Construction of 3D-Printed Sodium Alginate/Chitosan/Halloysite Nanotube Composites as Adsorbents of Methylene Blue
Abstract
1. Introduction
2. Results
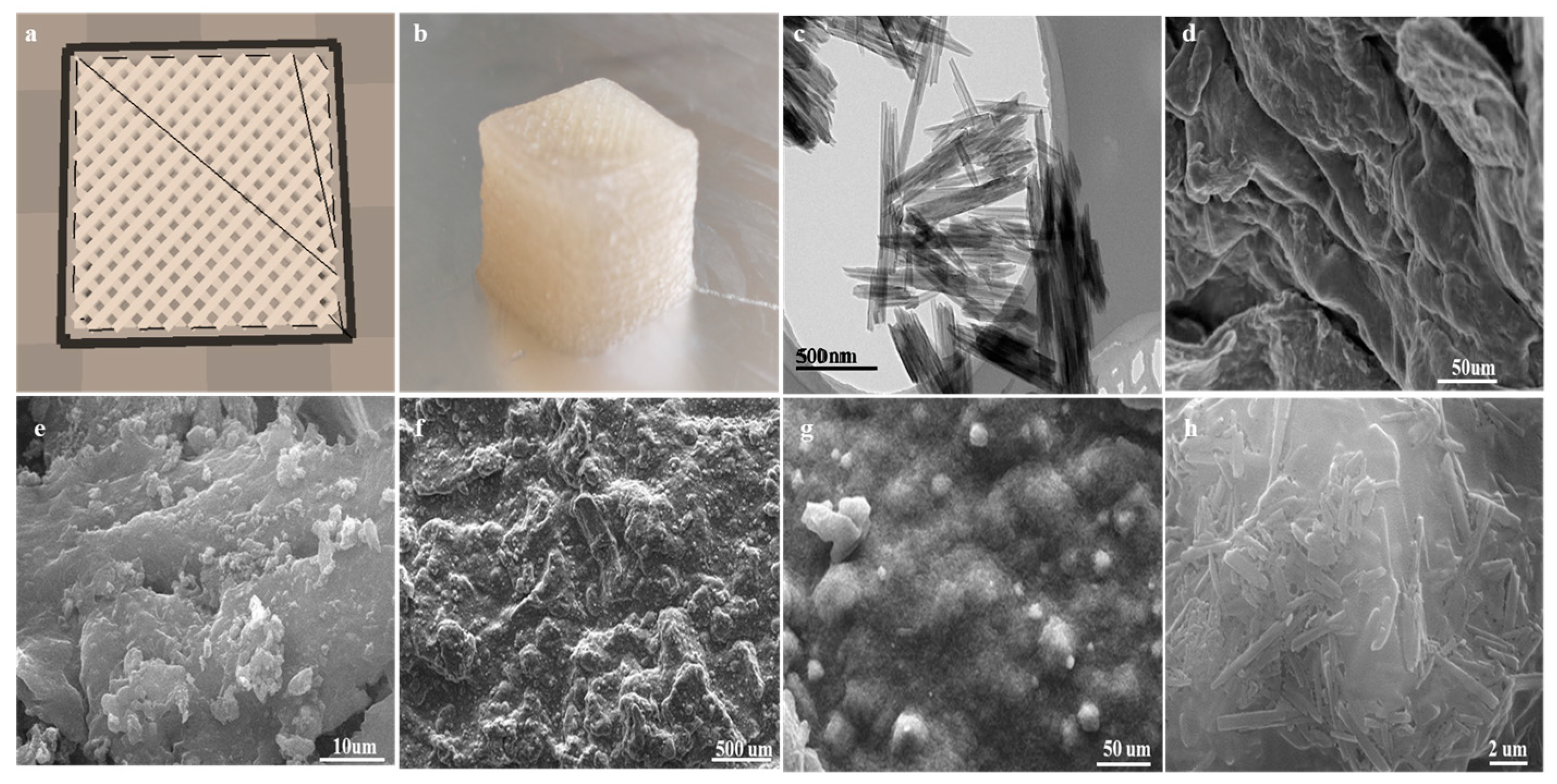
2.1. The Morphology of Composited Adsorbents
2.2. FTIR Analysis
2.3. XRD Analysis
2.4. DSC Analysis
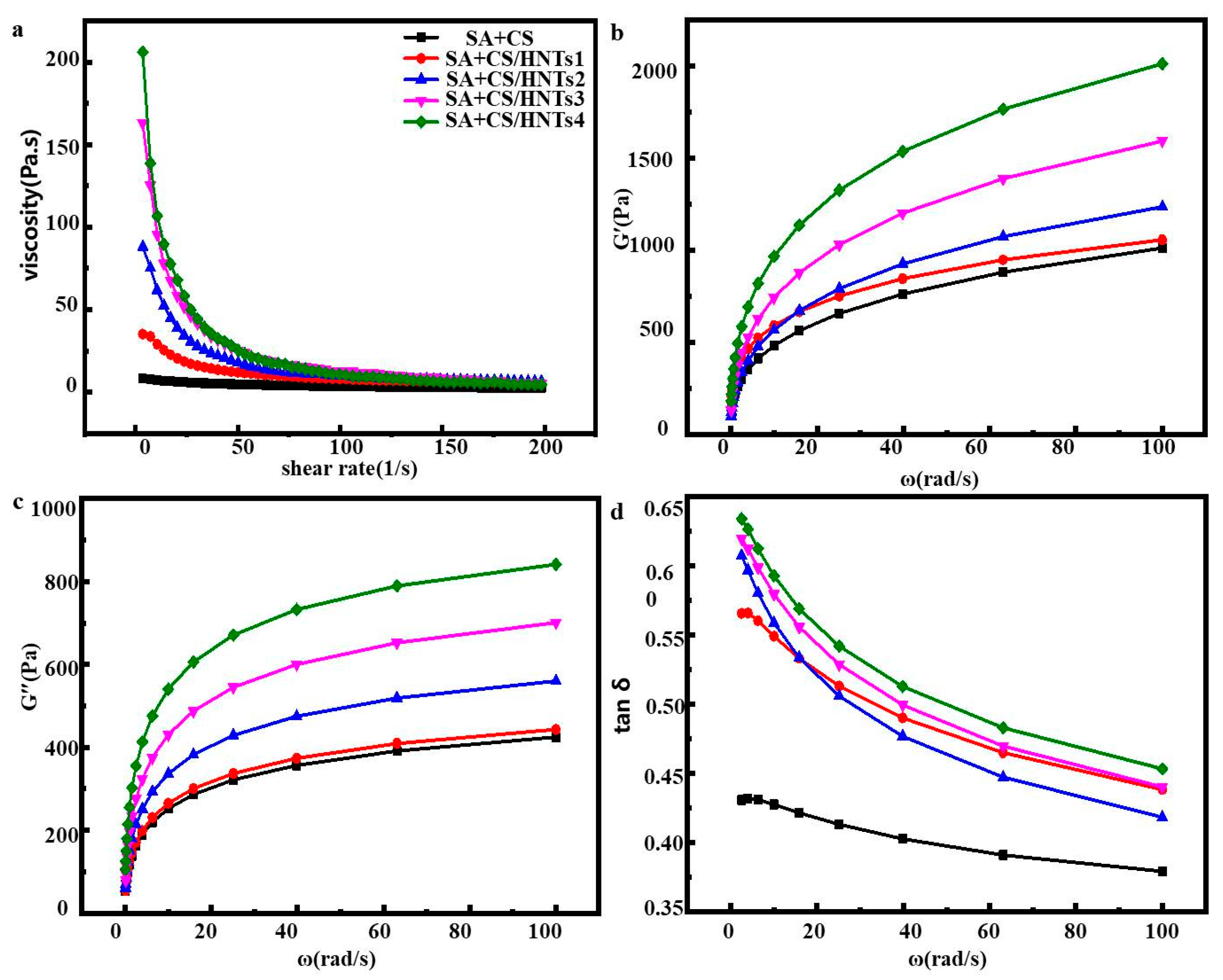
2.5. Rheological Behavior
2.6. Adsorption Performance
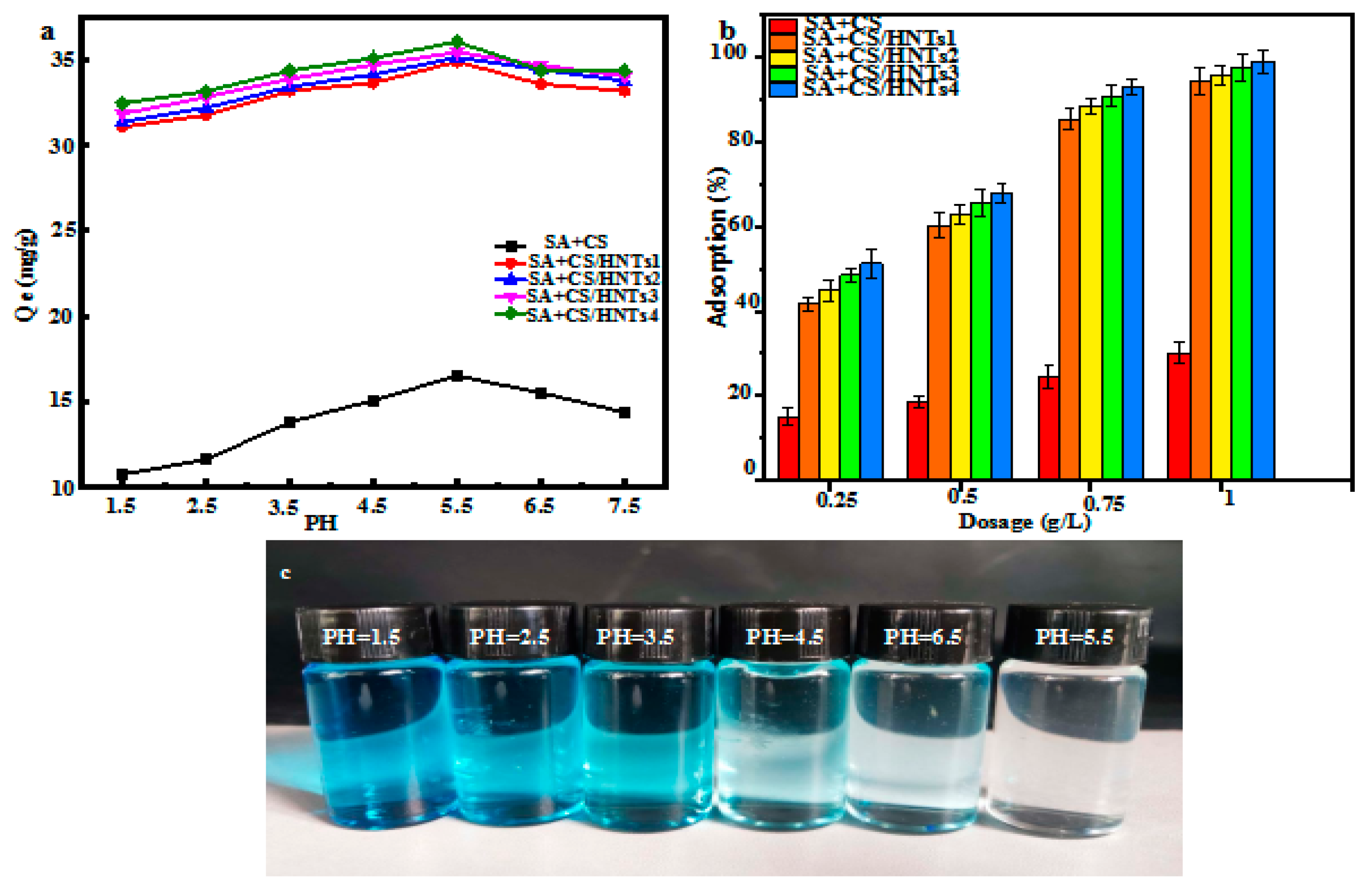
2.6.1. Effect of pH
2.6.2. Effect of Adsorbent Loading
2.6.3. Effect of Initial MB Concentration
2.7. Adsorption Isotherms
2.8. Adsorption Kinetics
2.9. Adsorption Mechanism
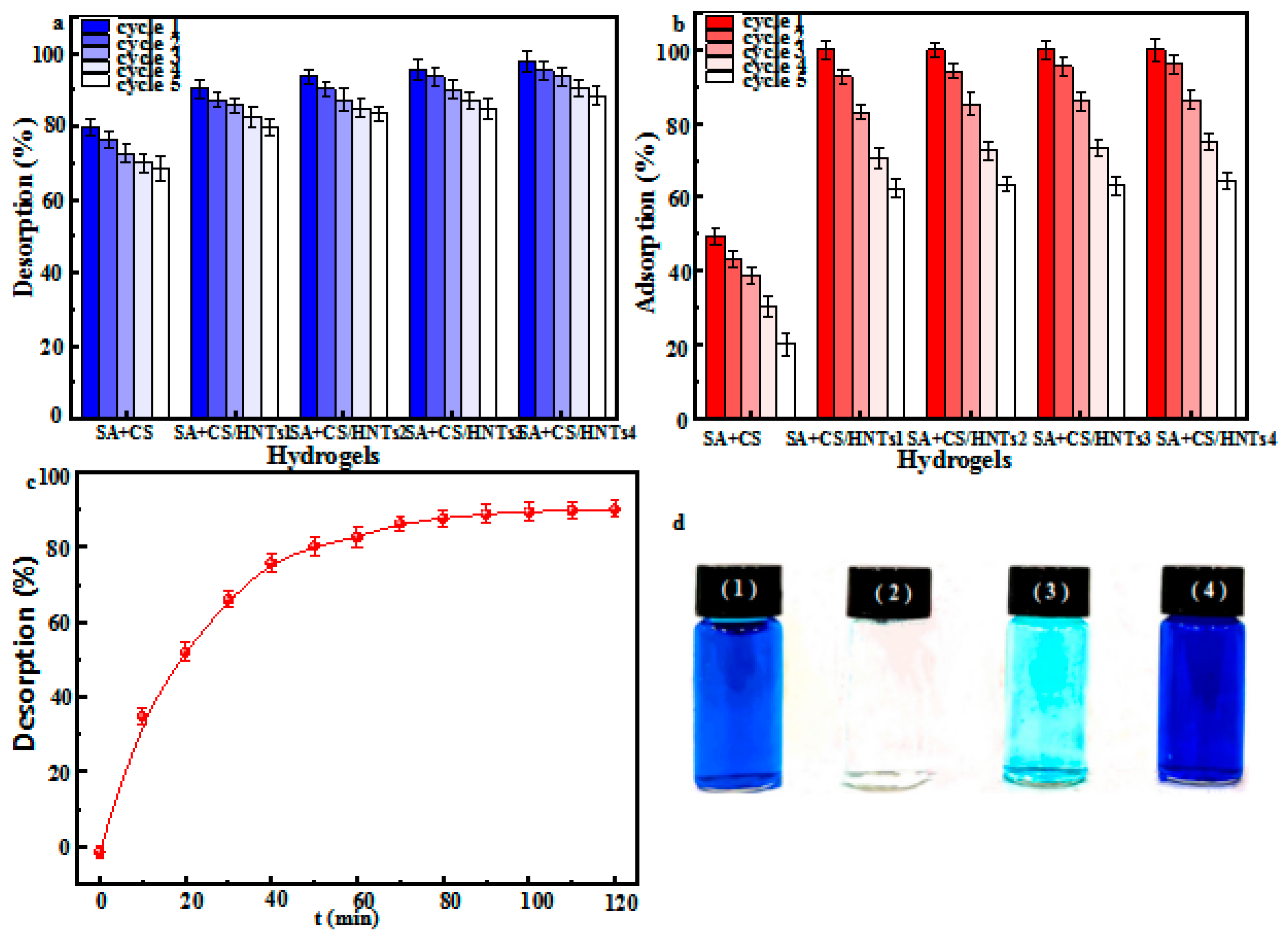
2.10. Adsorbent Regeneration and Reusability
3. Materials and Methods
3.1. Materials
3.2. Preparation of SA+CS/HNT Composites
3.3. Preparation of 3D-Printed Sodium Alginate/Chitosan/Halloysite Nanotube Composites
3.4. MB Adsorption onto 3D-Printed Composites
3.5. Transmission Electron Microscopy (TEM)
3.6. Scanning Electron Microscopy (SEM)
3.7. Fourier Transform Infrared (FTIR) Spectroscopy
3.8. Rheological Measurements
3.9. Differential Scanning Calorimetry (DSC)
3.10. X-ray Diffraction (XRD) Analysis
3.11. Adsorption Performance
3.12. Desorption and Recovery Experiments
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2018, 364, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Liu, L.; Li, W.; Yang, Y. High sorption of reactive dyes onto cotton controlled by chemical potential gradient for reduction of dyeing effluents. J. Environ. Manag. 2019, 239, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, J.; Zhang, H.; Zhao, J.; Liu, H.; Wang, J.; Li, G.; Liang, H. Membrane-based technology in water and resources recovery from the perspective of water social circulation: A review. Sci. Total Environ. 2024, 908, 168277. [Google Scholar] [CrossRef]
- Okasha, A.T.; Abdel-Khalek, A.A.; Alenazi, N.A.; AlHammadi, A.A.; Zoubi, W.A.; Alhammadi, S.; Ko, Y.G.; Abukhadra, M.R. Progress of synthetic cyclodextrins-based materials as effective adsorbents of the common water pollutants: Comprehensive review. J. Environ. Chem. Eng. 2023, 11, 109824. [Google Scholar] [CrossRef]
- Dong, M.; Guo, J.; Liu, Q.; Zeng, J.; Xiong, X.; Gai, X.; Wang, Y.; Wu, Y. Low-pressure carbon nanotube membrane with different surface properties for the removal of organic dyes and PPCPs. J. Environ. Chem. Eng. 2023, 11, 110131. [Google Scholar] [CrossRef]
- Chen, W.L.; Cheng, C.C.; Lu, C.H.; Chen, J.K. Fabrication of sandwich-structured capacitor containing core@shell polystyrene@graphene oxide microspheres for switchable removal of dyes from water by dielectrophoresis force. Sep. Purif. Technol. 2024, 331, 125600. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Sultan, S.; Mathew, A.P. Binder-free Three-dimensional (3D) printing of Cellulose-ZIF8 (CelloZIF-8) for water treatment and carbon dioxide (CO2) adsorption. Chem. Eng. J. 2023, 468, 143567. [Google Scholar] [CrossRef]
- Godoi, F.C.D.; Prakash, S.; Bhandari, B. 3d printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef]
- Shao, Y.; Chaussy, D.; Grosseau, P.; Beneventi, D. Use of microfibrillated cellulose/lignosulfonate blends as carbon precursors: Impact of composite rheology on 3D printing. Ind. Eng. Chem. Res. 2015, 54, 10575–10582. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Su, S.; Feng, S.; Wang, J. Ag2O/sodium alginate-reduced graphene oxide aerogel beads for efficient visible light driven photocatalysis. Appl. Surf. Sci. 2018, 430, 155–164. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, S.; Jing, Y.; Yao, Y.; Wang, X.; Jia, Y. Preparation of halloysite@graphene oxide composite and its application for high-efficient decontamination of U(VI) from aqueous solution. J. Mol. Liq. 2016, 220, 304–310. [Google Scholar] [CrossRef]
- Urmila, C.; Gurpreet, K.; Horst-Günter, R.; Ajeet, K.; Ganga, R.C.; Yogendra, K.M. Advanced metal oxides nanostructures to recognize and eradicate water pollutants. Prog. Mater. Sci. 2023, 139, 101169. [Google Scholar]
- Yang, F.; Zhang, M.; Bhandari, B.; Liu, Y. Investigation on lemon juice gel as food material for 3D printing and optimization of printing parameters. LWT-Food Sci. Technol. 2018, 87, 67–76. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, I.J.; Park, S.M.; Lee, J.H.; Park, H.J. Effect of hydrocolloid addition on dimensional stability in post-processing of 3D printable cookie dough. LWT-Food Sci. Technol. 2018, 101, 69–75. [Google Scholar] [CrossRef]
- Dong, F.; Wang, J.; Wang, Y.; Ren, S. Synthesis and humidity controlling properties of halloysite/poly(sodium acrylate-acrylamide) composite. J. Mater. Chem. 2012, 22, 11093–11100. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Y.; Wang, A. Preparation of granular composite by the redox couple for efficient and fast adsorption of La(III) and Ce(III). J. Environ. Chem. Eng. 2015, 3, 1416–1425. [Google Scholar] [CrossRef]
- Godiya, C.B.; Xiao, Y.; Lu, X. Amine functionalized sodium alginate composite for efficient and rapid removal of methyl blue in water. Int. J. Biol. Macromol. 2020, 144, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, W.; Yan, L.; Huang, D.; Lin, L. Extrusion-based food printing for digitalized food design and nutrition control. J. Food Eng. 2018, 220, 1–11. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B.; Yang, C. Impact of rheological properties of mashed potatoes on 3D printing. J. Food Eng. 2017, 220, 76–82. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, A.; Wang, C.; Qin, W.; Zhao, N.; Song, H.; Gao, J. Liquid crystalline phase behavior and fiber spinning of cellulose/ionic liquid/halloysite nanotubes dispersions. J. Mater. Chem. 2014, 2, 7327–7336. [Google Scholar] [CrossRef]
- Riela, S.; Massaro, M.; Colletti, C.G.; Bommarito, A.; Giordano, C.; Milioto, S.; Noto, R.; Poma, P.; Lazzara, G. Development and characterization of co-loaded curcumin/triazole-halloysite systems and evaluation of their potential anticancer activity. Int. J. Pharmaceut. 2014, 475, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Niu, Y.; Gong, M.; Shi, R.; Chen, D.; Zhang, L.; Lvov, Y. Electrospun microfiber membranes embedded with drug-loaded clay nanotubes for sustained antimicrobial protection. ACS Nano 2015, 9, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Kurczewska, J.; Cegłowski, M.; Schroeder, G. Alginate/PAMAM dendrimer-Halloysite beads for removal of cationic and anionic dyes. Int. J. Biol. Macromol. 2018, 123, 398–408. [Google Scholar] [CrossRef]
- Li, C.; Li, J. Preparation of chitosan-ferulic acid conjugate: Structure characterization and in the application of pharmaceuticals. Int. J. Biol. Macromol. 2017, 105, 1539–1543. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate-chitosan complex formed with different polymers ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, J.; Wang, A. Alkali activation of halloysite for adsorption and release of ofloxacin. Appl. Surf. Sci. 2013, 287, 54–61. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Liu, X.; Tu, H.; Zhang, J.; Shi, X.; Deng, H.; Du, Y. Layer-by-layer immobilization of quaternized carboxymethyl chitosan/organic rectorite and alginate onto nanofibrous mats and their antibacterial application. Carbohyd. Polym. 2015, 121, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.; Ray, S.K. Chitosan based nano composite adsorbent-Synthesis, characterization and application for adsorption of binary mixtures of Pb(II) and Cd(II) from water. Carbohyd. Polym. 2018, 182, 159–171. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Qiao, C.; Li, Y.; Li, T.; Xu, C. Effects of chitosan quaternary ammonium salt on the physicochemical properties of sodium carboxymethyl cellulose-based films. Carbohyd. Polym. 2018, 184, 37–46. [Google Scholar] [CrossRef]
- Chen, J.; Mu, T.; Goffin, D.; Blecker, C.; Richard, G.; Richel, A.; Haubruge, E. Application of soy protein isolate and hydrocolloids-based mixtures as promising food material in 3D food printing. J. Food Eng. 2019, 26, 76–86. [Google Scholar] [CrossRef]
- Fischer, P.; Windhab, E.J. Rheology of food material. Curr. Opin. Colloid. In. 2011, 16, 36–40. [Google Scholar] [CrossRef]
- Yang, F.; Guo, C.; Zhang, M.; Bhandari, B.; Liu, Y. Improving 3D printing process of lemon juice gel based on fluid flow numerical simulation. LWT-Food Sci. Technol. 2019, 102, 89–99. [Google Scholar] [CrossRef]
- Ioannis, A.; Alok, M.; Muhammad, U.; Jyoti, M.; Guanghui, Y.; Avelino, N.; Michael, K. A review on halloysite-based adsorbents to remove pollutants in water and wastewater. J. Mol. Liq. 2018, 269, 855–868. [Google Scholar]
- Wang, H.; Jia, S.; Wang, H.; Li, B.; Liu, W.; Li, N.; Qiao, J.; Li, C. A novel-green adsorbent based on betaine-modified magnetic nanoparticles for removal of methyl blue. Sci. Bull. 2017, 62, 319–325. [Google Scholar] [CrossRef]
- Arora, C.; Soni, S.; Sahu, S.; Mittal, J.; Kumar, P.; Bajpai, P.K. Iron based metal organic framework for efficient removal of methylene blue dye from industrial waste. J. Mol. Liq. 2019, 284, 343–352. [Google Scholar] [CrossRef]
- Nakhjiri, M.T.; Marandi, G.B.; Kurdtabar, M. Poly(AA-co-VPA) composite cross-linked with N-maleyl chitosan as dye adsorbent: Isotherms, kinetics and thermodynamic investigation. Inter. J. Biol. Macromol. 2018, 117, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Sebeia, N.; Jabli, M.; Ghith, A.; El Ghoul, Y.; Alminderej, F.M. Populus tremula, Nerium oleander and Pergularia tomentosa seed fibers as sources of cellulose and lignin for the bio-sorption of methylene blue. Int. J. Biol. Macromol. 2019, 121, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dai, Y.; Wu, Y.; Lu, G.; Cao, Z.; Cheng, J.; Wang, K.; Yang, H.; Xia, Y.; Wen, X.; et al. Facile preparation of polyacrylamide/chitosan/Fe3O4 composite hydrogels for effective removal of methylene blue from aqueous solution. Carbohyd. Polym. 2020, 234, 115882. [Google Scholar] [CrossRef]
- Qiu, J.; Fan, P.; Feng, Y.; Liu, F.; Ling, C.; Li, A. Comparison of the adsorption behaviors for methylene blue on two renewable gels with different physical state. Environ. Pollut. 2019, 254, 113–117. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H. Removal of anionic dyes from an aqueous solution by a magnetic cationic adsorbent modified with DMDAAC. New J. Chem. 2018, 42, 7262–7271. [Google Scholar] [CrossRef]
- Duan, N.; Li, Q.; Liu, J.; Xiao, H. Enhanced adsorption performance of CuO-Al2O3 composite derived from cotton template. Can. J. Chem. Eng. 2015, 93, 2015–2023. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Gu, X.; Lu, J. Competitive adsorption of methylene blue and Cu2+ onto citric acid modified pine sawdust. Clean-Soil Air Water 2015, 43, 96–103. [Google Scholar] [CrossRef]
- Ghaffar, A.; Younis, M.N. Interaction and thermodynamics of methylene blue adsorption on oxidized multi-walled carbon nanotubes. Green Process. Synth. 2015, 4, 209–217. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S. A study on the adsorption of methylene blue onto gum ghatti/TiO2 nanoparticles-based composite nanocomposite. Inter. J. Biol. Macromol. 2016, 88, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chang, P.; Wang, S.; Yu, J.; Ma, X. Preparation and properties of halloysite nanotubes/plasticized Dioscorea opposita Thunb. starch composites. Carbohyd. Polym. 2011, 83, 186–191. [Google Scholar] [CrossRef]
- Wang, L.; Highley, C.B.; Yeh, Y.C.; Galarraga, J.H.; Uman, S.; Burdick, J.A. Three-dimensional extrusion bioprinting of single-and double-network composites containing dynamic covalent crosslinks. J. Biomed. Mater. Res. A 2018, 106, 865–875. [Google Scholar] [CrossRef]



| Model | Parameters | Adsorbents | ||||
|---|---|---|---|---|---|---|
| SA+CS | SA+CS/HNTs1 | SA+CS/HNTs2 | SA+CS/HNTs3 | SA+CS/HNTs4 | ||
| Langmuir model | R2 | 0.99926 | 0.99967 | 0.99968 | 0.99965 | 0.99961 |
| Qm (mg/g) | 89.7 | 267.4 | 321.7 | 357.3 | 376.3 | |
| KL (L/mg) | 3.35 | 3.71 | 5.15 | 7.43 | 9.02 | |
| Freundlich model | R2 | 0.85891 | 0.49118 | 0.52719 | 0.55996 | 0.58077 |
| 1/n | 0.154 | 0.167 | 0.187 | 0.197 | 0.21 | |
| KF (L/mg) | 19.19 | 39.77 | 40.85 | 42.26 | 43.46 | |
| Pseudo-first-order kinetics | R2 | 0.8920 | 0.95957 | 0.88096 | 0.96379 | 0.9647 |
| K1 (min−1) | 0.04422 | 0.05603 | 0.05866 | 0.05963 | 0.06188 | |
| Qe (L/mg) | 17.22 | 35.60 | 36.52 | 36.82 | 37.90 | |
| Pseudo-second-order kinetics | R2 | 0.99771 | 0.99749 | 0.99773 | 0.99791 | 0.99794 |
| K2 (g/mg/min) | 1224.46 | 11,865.65 | 14,653.27 | 14,560.60 | 1615.30 | |
| Qe (L/mg) | 17.8031 | 36.7918 | 37.5940 | 37.9939 | 39.1236 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Ji, A.; Xia, G.; Liu, L.; Yan, J. Construction of 3D-Printed Sodium Alginate/Chitosan/Halloysite Nanotube Composites as Adsorbents of Methylene Blue. Molecules 2024, 29, 1609. https://doi.org/10.3390/molecules29071609
Luo J, Ji A, Xia G, Liu L, Yan J. Construction of 3D-Printed Sodium Alginate/Chitosan/Halloysite Nanotube Composites as Adsorbents of Methylene Blue. Molecules. 2024; 29(7):1609. https://doi.org/10.3390/molecules29071609
Chicago/Turabian StyleLuo, Jinjie, Anping Ji, Guofeng Xia, Lizi Liu, and Juan Yan. 2024. "Construction of 3D-Printed Sodium Alginate/Chitosan/Halloysite Nanotube Composites as Adsorbents of Methylene Blue" Molecules 29, no. 7: 1609. https://doi.org/10.3390/molecules29071609
APA StyleLuo, J., Ji, A., Xia, G., Liu, L., & Yan, J. (2024). Construction of 3D-Printed Sodium Alginate/Chitosan/Halloysite Nanotube Composites as Adsorbents of Methylene Blue. Molecules, 29(7), 1609. https://doi.org/10.3390/molecules29071609






