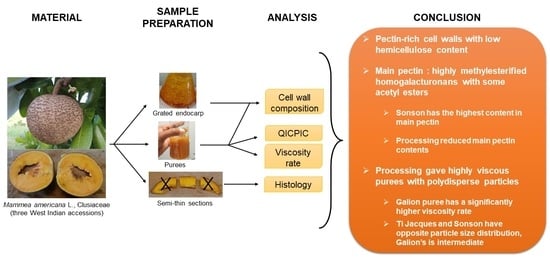
Characterization of Crude and Processed Pulp Cell Walls of Three Selected Mamey Accessions (Mammea americana L.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Cell Walls from Mamey Fruit Pulp
2.1.1. Cell Wall Yields and Composition
2.1.2. Yields and Sugar Composition of Water-Soluble and -Insoluble Polysaccharides
2.1.3. Yields and Sugar Composition of Water-Soluble and -Insoluble Polysaccharides
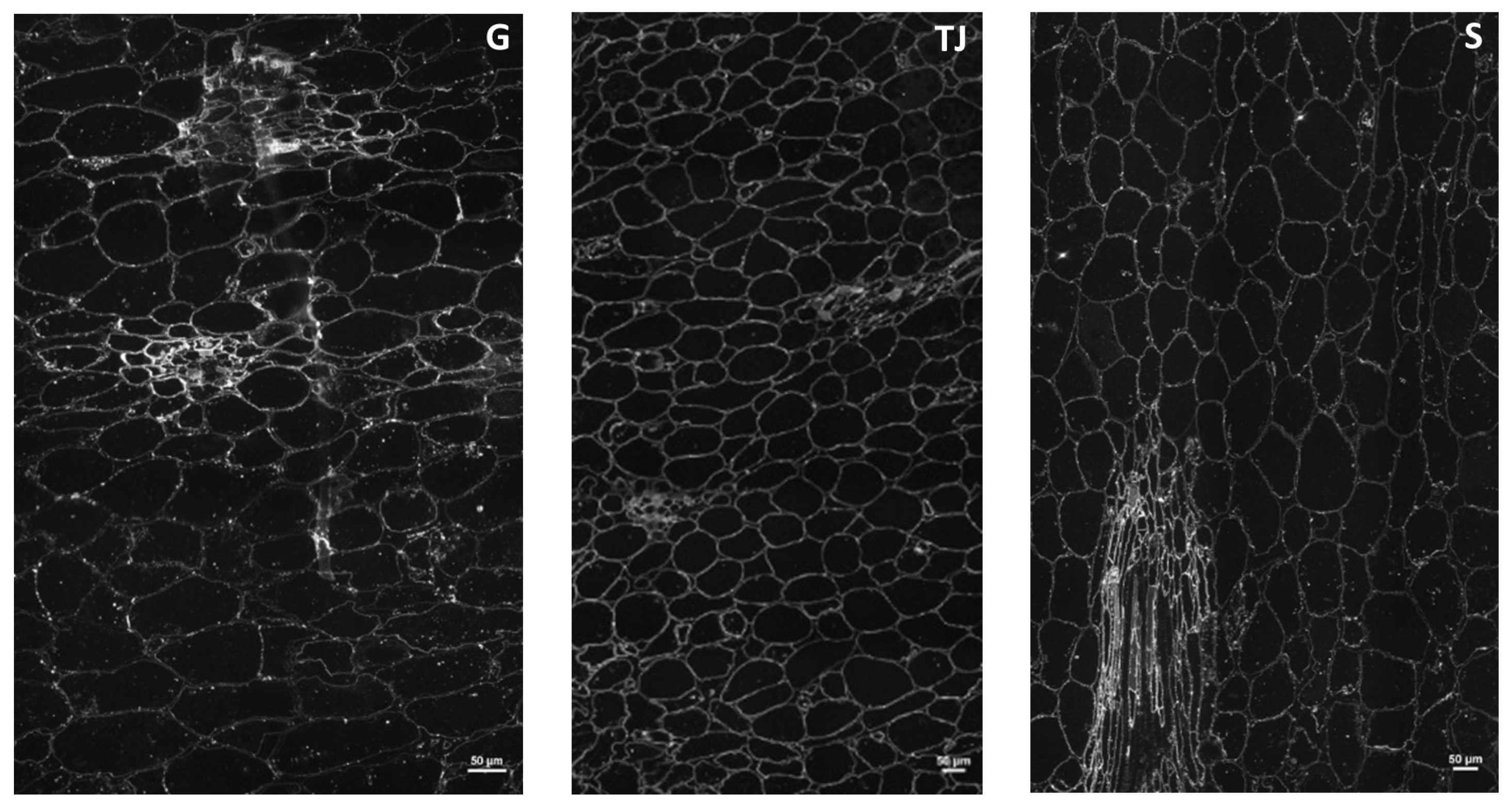
2.1.4. Immunohistochemical Characterization of Cell Wall Polysaccharides
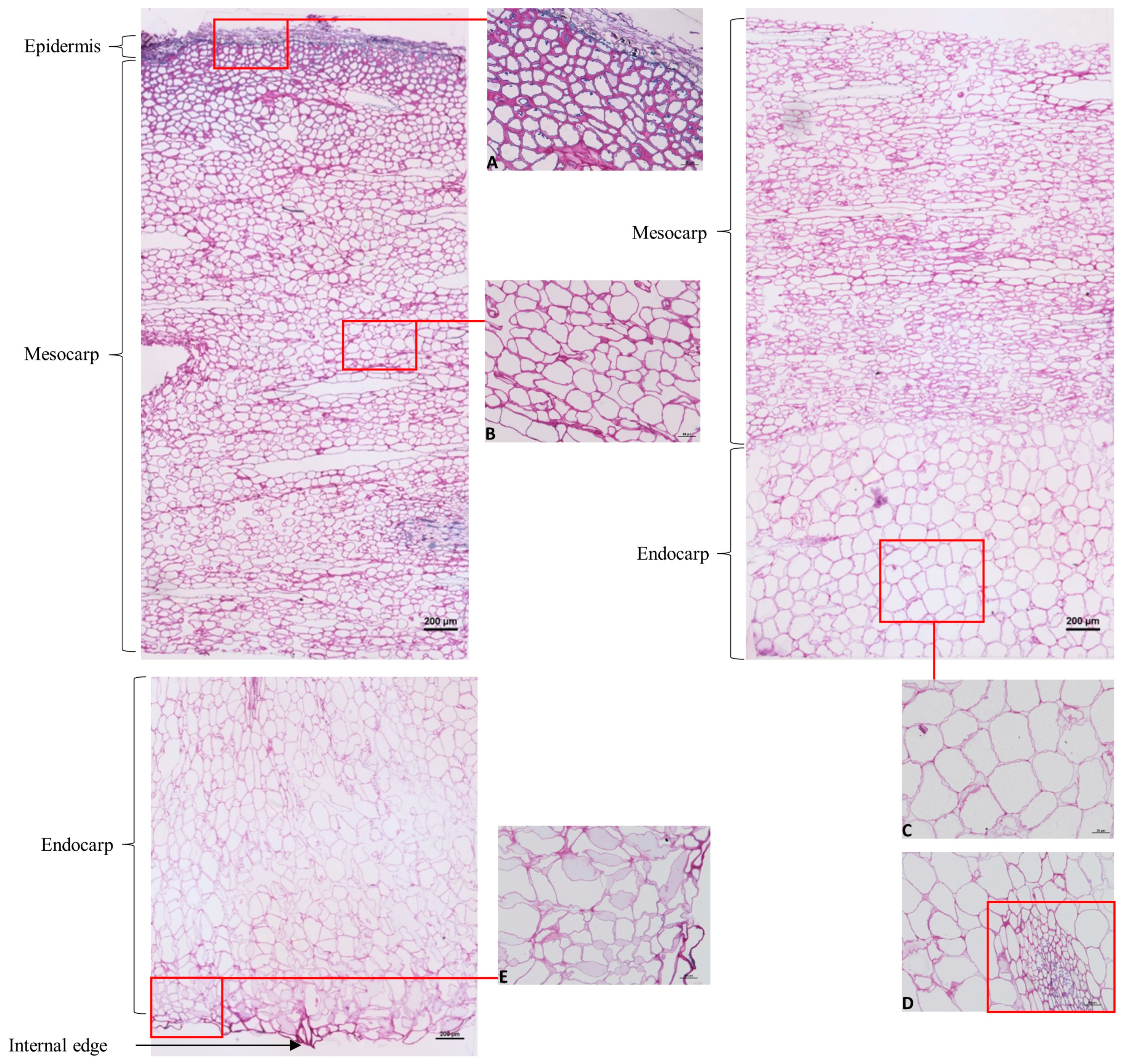
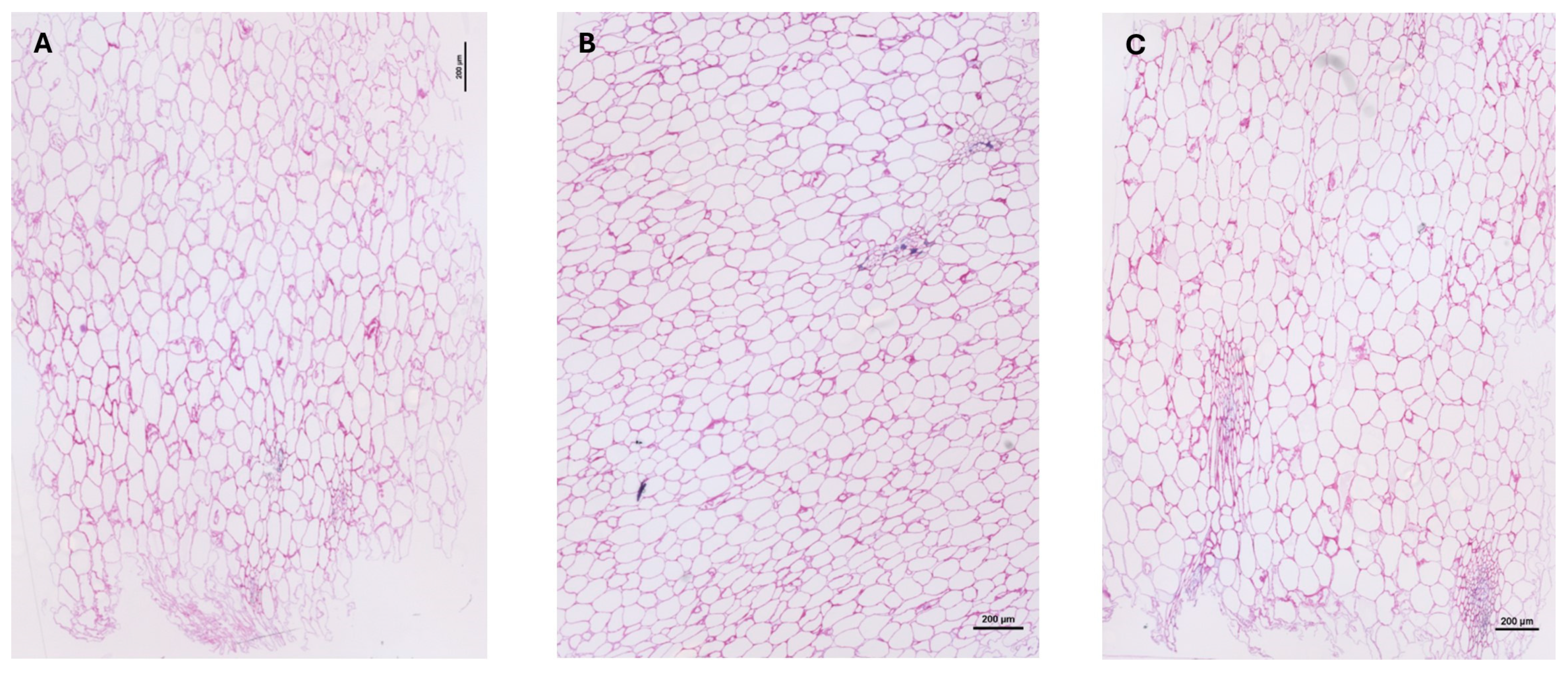
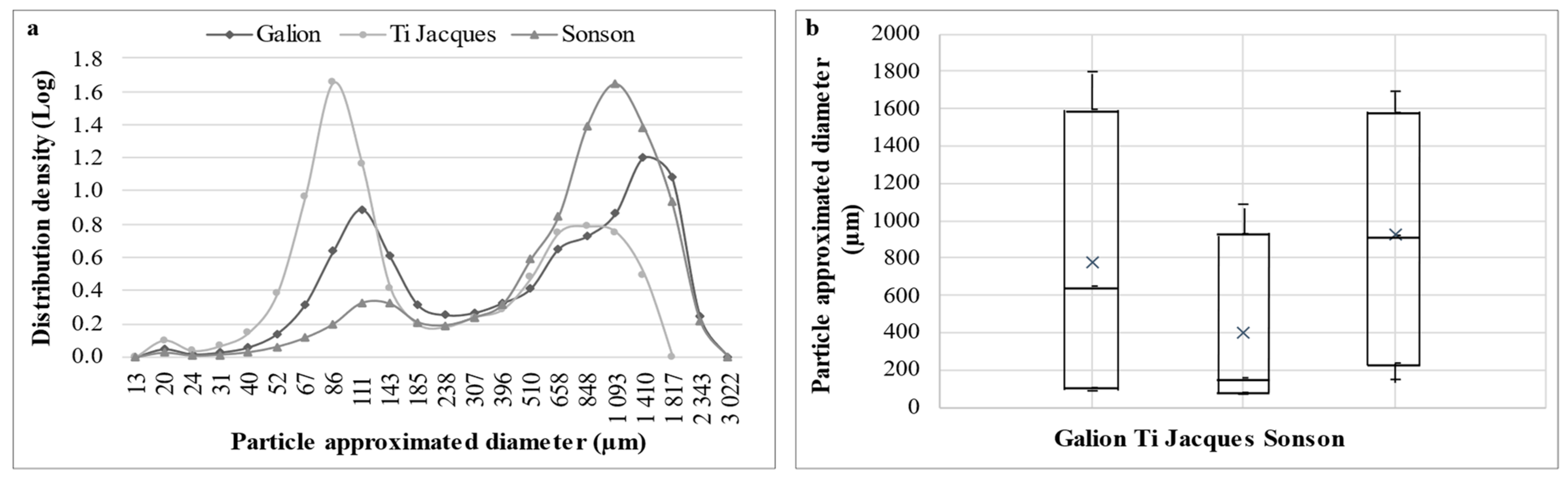
2.2. Rheological and Histological Characterization of Mamey Puree
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Standards
3.3. Plant Material
3.4. Preparation of Puree and Grated Endocarp
3.5. Preparation of Alcohol-Insoluble Residue (AIR)
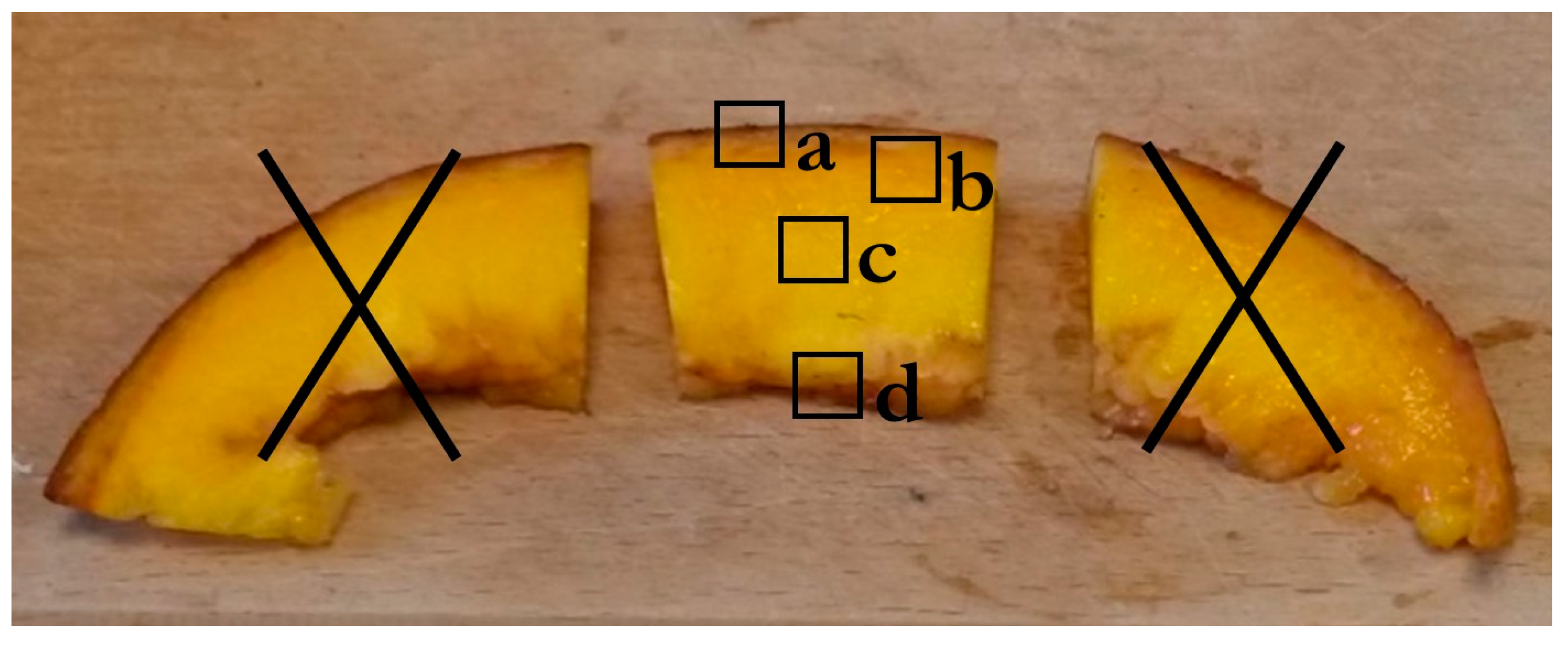
3.6. Cell Wall Immunohistochemistry
3.6.1. Sample Preparation
3.6.2. Coloration for Observation under Macroscope
3.6.3. Immunolabeling on Endocarps
3.7. Cell Wall Chemical Composition
3.7.1. Sample Preparation
3.7.2. Quantitative Analysis of Sugars
3.7.3. Quantitative Analysis of Methanol and Acetic Acid
3.7.4. Molecular Weight of Polysaccharides
3.8. Rheological and Histological Characterization of Purees
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lémus, C.; Smith-Ravin, J.; Marcelin, O. Mammea americana: A review of traditional uses, phytochemistry and biological activities. J. Herb. Med. 2021, 29, 100466. [Google Scholar] [CrossRef]
- Gervais, L.; Lavigne, C. Mamey (Mammea americana L.) in Martinique Island: An inheritance to be developed. Fruits 2007, 62, 237–246. [Google Scholar] [CrossRef]
- Péroumal, A. Caractérisation des Fruits et de la Pulpe de Six Accessions de Mammea Americana. Aptitude à la Transformation des Fruits et Caractérisation des Composés Phénoliques de la Pulpe. Ph.D. Thesis, Université des Antilles et de la Guyane, Pointe-à-Pitre, Guadeloupe, 2014. [Google Scholar]
- de Rosso, V.; Mercadante, A. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Giuffrida, D.; Menchaca, D.; Dugo, P.; Donato, P.; Cacciola, F.; Murillo, E. Study of the carotenoid composition in membrillo, guanabana toreta, jobo and mamey fruits. Fruits 2015, 70, 163–172. [Google Scholar] [CrossRef]
- Péroumal, A.; Adenet, S.; Rochefort, K.; Fahrasmane, L.; Aurore, G. Variability of traits and bioactive compounds in the fruit and pulp of six mamey apple (Mammea americana L.) accessions. Food Chem. 2017, 234, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.d.S.; Rodrigues, A.M.d.C.; da Silva, L.H.M. Development of a dehydrated product with edible film characteristics from mammee apple (Mammea americana L.) using refractance window drying. Food Sci. Technol. 2019, 40, 245–249. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Martínez-Álvarez, G.M.; Vázquez-Riascos, A.M. Effect of processing on the physicochemical and sensory properties of mammee apple (Mammea americana L.) fruit. Agrociencia 2014, 48, 377–385. [Google Scholar]
- Palmont, D.; Mazaloubeaud, C.; Gervais, B.; Cécilia-Joseph, E.; Smith-Ravin, E.J.; Marcelin, O. Assessment of the suitability for primary processing of eight accessions of mamey (Mammea americana L.) cultivated in Martinique. IOSR J. Environ. Sci. Toxicol. Food Technol. 2020, 14, 34–46. [Google Scholar]
- Péroumal, A.; Vingadassalon, A.; Lawrence, G.; Adenet, S.; Rochefort, K. Nutritional characteristics and phenolic compounds of ripe fruit pulp from six accessions of Mammea americana L. Int. J. Agric. Sci. Food Technol. 2022, 8, 033–037. [Google Scholar]
- Caamaño-Murillo, M.E.; Macías-Catagua, A.D.; Mera-Zambrano, C.M.; Roldán-Vera, M.D. Evaluación del uso de Mammea americana (Mamey) en repostería actual. Rev. Gastron. Cocina 2023, 2, 30–37. [Google Scholar] [CrossRef]
- Brito, B.; Vaillant, F. Enzymatic liquefaction of cell-walls from Kent and Tommy Atkins mango fruits. Int. J. Food Sci. Nutr. Eng. 2012, 2, 76–84. [Google Scholar] [CrossRef]
- Marcelin, O.; Smith, A.B.; Bonnin, E.; Brillouet, J.-M. Enzymatic breakdown of cell wall polysaccharides of guava (Psidium guajava L.) puree. IOSR J. Environ. Sci. Toxicol. Food Technol. 2017, 11, 14–23. [Google Scholar] [CrossRef]
- Dheilly, E.; Gall, S.L.; Guillou, M.-C.; Renou, J.-P.; Bonnin, E.; Orsel, M.; Lahaye, M. Cell wall dynamics during apple development and storage involves hemicellulose modifications and related expressed genes. BMC Plant Biol. 2016, 16, 201. [Google Scholar] [CrossRef]
- Gross, K.C. Fractionation and partial characterization of cell walls from normal and non-ripening mutant tomato fruit. Physiol. Plant. 1984, 62, 25–32. [Google Scholar] [CrossRef]
- Nunan, K.J.; Sims, I.M.; Bacic, A.; Robinson, S.P.; Fincher, G.B. Changes in cell wall composition during ripening of grape berries. Plant Physiol. 1998, 118, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, O.; Mazaloubeaud, C.; Priam, F.; Marcus, R.; Belloir, F.; Vrabié, V.; Smith-Ravin, E.J. An integrative analytical study of the functional and antioxidant properties of selected varieties of pink guava Psidium guajava L. IOSR J. Environ. Sci. Toxicol. Food Technol. 2017, 11, 57–67. [Google Scholar] [CrossRef]
- Renard CM, G.C.; Ginies, C. Comparison of the cell wall composition for flesh and skin from five different plums. Food Chem. 2009, 114, 1042–1049. [Google Scholar] [CrossRef]
- Kurz, C.; Carle, R.; Schieber, A. Characterisation of cell wall polysaccharide profiles of apricots (Prunus armeniaca L.), peaches (Prunus persica L.), and pumpkins (Cucurbita sp.) for the evaluation of fruit product authenticity. Food Chem. 2008, 106, 421–430. [Google Scholar] [CrossRef]
- Hwang, J.; Kokini, J.L. Contribution of the side branches to rheological properties of pectins. Carbohydr. Polym. 1992, 19, 41–50. [Google Scholar] [CrossRef]
- Kaya, M.; Sousa, A.G.; Crépeau, M.-J.; Sørensen, S.O.; Ralet, M.-C. Characterization of citrus pectin samples extracted under different conditions: Influence of acid type and pH of extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef]
- Mourão, K.S.M.; Beltrati, C.M. Morphology and anatomy of developing fruits and seeds of Mammea americana L. (Clusiaceae). Rev. Bras. Biol. 2000, 60, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.J.; Fulcher, R.G. Interaction of some dyes with cereal beta-glucans. Cereal Chem. 1978, 55, 952–966. [Google Scholar]
- Albersheim, P.; Darvill, A.; Roberts, K.; Sederoff, R.; Staehelin, A. Plant Cell Walls; Garland Science: New York, NY, USA, 2010. [Google Scholar]
- Leverrier, C.; Almeida, G.; Espinosa-Munoz, L.; Cuvelier, G. Influence of particle size and concentration on rheological behaviour of reconstituted apple purees. Food Biophys. 2016, 11, 235–247. [Google Scholar] [CrossRef]
- Leverrier, C.; Almeida, G.; Menut, P.; Cuvelier, G. Design of model apple cells suspensions: Rheological properties and impact of the continuous phase. Food Biophys. 2017, 12, 383–396. [Google Scholar] [CrossRef]
- Brat, P.; Olle, D.; Reynes, M.; Cogat, P.-O.; Brillouet, J.-M. Preparation of passion fruit puree by flash vacuum-expansion. J. Food Sci. 2001, 66, 542–547. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Williams MA, K.; Tanhatan-Nasseri, A.; Ropartz, D.; Quéméner, B.; Bonnin, E. Innovative enzymatic approach to resolve homogalacturonans based on their methylesterification pattern. Biomacromolecules 2012, 13, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Ngolong Ngea, G.L.; Guillon, F.; Essia Ngang, J.J.; Bonnin, E.; Bouchet, B.; Saulnier, L. Modification of cell wall polysaccharides during retting of cassava roots. Food Chem. 2016, 213, 402–409. [Google Scholar] [CrossRef] [PubMed]
- DuBois Michel Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Tranquet, O.; Poulain, D.; Moïse, A.; Guillon, F. Monoclonal antibodies to rhamnogalacturonan I backbone. Planta 2010, 231, 1373–1383. [Google Scholar] [CrossRef]
- Thibault, J.-F. Automatisation du dosage des substances pectiques par la méthode au metahydroxydiphenyle. Lebensm. Wiss. Technol. 1979, 12, 247–251. [Google Scholar]
- Englyst, H.; Cummings, J. Improved method for measurement of dietary fiber as non-starch polysaccharides in plant foods. J. Assoc. Off. Anal. Chem. 1988, 71, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Levigne, S.; Thomas, M.; Ralet, M.-C.; Quemener, B.; Thibault, J.-F. Determination of the degrees of methylation and acetylation of pectins using a C18 column and internal standards. Food Hydrocoll. 2002, 16, 547–550. [Google Scholar] [CrossRef]
| Sample | Accession | Yield a | Proteins b | Ashes b | UA b | Methanol (DM) b | Acetic Acid b | Cel Glc c | Non-Cel Glc d | Ara e | Gal e | Xyl e | Man e | Rha e | Fuc e |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh pulp | Galion | 3.4 ± 0.1 | 11.2 ± 0.3 | 1.1 ± 0.1 | 30.8 ± 0.1 | 4.0 ± 0.2 (73.1 ± 2.8) | 2.7 ± 0.1 | 16.3 ± 0.6 | 0.7 ± 0.1 | 8.8 ± 0.3 | 6.1 ± 0.5 | 2.9 ± 0.1 | 1.4 ± 0.0 | 1.1 ± 0.1 | 0.4 ± 0.0 |
| Ti Jacques | 3.5 ± 0.0 | 7.8 ± 0.3 | 1.0 ± 0.1 | 28.8 ± 0.1 | 3.7 ± 0.1 (72.5 ± 1.2) | 2.9 ± 0.0 | 15.1 ± 0.8 | 0.6 ± 0.0 | 9.1 ± 0.0 | 6.3 ± 0.2 | 3.0 ± 0.3 | 1.3 ± 0.1 | 1.0 ± 0.1 | 0.4 ± 0.0 | |
| Sonson | 3.0 ± 0.4 | 8.4 ± 0.3 | 1.2 ± 0.1 | 33.6 ± 0.9 | 4.6 ± 0.0 (76.7 ± 0.2) | 2.7 ± 0.1 | 14.8 ± 0.1 | 0.9 ± 0.1 | 8.2 ± 0.8 | 4.4 ± 0.8 | 2.5 ± 0.1 | 1.7 ± 0.2 | 0.9 ± 0.0 | 0.4 ± 0.0 | |
| Puree | Galion | 2.3 | 12.3 ± 0.6 | 1.9 ± 0.0 | 29.0 ± 0.0 | 3.5 ± 0.0 (67.4 ± 0.1) | 2.6 ± 0.0 | 17.6 | 0.8 ± 0.1 | 9.1 ± 0.0 | 6.3 ± 0.0 | 2.8 ± 0.0 | 1.5 ± 0.0 | 1.2 ± 0.0 | 0.4 ± 0.0 |
| Ti Jacques | 3.8 | 10.7 ± 1.7 | 1.3 ± 0.1 | 26.0 ± 0.1 | 3.1 ± 0.1 (66.2 ± 1.0) | 2.8 ± 0.1 | 18.6 | 0.8 ± 0.1 | 9.0 ± 0.1 | 6.8 ± 0.0 | 3.3 ± 0.0 | 1.6 ± 0.0 | 1.3 ± 0.1 | 0.4 ± 0.0 | |
| Sonson | 3.1 | 10.8 ± 0.5 | 1.1 ± 0.0 | 32.7 ± 0.3 | 4.2 ± 0.1 (72.1 ± 1.6) | 2.5 ± 0.1 | 19.3 | 0.8 ± 0.1 | 8.6 ± 0.1 | 6.1 ± 0.2 | 2.6 ± 0.1 | 1.8 ± 0.0 | 1.2 ± 0.1 | 0.5 ± 0.0 |
| Accession | Sample | Yield a | UA b | Methanol (DM) b | Acetic Acid b | Cel Glc c | Non-Cel Glc d | Ara e | Gal e | Xyl e | Man e | Rha e | Fuc e | UA/Rha |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WSF | ||||||||||||||
| Galion | Fresh pulp | 20.7 ± 0.4 | 73.4 ± 2.5 | 9.2 ± 0.8 (68.4 ± 3.4) | 2.2 ± 0.2 | nd | 0.9 ± 0.1 | 6.3 ± 0.4 | 3.4 ± 0.3 | 1.8 ± 0.0 | <dL | 1.5 ± 0.1 | 0.2 ± 0.0 | 41.2 ± 0.2 |
| Puree | 19.0 | 70.9 ± 1.7 | 9.1 ± 0.2 (70.3 ± 0.0) | 2.4 ± 0.0 | nd | 0.8 ± 0.0 | 5.7 ± 0.1 | 3.1 ± 0.0 | 1.6 ± 0.0 | <dL | 1.4 ± 0.0 | 0.2 ± 0.0 | 42.0 | |
| Ti Jacques | Fresh pulp | 17.4 ± 0.7 | 81.6 ± 3.6 | 9.7 ± 0.2 (65.7 ± 4.2) | 1.9 ± 0.1 | nd | 0.8 ± 0.1 | 6.0 ± 0.0 | 3.0 ± 0.1 | 1.3 ± 0.1 | <dL | 1.2 ± 0.1 | 0.2 ± 0.0 | 56.9 ± 1.5 |
| Puree | 11.8 | 70.5 ± 1.2 | 8.9 ± 0.3 (69.7 ± 2.3) | 1.9 ± 0.0 | nd | 0.9 ± 0.0 | 6.3 ± 0.5 | 3.1 ± 0.2 | 1.5 ± 0.1 | 0.3 ± 0.0 | 1.5 ± 0.0 | 0.2 ± 0.0 | 38.5 | |
| Sonson | Fresh pulp | 22.7 ± 0.4 | 73.7 ± 2.3 | 9.1 ± 0.5 (67.5 ± 1.6) | 1.6 ± 0.1 | nd | 0.9 ± 0.0 | 5.4 ± 1.8 | 2.2 ± 0.3 | 0.8 ± 0.2 | <dL | 1.2 ± 0.4 | 0.2 ± 0.1 | 56.3 ± 16.0 |
| Puree | 19.0 | 66.4 ± 0.3 | 9.6 ± 0.1 (79.2 ± 0.7) | 1.9 ± 0.0 | nd | 1.0 ± 0.1 | 5.7 ± 0.2 | 3.0 ± 0.1 | 0.9 ± 0.1 | 0.3 ± 0.0 | 1.2 ± 0.0 | 0.2 ± 0.0 | 47.0 | |
| WAIR | ||||||||||||||
| Galion | Fresh pulp | 75.2 ± 0.4 | 24.5 ± 0.6 | 2.6 ± 0.0 (58.5 ± 1.2) | 2.5 ± 0.0 | 35.8 ± 1.0 | 1.0 ± 0.2 | 11.3 ± 0.7 | 8.4 ± 1.1 | 4.6 ± 0.0 | 2.0 ± 0.1 | 1.8 ± 0.1 | 0.7 ± 0.0 | 11.0 ± 0.8 |
| Puree | 77.8 | 23.7 ± 0.4 | 2.4 ± 0.0 (56.7 ± 1.0) | 2.5 ± 0.1 | 34.8 | 0.9 ± 0.0 | 11.0 ± 0.0 | 8.5 ± 0.0 | 4.2 ± 0.0 | 2.0 ± 0.0 | 1.8 ± 0.1 | 0.7 ± 0.0 | 11.1 | |
| Ti Jacques | Fresh pulp | 79.5 ± 0.7 | 23.4 ± 1.2 | 2.7 ± 0.2 (63.8 ± 0.3) | 2.9 ± 0.1 | 38.7 ± 0.7 | 0.9 ± 0.0 | 12.3 ± 0.2 | 9.5 ± 0.5 | 5.3 ± 0.3 | 2.0 ± 0.0 | 1.9 ± 0.1 | 0.7 ± 0.0 | 10.3 ± 1.0 |
| Puree | 76.8 | 18.8 ± 0.0 | 1.9 ± 0.0 (54.8 ± 1.1) | 2.7 ± 0.1 | 33.1 | 1.0 ± 0.0 | 11.2 ± 0.3 | 9.2 ± 0.5 | 4.7 ± 0.3 | 2.1 ± 0.2 | 1.7 ± 0.3 | 0.6 ± 0.1 | 9.2 | |
| Sonson | Fresh pulp | 73.6 ± 0.7 | 24.6 ± 0.4 | 2.8 ± 0.1 (62.9 ± 1.3) | 2.6 ± 0.0 | 40.2 ± 0.0 | 1.3 ± 0.0 | 11.7 ± 0.7 | 7.0 ± 1.5 | 4.8 ± 0.0 | 3.0 ± 0.3 | 1.6 ± 0.1 | 0.8 ± 0.0 | 13.2 ± 1.0 |
| Puree | 75.8 | 26.0 ± 0.2 | 3.0 ± 0.1 (62.8 ± 1.6) | 2.6 ± 0.1 | 40.9 | 0.9 ± 0.1 | 11.2 ± 0.1 | 8.5 ± 0.1 | 4.4 ± 0.0 | 3.7 ± 1.4 | 1.8 ± 0.0 | 0.8 ± 0.0 | 12.1 | |
| Sample | Accession | Mw a | IV b | I c | (Ara + Gal)/Rha d |
|---|---|---|---|---|---|
| Fresh pulp | Galion | 350 | 8.5 | 2.3 | 6.7 |
| Ti Jacques | 350 | 11.0 | 2.1 | 7.8 | |
| Sonson | 334 | 10.3 | 1.8 | 7.1 | |
| Puree | Galion | 392 | 9.0 | 2.3 | 6.5 |
| Ti Jacques | 295 | 8.1 | 2.3 | 6.4 | |
| Sonson | 355 | 10.3 | 1.9 | 7.6 |
| Probes | Suppliers | Origin | Targets | Dilution | Sample Pretreatments a |
|---|---|---|---|---|---|
| INRA-RU1 | INRAE Nantes [31] | Mouse | Rhamnogalacturonan I | 1/3 | Alkali: incubation in 50 nM NaOH for 20 min at 4 °C |
| LM5 | PlantProbes | Rat | (1–4)-β-D-galactan | 1/10 | Alkali + Enzymatic: incubation overnight at 38 °C Mixture of galactanase (50 U/mL) and endo-arabinanase (50 U/mL) |
| LM6 | Linear (1–5)-α-L-arabinan | 1/10 | |||
| LM19 | Weakly methyl-esterified homogalacturonan | 1/10 | Enzymatic:
| ||
| LM20 | Strongly methyl-esterified homogalacturonan | 1/10 | |||
| 2F4 | Weakly methyl-esterified homogalacturonan chelated by Ca2+ | 1/5 | None | ||
| LM15 | Xyloglucan | 1/5 | Enzymatic:
| ||
| LM21 | Mannan | 1/5 | |||
| CBM3a | Clostridium thermocellum | Crystalline cellulose | 1/975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmont, D.; Bonnin, E.; Smith Ravin, E.J.; Lahaye, M.; Marcelin, O. Characterization of Crude and Processed Pulp Cell Walls of Three Selected Mamey Accessions (Mammea americana L.). Molecules 2024, 29, 1596. https://doi.org/10.3390/molecules29071596
Palmont D, Bonnin E, Smith Ravin EJ, Lahaye M, Marcelin O. Characterization of Crude and Processed Pulp Cell Walls of Three Selected Mamey Accessions (Mammea americana L.). Molecules. 2024; 29(7):1596. https://doi.org/10.3390/molecules29071596
Chicago/Turabian StylePalmont, Déborah, Estelle Bonnin, Emilie J. Smith Ravin, Marc Lahaye, and Odile Marcelin. 2024. "Characterization of Crude and Processed Pulp Cell Walls of Three Selected Mamey Accessions (Mammea americana L.)" Molecules 29, no. 7: 1596. https://doi.org/10.3390/molecules29071596
APA StylePalmont, D., Bonnin, E., Smith Ravin, E. J., Lahaye, M., & Marcelin, O. (2024). Characterization of Crude and Processed Pulp Cell Walls of Three Selected Mamey Accessions (Mammea americana L.). Molecules, 29(7), 1596. https://doi.org/10.3390/molecules29071596