Highly Sensitive and Selective Toluene Gas Sensors Based on ZnO Nanoflowers Decorated with Bimetallic AuPt
Abstract
1. Introduction
2. Results and Discussion
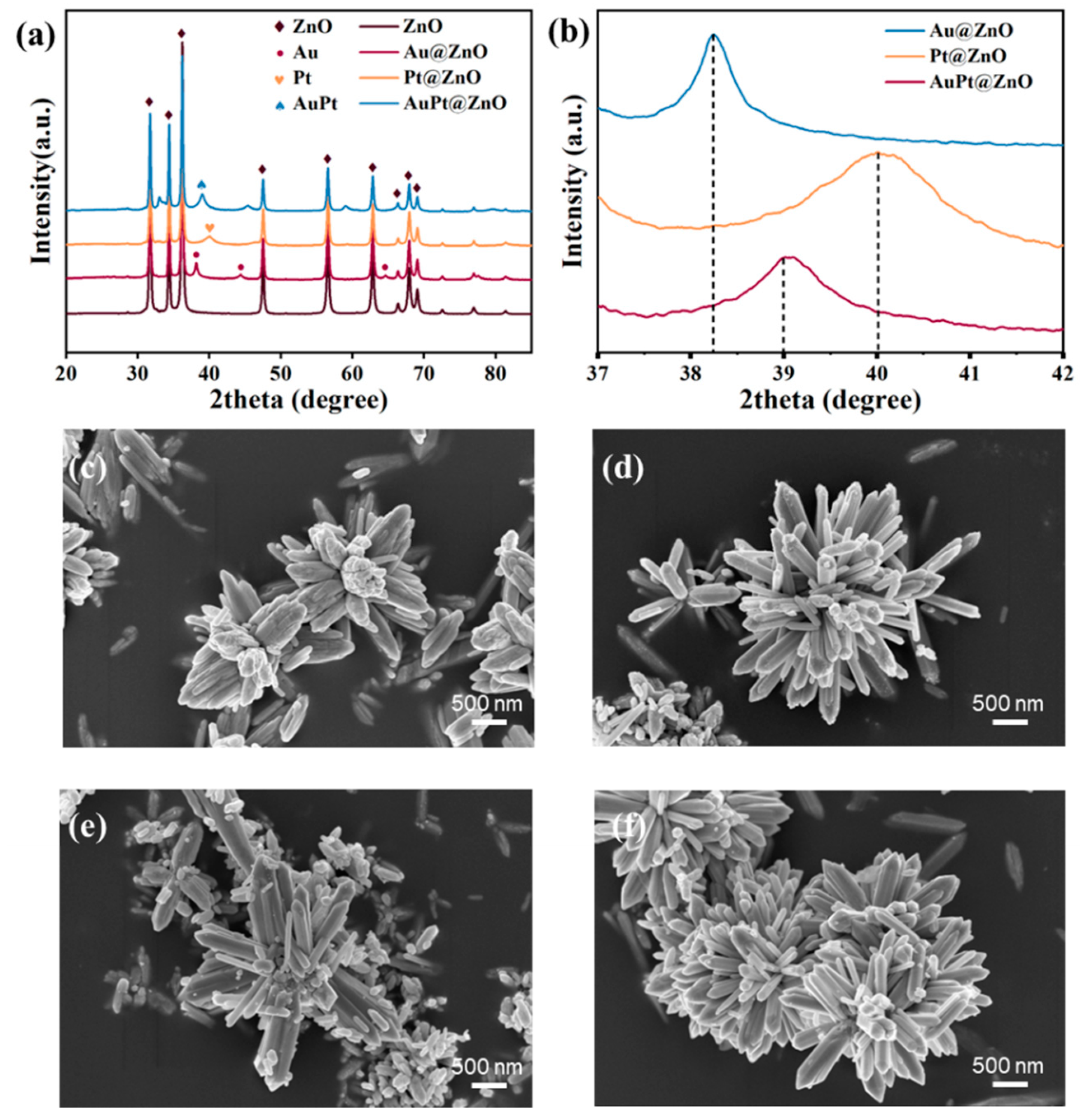
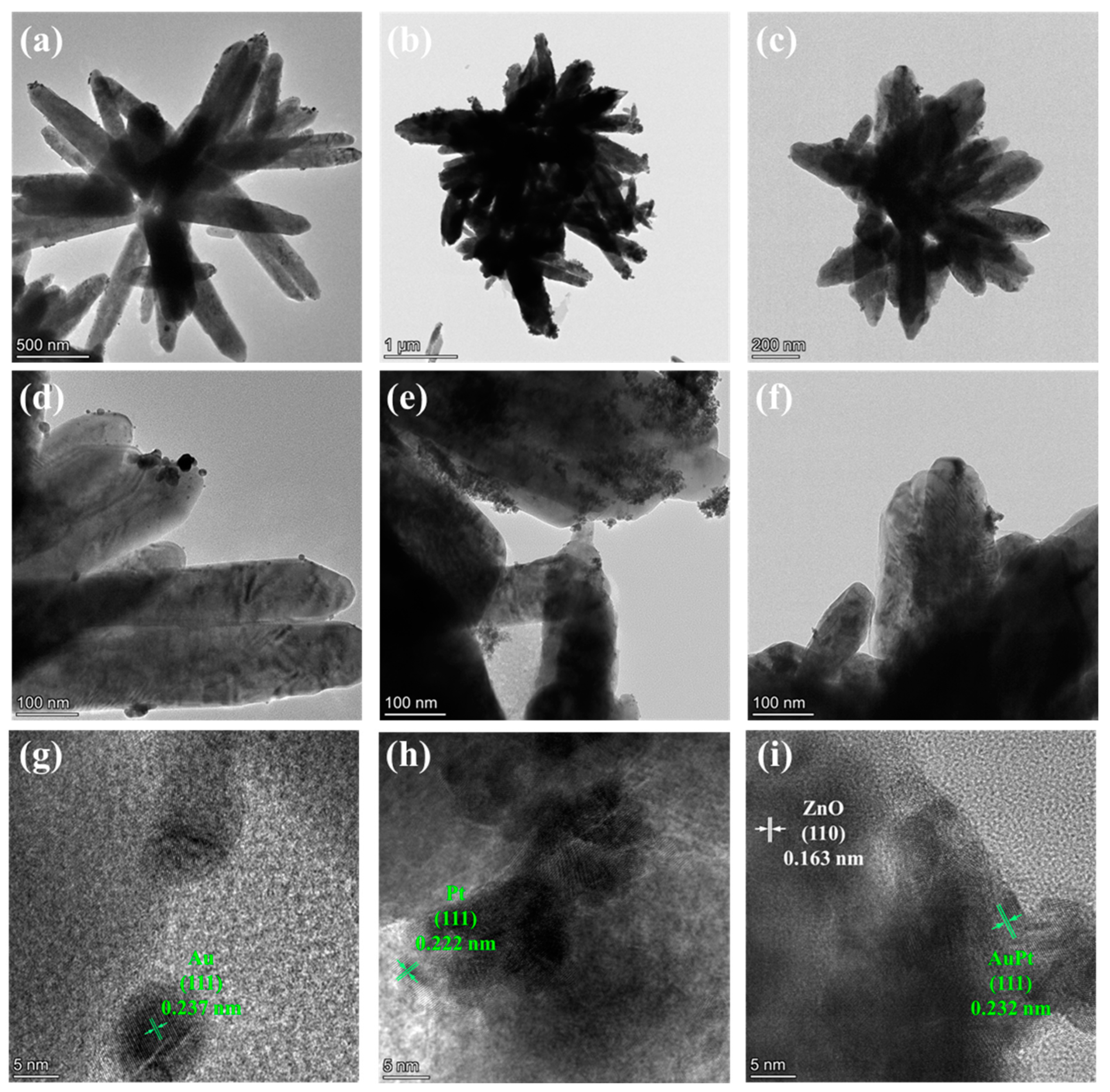
2.1. Morphology and Structure Properties
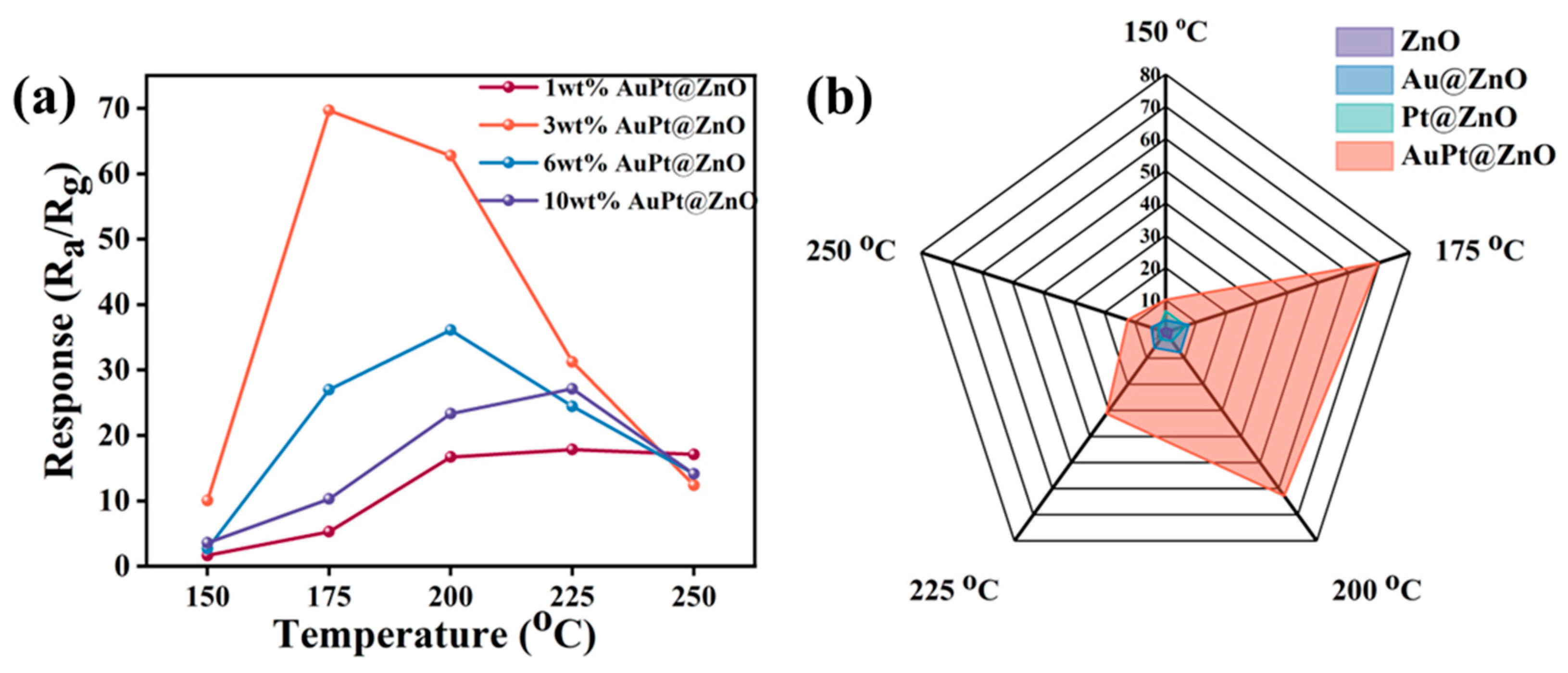
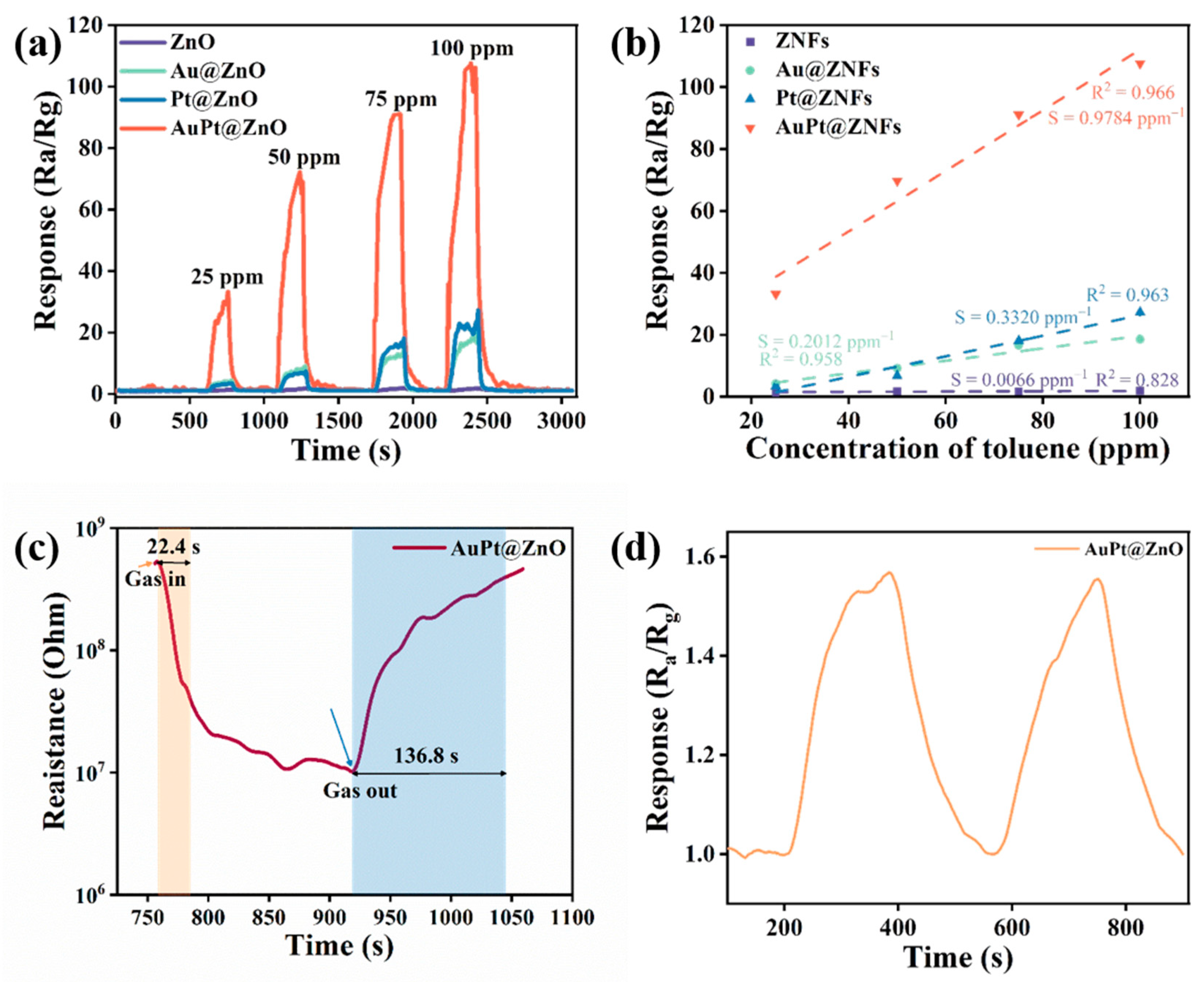
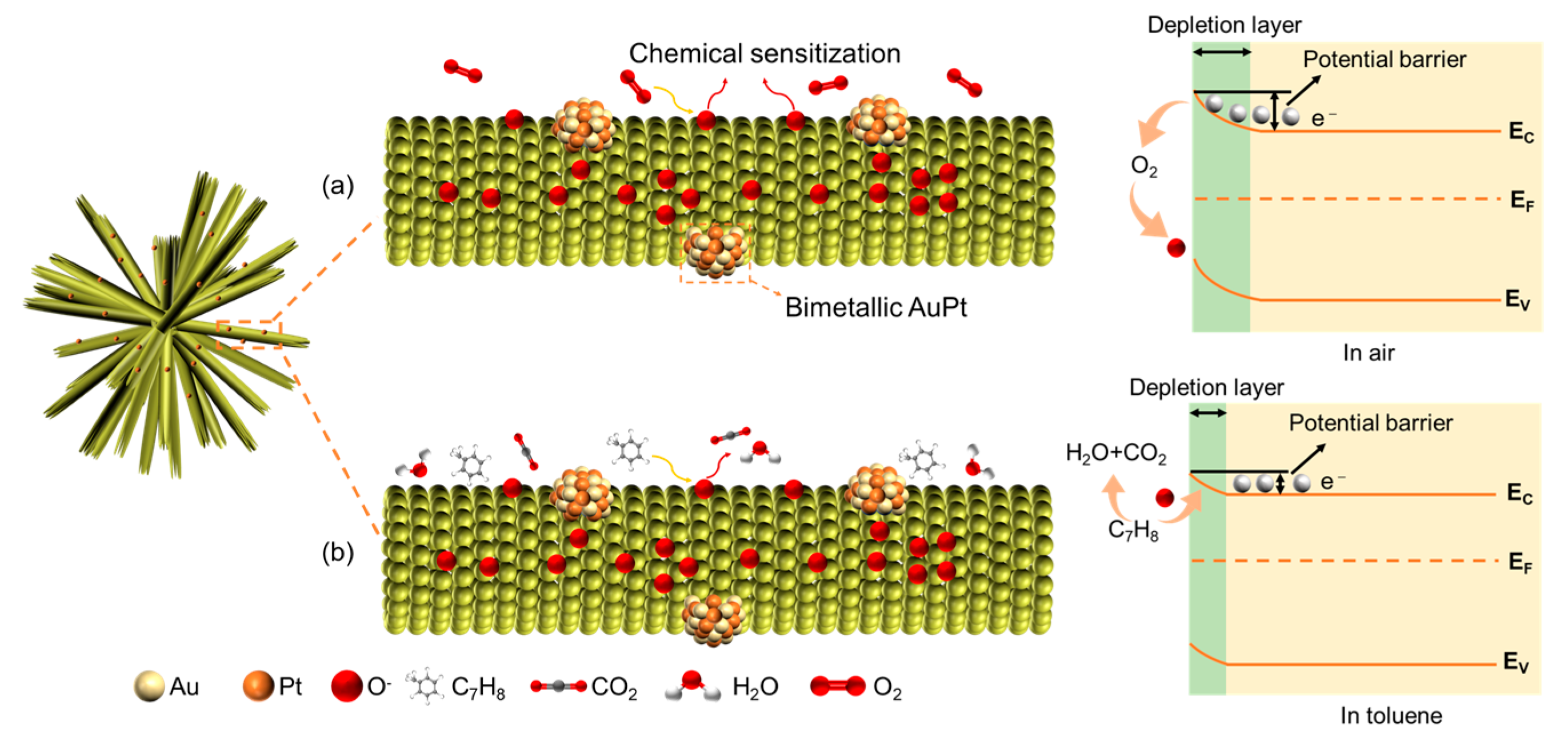
2.2. Performance of the Gas Sensors
2.3. Performance of the Gas Sensors
3. Materials and Methods
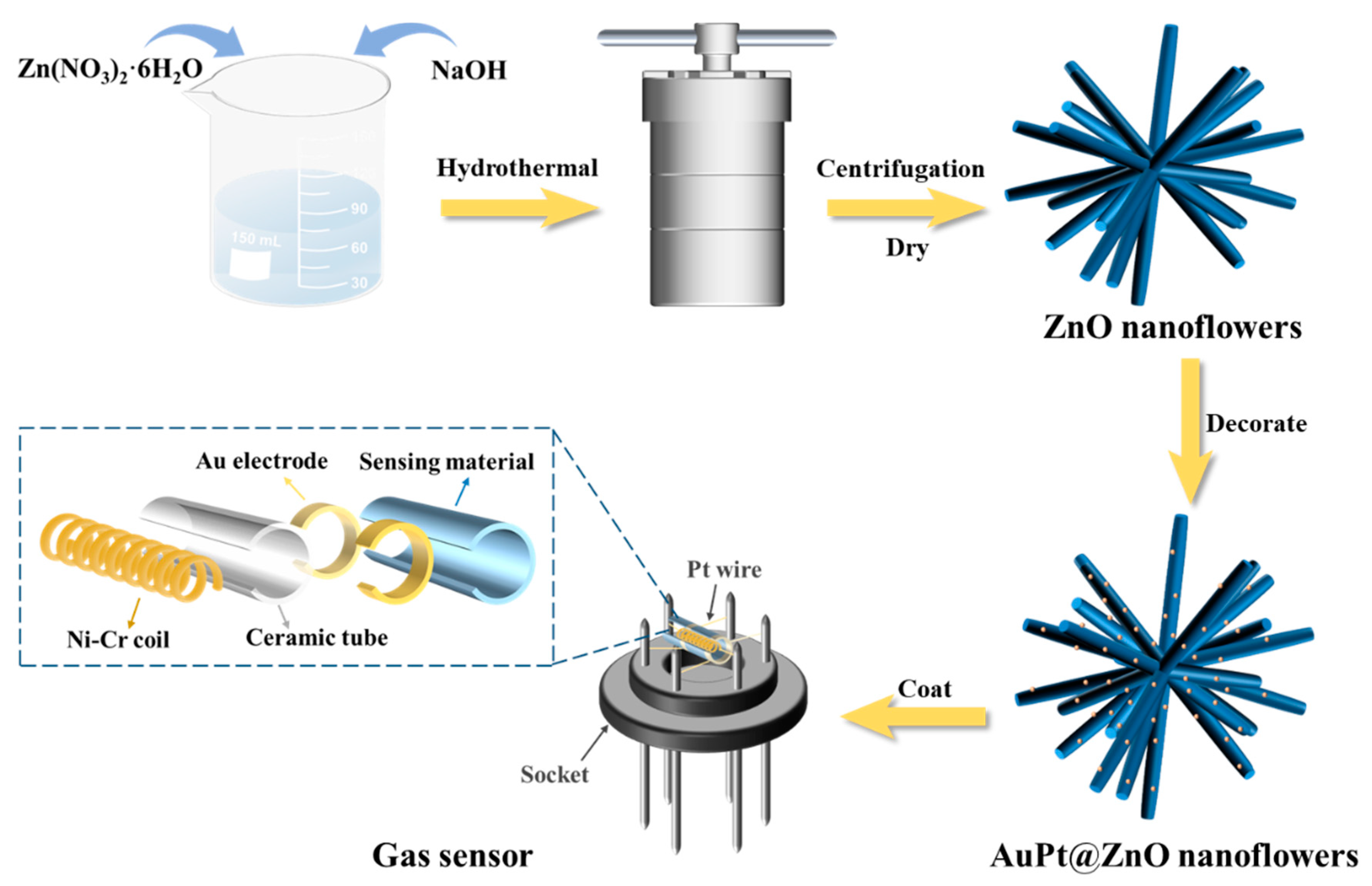
3.1. Preparation of ZNFs and ZNFs Decorated with Au, Pt, and AuPt Alloy
3.2. Characterization of Morphology and Structure
3.3. Fabrication and Measurements of Gas Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Hu, J.; Li, M.; Li, Z.; Yuan, Y.; Yang, X.; Guo, L. Highly efficient toluene gas sensor based on spinel structured hollow urchin-like core-shell ZnFe2O4 spheres. Sens. Actuators B Chem. 2021, 349, 130734. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Sharma, B.; Vikraman, D.; Jo, E.-B.; Sivakumar, P.; Kim, H.-S. Switchable p-n gas response for 3D-hierarchical NiFe2O4 porous microspheres for highly selective and sensitive toluene gas sensors. J. Alloys Compd. 2021, 886, 161281. [Google Scholar] [CrossRef]
- Su, Z.; Yang, W.; Wang, C.; Xiong, S.; Cao, X.; Peng, Y.; Si, W.; Weng, Y.; Xue, M.; Li, J. Roles of oxygen vacancies in the bulk and surface of CeO2 for toluene catalytic combustion. Environ. Sci. Technol. 2020, 54, 12684–12692. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Deng, J.; Impeng, S.; Li, S.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Boosting Toluene Combustion by Engineering Co–O Strength in Cobalt Oxide Catalysts. Environ. Sci. Technol. 2020, 54, 10342–10350. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.J.; Hannigan, J.H.; Bowen, S.E. Effects of inhaled combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an environmental exposure model. Environ. Toxicol. Pharmacol. 2021, 81, 103518. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, S.; Sun, P.; Wang, Y.; Shimanoe, K.; Lu, G. Unexpected and enhanced electrostatic adsorption capacity of oxygen vacancy-rich cobalt-doped In2O3 for high-sensitive MEMS toluene sensor. Sens. Actuators B Chem. 2021, 342, 129949. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, S.; Zhou, T.; Tu, J.; Zhang, T. Facile preparation of hierarchical structure based on p-type Co3O4 as toluene detecting sensor. Appl. Surf. Sci. 2020, 503, 144167. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jeong, S.-Y.; Moon, Y.K.; Lee, J.-H. Dual-mode gas sensor for ultrasensitive and highly selective detection of xylene and toluene using Nb-doped NiO hollow spheres. Sens. Actuators B Chem. 2019, 301, 127140. [Google Scholar] [CrossRef]
- Seekaew, Y.; Wisitsoraat, A.; Phokharatkul, D.; Wongchoosuk, C. Room temperature toluene gas sensor based on TiO2 nanoparticles decorated 3D graphene-carbon nanotube nanostructures. Sens. Actuators B Chem. 2019, 279, 69–78. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Suematsu, K.; Zhang, W.; Zhang, W.; Zhuiykov, S.; Shimanoe, K.; Hu, J. MOF-derived Au-NiO/In2O3 for selective and fast detection of toluene at ppb-level in high humid environments. Sens. Actuators B Chem. 2022, 360, 131631. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Park, Y.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Toluene- and benzene-selective gas sensors based on Pt- and Pd-functionalized ZnO nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 294, 78–88. [Google Scholar] [CrossRef]
- Su, X.; Zhang, X.; Pei, J.; Deng, M.; Pan, L.; Liu, J.; Cui, M.; Zhan, C.; Wang, J.; Wu, Y.; et al. Working memory-related alterations in neural oscillations reveal the influence of in-vehicle toluene on cognition at low concentration. Environ. Sci. Pollut. Res. 2023, 30, 21723–21734. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, Z.; Liu, Y.; Gao, J.; Wang, X.; Suo, H.; Yang, X.; Zhao, C.; Liu, F. Gas sensor based on Ni foam: SnO2-decorated NiO for Toluene detection. Sens. Actuators B Chem. 2020, 318, 128167. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J. Mechanism for toluene detection of flower-like ZnO sensors prepared by hydrothermal approach: Charge transfer. Sens. Actuators B Chem. 2015, 207, 66–73. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Swart, H.C.; Hillie, K.T.; Motaung, D.E. Engineering of rare-earth Eu3+ ions doping on p-type NiO for selective detection of toluene gas sensing and luminescence properties. Sens. Actuators B Chem. 2021, 347, 130530. [Google Scholar] [CrossRef]
- Hermawan, A.; Zhang, B.; Taufik, A.; Asakura, Y.; Hasegawa, T.; Zhu, J.; Shi, P.; Yin, S. CuO nanoparticles/Ti3C2Tx MXene hybrid nanocomposites for detection of toluene gas. ACS Appl. Nano Mater. 2020, 3, 4755–4766. [Google Scholar] [CrossRef]
- Hermawan, A.; Asakura, Y.; Inada, M.; Yin, S. One-step synthesis of micro-/mesoporous SnO2 spheres by solvothermal method for toluene gas sensor. Ceram. Int. 2019, 45, 15435–15444. [Google Scholar] [CrossRef]
- Moiz, M.A.; Mumtaz, A.; Salman, M.; Husain, S.W.; Baluch, A.H.; Ramzan, M. Band gap Engineering of ZnO via transition metal Doping: An ab initio study. Chem. Phys. Lett. 2021, 781, 138979. [Google Scholar] [CrossRef]
- Teke, A.; Özgür, Ü.; Doğan, S.; Gu, X.; Morkoç, H.; Nemeth, B.; Nause, J.; Everitt, H.O. Excitonic fine structure and recombination dynamics in single-crystalline ZnO. Phys. Rev. B 2004, 70, 195207. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.-G.; Xi, R.; Zhang, L.; Zhang, S.-H.; Wang, L.-J.; Pan, G.-B. In situ synthesis of flower-like ZnO on GaN using electrodeposition and its application as ethanol gas sensor at room temperature. Sens. Actuators B Chem. 2019, 292, 270–276. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Maboudian, R.; Carraro, C.; Han, C.; Liu, W.; Wei, D. Facile synthesis of ZnO-SnO2 hetero-structured nanowires for high-performance NO2 sensing application. Sens. Actuators B Chem. 2021, 333, 129613. [Google Scholar] [CrossRef]
- Gupta, S.; Knoepfel, A.; Zou, H.; Ding, Y. Investigations of methane gas sensor based on biasing operation of n-ZnO nanorods/p-Si assembled diode and Pd functionalized Schottky junctions. Sens. Actuators B Chem. 2023, 392, 134030. [Google Scholar] [CrossRef]
- Cai, L.-X.; Chen, L.; Sun, X.-Q.; Geng, J.; Liu, C.-C.; Wang, Y.; Guo, Z. Ultra-sensitive triethylamine gas sensors based on polyoxometalate-assisted synthesis of ZnWO4/ZnO hetero-structured nanofibers. Sens. Actuators B Chem. 2022, 370, 132422. [Google Scholar] [CrossRef]
- Nataraj, N.; Chen, T.W.; Gan, Z.W.; Chen, S.M.; Lou, B.S.; Ali, M.A.; Al-Hemaid, F.M. Two-dimensional copper oxide/zinc oxide nanoflakes with three-dimensional flower-like heterostructure enhanced with electrocatalytic activity toward nimesulide detection. Mater. Today Chem. 2022, 24, 100768. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Zhu, B.; Yu, J. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sens. Actuators B Chem. 2021, 331, 129425. [Google Scholar] [CrossRef]
- Agarwal, S.; Rai, P.; Gatell, E.N.; Llobet, E.; Güell, F.; Kumar, M.; Awasthi, K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens. Actuators B Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Ma, Q.; Chu, S.; Li, H.; Guo, J.; Zhang, Q.; Lin, Z. Flower-like In2O3/ZnO heterostructure with accelerated multi-orientation electron transport mechanism for superior triethylamine detection. Appl. Surf. Sci. 2021, 569, 151074. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-surfactant-assisted hydrothermal synthesis of one-dimensional ZnO nanorods for high-performance ethanol gas sensor. Sens. Actuators B Chem. 2019, 286, 501–511. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, M.; Liu, S.; Hassan, M.; Zhang, X.; Lei, S.; Qiao, G.; Liu, G. One-pot hydrothermal synthesis of self-assembled MoS2/WS2 nanoflowers for chemiresistive room-temperature NO2 sensors. Sens. Actuators B Chem. 2024, 403, 135215. [Google Scholar] [CrossRef]
- Jamnani, S.R.; Moghaddam, H.M.; Leonardi, S.G.; Neri, G.; Ferlazzo, A. VOCs sensing properties of samarium oxide nanorods. Ceram. Int. 2024, 50, 403–411. [Google Scholar] [CrossRef]
- Lin, L.; Liu, T.; Yu, W.; Gou, Z.; Zeng, W. Synthesis of multifarious hierarchical flower-like NiO and their gas-sensing properties. Mater. Res. Bull. 2013, 48, 2730–2736. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Sung, S.-H.; Lee, G.; Kim, J.; Seong, J.; Shim, Y.-S.; Jun, S.C.; Jeon, S. High-performance gas sensor array for indoor air quality monitoring: The role of Au nanoparticles on WO3, SnO2, and NiO-based gas sensors. J. Mater. Chem. A 2021, 9, 1159–1167. [Google Scholar] [CrossRef]
- Mardare, C.C.; Hassel, A.W. Review on the Versatility of Tungsten Oxide Coatings. Phys. Status Solidi a 2019, 216, 1900047. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Suman, P.H.; Kim, J.J.; Tuller, H.L.; Orlandi, M.O. Investigation of electronic and chemical sensitization effects promoted by Pt and Pd nanoparticles on single-crystalline SnO nanobelt-based gas sensors. Sens. Actuators B Chem. 2019, 301, 127055. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.-Y.; Feng, P.; Dang, C.; Li, M.; Lu, H.-L.; Gao, L. Bimetallic AuPt alloy nanoparticles decorated on ZnO nanowires towards efficient and selective H2S gas sensing. Sens. Actuators B Chem. 2022, 367, 132024. [Google Scholar] [CrossRef]
- Ahemad, M.J.; Le, T.D.; Kim, D.-S.; Yu, Y.-T. Bimetallic AgAualloy@ZnO core-shell nanoparticles for ultra-high detection of ethanol: Potential impact of alloy composition on sensing performance. Sens. Actuators B Chem. 2022, 359, 131595. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, S.; Qu, F.; Gong, S.; Wang, C.; Qiu, L.; Yang, M.; Cheng, W. High performance acetone sensor based on ZnO nanorods modified by Au nanoparticles. J. Alloys Compd. 2019, 797, 246–252. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Zhou, P.; Zhong, X.; Li, G.; Han, C.; Wei, D.; Li, S. Bimetallic Au/Pd nanoparticles decorated ZnO nanowires for NO2 detection. Sens. Actuators B Chem. 2019, 289, 160–168. [Google Scholar] [CrossRef]
- Kim, M.-H.; Jang, J.-S.; Koo, W.-T.; Choi, S.-J.; Kim, S.-J.; Kim, D.-H.; Kim, I.-D. Bimodally porous WO3 microbelts functionalized with Pt catalysts for selective H2S sensors. ACS Appl. Mater. Interfaces 2018, 10, 20643–20651. [Google Scholar] [CrossRef]
- Le, H.-J.; Van Dao, D.; Yu, Y.-T. Superfast and efficient hydrogen gas sensor using PdAualloy@ZnO core–shell nanoparticles. J. Mater. Chem. A 2020, 8, 12968–12974. [Google Scholar] [CrossRef]
- Kolmakov, A.; Klenov, D.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Neri, G. Metal-core@ metal oxide-shell nanomaterials for gas-sensing applications: A review. J. Nanoparticle Res. 2015, 17, 371. [Google Scholar] [CrossRef]
- Theka, T.J.; Thamaga, B.R.J.; Tshabalala, Z.P.; Motsoeneng, R.G.; Swart, H.C.; Motaung, D.E. Fabrication of metal-organic frameworks derived Co3O4 loaded on TiO2: Influence of Fe loading on the Co3O4/TiO2 heterostructure for low-ppm benzene detection. Appl. Surf. Sci. 2024, 644, 158789. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Xu, M.; Hu, X.; Zhang, H.; Wang, Y.; Huang, W. A Au-functionalized ZnO nanowire gas sensor for detection of benzene and toluene. Phys. Chem. Chem. Phys. 2013, 15, 17179–17186. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, H.W.; Kim, S.S. Self-heating effects on the toluene sensing of Pt-functionalized SnO2–ZnO core–shell nanowires. Sens. Actuators B Chem. 2017, 251, 781–794. [Google Scholar] [CrossRef]
- Shi, J.; Xiong, J.; Qiao, L.; Liu, C.; Zeng, Y. Facile MOF-on-MOF isomeric strategy for ZnO@Co3O4 single-shelled hollow cubes with high toluene detection capability. Appl. Surf. Sci. 2023, 609, 155271. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Sheng, K.; Zhou, X.; Zhang, X.; Chen, C.; Dong, B.; Bai, X.; Geyu, L.; Song, H. Facile synthesis of controllable TiO2 composite nanotubes via templating route: Highly sensitive detection of toluene by double driving from Pt@ZnO NPs. Sens. Actuators B Chem. 2018, 273, 1676–1686. [Google Scholar] [CrossRef]
- Kim, H.W.; Kwon, Y.J.; Mirzaei, A.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S. Synthesis of zinc oxide semiconductors-graphene nanocomposites by microwave irradiation for application to gas sensors. Sens. Actuators B Chem. 2017, 249, 590–601. [Google Scholar] [CrossRef]
- Cai, Z.; Park, J.; Park, S. Porous In2O3–ZnO nanofiber-based sensor for ultrasensitive room-temperature detection of toluene gas under UV illumination. J. Mater. Res. Technol. 2023, 24, 2482–2499. [Google Scholar] [CrossRef]
- Dey, S.; Nag, S.; Santra, S.; Ray, S.K.; Guha, P.K. Voltage-controlled NiO/ZnO p–n heterojunction diode: A new approach towards selective VOC sensing. Microsyst. Nanoeng. 2020, 6, 35. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Wang, P.; Dong, T.; Jia, C.; Yang, P. Ultraselective acetone-gas sensor based ZnO flowers functionalized by Au nanoparticle loading on certain facet. Sens. Actuators B Chem. 2019, 288, 1–11. [Google Scholar] [CrossRef]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Han, S.; Lee, H.Y.; Choi, S.-W.; Kim, S.S.; Kim, H.W. Hybridization of silicon nanowires with TeO2 branch structures and Pt nanoparticles for highly sensitive and selective toluene sensing. Appl. Surf. Sci. 2020, 525, 146620. [Google Scholar] [CrossRef]
- Qiao, L.; Bing, Y.; Wang, Y.; Yu, S.; Liang, Z.; Zeng, Y. Enhanced toluene sensing performances of Pd- loaded SnO2 cubic nanocages with porous nanoparticle-assembled shells. Sens. Actuators B Chem. 2017, 241, 1121–1129. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low-Voltage-Driven Sensors Based on ZnO Nanowires for Room-Temperature Detection of NO2 and CO Gases. ACS Appl. Mater. Interfaces 2019, 11, 24172–24183. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Dey, S.; Das, D.; Guha, P.K. Adsorption-Mediated n-Type ZnO Surface Reconstruction for Optically Enhanced Volatile Organic Compound Sensing. ACS Appl. Electron. Mater. 2022, 4, 3825–3833. [Google Scholar] [CrossRef]
- Dong, C.; Liu, X.; Xiao, X.; Du, S.; Wang, Y. Monodisperse ZnFe2O4 nanospheres synthesized by a nonaqueous route for a highly slective low-ppm-level toluene gas sensor. Sens. Actuators B Chem. 2017, 239, 1231–1236. [Google Scholar] [CrossRef]
- Asgari, M.; Saboor, F.H.; Amouzesh, S.P.; Coull, M.W.; Khodadadi, A.A.; Mortazavi, Y.; Hyodo, T.; Shimizu, Y. Facile ultrasonic-assisted synthesis of SiO2/ZnO core/shell nanostructures: A selective ethanol sensor at low temperatures with enhanced recovery. Sens. Actuators B Chem. 2022, 368, 132187. [Google Scholar] [CrossRef]
- Li, C.; Choi, P.G.; Kim, K.; Masuda, Y. High performance acetone gas sensor based on ultrathin porous NiO nanosheet. Sens. Actuators B Chem. 2022, 367, 132143. [Google Scholar] [CrossRef]
- Bai, H.; Guo, H.; Wang, J.; Dong, Y.; Liu, B.; Xie, Z.; Guo, F.; Chen, D.; Zhang, R.; Zheng, Y. A room-temperature NO2 gas sensor based on CuO nanoflakes modified with rGO nanosheets. Sens. Actuators B Chem. 2021, 337, 129783. [Google Scholar] [CrossRef]
- Wang, T.; Liu, G.; Zhang, D.; Wang, D.; Chen, F.; Guo, J. Fabrication and properties of room temperature ammonia gas sensor based on SnO2 modified WSe2 nanosheets heterojunctions. Appl. Surf. Sci. 2022, 597, 153564. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Myung, J.-h. Selective ppb-level NO2 gas sensor based on SnO2-boron nitride nanotubes. Sens. Actuators B Chem. 2021, 331, 129464. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Ellison, G.B. Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-F.; Liu, W.-K.; Li, S.; Li, Y.; Shi, Z.-F.; Tian, Y.-T.; Li, X.-J. High-performance methanol sensor based on GaN nanostructures grown on silicon nanoporous pillar array. Sens. Actuators B Chem. 2017, 250, 518–524. [Google Scholar] [CrossRef]



| Samples | Oc (%) | OL (%) |
|---|---|---|
| ZnO | 54.92 | 45.08 |
| Au@ZnO | 61.09 | 38.91 |
| Pt@ZnO | 59.19 | 40.81 |
| AuPt@ZnO | 65.81 | 34.19 |
| Materials | Morphology | Concentration (ppm) | Response (Ra/Rg) | Operating Temperature (°C) | Response/Recovery Time (s) | Reference |
|---|---|---|---|---|---|---|
| Au-ZnO | Nanowire | 100 | 8.623 | 340 | 60/180 | [44] |
| Pt-coated SnO2-ZnO | Core–shell nanofibers | 50 | 3.14 (Voltage 20 V) | RT | -/- | [45] |
| ZnO/ZnFe2O4 | Spherical urchin-like core–shell structure | 100 | 59.41 | 275 | 3/189 | [1] |
| ZnO@Co3O4 | Hollow cubes | 100 | 26.4 | 290 | 11.2/12.5 | [46] |
| Pt@ZnO-TiO2 | Nanotube | 5 | 25 | 300 | 7.5/20.1 | [47] |
| ZnO/graphene | Nanoparticle | 1 | 12.57 | 300 | -/- | [48] |
| In2O3-ZnO | Nanofiber | 100 | 14.63 | RT | 14/201 | [49] |
| NiO/ZnO | Nanoflower | 95 | 19.1 | 300 | 70/55 | [50] |
| AuPt@ZnO | Nanoflower | 50 | 69.7 | 175 | 22.4/136.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Liu, Y.; Shen, Y.; Xu, L.; Lu, J.; Li, M.; Lu, H.-L.; Gao, L. Highly Sensitive and Selective Toluene Gas Sensors Based on ZnO Nanoflowers Decorated with Bimetallic AuPt. Molecules 2024, 29, 1657. https://doi.org/10.3390/molecules29071657
Peng H, Liu Y, Shen Y, Xu L, Lu J, Li M, Lu H-L, Gao L. Highly Sensitive and Selective Toluene Gas Sensors Based on ZnO Nanoflowers Decorated with Bimetallic AuPt. Molecules. 2024; 29(7):1657. https://doi.org/10.3390/molecules29071657
Chicago/Turabian StylePeng, Huiting, Yiping Liu, Yinfeng Shen, Ling Xu, Jicun Lu, Ming Li, Hong-Liang Lu, and Liming Gao. 2024. "Highly Sensitive and Selective Toluene Gas Sensors Based on ZnO Nanoflowers Decorated with Bimetallic AuPt" Molecules 29, no. 7: 1657. https://doi.org/10.3390/molecules29071657
APA StylePeng, H., Liu, Y., Shen, Y., Xu, L., Lu, J., Li, M., Lu, H.-L., & Gao, L. (2024). Highly Sensitive and Selective Toluene Gas Sensors Based on ZnO Nanoflowers Decorated with Bimetallic AuPt. Molecules, 29(7), 1657. https://doi.org/10.3390/molecules29071657








