Abstract
Oral cancer is a common malignancy with a high mortality rate. Although surgery is the best treatment option for patients with cancer, this approach is ineffective for advanced metastases. Molecular agents are irreplaceable in preventing and treating distant metastases. This review aims to summarise the molecular agents used for the treatment of oral cancer in the last decade and describe their sources and curative effects. These agents are classified into phenols, isothiocyanates, anthraquinones, statins, flavonoids, terpenoids, and steroids. The mechanisms of action of these agents include regulating the expression of cell signalling pathways and related proteases to affect the proliferation, autophagy, migration, apoptosis, and other biological aspects of oral cancer cells. This paper may serve as a reference for subsequent studies on the treatment of oral cancer.
1. Introduction
Oral diseases are a global public health problem, affecting the health and quality of life of 3–5 billion people [1,2,3]. If untreated, they may even lead can to systemic diseases [4,5]. Thus, many studies have focused on the aetiology, pathogenesis, and therapeutic regimens of oral cancer [6,7,8].
Oral cancer often arises from pre-existing white spots and oral submucosal fibrosis, and its incidence has increased with the increasing consumption of carcinogens, such as tobacco and alcohol [9,10,11]. The types of oral cancer include cancer of the palate, tongue, floor of the mouth, lip, buccal mucosa, etc., and vary according to the location of the infected cancer cells [12]. Physiological disorders are caused by the disease itself or traditional treatment, such as pain, paraesthesia, dysphagia, dysphagia, infection, ulceration, maxillofacial deformity, and other complications. Oral cancers have a significant genetic diversity, and these subgroups include p53-independent tumours, subtypes with multiple tumour suppressor l (MTS1), oral leucoplakia, etc. [13]. Tongue cancer is mainly caused by cell cycle-related gene cyclin D1 changes [14]. These oncogenes influence the clinicopathological features of oral squamous cell carcinoma, including poor tumour differentiation, lymph node involvement, and poor survival [15]. Oral squamous cell carcinoma (OSCC) is the leading cause of cancer-related deaths, and its incidence and mortality are increasing considerably annually [16,17,18]. Despite advances in diagnostic imaging, surgery, radiation, and chemotherapy, oral cancer is often diagnosed at a later stage of disease development, leading to poor prognosis and high mortality. Moreover, many patients with oral cancer are resistant to standard treatments owing to heterogeneity within the tumour or a genetic mutation which occurs during treatment, resulting in the high recurrence rate of this disease [19,20,21]. Oral cancer metastasises to various tissues or organs of the body through the lymphatic system or blood, and generally has no specific location, but it is more likely to metastasise to the head and neck area [20]. Thus, the development of safe and reliable drugs is crucial for the effective treatment of oral cancer. Different cell death pathways, immunotherapy, and the targeted inhibition of tumour cells have been explored for the treatment of malignant tumours [12,22]. Additionally, target discovery and validation are the key steps in developing molecular agents for the treatment of oral cancer. Multiple signalling pathways are involved in the progression of oral cancer, such as Toll-like receptor 4 signalling (TLR4), phosphoinositide 3-kinase (PI3K) pathway, janus kinase (JAK)–signal transduction and activator of transcription (STAT) pathway, etc. [23]. The study of Kenison et al. indicates that it is of great significance to develop immune checkpoint inhibitors targeting aromatic hydrocarbon receptors for oral cancer immunotherapy [24]. Current research is focused on discovering new targets of oral cancer drugs and in verifying targets of traditional drugs [25].
Various oral cancer drugs have been launched with the recent rise in the occurrence, development, and diagnosis, of oral cancer; further, the continuous development of clinical trials on molecular targeted therapies has accelerated this process. This review summarises the molecular agents used to treat oral cancer and their mechanisms of action, pharmacological advantages, and development strategies. It also discusses research progress in oral cancer drugs and candidates. This paper may serve as a reference for designing novel oral cancer drugs with simple structures and good efficacy.
2. Polyphenols
Natural polyphenols (Figure 1) have emerged as promising chemopreventive and anti-cancer agents [26,27,28]. They exert anti-proliferative, anti-metastatic, and pro-apoptotic effects on tumour cells. Natural polyphenols can function synergistically with chemotherapy drugs to overcome drug resistance. Considering the anti-cancer, anti-metastatic, and chemopreventive effects of natural polyphenols on oral cancer, several researchers investigated the mechanisms of action of these agents [29]. Kapoor et al. [30] found that [6]-gingerol (1) can significantly inhibit the proliferation of oral cancer cells (OCCs) by inducing apoptosis and G2/M phase arrest. 6-Gingerol can also inhibit OCC migration and invasion by regulating N-cadherin and vimentin, inducing AMPK activation in Ca9-22 cells, and inhibiting the AKT/mTOR signalling pathway. Liu et al. [31] found that platyphyllenone (2) induces OCC autophagy and apoptosis by regulating the serine/threonine protein kinase B (AKT) and c-Jun N-terminal kinase (JNK) pathways. Resveratrol (3) inhibits OCC proliferation by inhibiting the transactivation of the element binding protein 1 (SREBP1), subsequently down-regulating the expression of epidermal fatty acid-binding protein (E-FABP), blocking the proliferation of Ca9-22 cells, and finally inducing autophagy [32,33]. Yang et al. [34] found that phloretin (4) exerts anti-proliferative activity against human OCC through reactive oxygen species (ROS)-mediated apoptosis and G0/G1 phase arrest. Piperlongumine (PL, 5) inhibits the production of tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) and the activation of nuclear factor-κB (NF-κB) in pro-inflammatory response [35,36]. Moreover, PL prevents plaque formation, thereby inhibiting the development of malignant phenotypes and the formation of tumour stem cells [35]. At the molecular level, in vitro studies have shown that curcumin (CUR, 6) suppresses OCC growth by inhibiting SCC-25 cell proliferation and inducing G2/M phase arrest in a dose-dependent manner [37]. A novel synthetic CUR analogue, GO-Y078 (7), induces caspase-mediated apoptosis in OCC by up-regulating apoptosis regulatory proteins SMAC/DIABLO and haem oxygenase (HO)-1 [38]. Semlali et al. [39] demonstrated that curcumin analogue (PAC, 8) dose-dependently inhibits the proliferation of OCC by disrupting cell cycle distribution, down-regulating the expression of oncogenes (cyclin D1) and cyclin-dependent kinase inhibitors (p21WAF1), and increasing the apoptosis, autophagy, and oxidative stress of OCC. Caffeic acid phenethyl ester (CAPE, 9) dose-dependently inhibits the proliferation of TW2.6 cells by up-regulating the expression of Bax and Puma, activating the Bax protein, and causing conformational changes, mitochondrial translocation, and oligomerisation [40]. Rosmarinic acid (10) exerts anti-cancer effects on different human cancer cell lines by inducing apoptosis and G2/M phase arrest, causing endoplasmic reticulum (ER) stress and decreasing the migration potential of cancer cells in a concentration-dependent manner [41,42]. Delta-8- and delta-9-tetrahydrocannabinol (11 and 12) inhibits the growth of OCC through various mechanisms, such as inhibiting the expression of epithelial–mesenchymal transition (EMT) markers (such as E-cadherin), reducing the production of ROS, and increasing the expression of glutathione and glutathione [43]. Yang et al. [44] confirmed that pterostilbene (14) inhibits the growth of SAS and OECM-1 cell lines and induces autophagy by inhibiting Akt, p38, and extracellular signal-regulated kinase ½ (ERK1/2) and activating the c-Jun N-terminal kinase (JNK) pathways. Huang et al. [45] designed and synthesised a series of bis(hydroxymethyl)propionate analogue prodrugs using natural rosewood stilbene as the lead compound. They screened the anti-proliferative effects of all derivatives on cisplatin-resistant oral squamous cells (CARs) and found that several compounds show stronger antitumour activities than rosewood stilbene and resveratrol.
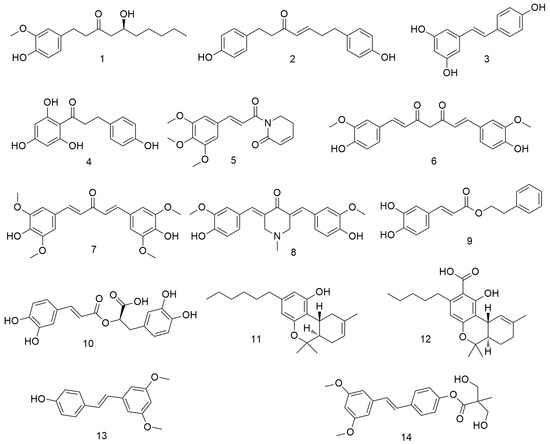
Figure 1.
Polyphenol agents.
Flavonoids (Figure 2) are found in plants, including vegetables, fruits, and other foods. These agents prevent the carcinogenesis and proliferation of tumours via various mechanisms, such as regulating the apoptosis and autophagy pathways and causing cell cycle arrest [46,47,48]. 7,8-Dihydroxyflavone (15) can induce the apoptosis of OCC by inducing G-phase arrest in OSCC cells and down-regulating specificity protein 1 (Sp1) levels in HN22 and HSC4 cells, indicating that it plays an important antitumour role in OSCC [49]. Liquiritigenin (LQ, 16) is inactivated via the PI3K/AKT/mTOR pathway, which largely limits tumour growth and enhances apoptosis and autophagy, thereby inhibiting the progression of OCC. In addition, LQ inhibits AKT phosphorylation in tumour tissues [50]. Chrysin (17) regulates the apoptosis and autophagy of MC3 cells by inducing MAPK/extracellular signalling, reducing the activity of human mucoepidermoid carcinoma MC3 OCC, and causing morphological changes in MC3 cells [51]. Fisetin (3,3-,4-,7-tetrahydroxyflavone, 18) is a naturally occurring flavonoid with antioxidant, anti-inflammatory, and anti-cancer properties [52]. This flavonoid enhances the apoptosis of Ca9-22 cells at the human tongue scale through the mitochondrial pathway and inhibition of autophagy. In addition, it can cause cell cycle arrest by disrupting Wnt, mTOR, and NF-xB signals and preventing the invasion and migration of cancer cells. Quercetin (19) can cause mitochondrial dysfunction and inhibit the viability, migration, and invasion of OCC via the mitochondrial apoptosis pathway [53,54]. Baicalein (20) induces the apoptosis, causes the GO/G1 phase arrest, and reduces the NF-κB activity of OSCC cells. In addition, baicalein inhibits the proliferation of OSCC in vivo and in vitro by down-regulating the relative mRNA levels of the transcription factors Sp1, p65, and p50 [55]. Tu et al. [56] found that luteolin (21) combined with radiotherapy reduces the tumourigenicity of OCSC by inactivating the IL-6/STAT3 signalling pathway. Moreover, luteolin treatment reduces the proliferation and self-renewal ability of enriched OCSCs. Huang et al. [57] reported that hydroxygenkwanin (22) inhibits cell cycle, cell colony formation, and cell motility by activating p21 and the intrinsic apoptosis pathway. Moreover, apigenin (23) can induce the apoptosis of tongue and oral carcinoma-derived cell line SCC-25 and regulate the expression of cyclin D and E, inactivation of cyclin dependent kinase 1 (CDK1), and cell cycle arrest at the G0/G and G2/M phases [58]. Hesperidin (24) exerts anti-cancer effects on OCC by inactivating transcriptional actvator 1 (STAT1) and STAT3 signalling molecules and inhibiting programmed cell death 1 ligand 1 (PD-L1) expression [59].Velmurugan et al. [60] demonstrated for the first time that luteosin-7-O-glucoside (25) inhibits the invasion and migration of OCC by regulating matrix metalloproteinase-2 (MMP-2) expression and the extracellular signal-regulated kinase pathway and significantly reduces the metastasis of oral cancer by alleviating the P38-induced increase in MMP-2 expression.
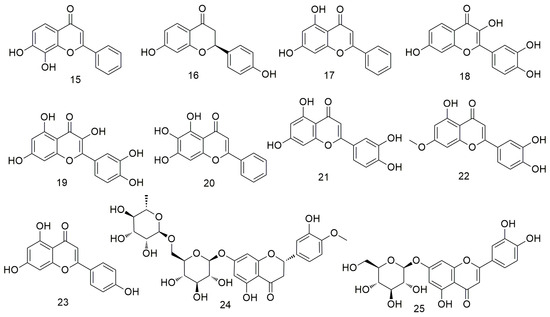
Figure 2.
Flavonoid agents.
3. Isothiocyanates
Isothiocyanates (Figure 3) are natural phytochemical compounds derived from plants, such as broccoli, cabbage, papaya, and wasabi, which demonstrate many biological effects, including neuroprotective, anti-inflammatory, and anti-cancer effects. Tsai et al. [61] reported that cathepsin S inhibitors can be used to prevent or delay cancer metastasis. Chen et al. [62] observed that sulforaphane (26) reduces the motility and aggressiveness of SCC-9 and SCC-14 cells by decreasing the expression of cathepsin S and inhibits the migration of OCC by regulating the expression of cathepsin S and its downstream target LC3. Varadarajan et al. [63] found that benzyl isothiocyanate (27) shows anti-cancer effects on the SCC-25 cell line through G2/M phase blockade and apoptosis induction. 6-MITC (28), a wasabi compound, can enhance the sensitivity of OCC cells to the growth inhibitory effect of anti-cancer drugs [64]. Furthermore, 6-MITC and its derivatives 17,447 (29) and 17,557 (30) inhibit OCC growth in a dose-dependent manner [64].
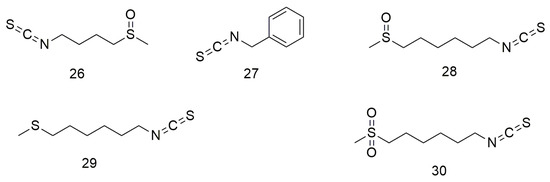
Figure 3.
Isothiocyanate agents.
4. Quinones
Anthraquinones (Figure 4) are a class of quinone compounds that can occur naturally or synthesised artificially. These drugs have various effects, including haemostatic, anti-bacterial, and antitumour. Hsu et al. [65] demonstrated that chrysophanol (31) inhibits the proliferation and metastasis and increases the apoptosis of FaDu and SAS cell lines by promoting ROS production and cell cycle G1 arrest. Meanwhile, aloe emodin (32) reduces the viability of SCC15 cells and induces apoptosis by regulating the expression of caspase-3/9 [66]. Lin et al. [67] showed that plumbagin (33) reduces the viability of CR-SAS cells and induces apoptosis. In addition, plumbagin increases ROS production, leading to mitochondrial dysfunction and ER stress. Animal experiments have also been conducted to demonstrate the in vivo anti-cancer effects of plumbagin on drug-resistant OCC. Shikonin (34) enhances the sensitivity of OCC cells to cisplatin. It also inhibits the activity and malignant proliferation of OCC by down-regulating the expression of β-catenin [68]. Acetylshikonin (35) significantly inhibits the invasion of YD10B OCC with porphyrin gingival infection by inhibiting IL-8- and IL-8-dependent MMP release [69]. Acetylshikonin (35) enhances the phosphorylation of JNK and p38 MAPK via ROS production and triggers apoptosis in Ca9-22 cells [70]. Therefore, acetylshikonin is a strong candidate for a selective chemotherapeutic agent for the treatment of OSCC.
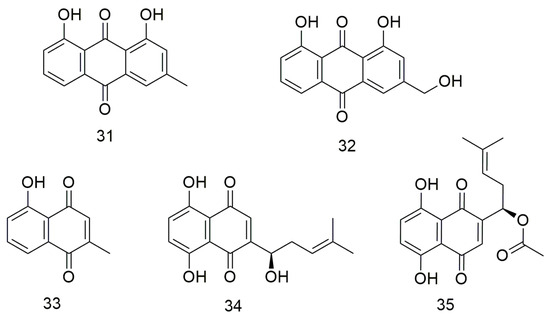
Figure 4.
Anthraquinone agents.
5. Statins
Statins (Figure 5) inhibit cholesterol biosynthesis by blocking the activity of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase and preventing the conversion of HMG-CoA to methanate [71,72,73]. Atorvastatin (36) suppresses NADPH oxidase activity and ROS formation by inhibiting Racl activity and induces angiogenesis by increasing VEGF-A expression after ROS formation [74]. In addition, atorvastatin reportedly inhibits the growth of oral tumours by reducing cell migration. This drug creates a toxic microenvironment and inhibits the metastasis of oral squamous cancer cells by increasing intracellular oxidative stress [74]. Lovastatin (37) and simvastatin (38) inhibit the proliferation of tumour cells by enhancing the response of PD-1 ICB and inducing T cells to kill tumour cells [75]. Combined treatment with daily oral simvastatin (38) or lovastatin and PD-1 blocking enhances tumour control and prolongs survival, suggesting that statins may enhance the response to PD-1 checkpoint blocking and other HNSCC immunotherapies [75]. Huang et al. [76] found that statin (37–42) use significantly decreases the incidence of OCSCC among betel nut chewers.
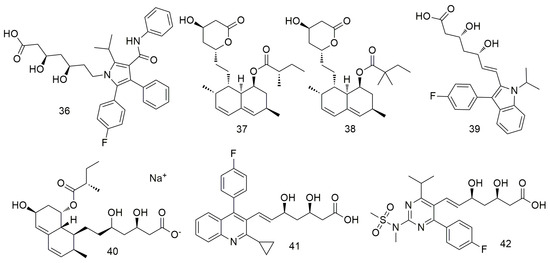
Figure 5.
Statin agents.
6. Terpenoids and Steroids
Zhang et al. [77] showed that linalool (43, Figure 6) monoterpene exerts its antitumour effect by reducing the mitochondrial membrane potential and inhibiting the cell cycle and PI3K/AKT signalling pathway. Dehydroandrographolide (44) induces autophagy in human OCC by regulating the expression of p53, activating JNK1/2 and inhibiting Akt and p38 expression [78]. It can also effectively inhibit tumour formation in vivo in xenotransplantation models of oral cancer. Coronarin D (45) can significantly reduce cancer cell viability by increasing the loss of mitochondrial membrane potential and the expression of death receptors, resulting in the activation of caspase-3/8/9 [79]. It also induces the apoptosis of human SCC-9 and SAS cells by causing G2/M phase arrest, decreasing the activation of ERK1/2, p-38, and AKT, and increasing the activation of JNK1/2. Costunolide (46) triggers cell apoptosis by inhibiting AKT activity and significantly promoting ROS production [80]. In addition, an in vivo mouse model analysis showed that costunolide strongly inhibits the growth of cell-derived xenograft oral cancer. 4-Carbomethoxyl-10-epigyrosanoldie E (47) induces ROS production in OCC, thereby initiating multiple cellular pathways, including ER stress and mitochondria-induced apoptotic pathway dysfunction, ultimately leading to autophagy [81]. Sinularin (48) exerts oxidative stress-mediated anti-proliferative, G2/M-blocking, and apoptotic effects on OCC and is associated with ROS production, making it a potential marine drug against oral cancer [82]. Yang et al. [83] confirmed that dihydrosinularin (49) exerts its anti-proliferative effect on OCC by inducing apoptosis, double-strand breaks, and DNA oxidative damage without causing cytotoxicity to non-malignant oral cells. Trichodermin (50) inhibits the migration and invasion of OSCC Ca922 and HSC-3 cells by down-regulating the expression of MMP-9. In addition, trichodermin can reduce the mitochondrial membrane potential and mitochondrial oxidative phosphorylation of OSCC cells and regulate the expression levels of histone deacetylase 2 and downstream proteins [84]. Triptolide (51) significantly inhibits the proliferation, cell cycle arrest, and apoptosis of taxol-resistant SAS/Taxol cells. Kuo et al. [85] found that triptolide inhibits the growth of oral cancer tumour and proliferation of OSCC cells by down-regulating PD-L1 expression. The antibiotic antimycin A (52) mediates the apoptosis of OCC CAL27 and Ca9-22 cells by increasing oxidative stress and ROS production [86]. Nitrated [6,6,6] tricycle (53)-derived compounds induce apoptosis and DNA damage in OCC by inducing oxidative stress [87]. Meanwhile, pseudolaric acid B (54) significantly inhibits the caspase-dependent apoptosis of HN22 cells [88].
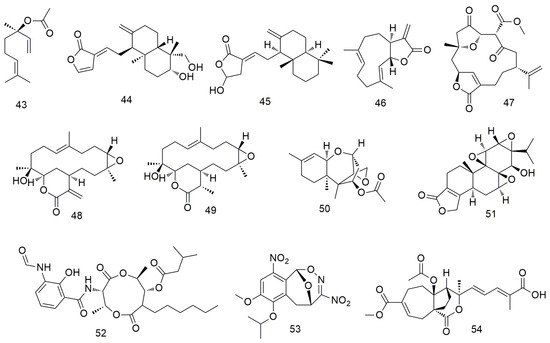
Figure 6.
Terpenoid agents.
Gambogic acid causes the G1 arrest of OSCC cells. In addition, gambogic acid (55, Figure 7) can pharmacologically inhibit p38 kinase, significantly reduce haem oxygenase 1 (HO-1) expression, induce caspase cleavage, and promote cell apoptosis [89]. Paclitaxel (56) significantly inhibits the activity and proliferation of OCC by increasing the expression of Bim, Bid, MMP-2, and MMP-9. In addition, paclitaxel inhibits the growth of oral cancer cell lines by inhibiting the EGFR signalling pathway [90]. Paclitaxel combined with lupeol inhibits the simulation of hypoxia-induced angiogenesis [91]. Ursolic acid (57) induces caspase-dependent cell apoptosis by down-regulating the expression of multiple biomarkers, including Akt/mTOR/NF-xB signalling [92]. It also inhibits angiogenesis by preventing the migration/invasion of Ca922 OCC and blocking the secretion of MMP-2. Cis-3-o-p-hydroxycinnamyl ursolic acid (58) inhibits the stagnation of oral cancer cell lines (Ca9-22 and SAS cells) in the G1 phase in a concentration-dependent manner [93]. Additionally, cis-3-O-p-hydroxycinnamoyl ursolic acid triggers the production of intracellular ROS and mediates mitochondrial apoptosis by inducing ROS dependence and p53. Sharifi et al. [94] found that the cytotoxic mechanism of thistle saponins IV (59) and IVa may be mediated through the mitochondrial apoptosis pathway and that both saponins can reduce the migration, invasion potential, and metastasis of HN-5 cancer cells. Ursodeoxycholic acid (60) induces the apoptosis of cancer cells by promoting the expression of caspase-3/8/9 and reducing the expression of pro-apoptotic proteins [95]. Betulinic acid (61) inhibits the proliferation of OSCC cells by regulating ROS and p53 signalling, making it a potential drug for the treatment of oral cancer [96]. Lupeol (62) can promote the apoptosis and inhibit the proliferation of OSCC cells by inducing the phosphorylation of EGFR and inhibiting the activation of downstream molecules, such as protein kinase B (or AKT) and NF-κB [91,97]. Zhang et al. [98] found that 20(S)-ginsenoside Rh2 (63) induces the apoptosis and inhibits the growth of OCC by inducing G0/G phase arrest and significantly down-regulating the levels of p-Src, p-B-Raf, and p-ERK1/2 proteins. Li et al. [99] found that ginsenoside M1 induces cell apoptosis by increasing the expression of pro-apoptotic protein p53, promoting DNA breakage, and inhibiting the cell cycle. In addition, ginsenoside M1 (64) dose-dependently inhibits the colony formation and migration of SAS and OEC-M1 cells and reduces the expression of the transfer-related protein vimentin. Li et al. [100] found that riparsaponin (65) inhibits OSCC metastasis by down-regulating the expression of cellular-mesenchymal epithelial transition factor (c-MET), MMP-2, and MMP-9 and by up-regulating the expression of E-cadherin; it also shows significant anti-OSCC activity by inducing mitochondria-mediated apoptosis.
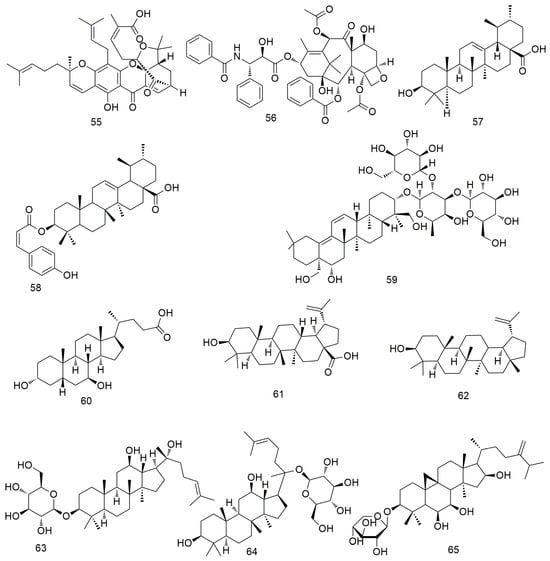
Figure 7.
Terpenoid and steroid agents.
7. Other Compounds
In addition to the above compounds, many other compounds (Figure 8 and Figure 9) induce oral cancer cell cycle arrest, promote cell apoptosis, and inhibit tumour cell metastasis. Cordycepin (66) not only regulates the OEC-MI cell cycle but also exerts anti-cancer effects on human OSCC cells when combined with irradiation [101]. Cordycepin (66) and IR synergistically induce ATG5 and p21 to inhibit cell proliferation in an autophagy cascade-dependent manner [102]. Li et al. [103] found that doxazosin (67) has obvious antioxidant and protective effects on normal cells and can effectively induce the death of oral cancer KB cells by inducing apoptotic signalling. Methylnaltrexone (68) strongly inhibits the proliferation, cloning activity, invasion, and migration of FaDu and MDA686Tu cells and inhibits tumour growth in HNSCC-bearing mice [104]. 4-Nitroquinoline (69) induces the expression of cancer stem cell (CSC) markers in rat tongue cancer, and candidate CSCs increase in infiltrating areas after SCC [105,106,107]. Dasatinib (70) exhibits strong anti-growth, anti-angiogenic, and pro-apoptotic effects on two types of OCC cells (YD-38 and HSC-3) by regulating multiple cell targets and pathways [108]. Ligustilid (71) e inhibits the migration of anoxic TW2.6 cells and induces caspase-dependent apoptosis. Hsu et al. [109] demonstrated that ligustilide induces C-MYC-dependent apoptosis in hypoxic oral cancer cell lines (including TW2.6 and OML1) via ER stress signalling. Anlotinib induces G2/M arrest and apoptosis in two oral cancer cell lines, Cal-27 and SCC-25, by targeting the antiangiogenic activity of several tyrosine kinases, including vascular endothelial growth factor receptor, fibroblast growth factor receptor, and platelet-derived growth factor receptor [110]. Olaparib (74) treatment significantly reduces the proliferation, migration, invasion, and adhesion of OSS cells. Olaparib inhibits the mRNA expression of markers related to tumourigenesis and EMT, and significantly inhibits tumourigenesis and bone invasion [111]. Orlistat (75) induces the apoptosis and cell cycle arrest of HSC-3 cells in the G2/M phase by decreasing the expression of cyclins D1 and E and increasing the phosphorylation of CDK1 [112]. Ricinine (76) analogues exert anti-cancer activity by down-regulating protein tyrosine phosphatase (PTP1B) and cyclooxygenase-2 (COX-2) enzymes through highly activated PTP1B protein [113]. Entinostat (77) reduces the proliferation and promotes the apoptosis of OSCC cells by causing GO/G1 phase arrest. It can also increase the expression of acetylated histones H3 and H4 and alter the expression of cell cycle-related proteins, such as p21 [114]. Dibenzylideneacetone (78) inhibits cell viability and induces apoptosis by degrading specific Spl [115]. It also increases Bax expression, resulting in conformational changes, translocation to the mitochondria, and oligomerisation. In addition, siRNA and miramycin A induce Bax protein expression to increase apoptosis by down-regulating Spl expression.
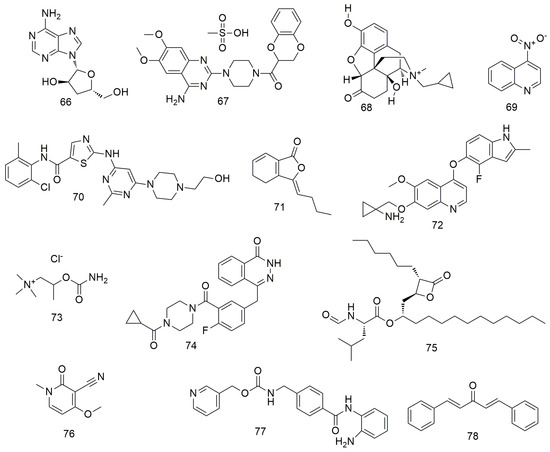
Figure 8.
Other agents.
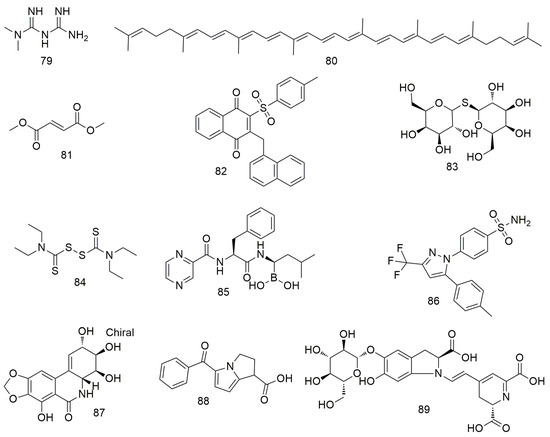
Figure 9.
Other agents.
Metformin (79) inhibits the growth and metastasis of oral cancer by down-regulating the expression of Aurora-A and Late SV40 Factor. It also suppresses tumourigenesis in xenotransplantation models [116]. The inhibitory effect of metformin on oral cancer is associated with the decreased expression of OrorA-A. Lycopene (80) inhibits the migration and promotes the apoptosis of OSCC cells by blocking the insulin-like growth factor 1 pathway [117]. Dimethyl fumarate (81) slows the progression and growth of OSCC by regulating apoptosis and reducing oxidative stress. It also reduces the migration ability of tumour cells by regulating the expression of EMT markers N-cadherin and E-cadherin [118,119]. Tang et al. [120] found that CHW09 (82) induces the apoptosis, oxidative stress, and DNA damage of OCC without exerting cytotoxicity to normal cells. Thiodigalactoside (83) significantly inhibit the growth, induce the cell cycle arrest and apoptosis, and prevent the angiogenesis of OSCC cells [121]. CuCl2 alone or in combination with disulfiram (84) significantly reduces ROS levels in the mitochondria of OECM-1 and SG cells [122]. In addition, the binding of disulfiram to Cu2+ significantly increases the cytotoxicity of OECM-1 OCC. Bortezomib (85) reduces TRAF6 expression via autophagy-mediated lysosomal degradation, which weakens the tumourigenicity of OSCC cells [123]. Celecoxib (86) inhibits oral EMT and cell migration by reducing the expression of transcription factors [124]. Narciclasine (87) inhibits oral cancer metastasis by regulating ERK pathways and cathepsin B [125]. Ketorolac (88) down-regulates DDX3 expression in the human OSCC cell line (H357) and directly inhibits ATP hydrolysis with DDX3 [126]. In addition, treatment with ketoate decreases the number and severity of tongue tumour lesions in a mouse model of carcinogen-induced tongue tumour. Betanin (89) can inhibit cell viability, MMP, and inflammation via the NF-kB/PI3K/Akt pathway and increase ROS levels in SCC131 and SCC4 OCC to induce apoptosis [127].
8. Conclusions
The incidence and mortality of oral cancer are serious threats to human life and health. This review summarises different types of oral cancer drugs and describes their sources, curative effects, and mechanisms of action, which include inhibiting the proliferation and migration, blocking the cell cycle, and enhancing the autophagy and apoptosis of oral cancer cells (Table 1), but their mechanisms of action are complex and their targets are different. Among many signalling pathways, the AKT/mTOR pathway has been studied the most, which is targeted by 6-gingerol, liquiritigenin, linalool, etc. The anti-oral mechanism of curcumin, phloretin, 6-MITC, and entinostat is through the inhibition of cell cycle. In addition, the promotion of apoptosis and autophagy is also the focus of antitumour small-molecule drug research and development, such as PAC, which has both capabilities. In particular, the IC50 value of entinostat is 0.54 µM, which is the best antitumour proliferation activity among these molecular agents. It is worth noting that dasatinib acts as an anti-growth, anti-angiogenesis, and pro-apoptotic agent by regulating multiple targets, including Src, EGFR, STAT-3, STAT-5, PKB, ERK-1/2, S6, eIF-2α, GRP78, caspase-9/3, Mcl-1, and HIF-1α. Therefore, dasatinib can be used as the first choice of anti-oral drugs.

Table 1.
Anti-oral cancer small-molecule agents.
The research on the mechanism of small-molecule anti-oral cancer is still mostly at the characterisation level, and the research on upstream and downstream signal transduction pathways needs to be further deepened. Although there are many studies on small-molecule drugs for oral cancer, there are few clinical studies reported. Therefore, how to improve the availability of drugs, enhance the targeting and accuracy of drugs, so as to better apply in clinical research, is the focus of follow-up research of small-molecule drugs. The application of disulfiram in the treatment of oral cancer provides us with a new idea for the development of antitumour drugs. The new use of old drugs can perfectly avoid the key problems of cancer drug research and development, such as long research and development cycle, high cost, and low success rate. Exploring and understanding the mechanism of action of known active anti-oral cancer compounds is of great significance for the search for new anti-oral cancer drug targets and designing anti-oral cancer drugs with strong effect, good effect, and small side effects.
Author Contributions
Writing—original draft, K.W. and W.Z.; writing—review and editing, Y.K., X.Z. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Science and Technology Public Relations Project of Henan Province (232102310372), Key scientific research project plan of colleges and universities in Henan Province (24B320013 and 24A350009), Natural Science Foundation of Henan (232300420411).
Acknowledgments
We are grateful to Weihua Hou for her contributions in the article writing-review and editing process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Petrariu, O.A.; Barbu, I.C.; Niculescu, A.G.; Constantin, M.; Grigore, G.A.; Cristian, R.E.; Mihaescu, G.; Vrancianu, C.O. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front. Microbiol. 2023, 14, 1296447. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.; Balaha, H.M.; Maklad, A.S.; Almars, A.M.; Elhosseini, M.A. Revolutionizing Oral Cancer Detection: An Approach Using Aquila and Gorilla Algorithms Optimized Transfer Learning-Based CNNs. Biomimetics 2023, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Sansare, K.; Jadhav, T.S.; Venkatraman, S.; Vahanwala, S. Oral cancer in pregnancy: A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101647. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Yoshida, M.; Kanamori, D.; Kobayashi, Y.; Nakajima, Y.; Murai, M.; Usui, M. Changes in oral health status in terminal cancer patients during the last weeks of life. Ann. Palliat. Med. 2023, 13, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, A., Jr.; Barman, I., Jr.; Roy, D.; Das, A.; Mahanta, P., Sr. Oral Cancer Hazards Related to Tobacco Use and a Transtheoretical Model Assessment of Preparedness of Individuals With Oral Potentially Malignant Disorders to Quit Tobacco Use. Cureus 2023, 15, e48125. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kamarajan, P.; Cho, A.; Wang, S.; Hung, G.C.; Najarzadegan, F.; Wong, D.T.; Ton-That, H.; Wang, C.Y.; Kapila, Y.L. Biological biomarkers of oral cancer. Periodontol. 2000 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.R.S.; Ferraz, D.L.F.; de Oliveira, C.R.G.; Evangelista, K.; Silva, M.A.G.; Silva, F.P.Y.; Silva, B.S.F. Risk and prevalence of oral cancer in patients with different types of lupus erythematosus: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 136, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.; Maddineni, S.; Arnaud, E.H.; Divi, V.; Megwalu, U.C.; Topf, M.C.; Sunwoo, J.B. Oral cavity cancer in young, non-smoking, and non-drinking patients: A contemporary review. Crit. Rev. Oncol. Hematol. 2023, 190, 104112. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.; Deloumeaux, J.; Joachim, C.; Gaete, S.; Michineau, L.; Herrmann-Storck, C.; Duflo, S.; Luce, D. Joint effect of tobacco, alcohol, and oral HPV infection on head and neck cancer risk in the French West Indies. Cancer Med. 2020, 9, 6854–6863. [Google Scholar] [CrossRef]
- Lissoni, A.; Agliardi, E.; Peri, A.; Marchioni, R.; Abati, S. Oral microbiome and mucosal trauma as risk factors for oral cancer: Beyond alcohol and tobacco. A literature review. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. S3), 11–18. [Google Scholar]
- Sujatha, G.; Veeraraghavan, V.P.; Alamoudi, A.; Bahammam, M.A.; Bahammam, S.A.; Alhazmi, Y.A.; Alharbi, H.S.; Alzahrani, K.J.; Al-Ghamdi, M.S.; Alzahrani, F.M.; et al. Role of Toothbrushes as Gene Expression Profiling Tool for Oral Cancer Screening in Tobacco and Alcohol Users. Int. J. Environ. Res. Public Health 2022, 19, 8052. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Mahootchi, P.; Rastegar, Z.; Abbasi, B.; Alam, M.; Abbasi, K.; Fani-Hanifeh, S.; Amookhteh, S.; Sadeghi, S.; Soufdoost, R.S.; et al. Photodynamic Therapy in Oral Cancer: A Narrative Review. Photobiomodul Photomed. Laser Surg. 2023, 41, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S. Molecular, genomic and mutational landscape of oral leukoplakia. Oral Dis. 2021, 27, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; Jessri, M.; Bennett, N.C.; Dalley, A.J.; Shearston, K.D.; Fox, S.A. Exome sequencing of oral leukoplakia and oral squamous cell carcinoma implicates DNA damage repair gene defects in malignant transformation. Oral Oncol. 2019, 96, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Masse, R.; Duvernay, J.; Korbi, S.; Majoufre, C.; Schlund, M. Oral carcinoma cuniculatum, a rare variant of squamous cell carcinoma. J. Stomatol. Oral Maxillofac. Surg. 2023, 125, 101729. [Google Scholar] [CrossRef]
- Pangarkar, M.; Wagh, U.; Pathak, A. Autophagy indicators in oral squamous cell carcinoma. Pathology 2024, 56, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, Z.; Lu, M.; Zhang, Y.; Jin, L.; Ye, W. Poor oral health is associated with an increased risk of esophageal squamous cell carcinoma—A population-based case-control study in China. Int. J. Cancer 2017, 140, 626–635. [Google Scholar] [CrossRef]
- Fleshner, N.E.; Alibhai, S.M.H.; Connelly, K.A.; Martins, I.; Eigl, B.J.; Lukka, H.; Aprikian, A. Adherence to oral hormonal therapy in advanced prostate cancer: A scoping review. Ther. Adv. Med. Oncol. 2023, 15, 17588359231152845. [Google Scholar] [CrossRef]
- Liao, Y.H.; Chou, W.Y.; Chang, C.W.; Lin, M.C.; Wang, C.P.; Lou, P.J.; Chen, T.C. Chemoprevention of oral cancer: A review and future perspectives. Head Neck 2023, 45, 1045–1059. [Google Scholar] [CrossRef]
- Shigeishi, H. Association between human papillomavirus and oral cancer: A literature review. Int. J. Clin. Oncol. 2023, 28, 982–989. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Namanloo, R.A.; Aghili, S.S.; Maskani, P.; Alam, M.; Abbasi, K.; Nouri, F.; Tahmasebi, E.; Yazdanian, M.; Tebyaniyan, H. Photodynamic therapy in oral cancer: A review of clinical studies. Med. Oncol. 2023, 40, 91. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, Y.; Zhu, W.; Bai, X.-g.; Qi, J. Advances in molecular agents targeting toll-like receptor 4 signaling pathways for potential treatment of sepsis. Eur. J. Med. Chem. 2024, 268, 116300. [Google Scholar] [CrossRef] [PubMed]
- Kenison, J.E.; Wang, Z.; Yang, K.; Snyder, M.; Quintana, F.J.; Sherr, D.H. The aryl hydrocarbon receptor suppresses immunity to oral squamous cell carcinoma through immune checkpoint regulation. Proc. Natl. Acad. Sci. USA 2021, 118, e2012692118. [Google Scholar] [CrossRef]
- Thiruvengadam, R.; Kim, J.H. Therapeutic strategy for oncovirus-mediated oral cancer: A comprehensive review. Biomed. Pharmacother. 2023, 165, 115035. [Google Scholar] [CrossRef] [PubMed]
- Antoniraj, M.G.; Devi, K.P.; Berindan-Neagoe, I.; Nabavi, S.F.; Khayat Kashani, H.R.; Aghaabdollahian, S.; Afkhami, F.; Jeandet, P.; Lorigooini, Z.; Khayatkashani, M.; et al. Oral microbiota in cancer: Could the bad guy turn good with application of polyphenols? Expert. Rev. Mol. Med. 2022, 25, e1. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Silva, M.; Pinillos, I.; Bartolome, B.; Moreno-Arribas, M.V. Interplay between Dietary Polyphenols and Oral and Gut Microbiota in the Development of Colorectal Cancer. Nutrients 2020, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Ogawa, T.; Niwano, Y.; Sasaki, K.; Tachibana, K. Effects of polyphenols on doxorubicin-induced oral keratinocyte cytotoxicity and anticancer potency against oral cancer cells. J. Oral Pathol. Med. 2018, 47, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Siddiqui, I.A.; Ahmad, N.; Gupta, S.; Mukhtar, H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004, 64, 8715–8722. [Google Scholar] [CrossRef]
- Kapoor, V.; Aggarwal, S.; Das, S.N. 6-Gingerol Mediates its Anti Tumor Activities in Human Oral and Cervical Cancer Cell Lines through Apoptosis and Cell Cycle Arrest. Phytother. Res. 2016, 30, 588–595. [Google Scholar] [CrossRef]
- Liu, Y.T.; Ho, H.Y.; Lin, C.C.; Chuang, Y.C.; Lo, Y.S.; Hsieh, M.J.; Chen, M.K. Platyphyllenone Induces Autophagy and Apoptosis by Modulating the AKT and JNK Mitogen-Activated Protein Kinase Pathways in Oral Cancer Cells. Int. J. Mol. Sci. 2021, 22, 4211. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Ogasawara, Y.; Hayashi, H.; Inoue, K.; Sakashita, H. Resveratrol Inhibits Proliferation and Induces Autophagy by Blocking SREBP1 Expression in Oral Cancer Cells. Molecules 2022, 27, 8250. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, G.; Di Prima, G.; Belfiore, E.; Campisi, G.; De Caro, V. Chemopreventive and Anticancer Role of Resveratrol against Oral Squamous Cell Carcinoma. Pharmaceutics 2023, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yin, X.; Ma, D.; Su, Z. Anticancer activity of Phloretin against the human oral cancer cells is due to G0/G1 cell cycle arrest and ROS mediated cell death. J. BUON 2020, 25, 344–349. [Google Scholar] [PubMed]
- Chen, Y.J.; Kuo, C.C.; Ting, L.L.; Lu, L.S.; Lu, Y.C.; Cheng, A.J.; Lin, Y.T.; Chen, C.H.; Tsai, J.T.; Chiou, J.F. Piperlongumine inhibits cancer stem cell properties and regulates multiple malignant phenotypes in oral cancer. Oncol. Lett. 2018, 15, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Yoo, H.; Kim, J.A.; Lee, S.; Jee, J.G.; Lee, M.Y.; Lee, Y.M.; Bae, J.S. Barrier protective effects of piperlonguminine in LPS-induced inflammation in vitro and in vivo. Food Chem. Toxicol. 2013, 58, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Sharma, N.K.; Mishra, N.; Mahajan, A.; Krishnan, A.; Rajpoot, R.; Kumar, J.A.; Pandey, A. Effects of curcumin on oral cancer at molecular level: A systematic review. Natl. J. Maxillofac. Surg. 2023, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-H.; Shih, P.-C.; Ding, Y.-F.; Chen, L.-H.; Hsieh, F.-K.; Tsai, M.-Y.; Li, P.-Y.; Lin, C.-W.; Yang, S.-F. Curcumin analog, GO-Y078, induces HO-1 transactivation-mediated apoptotic cell death of oral cancer cells by triggering MAPK pathways and AP-1 DNA-binding activity. Expert. Opin. Ther. Targets 2022, 26, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Semlali, A.; Contant, C.; Al-Otaibi, B.; Al-Jammaz, I.; Chandad, F. The curcumin analog (PAC) suppressed cell survival and induced apoptosis and autophagy in oral cancer cells. Sci. Rep. 2021, 11, 11701. [Google Scholar] [CrossRef]
- Yu, H.J.; Shin, J.A.; Yang, I.H.; Won, D.H.; Ahn, C.H.; Kwon, H.J.; Lee, J.S.; Cho, N.P.; Kim, E.C.; Yoon, H.J.; et al. Apoptosis induced by caffeic acid phenethyl ester in human oral cancer cell lines: Involvement of Puma and Bax activation. Arch. Oral Biol. 2017, 84, 94–99. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.Y.; Jim, W.T.; Su, L.C.; Chung, C.J.; Lin, C.Y.; Huo, C.; Tseng, J.C.; Huang, S.H.; Lai, C.J.; Chen, B.C.; et al. Caffeic Acid phenethyl ester is a potential therapeutic agent for oral cancer. Int. J. Mol. Sci. 2015, 16, 10748–10766. [Google Scholar] [CrossRef] [PubMed]
- Semlali, A.; Beji, S.; Ajala, I.; Rouabhia, M. Effects of tetrahydrocannabinols on human oral cancer cell proliferation, apoptosis, autophagy, oxidative stress, and DNA damage. Arch. Oral Biol. 2021, 129, 105200. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-P.; Lin, C.-W.; Chen, M.-K.; Yang, S.-F.; Chiou, H.-L.; Hsieh, M.-J. Pterostilbene induce autophagy on human oral cancer cells through modulation of Akt and mitogen-activated protein kinase pathway. Oral Oncol. 2015, 51, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.T.; Huang, L.J.; Wu, T.S.; Lin, H.Y.; Morris-Natschke, S.L.; Lee, K.H.; Kuo, S.C. Synthesis and antitumor activity of bis(hydroxymethyl)propionate analogs of pterostilbene in cisplatin-resistant human oral cancer cells. Bioorg. Med. Chem. 2018, 26, 3909–3916. [Google Scholar] [CrossRef]
- Eltahir, S.; Ahmad, A. Flavonoids on the Frontline against Cancer Metastasis. Cancers 2023, 15, 4139. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Mishra, K.P. Role of Plant-Derived Flavonoids in Cancer Treatment. Nutr. Cancer 2023, 75, 430–449. [Google Scholar] [CrossRef]
- Gu, Y.; Zheng, Q.; Fan, G.; Liu, R. Advances in Anti-Cancer Activities of Flavonoids in Scutellariae radix: Perspectives on Mechanism. Int. J. Mol. Sci. 2022, 23, 11042. [Google Scholar] [CrossRef]
- Lee, R.H.; Shin, J.C.; Kim, K.H.; Choi, Y.H.; Chae, J.I.; Shim, J.H. Apoptotic effects of 7,8-dihydroxyflavone in human oral squamous cancer cells through suppression of Sp1. Oncol. Rep. 2015, 33, 631–638. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Jin, Y.; Yu, H.; Fang, J. Liquiritigenin exerts the anti-cancer role in oral cancer via inducing autophagy-related apoptosis through PI3K/AKT/mTOR pathway inhibition in vitro and in vivo. Bioengineered 2021, 12, 6070–6082. [Google Scholar] [CrossRef]
- Jung, G.H.; Lee, J.H.; Han, S.H.; Woo, J.S.; Choi, E.Y.; Jeon, S.J.; Han, E.J.; Jung, S.H.; Park, Y.S.; Park, B.K.; et al. Chrysin Induces Apoptosis via the MAPK Pathway and Regulates ERK/mTOR-Mediated Autophagy in MC-3 Cells. Int. J. Mol. Sci. 2022, 23, 15747. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Choi, N.E.; Lee, J.H.; Kang, H.M.; Yu, S.B.; Kim, H.J.; Kang, H.K.; Kim, I.R. Crosstalk between Fisetin-induced Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma. J. Cancer 2019, 10, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.S.; Yao, C.N.; Liu, H.C.; Yu, F.S.; Lin, J.J.; Lu, K.W.; Liao, C.L.; Chueh, F.S.; Chung, J.G. Quercetin induced apoptosis of human oral cancer SAS cells through mitochondria and endoplasmic reticulum mediated signaling pathways. Oncol. Lett. 2018, 15, 9663–9672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fang, Z.; Zha, Z.; Sun, Q.; Wang, H.; Sun, M.; Qiao, B. Quercetin inhibits cell viability, migration and invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur. J. Pharmacol. 2019, 847, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, Y.; Zhou, H.; Lv, J. Baicalein inhibits the growth of oral squamous cell carcinoma cells by downregulating the expression of transcription factor Sp1. Int. J. Oncol. 2020, 56, 273–282. [Google Scholar] [CrossRef]
- Tu, D.G.; Lin, W.T.; Yu, C.C.; Lee, S.S.; Peng, C.Y.; Lin, T.; Yu, C.H. Chemotherapeutic effects of luteolin on radio-sensitivity enhancement and interleukin-6/signal transducer and activator of transcription 3 signaling repression of oral cancer stem cells. J. Formos. Med. Assoc. 2016, 115, 1032–1038. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lee, P.C.; Wang, J.J.; Hsu, Y.C. Anticancer Effect and Mechanism of Hydroxygenkwanin in Oral Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, D.; Garavello, W.; Rigolio, R.; Pignataro, L.; Gaini, R.; Nicolini, G. Apigenin impairs oral squamous cell carcinoma growth in vitro inducing cell cycle arrest and apoptosis. Int. J. Oncol. 2013, 43, 1675–1682. [Google Scholar] [CrossRef]
- Wudtiwai, B.; Makeudom, A.; Krisanaprakornkit, S.; Pothacharoen, P.; Kongtawelert, P. Anticancer Activities of Hesperidin via Suppression of Up-Regulated Programmed Death-Ligand 1 Expression in Oral Cancer Cells. Molecules 2021, 26, 5345. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Lin, J.T.; Mahalakshmi, B.; Chuang, Y.C.; Lin, C.C.; Lo, Y.S.; Hsieh, M.J.; Chen, M.K. Luteolin-7-O-Glucoside Inhibits Oral Cancer Cell Migration and Invasion by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway. Biomolecules 2020, 10, 502. [Google Scholar] [CrossRef]
- Tsai, J.Y.; Lee, M.J.; Chang, M.D.; Wang, H.C.; Lin, C.C.; Huang, H. Effects of novel human cathepsin S inhibitors on cell migration in human cancer cells. J. Enzym. Inhib. Med. Chem. 2014, 29, 538–546. [Google Scholar] [CrossRef]
- Chen, C.T.; Hsieh, M.J.; Hsieh, Y.H.; Hsin, M.C.; Chuang, Y.T.; Yang, S.F.; Yang, J.S.; Lin, C.W. Sulforaphane suppresses oral cancer cell migration by regulating cathepsin S expression. Oncotarget 2018, 9, 17564–17575. [Google Scholar] [CrossRef]
- Varadarajan, S.; Madapusi, B.T.; Narasimhan, M.; Pandian, C.D.; Dhanapal, S. Anticancer Effects of Carica papaya L. and Benzyl Isothiocyanate on an Oral Squamous Cell Carcinoma Cell Line: An In Vitro Study. J. Contemp. Dent. Pract. 2022, 23, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Tseng, W.S.; Lai, J.C.; Shieh, H.R.; Chi, C.W.; Chen, Y.J. Differential Pharmacological Activities of Oxygen Numbers on the Sulfoxide Moiety of Wasabi Compound 6-(Methylsulfinyl) Hexyl Isothiocyanate in Human Oral Cancer Cells. Molecules 2018, 23, 2427. [Google Scholar] [CrossRef]
- Hsu, P.C.; Cheng, C.F.; Hsieh, P.C.; Chen, Y.H.; Kuo, C.Y.; Sytwu, H.K. Chrysophanol Regulates Cell Death, Metastasis, and Reactive Oxygen Species Production in Oral Cancer Cell Lines. Evid. Based Complement. Altern. Med. 2020, 2020, 5867064. [Google Scholar] [CrossRef]
- Li, Q.; Wen, J.; Yu, K.; Shu, Y.; He, W.; Chu, H.; Zhang, B.; Ge, C. Aloe-emodin induces apoptosis in human oral squamous cell carcinoma SCC15 cells. BMC Complement. Altern. Med. 2018, 18, 296. [Google Scholar] [CrossRef]
- Lin, C.L.; Yu, C.I.; Lee, T.H.; Chuang, J.M.; Han, K.F.; Lin, C.S.; Huang, W.P.; Chen, J.Y.; Chen, C.Y.; Lin, M.Y.; et al. Plumbagin induces the apoptosis of drug-resistant oral cancer in vitro and in vivo through ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction. Phytomedicine 2023, 111, 154655. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Si, L.; Zheng, Y.; Wang, H. Shikonin enhances chemosensitivity of oral cancer through β-catenin pathway. Oral Dis. 2022, 12, 14458. [Google Scholar] [CrossRef]
- Cho, B.H.; Jung, Y.H.; Kim, D.J.; Woo, B.H.; Jung, J.E.; Lee, J.H.; Choi, Y.W.; Park, H.R. Acetylshikonin suppresses invasion of Porphyromonas gingivalis-infected YD10B oral cancer cells by modulating the interleukin-8/matrix metalloproteinase axis. Mol. Med. Rep. 2018, 17, 2327–2334. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, J.H.; Park, H.R.; Choi, Y.W. Acetylshikonin inhibits growth of oral squamous cell carcinoma by inducing apoptosis. Arch. Oral Biol. 2016, 70, 149–157. [Google Scholar] [CrossRef]
- Salar, T.; Jimenez, M.; Hameed, M.; Ocon, A. Chronic Anti-HMG-CoA Reductase Positive Necrotizing Myositis With Remote Exposure to Statins. Cureus 2023, 15, e40552. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Matyszewska, D.; Bilewicz, R. Model Lipid Raft Membranes for Embedding Integral Membrane Proteins: Reconstitution of HMG-CoA Reductase and Its Inhibition by Statins. Langmuir 2022, 38, 13888–13897. [Google Scholar] [CrossRef]
- Miyajima, C.; Hayakawa, Y.; Inoue, Y.; Nagasaka, M.; Hayashi, H. HMG-CoA Reductase Inhibitor Statins Activate the Transcriptional Activity of p53 by Regulating the Expression of TAZ. Pharmaceuticals 2022, 15, 1015. [Google Scholar] [CrossRef] [PubMed]
- Biselli-Chicote, P.M.; Lotierzo, A.T.; Biselli, J.M.; Paravino, E.C.; Goloni-Bertollo, E.M. Atorvastatin increases oxidative stress and inhibits cell migration of oral squamous cell carcinoma in vitro. Oral Oncol. 2019, 90, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kansal, V.; Burnham, A.J.; Kinney, B.L.C.; Saba, N.F.; Paulos, C.; Lesinski, G.B.; Buchwald, Z.S.; Schmitt, N.C. Statin drugs enhance responses to immune checkpoint blockade in head and neck cancer models. J. Immunother. Cancer 2023, 11, e005940. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chen, W.M.; Shia, B.C.; Chen, M.; Wu, S.Y. The effect of statin medication on the incidence of oral squamous cell carcinoma among betel-nut chewers. Head Neck 2023, 45, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhang, G. Linalool monoterpene exerts potent antitumor effects in OECM 1 human oral cancer cells by inducing sub-G1 cell cycle arrest, loss of mitochondrial membrane potential and inhibition of PI3K/AKT biochemical pathway. J. BUON 2019, 24, 323–328. [Google Scholar] [PubMed]
- Hsieh, M.J.; Lin, C.W.; Chiou, H.L.; Yang, S.F.; Chen, M.K. Dehydroandrographolide, an iNOS inhibitor, extracted from Andrographis paniculata (Burm.f.) Nees, induces autophagy in human oral cancer cells. Oncotarget 2015, 6, 30831–30849. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Hsieh, M.J.; Lin, J.T.; Chen, G.; Lin, C.C.; Lo, Y.S.; Chuang, Y.C.; Hsi, Y.T.; Chen, M.K.; Chou, M.C. Coronarin D induces human oral cancer cell apoptosis though upregulate JNK1/2 signaling pathway. Environ. Toxicol. 2019, 34, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yi, J.K.; Lim, S.G.; Park, S.; Zhang, H.; Kim, E.; Jang, S.; Lee, M.H.; Liu, K.; Kim, K.R.; et al. Costunolide Induces Apoptosis via the Reactive Oxygen Species and Protein Kinase B Pathway in Oral Cancer Cells. Int. J. Mol. Sci. 2021, 22, 7509. [Google Scholar] [CrossRef]
- She, Y.Y.; Lin, J.J.; Su, J.H.; Chang, T.S.; Wu, Y.J. 4-Carbomethoxyl-10-Epigyrosanoldie E Extracted from Cultured Soft Coral Sinularia sandensis Induced Apoptosis and Autophagy via ROS and Mitochondrial Dysfunction and ER Stress in Oral Cancer Cells. Oxid. Med. Cell Longev. 2022, 2022, 3017807. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Wu, C.Y.; Tang, J.Y.; Huang, C.Y.; Liaw, C.C.; Wu, S.H.; Sheu, J.H.; Chang, H.W. Sinularin induces oxidative stress-mediated G2/M arrest and apoptosis in oral cancer cells. Environ. Toxicol. 2017, 32, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.H.; Lin, Y.S.; Wang, S.C.; Lee, M.Y.; Tang, J.Y.; Chang, F.R.; Chuang, Y.T.; Sheu, J.H.; Chang, H.W. Soft Coral-Derived Dihydrosinularin Exhibits Antiproliferative Effects Associated with Apoptosis and DNA Damage in Oral Cancer Cells. Pharmaceuticals 2021, 14, 994. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lo, Y.H.; Lin, C.L.; Lee, T.H.; Leung, W.; Wang, S.W.; Lin, I.P.; Lin, M.Y.; Lee, C.H. Trichodermin inhibits the growth of oral cancer through apoptosis-induced mitochondrial dysfunction and HDAC-2-mediated signaling. Biomed. Pharmacother. 2022, 153, 113351. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.S.; Yang, C.Y.; Lin, C.K.; Lin, G.J.; Sytwu, H.K.; Chen, Y.W. Triptolide suppresses oral cancer cell PD-L1 expression in the interferon-gamma-modulated microenvironment in vitro, in vivo, and in clinical patients. Biomed. Pharmacother. 2021, 133, 111057. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.J.; Hsieh, C.Y.; Tang, J.Y.; Lin, L.C.; Huang, H.W.; Wang, H.R.; Yeh, Y.C.; Chuang, Y.T.; Ou-Yang, F.; Chang, H.W. Antimycin A shows selective antiproliferation to oral cancer cells by oxidative stress-mediated apoptosis and DNA damage. Environ. Toxicol. 2020, 35, 1212–1224. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chan, C.K.; Yen, C.Y.; Shiau, J.P.; Chang, M.Y.; Wang, C.C.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Antioral Cancer Effects by the Nitrated [6,6,6]Tricycles Compound (SK1) In Vitro. Antioxidants 2022, 11, 2072. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Ahn, C.H.; Yang, I.H.; Jin, B.; Lee, W.W.; Kim, J.H.; Ahn, M.H.; Swarup, N.; Hong, K.O.; Shin, J.A.; et al. Pseudolaric Acid B Induces Growth Inhibition and Caspase-Dependent Apoptosis on Head and Neck Cancer Cell lines through Death Receptor 5. Molecules 2019, 24, 3715. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Chen, Y.T.; Hsieh, Y.H.; Yang, W.E.; Su, C.W.; Chiu, W.Y.; Yang, S.F.; Lin, C.W. Gambogic Acid Induces HO-1 Expression and Cell Apoptosis through p38 Signaling in Oral Squamous Cell Carcinoma. Am. J. Chin. Med. 2022, 50, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, N.A.; Wang, R.; Huang, F.; Li, G. Paclitaxel induces apoptosis and reduces proliferation by targeting epidermal growth factor receptor signaling pathway in oral cavity squamous cell carcinoma. Oncol. Lett. 2015, 10, 2378–2384. [Google Scholar] [CrossRef]
- Saha, D.; Mitra, D.; Alam, N.; Sen, S.; Mustafi, S.M.; Majumder, P.K.; Majumder, B.; Murmu, N. Lupeol and Paclitaxel cooperate in hindering hypoxia induced vasculogenic mimicry via suppression of HIF-1alpha-EphA2-Laminin-5gamma2 network in human oral cancer. J. Cell Commun. Signal. 2023, 17, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Chin, H.K.; Lee, S.L.; Chiu, C.F.; Chung, J.G.; Lin, Z.Y.; Wu, C.Y.; Liu, Y.C.; Hsiao, Y.T.; Feng, C.H.; et al. Ursolic acid induces apoptosis and autophagy in oral cancer cells. Environ. Toxicol. 2019, 34, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Lin, C.S.; Hua, C.H.; Jou, Y.J.; Liao, C.R.; Chang, Y.S.; Wan, L.; Huang, S.H.; Hour, M.J.; Lin, C.W. Cis-3-O-p-hydroxycinnamoyl Ursolic Acid Induced ROS-Dependent p53-Mediated Mitochondrial Apoptosis in Oral Cancer Cells. Biomol. Ther. 2019, 27, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Naseri, N.; Fathiazad, F.; Asnaashari, S.; Hamedeyazdan, S. Anticancer effect of buddlejasaponin IV and buddlejasaponin IVa from Clinopodium umbrosum on oral cancer cells (HN-5). Toxicon 2022, 220, 106939. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Zhao, X.; Liu, W.; Deng, J.; Tan, X.; Qiu, L. Anticancer Effect of Ursodeoxycholic Acid in Human Oral Squamous Carcinoma HSC-3 Cells through the Caspases. Nutrients 2015, 7, 3200–3218. [Google Scholar] [CrossRef]
- Shen, H.; Liu, L.; Yang, Y.; Xun, W.; Wei, K.; Zeng, G. Betulinic Acid Inhibits Cell Proliferation in Human Oral Squamous Cell Carcinoma via Modulating ROS-Regulated p53 Signaling. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 1141–1152. [Google Scholar] [CrossRef]
- Rauth, S.; Ray, S.; Bhattacharyya, S.; Mehrotra, D.G.; Alam, N.; Mondal, G.; Nath, P.; Roy, A.; Biswas, J.; Murmu, N. Lupeol evokes anticancer effects in oral squamous cell carcinoma by inhibiting oncogenic EGFR pathway. Mol. Cell Biochem. 2016, 417, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yi, J.; Kim, E.; Choo, Y.; Hai, H.; Kim, K.; Kim, E.K.; Ryoo, Z.; Kim, M. 20(S)-Ginsenoside Rh2 Suppresses Oral Cancer Cell Growth by Inhibiting the Src-Raf-ERK Signaling Pathway. Anticancer Res. 2021, 41, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Wong, W.T.; Li, L.H.; Chu, L.J.; Menon, M.P.; Ho, C.L.; Chernikov, O.V.; Lee, S.L.; Hua, K.F. Ginsenoside M1 Induces Apoptosis and Inhibits the Migration of Human Oral Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9704. [Google Scholar] [CrossRef]
- Li, T.; Wang, L. Riparsaponin isolated from Homonoia riparia Lour induces apoptosis of oral cancer cells. Oncol. Lett. 2017, 14, 6841–6846. [Google Scholar] [CrossRef]
- Ho, S.Y.; Wu, W.S.; Lin, L.C.; Wu, Y.H.; Chiu, H.W.; Yeh, Y.L.; Huang, B.M.; Wang, Y.J. Cordycepin Enhances Radiosensitivity in Oral Squamous Carcinoma Cells by Inducing Autophagy and Apoptosis Through Cell Cycle Arrest. Int. J. Mol. Sci. 2019, 20, 5366. [Google Scholar] [CrossRef] [PubMed]
- Su, N.W.; Wu, S.H.; Chi, C.W.; Tsai, T.H.; Chen, Y.J. Cordycepin, isolated from medicinal fungus Cordyceps sinensis, enhances radiosensitivity of oral cancer associated with modulation of DNA damage repair. Food Chem. Toxicol. 2019, 124, 400–410. [Google Scholar] [CrossRef]
- Xing, D.; Li, L.; Meng, D.; Zhang, Y.; Ma, F. Anti-cell Proliferative Mechanism of Doxazosin on Human Oral Cancer Cells Through the Modulation of Antioxidant and Apoptotic Pathway. Appl. Biochem. Biotechnol. 2023, 195, 6824–6839. [Google Scholar] [CrossRef] [PubMed]
- Gorur, A.; Patino, M.; Shi, T.; Corrales, G.; Takahashi, H.; Rangel, R.; Gleber-Netto, F.O.; Pickering, C.; Myers, J.N.; Cata, J.P. Low doses of methylnaltrexone inhibits head and neck squamous cell carcinoma growth in vitro and in vivo by acting on the mu-opioid receptor. J. Cell Physiol. 2021, 236, 7698–7710. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Choi, H.; Kim, J.; Kim, S.; Jeon, S.; Ni, K.; Song, S.Y.; Oh, H.K.; Im, Y.; Lee, G.; et al. Expression of cancer stem cell marker during 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. J. Mol. Histol. 2014, 45, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.R.; Thakur, S.; Peroumal, D.; Utkalaja, B.G.; Dutta, A.; Kumari, P.; Subhadarsini, I.; Acharya, N. 4-nitroquinoline 1-oxide induces immune cells death to onset early immunosuppression during oral squamous cell carcinoma development. Front. Immunol. 2023, 14, 1274519. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Suchalatha, S.; Babu, P.V.; Devi, R.S.; Narayan, S.; Sabitha, K.E.; Shyamala Devi, C.S. Chemopreventive and therapeutic modulation of green tea polyphenols on drug metabolizing enzymes in 4-Nitroquinoline 1-oxide induced oral cancer. Chem. Biol. Interact. 2008, 172, 224–234. [Google Scholar] [CrossRef]
- Park, N.S.; Park, Y.K.; Yadav, A.K.; Shin, Y.M.; Bishop-Bailey, D.; Choi, J.S.; Park, J.W.; Jang, B.C. Anti-growth and pro-apoptotic effects of dasatinib on human oral cancer cells through multi-targeted mechanisms. J. Cell. Mol. Med. 2021, 25, 8300–8311. [Google Scholar] [CrossRef]
- Hsu, R.J.; Peng, K.Y.; Hsu, W.L.; Chen, Y.T.; Liu, D.W. Z-Ligustilide Induces c-Myc-Dependent Apoptosis via Activation of ER-Stress Signaling in Hypoxic Oral Cancer Cells. Front. Oncol. 2022, 12, 824043. [Google Scholar] [CrossRef]
- Deng, Z.; Liao, W.; Wei, W.; Zhong, G.; He, C.; Zhang, H.; Liu, Q.; Xu, X.; Liang, J.; Liu, Z. Anlotinib as a promising inhibitor on tumor growth of oral squamous cell carcinoma through cell apoptosis and mitotic catastrophe. Cancer Cell Int. 2021, 21, 37. [Google Scholar] [CrossRef]
- Nakamura, N.; Fujihara, H.; Kawaguchi, K.; Yamada, H.; Nakayama, R.; Yasukawa, M.; Kishi, Y.; Hamada, Y.; Masutani, M. Possible Action of Olaparib for Preventing Invasion of Oral Squamous Cell Carcinoma In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 2527. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, H.; Li, X. Orlistat treatment induces apoptosis and arrests cell cycle in HSC-3 oral cancer cells. Microb. Pathog. 2017, 112, 15–19. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.H.; Abdel Bar, F.M.; Harsha, C.; Monisha, J.; Shimizu, K.; Kunnumakkara, A.B.; Badria, F.A. Synthesis of new selective cytotoxic ricinine analogues against oral squamous cell carcinoma. Nat. Product. Res. 2019, 35, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.E.M.; do Nascimento Filho, C.H.V.; Marinho Bezerra, T.M.; Guerra, E.N.S.; Castilho, R.M.; Squarize, C.H. Entinostat is a novel therapeutic agent to treat oral squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Shin, J.A.; Nam, J.S.; Kang, B.S.; Cho, S.D. Apoptotic effect of dibenzylideneacetone on oral cancer cells via modulation of specificity protein 1 and Bax. Oral Dis. 2013, 19, 767–774. [Google Scholar] [CrossRef]
- Chen, C.H.; Tsai, H.T.; Chuang, H.C.; Shiu, L.Y.; Su, L.J.; Chiu, T.J.; Luo, S.D.; Fang, F.M.; Huang, C.C.; Chien, C.Y. Metformin disrupts malignant behavior of oral squamous cell carcinoma via a novel signaling involving Late SV40 factor/Aurora-A. Sci. Rep. 2017, 7, 1358. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Wang, X.; Li, C. Effect of Lycopene on Oral Squamous Cell Carcinoma Cell Growth by Inhibiting IGF1 Pathway. Cancer Manag. Res. 2021, 13, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Babukumar, S.; Vinothkumar, V.; Ramachandhiran, D. Modulating effect of hesperetin on the molecular expression pattern of apoptotic and cell proliferative markers in 7,12-dimethylbenz(a)anthracene-induced oral carcinogenesis. Arch. Physiol. Biochem. 2020, 126, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Basilotta, R.; Lanza, M.; Filippone, A.; Casili, G.; Mannino, D.; De Gaetano, F.; Chisari, G.; Colarossi, L.; Motta, G.; Campolo, M.; et al. Therapeutic Potential of Dimethyl Fumarate in Counteract Oral Squamous Cell Carcinoma Progression by Modulating Apoptosis, Oxidative Stress and Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2023, 24, 2777. [Google Scholar] [CrossRef]
- Tang, J.Y.; Wu, C.Y.; Shu, C.W.; Wang, S.C.; Chang, M.Y.; Chang, H.W. A novel sulfonyl chromen-4-ones (CHW09) preferentially kills oral cancer cells showing apoptosis, oxidative stress, and DNA damage. Environ. Toxicol. 2018, 33, 1195–1203. [Google Scholar] [CrossRef]
- Aggarwal, S.; Das, S.N. Thiodigalactoside shows antitumour activity by beta-galactoside-binding protein and regulatory T cells inhibition in oral squamous cell carcinoma. Oral Dis. 2016, 22, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chang, Y.L.; Liu, S.T.; Chen, G.S.; Lee, S.P.; Huang, S.M. Differential Cytotoxicity Mechanisms of Copper Complexed with Disulfiram in Oral Cancer Cells. Int. J. Mol. Sci. 2021, 22, 3711. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Wu, W.S.; Lin, L.C.; Liu, C.S.; Ho, S.Y.; Wang, B.J.; Huang, B.M.; Yeh, Y.L.; Chiu, H.W.; Yang, W.L.; et al. Bortezomib enhances radiosensitivity in oral cancer through inducing autophagy-mediated TRAF6 oncoprotein degradation. J. Exp. Clin. Cancer Res. 2018, 37, 91. [Google Scholar] [CrossRef]
- Chiang, S.L.; Velmurugan, B.K.; Chung, C.M.; Lin, S.H.; Wang, Z.H.; Hua, C.H.; Tsai, M.H.; Kuo, T.M.; Yeh, K.T.; Chang, P.Y.; et al. Preventive effect of celecoxib use against cancer progression and occurrence of oral squamous cell carcinoma. Sci. Rep. 2017, 7, 6235. [Google Scholar] [CrossRef] [PubMed]
- Shieu, M.K.; Ho, H.Y.; Lin, C.C.; Lo, Y.S.; Chuang, Y.C.; Hsieh, M.J.; Chen, M.K. Narciclasine suppresses oral cancer metastasis by modulating cathepsin B and extracellular signal-related kinase pathways. Biomed. Pharmacother. 2023, 158, 114159. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Routray, S.; Veeramachaneni, G.K.; Dash, R.; Botlagunta, M. Ketorolac salt is a newly discovered DDX3 inhibitor to treat oral cancer. Sci. Rep. 2015, 5, 9982. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yu, K.; Chu, X.; Yang, L. Betanin alleviates inflammation and ameliorates apoptosis on human oral squamous cancer cells SCC131 and SCC4 through the NF-kappaB/PI3K/Akt signaling pathway. J. Biochem. Mol. Toxicol. 2022, 36, e23094. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).