Metformin Loaded Zein Polymeric Nanoparticles to Augment Antitumor Activity against Ehrlich Carcinoma via Activation of AMPK Pathway: D-Optimal Design Optimization, In Vitro Characterization, and In Vivo Study
Abstract
1. Introduction
2. Results
2.1. Analysis of D-Optimal Design
2.2. Selection of the Optimum ZNs
2.3. Characterization of the Optimum ZNs
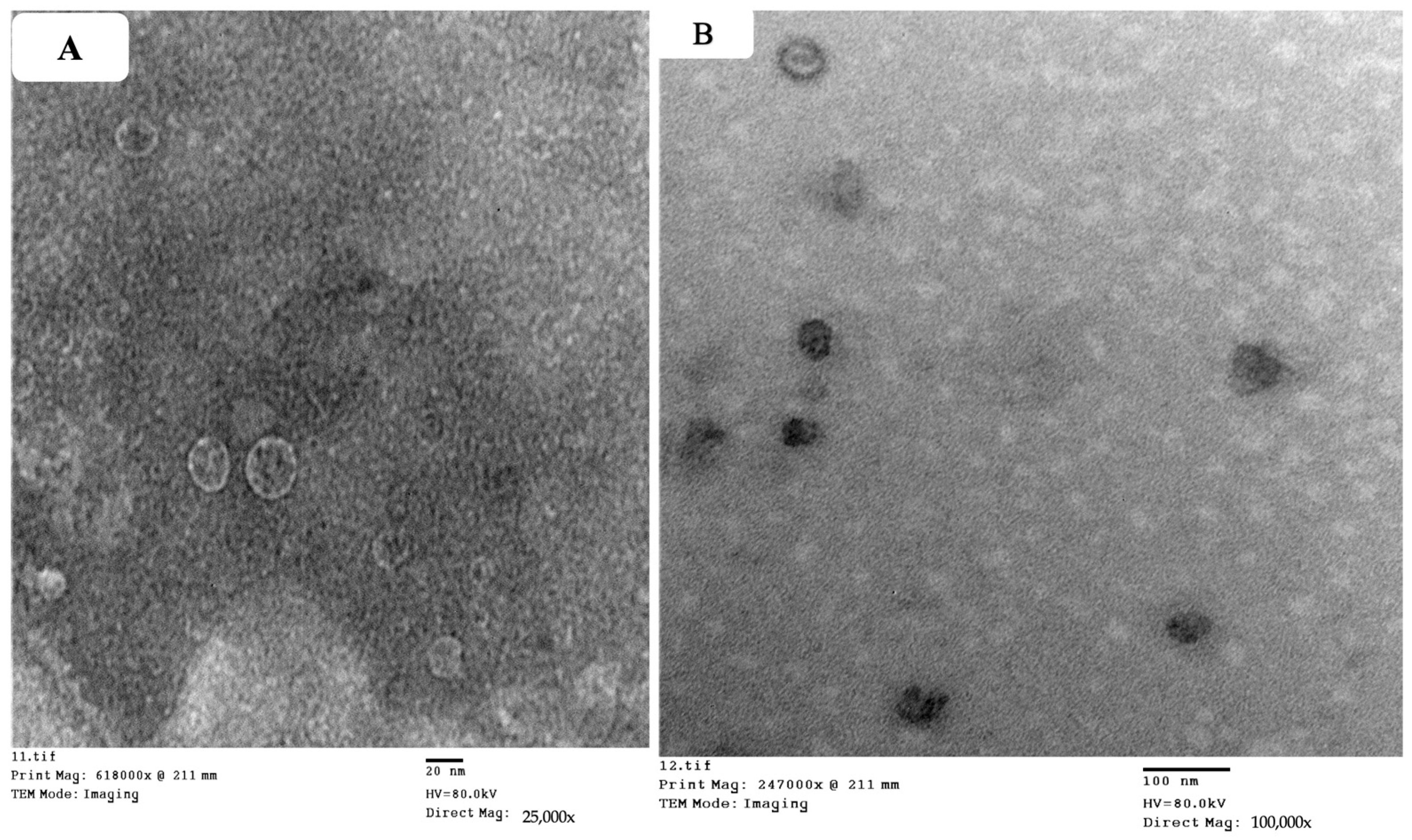
2.3.1. Transmission Electron Microscope (TEM)
2.3.2. In Vitro Release Study
2.3.3. Effect of Short-Term Storage
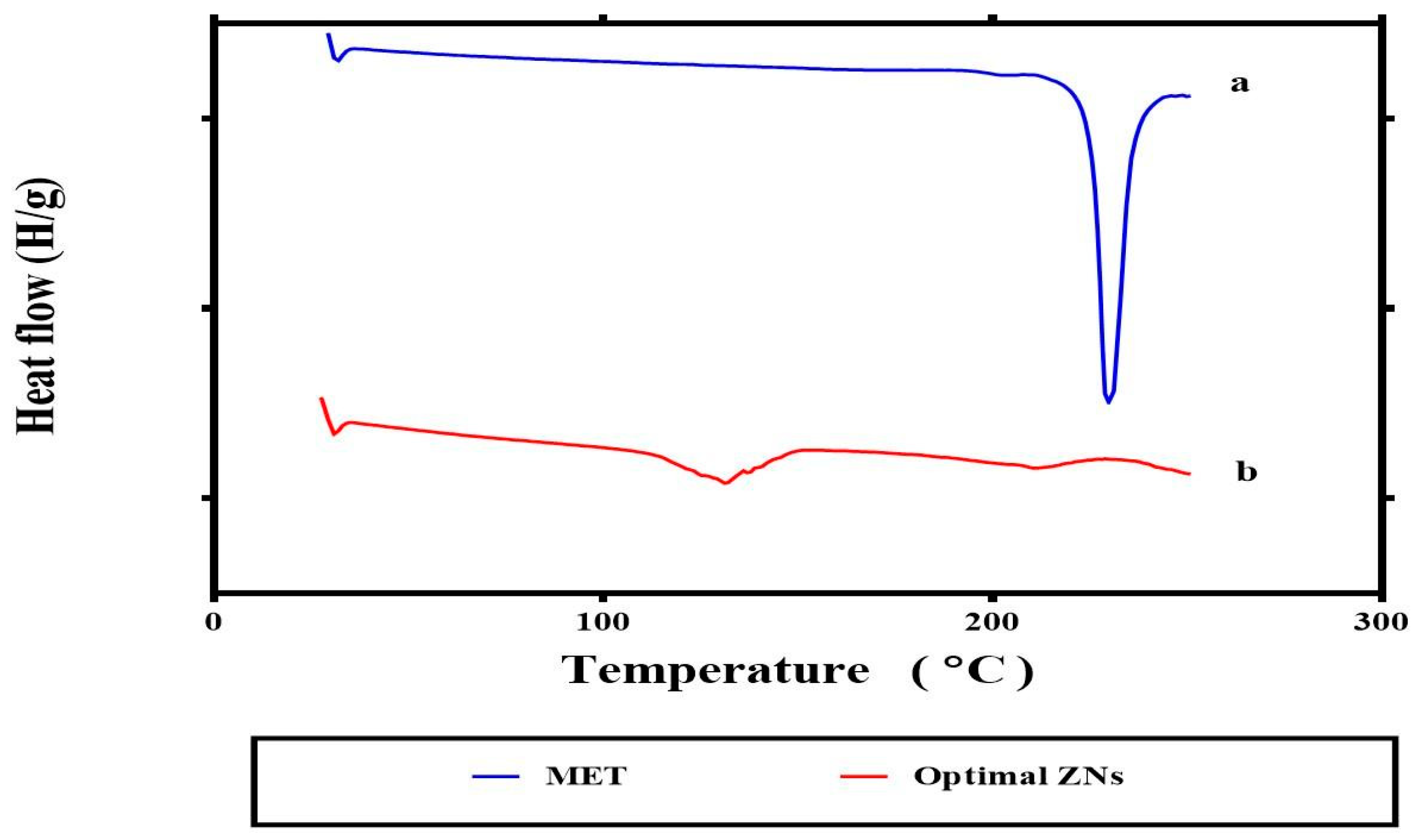
2.3.4. Differential Scanning Calorimetry
2.4. In Vivo Study
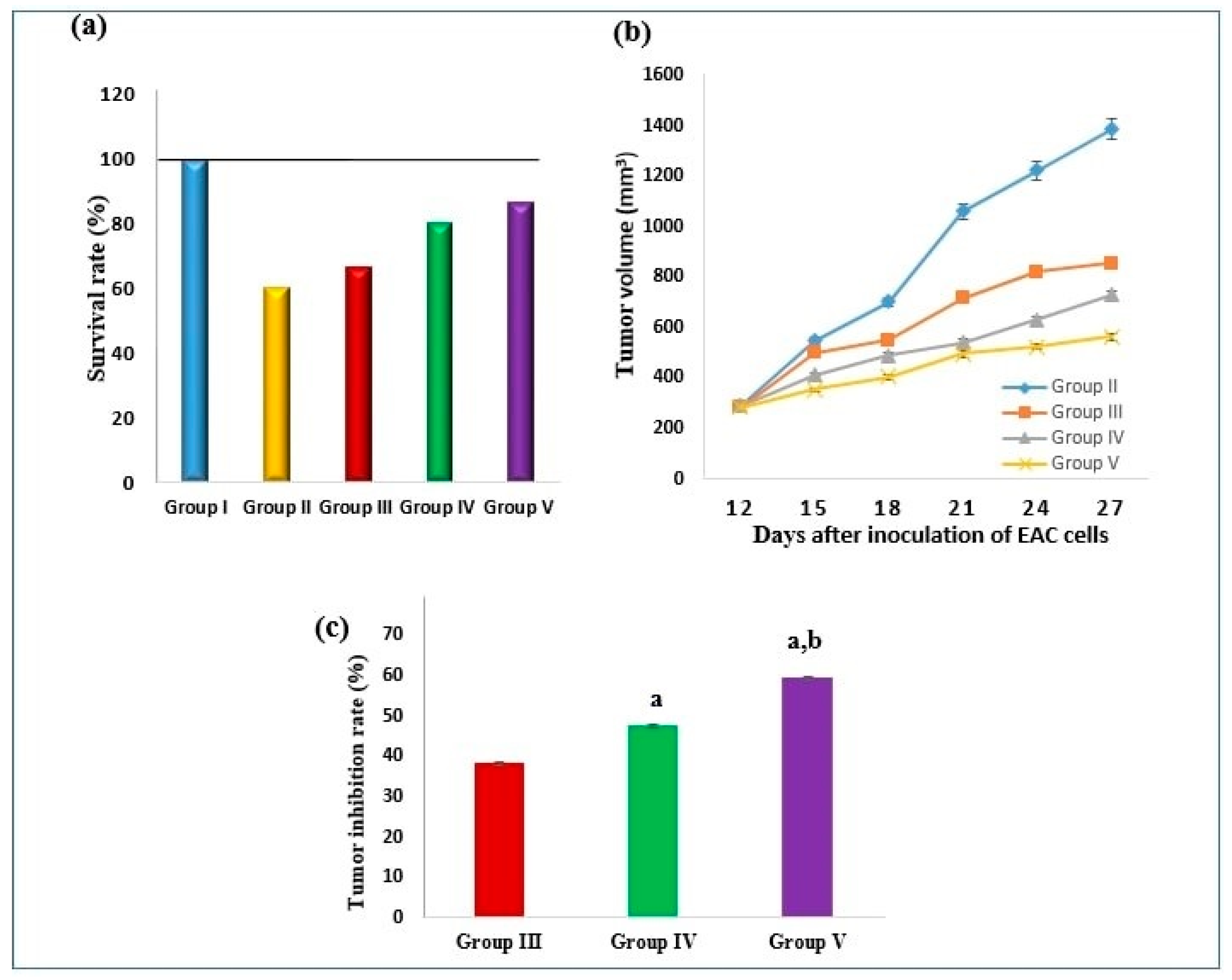
2.4.1. Effect on Survival Rate and Tumor Volume
2.4.2. Effect on Tumor P53, NF-κB Gene Expression, and pAMPK Level
2.4.3. Effect on Tumor Cyclin D1 and Caspase-3 Levels
2.4.4. Effect on Tumor COX-2 and PGE2 Levels
2.4.5. Effect on miRNA-191-5p and miRNA-543
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fabrication of MET-Loaded ZNs
4.3. In Vitro Characterization and Optimization of MET-Loaded ZNs
4.3.1. Calculation of Entrapment Efficiency Percent (EE%)
4.3.2. Calculation of Particle Size, Polydispersity Index, and Zeta Potential
4.3.3. Optimization of ZNs
4.3.4. Transmission Electron Microscope (TEM)
4.3.5. In Vitro Release Study
4.3.6. Effect of Short-Term Storage
4.3.7. Differential Scanning Calorimetry
4.4. In Vivo Study
4.4.1. Animals and Experimental Design
4.4.2. Assessment of Biochemical Parameters
4.4.3. Real-Time Polymerase Chain Reaction (RT-PCR) for P53, NF-κB, miRNA-191-5p, and miRNA-543
4.4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roshan, M.H.; Shing, Y.K.; Pace, N.P. Metformin as an adjuvant in breast cancer treatment. SAGE Open Med. 2019, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Dong, X.; Zhou, L.; Xiao, H.; Ho, P.Y.; Wong, M.S.; Wang, Y. Doxorubicin-loaded biodegradable self-assembly zein nanoparticle and its anti-cancer effect: Preparation, in vitro evaluation, and cellular uptake. Colloids Surf. B Biointerfaces 2016, 140, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Luo, J.; Yu, T.; Zhou, L.; Lv, H.; Shang, P. Anticancer mechanisms of metformin: A review of the current evidence. Life Sci. 2020, 254, 117717. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. Br. Med. J. 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [PubMed]
- Podhorecka, M. Metformin-its anti-cancer effects in hematologic malignancies. Oncol. Rev. 2021, 15, 514. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Paolino, D.; Iannone, M.; Palma, E.; Fresta, M.; Cosco, D. Sodium deoxycholate-decorated zein nanoparticles for a stable colloidal drug delivery system. Int. J. Nanomed. 2018, 13, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Liu, D.; Zhu, W.; Guan, S.; Fan, L.; Cai, D. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr. Polym. 2020, 240, 116325. [Google Scholar] [CrossRef] [PubMed]
- Regier, M.C.; Taylor, J.D.; Borcyk, T.; Yang, Y.; Pannier, A.K. Fabrication and characterization of DNA-loaded zein nanospheres. J. Nanobiotechnol. 2012, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Bonacci, S.; Paolino, D.; Celia, C.; Procopio, A.; Fresta, M.; Cosco, D. Paclitaxel-loaded sodium deoxycholate-stabilized zein nanoparticles: Characterization and in vitro cytotoxicity. Heliyon 2019, 5, e02422. [Google Scholar] [CrossRef]
- Shinde, P.; Agraval, H.; Srivastav, A.K.; Yadav, U.C.; Kumar, U. Physico-chemical characterization of carvacrol loaded zein nanoparticles for enhanced anticancer activity and investigation of molecular interactions between them by molecular docking. Int. J. Pharm. 2020, 588, 119795. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Chen, F.; Cheng, K.W. Hyaluronic Acid–Zein Core-Shell Nanoparticles Improve the Anticancer Effect of Curcumin Alone or in Combination with Oxaliplatin against Colorectal Cancer via CD44-Mediated Cellular Uptake. Molecules 2022, 27, 1498. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Ragaie, M.H.; Hassab, M.A.E.; El-Haggar, R.; Eldehna, W.M.; Al-Rashood, S.T.; Mosallam, S. Fenticonazole nitrate loaded trans-novasomes for effective management of tinea corporis: Design characterization, in silico study, and exploratory clinical appraisal. Drug Deliv. 2022, 29, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Elmahboub, Y.; Baraka, K.; Abdellatif, M.M.; Alaa-Eldin, A.A. Ultra-deformable liposomes containing terpenes (terpesomes) loaded fenticonazole nitrate for treatment of vaginal candidiasis: Box-Behnken design optimization, comparative ex vivo and in vivo studies. Drug Deliv. 2020, 27, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Nandi, G.; Changder, A.; Hudati, P.; Sarkar, S.; Ghosh, L.K. Sustained release gastroretentive tablet of metformin hydrochloride based on poly (acrylic acid)-grafted-gellan. Int. J. Biol. Macromol. 2017, 96, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Pardakhty, A.; Mohsen, S.; Baharanchi, H. Sorbitan monopalmitate-based proniosomes for transdermal delivery of chlorpheniramine maleate. Drug Deliv. 2005, 12, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Voci, S.; Salvatici, M.C.; Fresta, M.; Cosco, D. Brij-stabilized zein nanoparticles as potential drug carriers. Colloids Surf. B Biointerfaces 2021, 201, 111647. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Ali, A.A.; Khalil, N.M.; Elsisi, A.A. Evaluation of metformin hydrochloride tailoring bilosomes as an effective transdermal nanocarrier. Int. J. Nanomed. 2022, 17, 1185–1201. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Bilosomes as a novel carrier for the cutaneous delivery for dapsone as a potential treatment of acne: Preparation, characterization and in vivo skin deposition assay. J. Lip. Res. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Caster, J.M.; Stephanie, K.Y.; Patel, A.N.; Newman, N.J.; Lee, Z.J.; Warner, S.B.; Wagner, K.T.; Roche, K.C.; Tian, X.; Min, Y.; et al. Effect of particle size on the biodistribution, toxicity, and efficacy of drug-loaded polymeric nanoparticles in chemoradiotherapy. Nanomedicine 2017, 13, 1673–1683. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Whent, M.; Yu, L.L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surf. B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, J.; Kulkarni, R.; Chaudhari, R. In-vitro and ex-vivo evaluation of raloxifene hydrochloride delivery using nano-transfersome based formulations. J. Drug Deliv. Sci. Technol. 2018, 45, 151–158. [Google Scholar] [CrossRef]
- Hathout, R.M.; Elshafeey, A.H. Development and characterization of colloidal soft nano-carriers for transdermal delivery and bioavailability enhancement of an angiotensin II receptor blocker. Eur. J. Pharm. Biopharm. 2012, 82, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Hashem, F.M.; Al-Sawahli, M.M.; Nasr, M.; Ahmed, O.A. Optimized zein nanospheres for improved oral bioavailability of atorvastatin. Int. J. Nanomed. 2015, 10, 4059–4069. [Google Scholar] [CrossRef]
- Abd El-Alim, S.H.; Kassem, A.A.; Basha, M.; Salama, A. Comparative study of liposomes, ethosomes and transfersomes as carriers for enhancing the transdermal delivery of diflunisal: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 563, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Kim, A.; Oh, Y.K.; Kim, C.K. Effect of edge activators on the formation and transfection efficiency of ultradeformable liposomes. Biomaterials 2005, 26, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, J.; Yang, X.; Li, Y.; Wu, S.; Huang, Y.; Ye, S.; Xie, L.; Dai, L.; Hou, Z. Self-assembled nanoparticles based on amphiphilic anticancer drug–phospholipid complex for targeted drug delivery and intracellular dual-controlled release. ACS Appl. Mater. Interfaces 2015, 7, 17573–17581. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; El-Nabarawi, M.A.; Refai, H.; Abdelbary, A.A. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: In-vitro characterization, ex-vivo permeation and in-vivo assessment. Int. J. Nanomed. 2019, 2019, 6555–6574. [Google Scholar] [CrossRef]
- Gowda, N.G.S.; Shiragannavar, V.D.; Prabhuswamimath, S.C.; Tuladhar, S.; Chidambaram, S.B.; Santhekadur, P.K. Ehrlich Ascites carcinoma mice model for studying liver inflammation and fibrosis. Adv. Cancer Biol. Metastasis 2022, 4, 100029. [Google Scholar] [CrossRef]
- Jiralerspong, S.; Palla, S.L.; Giordano, S.H.; Meric-Bernstam, F.; Liedtke, C.; Barnett, C.M.; Hsu, L.; Hung, M.C.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 2009, 27, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xiao, J.; Liu, J.; Liu, J.; Shu, G.; Yin, G. Metformin suppresses the growth of colorectal cancer by targeting INHBA to inhibit TGF-β/PI3K/AKT signaling transduction. Cell Death Dis. 2022, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Iwama, H.; Yamashita, T.; Kobayashi, K.; Fujihara, S.; Fujimori, T.; Kamada, H.; Kobara, H.; Masaki, T. The anti-diabetic drug metformin inhibits pancreatic cancer cell proliferation in vitro and in vivo: Study of the microRNAs associated with the antitumor effect of metformin. Oncol. Rep. 2016, 35, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Lee, Y.H.; Koo, K.C. Current status and application of metformin for prostate cancer: A comprehensive review. Int. J. Mol. Sci. 2020, 21, 8540. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.B.; Rojas, E.A.; Misiewicz-Krzeminska, I.; Krzeminski, P.; Gutiérrez, N.C. Molecular mechanisms of p53 deregulation in cancer: An overview in multiple myeloma. Int. J. Mol. Sci. 2016, 17, 2003. [Google Scholar] [CrossRef] [PubMed]
- Ben Sahra, I.; Tanti, J.-F.; Bost, F. The combination of metformin and 2 deoxyglucose inhibits autophagy and induces AMPK-dependent apoptosis in prostate cancer cells. Autophagy 2010, 6, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, M.; Tichet, M.; Abbe, P.; Ohanna, M.; Lehraiki, A.; Rouaud, F.; Allegra, M.; Giacchero, D.; Bahadoran, P.; Bertolotto, C.; et al. Metformin blocks melanoma invasion and metastasis development in ampk/p53-dependent mannermetformin inhibits melanoma invasion. Mol. Cancer Ther. 2013, 12, 1605–1615. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Liu, Z.; Bi, M.H.; Zhang, J.J.; Han, Z.Q.; Han, X.; Wang, H.Y.; Sun, G.P.; Liu, H. Metformin induces apoptosis via a mitochondria-mediated pathway in human breast cancer cells in vitro. Exp. Ther. Med. 2016, 11, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Cejuela, M.; Martin-Castillo, B.; Menendez, J.A.; Pernas, S. Metformin and breast cancer: Where are we now? Int. J. Mol. Sci. 2022, 23, 2705. [Google Scholar] [CrossRef]
- Lu, C.-C.; Chiang, J.H.; Tsai, F.J.; Hsu, Y.M.; Juan, Y.N.; Yang, J.S.; Chiu, H.Y. Metformin triggers the intrinsic apoptotic response in human AGS gastric adenocarcinoma cells by activating AMPK and suppressing mTOR/AKT signaling. Int. J. Oncol. 2019, 54, 1271–1281. [Google Scholar] [CrossRef]
- Chen, K.; Qian, W.; Jiang, Z.; Cheng, L.; Li, J.; Sun, L.; Zhou, C.; Gao, L.; Lei, M.; Yan, B.; et al. Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer. Mol. Cancer 2017, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Aljofan, M.; Riethmacher, D. Anticancer activity of metformin: A systematic review of the literature. Future Sci. OA 2019, 5, FSO410. [Google Scholar] [CrossRef] [PubMed]
- Hadad, S.; Hardie, D.G.; Appleyard, V.; Thompson, A.M. Effects of metformin on breast cancer cell proliferation, the AMPK pathway and the cell cycle. Clin. Transl. Oncol. 2014, 16, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E.; Litchfield, L.M.; Mitra, A.K.; Nieman, K.M.; Mukherjee, A.; Zhang, Y.; Johnson, A.; Bradaric, M.; Lee, W.; Romero, I.L. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am. J. Obst. Gynecol. 2015, 212, 479.e1–479.e10. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Hu, X.; He, H.; Fang, W. Metformin suppresses breast cancer growth via inhibition of cyclooxygenase-2. Oncol. Let. 2021, 22, 615. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Miskimins, W.K. Metformin induces both caspase-dependent and poly (adp-ribose) polymerase-dependent cell death in breast cancer cellsmetformin cytotoxicity to breast cancer cells. Mol. Cancer Res. 2011, 9, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elmatty, D.M.; Ahmed, E.A.; Tawfik, M.K.; Helmy, S.A. Metformin enhancing the antitumor efficacy of carboplatin against Ehrlich solid carcinoma grown in diabetic mice: Effect on IGF-1 and tumoral expression of IGF-1 receptors. Int. Immunopharmacol. 2017, 44, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, J.N.; Zeng, C.; Li, X.; Zhou, Y.F.; Qi, Y.; Xue, Q. Metformin suppresses prostaglandin E2-induced cytochrome P450 aromatase gene expression and activity via stimulation of AMP-activated protein kinase in human endometriotic stromal cells. Reprod Sci. 2015, 22, 1162–1170. [Google Scholar] [CrossRef]
- Alimoradi, N.; Firouzabadi, N.; Fatehi, R. Metformin and insulin-resistant related diseases: Emphasis on the role of microRNAs. Biomed. Pharmacother. 2021, 139, 111662. [Google Scholar] [CrossRef]
- Sharma, S.; Nagpal, N.; Ghosh, P.C.; Kulshreshtha, R. P53-miR-191-SOX4 regulatory loop affects apoptosis in breast cancer. RNA 2017, 23, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Li, K.K.; Gao, L.; Li, S.Z.; Chen, K.; Zhang, J.B.; Wang, D.; Tu, R.F.; Zhang, J.X.; Tao, K.X.; et al. miR-191 promotes tumorigenesis of human colorectal cancer through targeting C/EBPβ. Oncotarget 2015, 6, 4144–4158. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xu, W.; Luo, Y.; Zhang, Y.; He, Y.; Yang, S.; Yuan, Z. MicroRNA 543 suppresses breast cancer cell proliferation, blocks cell cycle and induces cell apoptosis via direct targeting of ERK/MAPK. OncoTargets Ther. 2017, 10, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.A.A.; El-Maadawy, W.H.; Yousry, C.; ElMeshad, A.N.; Shoukri, R.A. Zein/phospholipid composite nanoparticles for successful delivery of gallic acid into ahscs: Influence of size, surface charge, and vitamin a coupling. Int. J. Nanomed. 2020, 15, 7995–8018. [Google Scholar] [CrossRef] [PubMed]
- Mosallam, S.; Ragaie, M.H.; Moftah, N.H.; Elshafeey, A.H.; Abdelbary, A.A. Use of novasomes as a vesicular carrier for improving the topical delivery of terconazole: In vitro characterization, in vivo assessment and exploratory clinical experimentation. Int. J. Nanomed. 2021, 16, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.M.; Abd El Malak, N.S.; Yehia, S.A.; Ahmed, M.A. Hyaluronic acid conjugated metformin-phospholipid sonocomplex: A biphasic complexation approach to correct hypoxic tumour microenvironment. Int. J. Nanomed. 2021, 16, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, M.M.; Khalil, I.A.; Khalil, M.A. Sertaconazole nitrate loaded nanovesicular systems for targeting skin fungal infection: In-vitro, ex-vivo and in-vivo evaluation. Int. J. Pharm. 2017, 527, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Fahmy, A.M.; Hamed, M.I.; Darwish, K.M.; El-Dahmy, R.M. Spironolactone hyaluronic acid enriched cerosomes (HAECs) for topical management of hirsutism: In silico studies, statistical optimization, ex vivo, and in vivo studies. Drug Deliv. 2021, 28, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.M.; Mosallam, S.; Mamdouh, M.A.; Hussein, M.A.; Abd El-Halim, S.M. Design and optimization of cranberry extract loaded bile salt augmented liposomes for targeting of MCP-1/STAT3/VEGF signaling pathway in DMN-intoxicated liver in rats. Drug Deliv. 2022, 29, 427–439. [Google Scholar] [CrossRef]
- Abdelbari, M.A.; El-Gazar, A.A.; Abdelbary, A.A.; Elshafeey, A.H.; Mosallam, S. Brij® integrated bilosomes for improving the transdermal delivery of niflumic acid for effective treatment of osteoarthritis: In vitro characterization, ex vivo permeability assessment, and in vivo study. Int. J. Pharm. 2023, 640, 123024. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mahmoud, O.A.; Ali, D.S.; Mamdouh, M.; Metwally, A.A. Modeling Drugs-PLGA Nanoparticles Interactions Using Gaussian Processes: Pharmaceutics Informatics Approach. J. Clus. Sci. 2021, 2021040524. [Google Scholar] [CrossRef]
- Albash, R.; Al-Mahallawi, A.M.; Hassan, M.; Alaa-Eldin, A.A. Development and optimization of terpene-enriched vesicles (terpesomes) for effective ocular delivery of fenticonazole nitrate: In vitro characterization and in vivo assessment. Int. J. Nanomed. 2021, 16, 609–621. [Google Scholar] [CrossRef]
- Albash, R.; Badawi, N.M.; Hamed, M.I.; Ragaie, M.H.; Mohammed, S.S.; Elbesh, R.M.; Darwish, K.M.; Lashkar, M.O.; Elhady, S.S.; Mosallam, S. Exploring the synergistic effect of bergamot essential oil with spironolactone loaded nano-phytosomes for treatment of acne vulgaris: In vitro optimization, in silico studies, and clinical evaluation. Pharmaceuticals 2023, 16, 128. [Google Scholar] [CrossRef]
- El-Naggar, M.M.; El-Nabarawi, M.A.; Teaima, M.H.; Hassan, M.; Hamed, M.I.; Elrashedy, A.A.; Albash, R. Integration of terpesomes loaded Levocetrizine dihydrochloride gel as a repurposed cure for Methicillin-Resistant Staphylococcus aureus (MRSA)-Induced skin infection; D-optimal optimization, ex-vivo, in-silico, and in-vivo studies. Int. J. Pharm. 2023, 633, 122621. [Google Scholar] [CrossRef]
- El-Nabarawi, M.; Khalil, I.; Saad, R. Impact of hydrophilic polymer solubilization on bioavailability enhancement of repaglinide by solid dispersion. Inven. Rapid Pharm. Tech. 2016, 3, 1–12. [Google Scholar]
- Hirsch, H.A.; Iliopoulos, D.; Joshi, A.; Zhang, Y.; Jaeger, S.A.; Bulyk, M.; Tsichlis, P.N.; Liu, X.S.; Struhl, K. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell 2010, 17, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; An, L.; Yan, D.; Matsuura, H.; Ding, W.; Zhang, M.; Xu, G.; Sun, Y.; Yuan, G.; Wang, M.; et al. Combined antitumor effects of P-5m octapeptide and 5-fluorouracil on a murine model of H22 hepatoma ascites. Exp. Ther. Med. 2018, 16, 1586–1592. [Google Scholar] [CrossRef]
- Cosetti, M.; Yu, G.-P.; Schantz, S.P. Five-year survival rates and time trends of laryngeal cancer in the US population. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.E.-M.M.; Ahmed, M.M.S.; Khayyal, M.T.E.D.; El-Merzabani, M.M. Hyperthermic potentiation of cisplatin cytotoxicity on solid Ehrlich carcinoma. Tumori J. 1993, 79, 268–272. [Google Scholar] [CrossRef]
- Salem, M.L.; Shoukry, N.M.; Teleb, W.K.; Abdel-Daim, M.M.; Abdel-Rahman, M.A. In vitro and in vivo antitumor effects of the Egyptian scorpion Androctonus amoreuxi venom in an Ehrlich ascites tumor model. Springerplus 2016, 5, 570. [Google Scholar] [CrossRef]
- Clotman, F.; Lannoy, V.J.; Reber, M.; Cereghini, S.; Cassiman, D.; Jacquemin, P.; Roskams, T.; Rousseau, G.G.; Lemaigre, F.P. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development 2002, 129, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
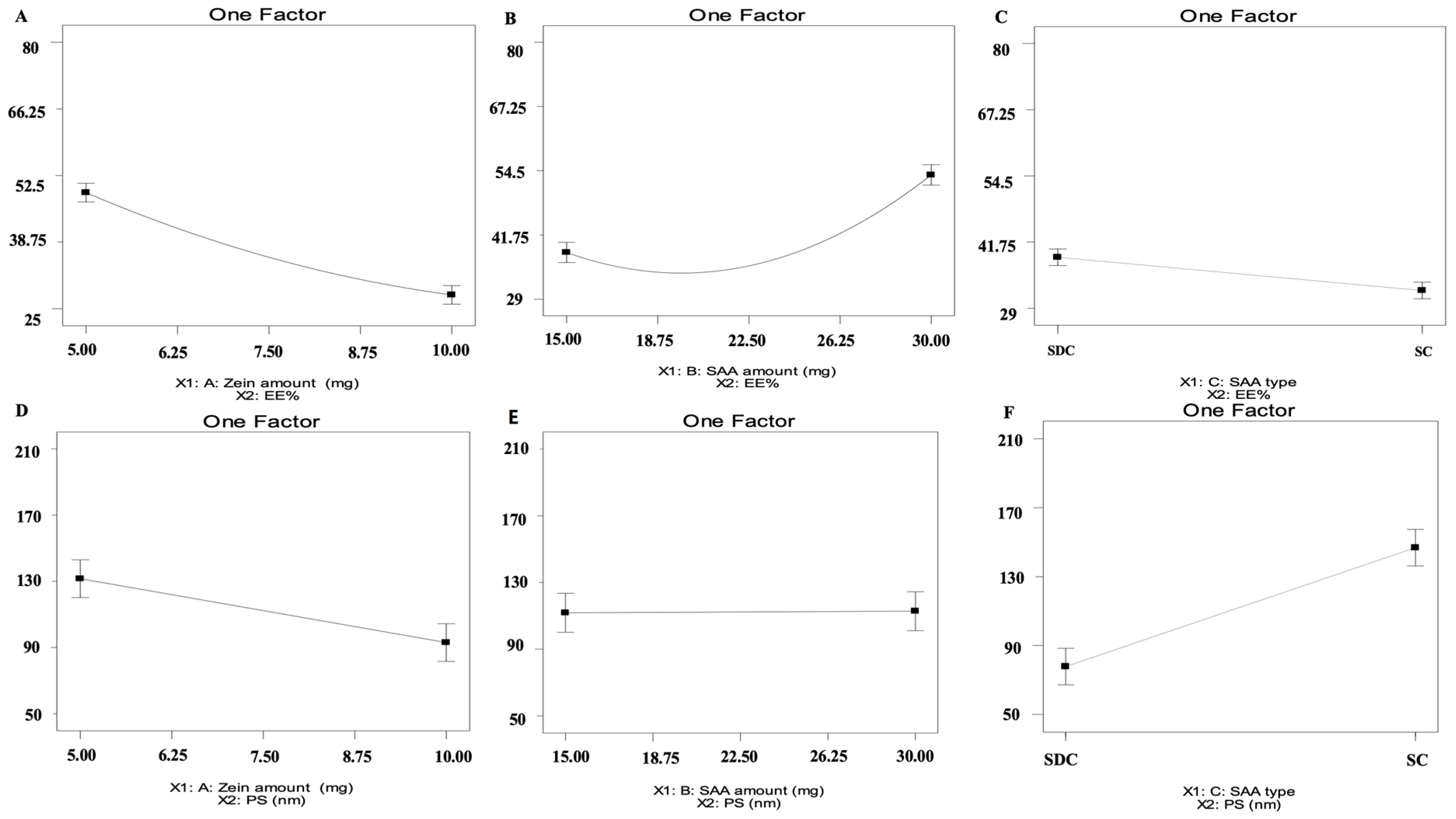
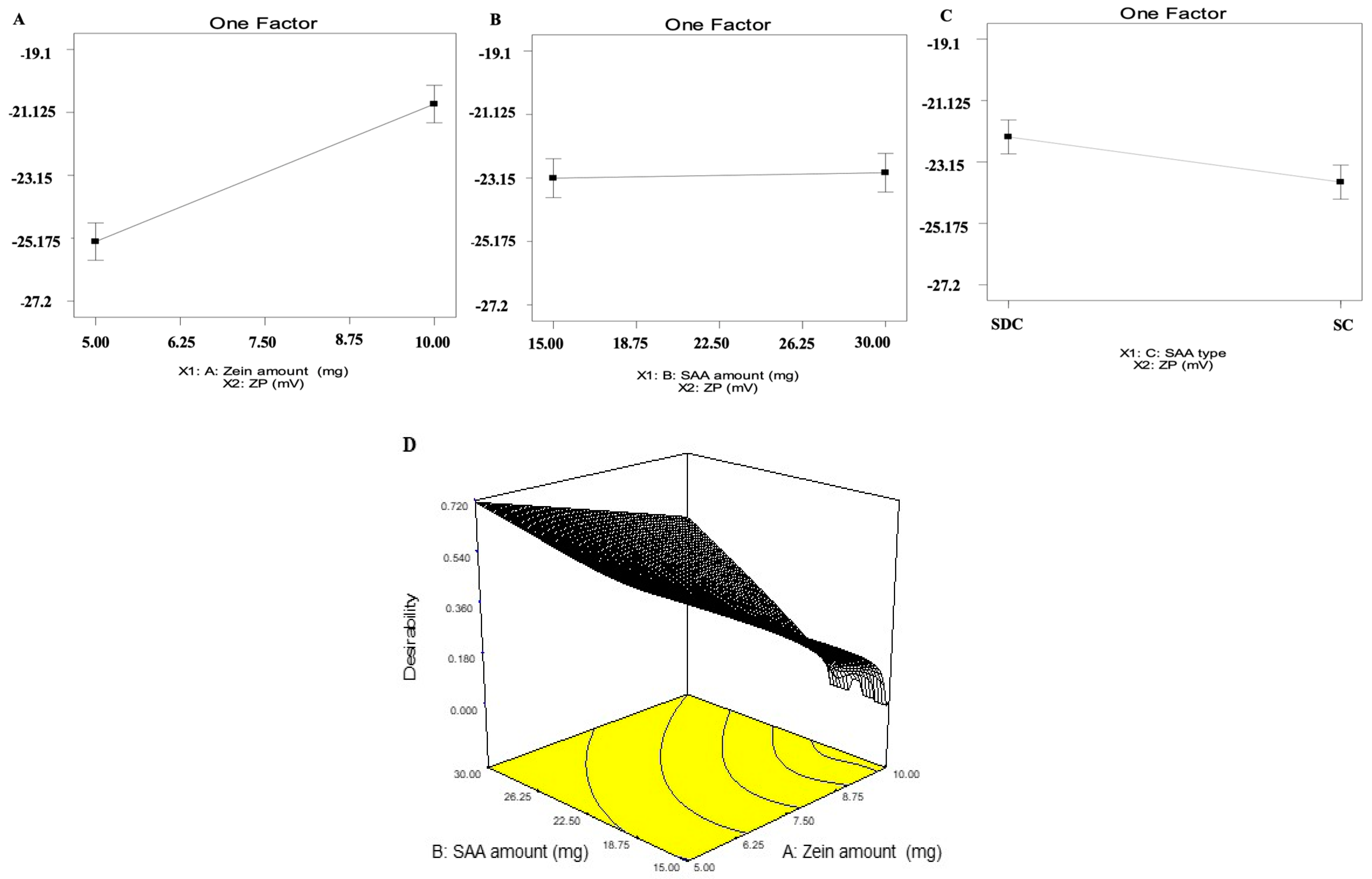


| Model | Adequate Precision | R2 | Adjusted R2 | Predicted R2 | p Value | |
|---|---|---|---|---|---|---|
| Y1: EE% | Quadratic | 51.973 | 0.99 | 0.99 | 0.98 | <0.0001 |
| Y2: PS (nm) | 2FI | 13.192 | 0.92 | 0.86 | 0.79 | 0.0005 |
| Y3: ZP (mV) | 2FI | 13.634 | 0.92 | 0.86 | 0.79 | 0.0005 |
| EE% | PS (nm) | ZP (mV) | ||||
| Predicted values of ZN 8 | 78.15 | 55.97 | −23.29 | |||
| Observed values of ZN 8 | 77.68 | 59.69 | −24.00 | |||
| Bias % | 0.60 | 6.23 | 2.95 | |||
| Zein Amount (mg) | Bile Salt Amount (mg) | Bile Salt Type | EE% | PS (nm) | PDI | ZP (mV) | |
|---|---|---|---|---|---|---|---|
| F1 | 10 | 15 | SDC | 38.77 ± 6.84 | 126.80 ± 2.46 | 0.43 ± 0.08 | −22.80 ± 3.23 |
| F2 | 5 | 15 | SDC | 43.53 ± 3.90 | 79.00 ± 8.35 | 0.76 ± 0.06 | −26.90 ± 1.06 |
| F3 | 10 | 30 | SDC | 52.55 ± 1.07 | 103.00 ± 2.98 | 0.73 ± 0.01 | −21.40 ± 0.61 |
| F4 | 10 | 30 | SDC | 52.55 ± 1.07 | 103.00 ± 2.98 | 0.73 ± 0.01 | −21.40 ± 0.61 |
| F5 | 5 | 30 | SDC | 77.68 ± 1.28 | 59.69 ± 1.79 | 0.49 ± 0.03 | −24.00 ± 0.80 |
| F6 | 7.5 | 22.5 | SDC | 32.75 ± 7.19 | 71.51 ± 22.90 | 0.34 ± 0.12 | −23.86 ± 1.23 |
| F7 | 7.5 | 15 | SDC | 40.31 ± 2.60 | 121.90 ± 3.86 | 0.99 ± 0.01 | −23.20 ± 1.26 |
| F8 | 5 | 30 | SDC | 77.68 ± 1.28 | 59.69 ± 1.79 | 0.49 ± 0.03 | −24.00 ± 0.80 |
| F9 | 5 | 15 | SC | 57.73 ± 1.39 | 87.33 ± 3.60 | 0.43 ± 0.01 | −26.70 ± 0.55 |
| F10 | 8.75 | 22.5 | SC | 37.39 ± 12.10 | 73.91 ± 1.17 | 0.51 ± 0.08 | −23.90 ± 0.51 |
| F11 | 5 | 22.5 | SC | 28.79 ± 6.54 | 69.21 ± 2.83 | 0.54 ± 0.11 | −24.10 ± 2.42 |
| F12 | 10 | 15 | SC | 40.78 ± 4.91 | 64.96 ± 1.95 | 0.36 ± 0.05 | −20.30 ± 0.32 |
| F13 | 10 | 30 | SC | 40.91 ± 7.17 | 62.55 ± 1.14 | 0.41 ± 0.01 | −21.40 ± 1.73 |
| F14 | 10 | 30 | SC | 40.91 ± 7.17 | 62.55 ± 1.14 | 0.41 ± 0.01 | −21.40 ± 1.73 |
| F15 | 7.5 | 15 | SC | 32.26 ± 9.97 | 62.33 ± 1.33 | 0.40 ± 0.01 | −20.10 ± 2.46 |
| No. | Group | P53 (Relative Expression) | NF-κB (Relative Expression) | pAMPK (ng/g Tissue) |
|---|---|---|---|---|
| I | Normal control | 1.01 ± 0.026 | 1.08 ± 0.022 | 10.73 ± 0.28 |
| II | SEC control | 0.67 ± 0.034 a,c,d,e | 3.53 ± 0.11 a,c,d,e | 5.61 ± 0.32 a,c,d,e |
| III | SEC bearing mice + MET | 1.15 ± 0.055 b,d,e | 2.95 ± 0.046 a,b,d,e | 8.07 ± 0.39 a,b |
| IV | SEC bearing mice + ZN8 | 4.78 ± 0.088 a,b,c,e | 1.61 ± 0.027 a,b,c,e | 9.06 ± 0.78 b |
| V | SEC bearing mice + 5-FU | 3.22 ± 0.059 a,b,c,d | 2.41 ± 0.033 a,b,c,d | 9.57 ± 0.48 b |
| No. | Group | Cyclin D1 (ng/g Tissue) | Caspase-3 (pg/g Tissue) | COX-2 (U/mg Tissue) | PGE2 (pg/g Tissue) |
|---|---|---|---|---|---|
| I | Normal control | 0.585 ± 0.026 | 11.27 ± 0.293 | 23.2 ± 0.928 | 103.7 ± 3.83 |
| II | SEC control | 4.37 ± 0.165 a,c,d,e | 0.87 ± 0.073 a,c,d,e | 82.10 ± 2.66 a,c,d,e | 924.5 ± 15.89 a,c,d,e |
| III | SEC bearing mice + MET | 3.11 ± 0.157 a,b,d,e | 3.15 ± 0.124 a,b,d,e | 63.56 ± 2.81 a,b,d,e | 631.5 ± 17.02 a,b,d,e |
| IV | SEC bearing mice + ZN8 | 1.42 ± 0.075 a,b,c,e | 5.4 ± 0.148 a,b,c,e | 42.84 ± 1.52 a,b,c,e | 232.9 ± 7.21 a,b,c,e |
| V | SEC bearing mice + 5-FU | 0.853 ± 0.063 b,c,d | 7.12 ± 0.274 a,b,c,d | 32.73 ± 1.11 a,b,c,d | 151.4 ± 4.78 a,b,c,d |
| Factors (Independent Variables) | Factor Type | Levels | ||
|---|---|---|---|---|
| (−1) | (+1) | |||
| X1: Zein amount (mg) | Numeric | 5 | 10 | |
| X2: Bile salt amount (mg) | Numeric | 15 | 30 | |
| X3: Bile salt type | Categoric | SDC | SC | |
| Responses (dependent variables) | Desirability Constraints | |||
| Y1: EE% | Maximize | |||
| Y2: PS (nm) | Minimize | |||
| Y3: ZP (mV) | Maximize (as absolute value) | |||
| Gene | Primer Sequence | Amplicon Size |
|---|---|---|
| p53 | F: 5′-AGTCTAGAGCCACCGTCCA-3′ R: 5′-TCTGACGCACACCTATTGCAAGC-3′ | 443 |
| NF-κB | F: 5′-GCTCAAGATCTGCCGAGTAAA-3′ R: 5′-GTCCCGTGAAATACACCTCAA-3′ | 113 |
| miR-191-5p | F: 5′CAACGGAATCCCAAAAGCAGCTG-3′ R: 5′TGTCGTGGAGTCGGC-3′ | 60 |
| miR-543 | F: 5- GGAAACATTCGCGGTGC-3′ R: 5-GTGCGTGTCGTGGAGTCG-3′ | 59 |
| β-Actin | F:5′-GTAGCCATCCAGGCTGTGTTG-3′ R: 5′-TGCCAGTGGTACGACCAGAG-3′ | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmahboub, Y.; Albash, R.; Magdy William, M.; Rayan, A.H.; Hamed, N.O.; Ousman, M.S.; Raslan, N.A.; Mosallam, S. Metformin Loaded Zein Polymeric Nanoparticles to Augment Antitumor Activity against Ehrlich Carcinoma via Activation of AMPK Pathway: D-Optimal Design Optimization, In Vitro Characterization, and In Vivo Study. Molecules 2024, 29, 1614. https://doi.org/10.3390/molecules29071614
Elmahboub Y, Albash R, Magdy William M, Rayan AH, Hamed NO, Ousman MS, Raslan NA, Mosallam S. Metformin Loaded Zein Polymeric Nanoparticles to Augment Antitumor Activity against Ehrlich Carcinoma via Activation of AMPK Pathway: D-Optimal Design Optimization, In Vitro Characterization, and In Vivo Study. Molecules. 2024; 29(7):1614. https://doi.org/10.3390/molecules29071614
Chicago/Turabian StyleElmahboub, Yasmina, Rofida Albash, Mira Magdy William, Amal H. Rayan, Najat O. Hamed, Mona S. Ousman, Nahed A Raslan, and Shaimaa Mosallam. 2024. "Metformin Loaded Zein Polymeric Nanoparticles to Augment Antitumor Activity against Ehrlich Carcinoma via Activation of AMPK Pathway: D-Optimal Design Optimization, In Vitro Characterization, and In Vivo Study" Molecules 29, no. 7: 1614. https://doi.org/10.3390/molecules29071614
APA StyleElmahboub, Y., Albash, R., Magdy William, M., Rayan, A. H., Hamed, N. O., Ousman, M. S., Raslan, N. A., & Mosallam, S. (2024). Metformin Loaded Zein Polymeric Nanoparticles to Augment Antitumor Activity against Ehrlich Carcinoma via Activation of AMPK Pathway: D-Optimal Design Optimization, In Vitro Characterization, and In Vivo Study. Molecules, 29(7), 1614. https://doi.org/10.3390/molecules29071614






