductive treatment of cellulose fibers. Cellulose 2018, 25, 3899–3911. [Google Scholar] [CrossRef]
- Dias, M.C.; Zidanes, U.L.; Martins, C.C.N.; de Oliveira, A.L.M.; Damásio, R.A.P.; de Resende, J.V.; de Barros Vilas Boas, E.V.; Belgacem, M.N.; Tonoli, G.H.D.; Ferreira, S.R. Influence of hemicellulose content and cellulose crystal change on cellulose nanofibers properties. Int. J. Biol. Macromol. 2022, 213, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Wang, W.; Cai, Z.; Xue, F.; Jin, Y.; Zhu, J.Y. Water retention value for characterizing fibrillation degree of cellulosic fibers at micro and nanometer scales. Cellulose 2018, 25, 2861–2871. [Google Scholar] [CrossRef]
- Ding, Q.; Zeng, J.; Wang, B.; Tang, D.; Chen, K.; Gao, W. Effect of Nanocellulose Fiber Hornification on Water Fraction Characteristics and Hydroxyl Accessibility during Dehydration. Carbohydr. Polym. 2018, 207, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sulaeva, I.; Klinger, K.M.; Amer, H.; Henniges, U.; Rosenau, T.; Potthast, A. Determination of molar mass distributions of highly oxidized dialdehyde cellulose by size exclusion chromatography and asymmetric flow field-flow fractionation. Cellulose 2015, 22, 3569–3581. [Google Scholar] [CrossRef]
- Chen, W.; Abe, K.; Uetani, K.; Yu, H.; Liu, Y.; Yano, H. Individual cotton cellulose nanofibers: Pretreatment and fibrillation technique. Cellulose 2014, 21, 1517–1528. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, W.; Liu, C.; Yan, F.; Wu, M.; Cui, Q.; Willför, S.; Xu, C.; Li, B. Preparation of Antibacterial Dialdehyde Nanocellulose Using LiBr·3H2O Non-Dissolving Pretreatment Promoted Periodate Oxidation. ACS Sustain. Chem. Eng. 2023, 11, 6641–6651. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Pettolino, F.; Flanagan, B.; Gidley, M.J.; Gilbert, E.P. Structure of cellulose microfibrils in mature cotton fibres. Carbohydr. Polym. 2017, 175, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, S.; Abidi, N. Molecular weight and organization of cellulose at different stages of cotton fiber development. Text. Res. J. 2019, 89, 726–738. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.; Fang, L.; Li, S.; Chen, N.; Pang, J.; Li, D. Effect of delignification technique on the ease of fibrillation of cellulose II nanofibers from wood. Cellulose 2018, 25, 7003–7015. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Zeng, J.; Li, J.; Wang, B.; Gao, W.; Xu, J.; Chen, K. Dynamic Covalent Bond Enabled Strong Bio-based Polyimide Materials with Thermally-driven Adaptivity, Healability and Recycling. Chem. Eng. J. 2023, 465, 143017. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, Y.; Wu, W.; Xiao, H. Dispersion Properties of Nanocellulose: A Review. Carbohydr. Polym. 2020, 250, 116892. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Wu, L.; Siciliano, A.P.; Chen, C.; Li, T.; Hu, L. The Critical Roles of Water in the Processing, Structure, and Properties of Nanocellulose. ACS Nano 2023, 17, 22196–22226. [Google Scholar] [CrossRef]
- Solhi, L.; Guccini, V.; Heise, K.; Solala, I.; Niinivaara, E.; Xu, W.; Mihhels, K.; Kröger, M.; Meng, Z.; Wohlert, J.; et al. Understanding Nanocellulose–Water Interactions: Turning a Detriment into an Asset. Chem. Rev. 2023, 123, 1925–2015. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Nakamura, K.; Hatakeyama, H. Determination of bound water content in polymers by DTA, DSC and TG. Thermochim. Acta 1988, 123, 153–161. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Iijima, M.; Hatakeyama, H. Role of bound water on structural change of water insoluble polysaccharides. Food Hydrocoll. 2016, 53, 62–68. [Google Scholar] [CrossRef]
- Lin, G.; Xu, J.; Wu, M.; Sun, Q.; Zhu, S.; Wang, B.; Zhang, W. Cellulose Nanofibril/Talc Composite Films with Excellent Barrier Properties by Alternate Hierarchical Method. ACS Appl. Polym. Mater. 2023, 5, 9180–9191. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Yang, J.; Zhu, R.; Zhang, Z.; Li, Y. Development and biocompatibility evaluation of biodegradable bacterial cellulose as a novel peripheral nerve scaffold. J. Biomed. Mater. Res. Part A 2018, 106, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.F.; Islam, M.A.; Khorshidi, B.; Tehrani-Bagha, A.; Sadrzadeh, M. Surface characterization of thin-film composite membranes using contact angle technique: Review of quantification strategies and applications. Adv. Colloid Interface Sci. 2021, 299, 102524. [Google Scholar] [CrossRef] [PubMed]
- López Durán, V.; Hellwig, J.; Larsson, P.T.; Wågberg, L.; Larsson, P.A. Effect of Chemical Functionality on the Mechanical and Barrier Performance of Nanocellulose Films. ACS Appl. Energy Mater. 2018, 1, 1959–1967. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.; Xu, Q.; Jin, L.; Wang, Y. Bio-based films with high antioxidant and improved water-resistant properties from cellulose nanofibres and lignin nanoparticles. Int. J. Biol. Macromol. 2022, 227, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, D. Determination of structural carbohydrates and lignin in biomass determination of structural carbohydrates and lignin in biomass. In Technical Report: NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, Colorado, 2011; pp. 1–15. [Google Scholar]
- Wada, M.; Okano, T.; Sugiyama, J. Synchrotron-radiated X-ray and neutron diffraction study of native cellulose. Cellulose 1997, 4, 221–232. [Google Scholar] [CrossRef]
- Kaewprasit, C.; Hequet, E.; Abidi, N.; Gourlot, J.P. Application of methylene blue adsorption to cotton fiber specific surface area measurement: Part I. Methodology. J. Cotton Sci. 1998, 2, 164–173. [Google Scholar]
- Park, S.; Venditti, R.A.; Jameel, H.; Pawlak, J.J. Changes in pore size distribution during the drying of cellulose fibers as measured by differential scanning calorimetry. Carbohydr. Polym. 2006, 66, 97–103. [Google Scholar] [CrossRef]

| Sample | CNFCF | DACNFCF | D-DACNFCF | CNFBH | DACNFBH | D-DACNFBH |
|---|---|---|---|---|---|---|
| WRV (%) | 311.38 ± 0.61 | 362.31 ± 7.90 | 354.44 ± 7.53 | 427.95 ± 7.14 | 521.15 ± 7.09 | 473.02 ± 7.21 |
| SSA (m2/g) | 249.26 ± 0.55 | 379.64 ± 2.75 | 292.09 ± 4.31 | 658.82 ± 0.28 | 793.27 ± 4.27 | 192.06 ± 11.56 |
| CrI (%) | 73.44 | 70.33 | 70.73 | 62.89 | 54.46 | 54.53 |
| Dhkl (nm) | 4.59 | 4.45 | 4.78 | 3.10 | 2.99 | 2.88 |
| Zeta potential (mV) | −14.96 ± 0.81 | −15.82 ± 0.31 | −14.10 ± 1.17 | −19.84 ± 0.33 | −23.97 ± 0.84 | −17.19 ± 0.14 |
| Surface charge density (eq/g) | (9.59 ± 0.07) × 10−5 | (4.43 ± 0.04) × 10−5 | (1.03 ± 0.01) × 10−5 | (2.75 ± 0.22) × 10−4 | (5.30 ± 0.02) × 10−5 | (0.86 ± 0.01) × 10−5 |
| Pulp | Chemical Composition (%) | ||
|---|---|---|---|
| Klason Lignin | Glucose | Hemicelluloses | |
| Cotton linter fiber | 0.45 ± 0.03 | 94.15 ± 0.12 | 0.83 ± 0.01 |
| BHKP | 0.74 ± 0.08 | 74.70 ± 0.64 | 14.90 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Q.; Gao, W.; Zhou, J.; Wu, J.; Zeng, J.; Wang, B.; Xu, J. Characteristics of Dialdehyde Cellulose Nanofibrils Derived from Cotton Linter Fibers and Wood Fibers. Molecules 2024, 29, 1664. https://doi.org/10.3390/molecules29071664
Tu Q, Gao W, Zhou J, Wu J, Zeng J, Wang B, Xu J. Characteristics of Dialdehyde Cellulose Nanofibrils Derived from Cotton Linter Fibers and Wood Fibers. Molecules. 2024; 29(7):1664. https://doi.org/10.3390/molecules29071664
Chicago/Turabian StyleTu, Qiyuan, Wenhua Gao, Junjie Zhou, Jinglin Wu, Jinsong Zeng, Bin Wang, and Jun Xu. 2024. "Characteristics of Dialdehyde Cellulose Nanofibrils Derived from Cotton Linter Fibers and Wood Fibers" Molecules 29, no. 7: 1664. https://doi.org/10.3390/molecules29071664
APA StyleTu, Q., Gao, W., Zhou, J., Wu, J., Zeng, J., Wang, B., & Xu, J. (2024). Characteristics of Dialdehyde Cellulose Nanofibrils Derived from Cotton Linter Fibers and Wood Fibers. Molecules, 29(7), 1664. https://doi.org/10.3390/molecules29071664






