A Red-Emission Fluorescent Probe for Intracellular Biothiols and Hydrogen Sulfide Imaging in Living Cells
Abstract
1. Introduction
2. Results
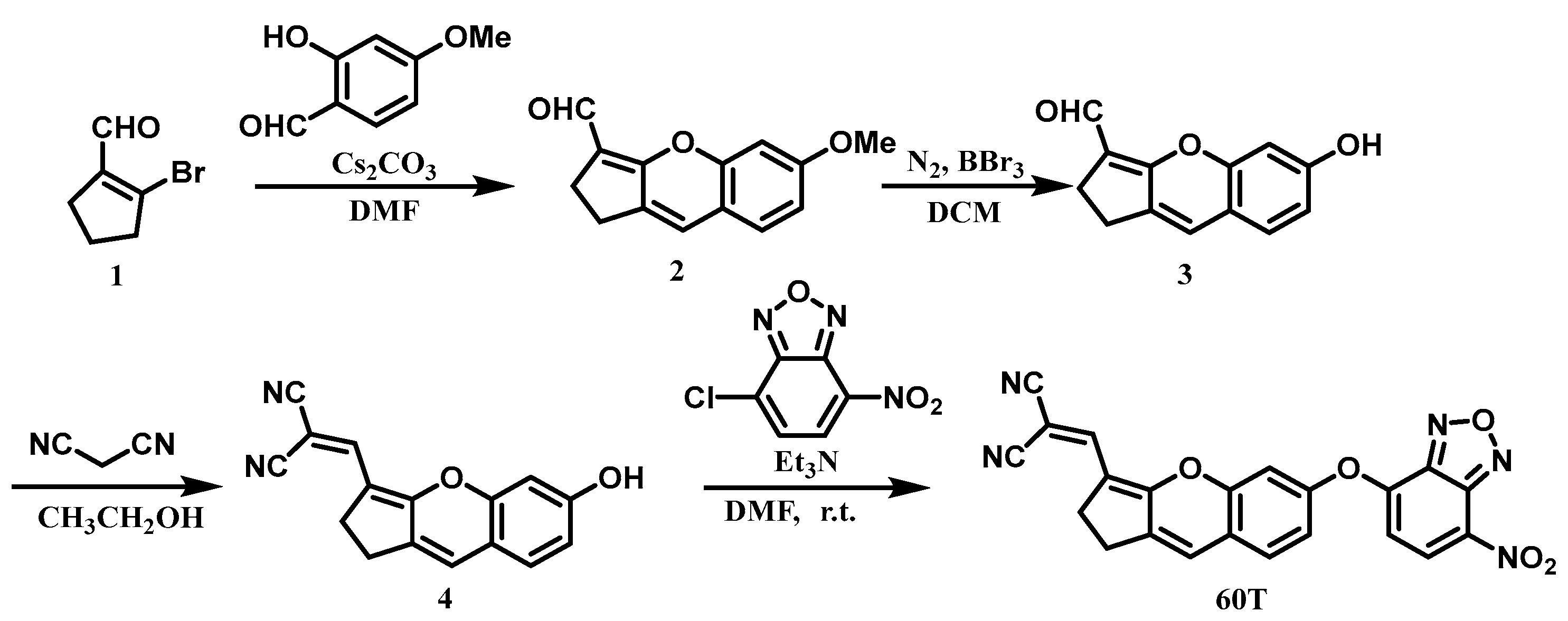
2.1. Design and Synthesis of Probe 60T
- Synthesis and characterization of compound 2. At 0 °C, 1.771 g (10 mmol) of compound 1 and 0.913 g (6 mmol) of 2-hydroxy-4-methoxybenzaldehyde were dissolved in 10 mL of DMF. Subsequently, 7.820 g (24 mmol) of cesium carbonate were added, and the reaction mixture was stirred at room temperature for 12 h. The reaction was quenched at 0 °C with dilute hydrochloric acid (2M). The organic layer was extracted with ethyl acetate (3 × 10 mL), and the combined organic layers were dried over anhydrous sodium sulfate. The crude product was then purified by silica gel column chromatography, yielding a yellow solid (1.1 g, 48% yield), known compound. 1H NMR (400 MHz, Chloroform-d) δ 10.02 (s, 1H), 7.06 (d, J = 8.4 Hz, 1H), 6.72 (d, J = 2.4 Hz, 1H), 6.68 (dd, J = 8.4, 2.5 Hz, 1H), 6.59 (s, 1H), 3.84 (s, 3H), 2.75–2.69 (m, 4H). 13C NMR (101 MHz, Chloroform-d) δ 184.5, 164.2, 161.0, 153.0, 137.5, 127.6, 122.4, 116.5, 115.6, 111.4, 101.6, 55.8, 24.4, 23.7 (Figures S1 and S2).
- Synthesis and characterization of compound 3. Under nitrogen protection, 457 mg (2 mmol) of compound 2 were dissolved in 4 mL of anhydrous dichloromethane (DCM). At 0 °C, boron tribromide (1.5 mL, 16 mmol) was introduced, and the reaction mixture was stirred at room temperature for 10 h. Quenching the reaction with water at 0 °C was followed by extraction of the organic layer with dichloromethane (3 × 10 mL). The combined organic layers were then dried over anhydrous sodium sulfate. Subsequently, the crude product underwent purification through silica gel column chromatography, yielding a yellow solid (352 mg, 83% yield), known compound. 1H NMR (400 MHz, DMSO-d6) δ 10.17 (s, 1H), 9.90 (s, 1H), 7.18 (d, J = 8.7 Hz, 1H), 6.85 (s, 1H), 6.63 (s, 2H), 2.73–2.70 (m, 2H), 2.55–2.53 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 182.8, 163.4, 159.1, 152.2, 135.6, 128.1, 123.0, 115.2, 113.8, 112.4, 102.7, 23.6, 23.3 (Figures S3 and S4).
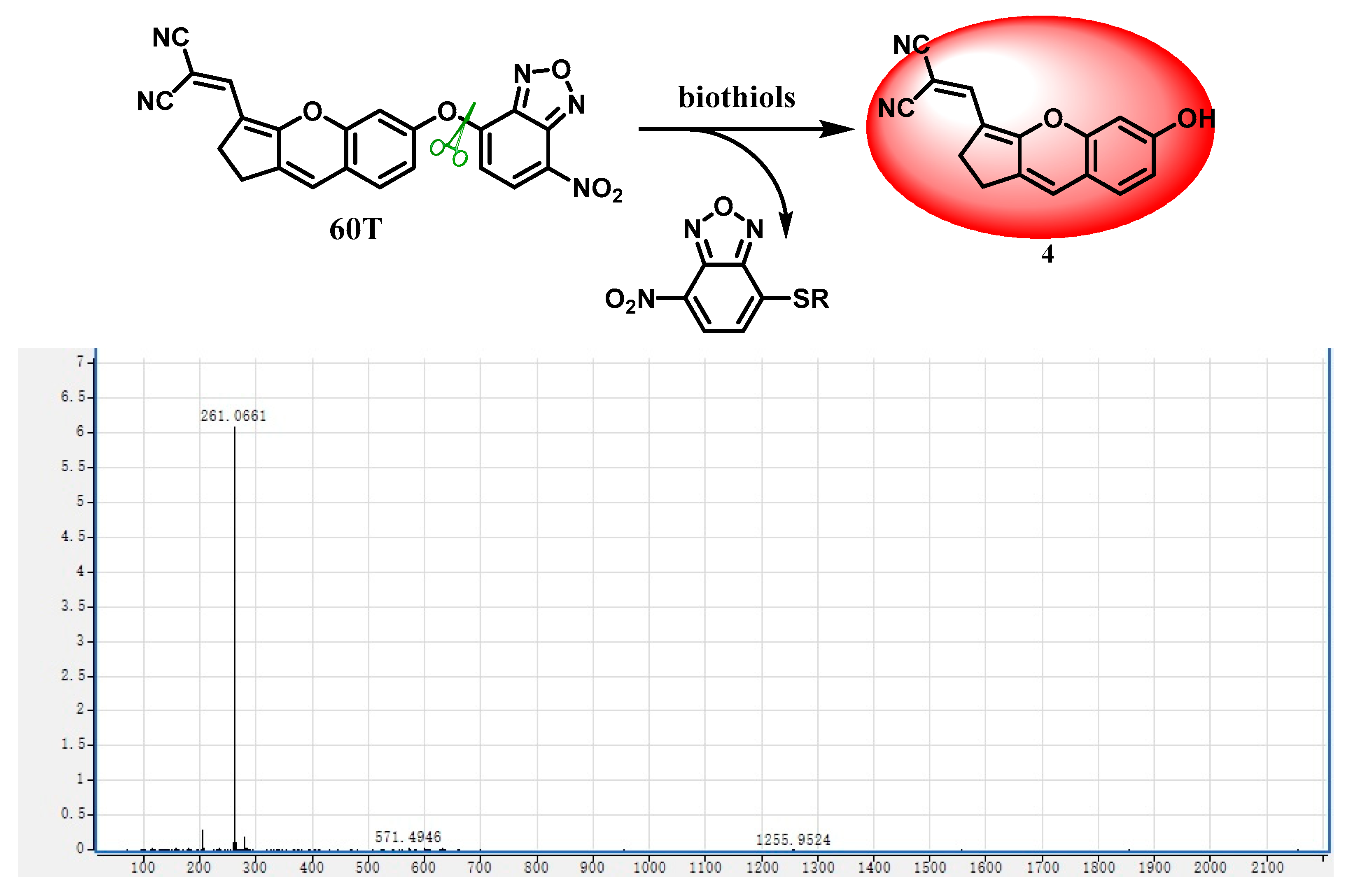
- Synthesis and characterization of compound Synthesis of compound 4. Compound 3 (352 mg, 1.6 mmol) was dissolved in 3 mL of anhydrous ethanol along with malononitrile (158 mg, 2.4 mmol). The reaction mixture was refluxed at 80 °C with stirring for 3 h. Following rotary evaporation, the residue was extracted with dichloromethane (3 × 10 mL). The combined organic layers were then dried over anhydrous sodium sulfate. The crude product underwent purification via silica gel column chromatography, yielding a red solid (345 mg, 82% yield). 1H NMR (400 MHz, DMSO-d6) δ 10.52 (s, 1H), 7.79 (s, 1H), 7.33 (d, J = 8.4 Hz, 1H), 7.22 (s, 1H), 6.78 (d, J = 2.2 Hz, 1H), 6.74 (dd, J = 8.0, 1.8 Hz, 1H), 2.94–2.92 (m, 2H), 2.86–2.83 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 164.9, 160.3, 153.1, 146.4, 134.1, 128.8, 127.7, 117.3, 116.2, 113.9, 113.3, 102.7, 66.0, 54.9, 24.8, 24.5 (Figures S5 and S6). HRMS (ESI−) m/z: [M-H]− Calculated: 261.0770; Found: 261.0661.
- Synthesis and characterization of probe 60T. Compound 4 (65 mg, 0.25 mmol) was dissolved in 5 mL of DMF, followed by the addition of 4-chloro-7-nitro-2,1,3-benzoxadiazole (158 mg, 2.4 mmol) and triethylamine (48 μL, 0.35 mmol). The reaction proceeded at room temperature for 4 h. The mixture was then extracted with ethyl acetate (3 × 10 mL), and the combined organic layers were dried over anhydrous sodium sulfate. The crude product underwent purification by silica gel column chromatography, resulting in a dark purple solid (49 mg, 48% yield). 1H NMR (400 MHz, DMSO-d6) δ 8.67 (d, J = 8.3 Hz, 1H), 7.84 (s, 1H), 7.67 (d, J = 8.5 Hz, 1H), 7.47 (d, J = 2.2 Hz, 1H), 7.34 (dd, J = 8.5, 2.4 Hz, 1H), 7.30 (s, 1H), 7.00 (d, J = 8.3 Hz, 1H), 3.01–2.98 (m, 2H), 2.95–2.92 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 163.3, 154.2, 152.4, 151.7, 145.4, 144.4, 138.6, 135.1, 131.1, 129.3, 125.2, 120.6, 117.6, 116.4, 115.3, 114.5, 111.8, 108.6, 69.7, 24.9, 24.7 (Figures S7 and S8). HRMS (ESI−) m/z: Calcd for C22H11N5O5 [M-H]−: 424.0687; found: 424.0679.
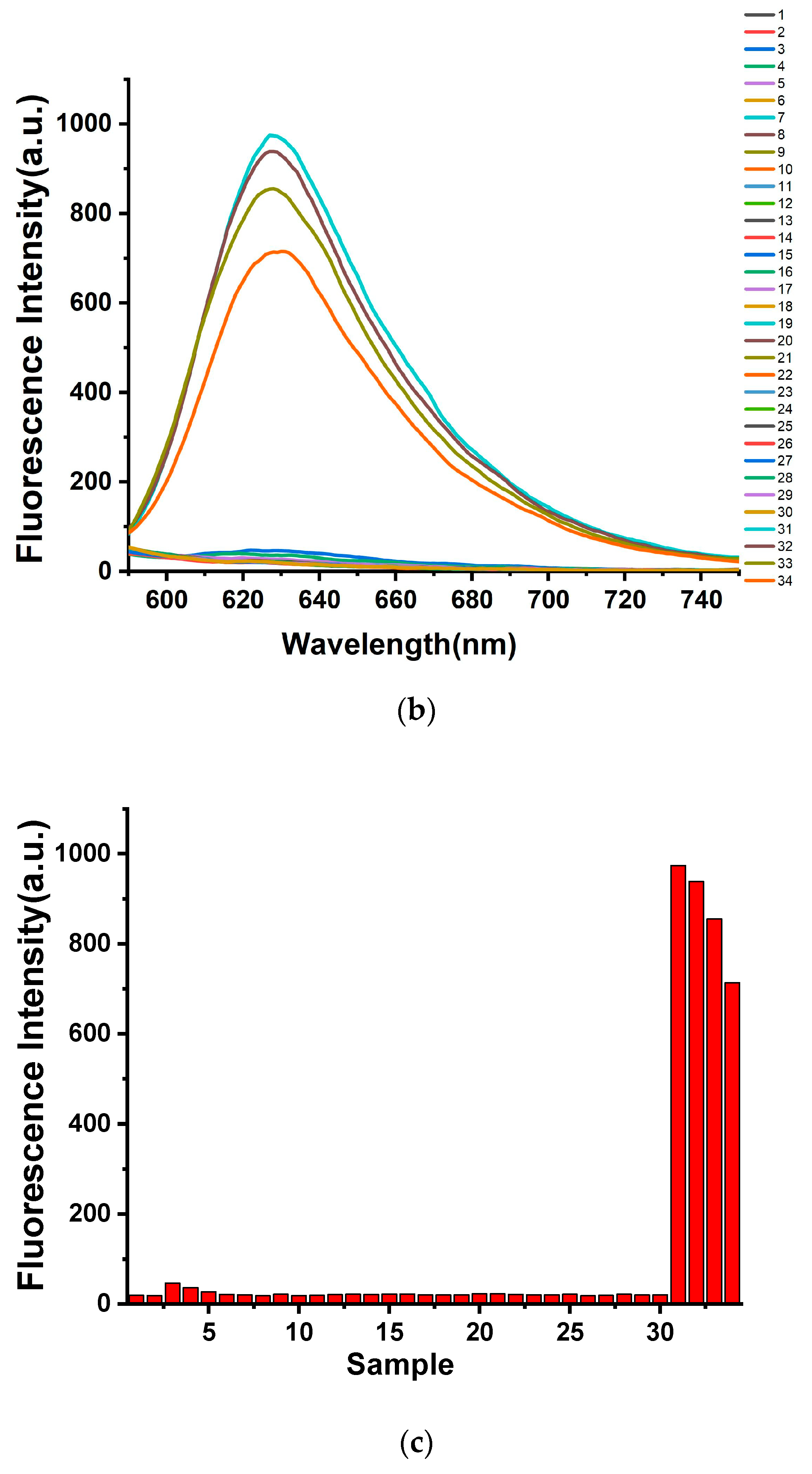
2.2. Selective Response of Thiol Probes
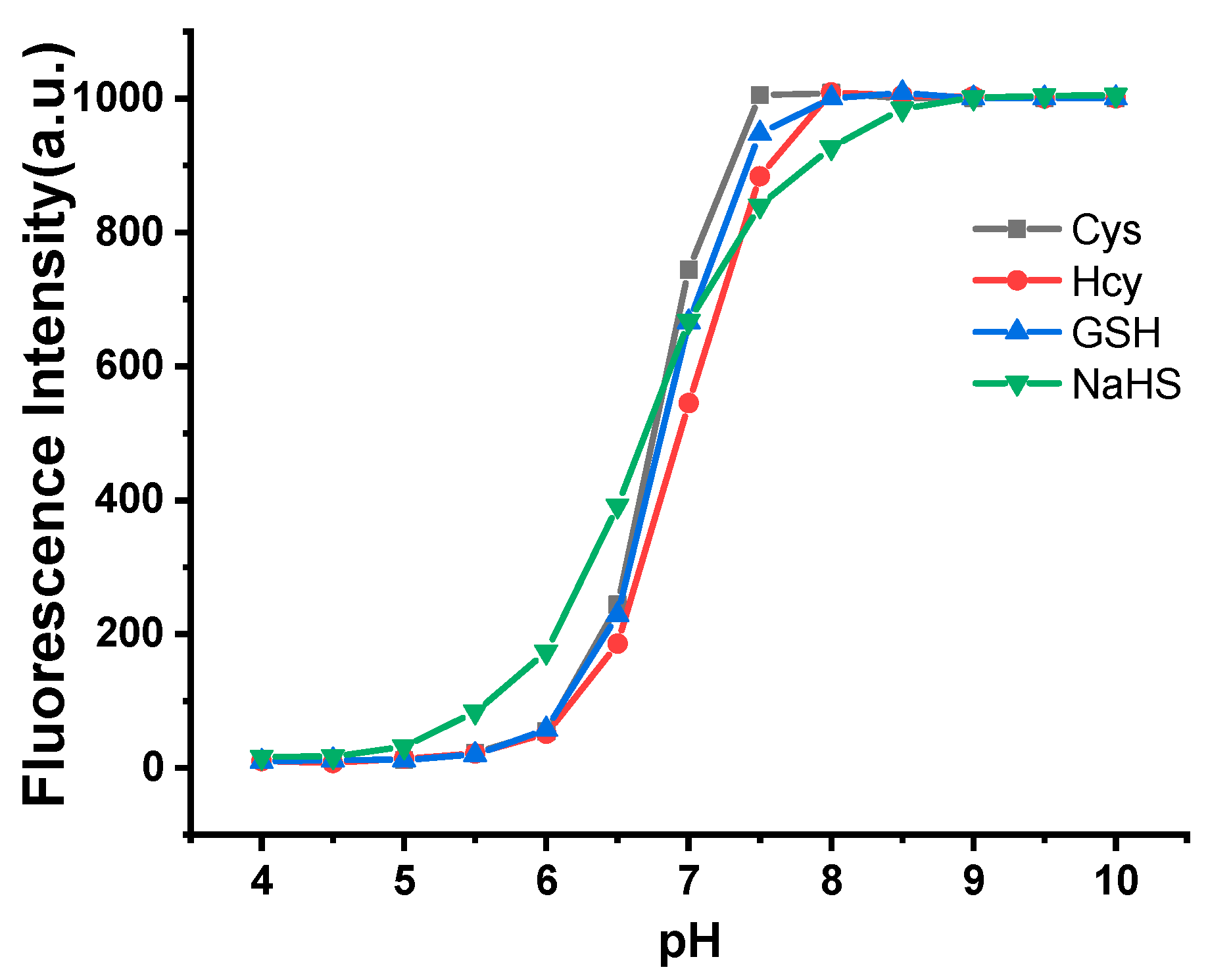
2.3. pH Dependence
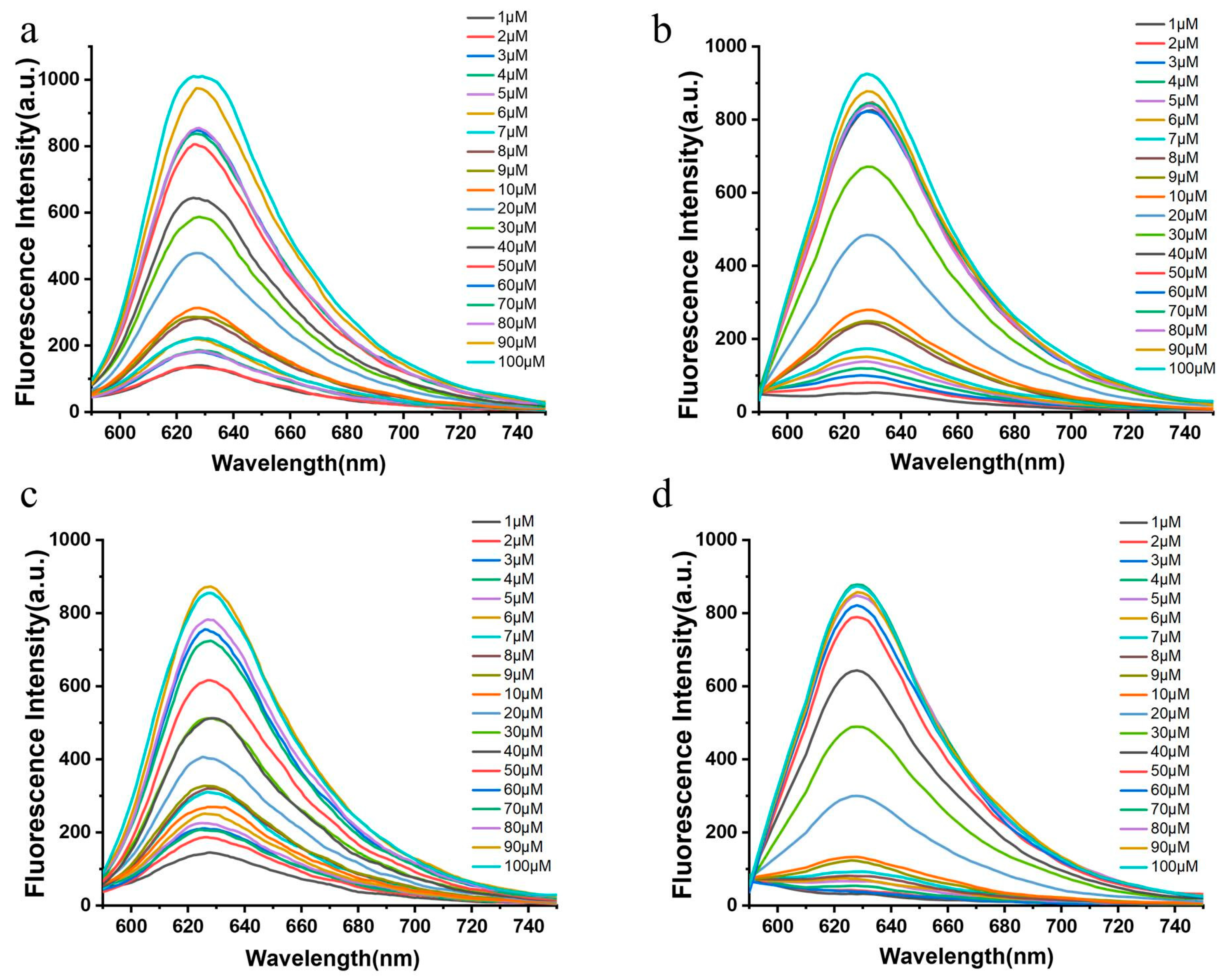
2.4. Concentration Dependence
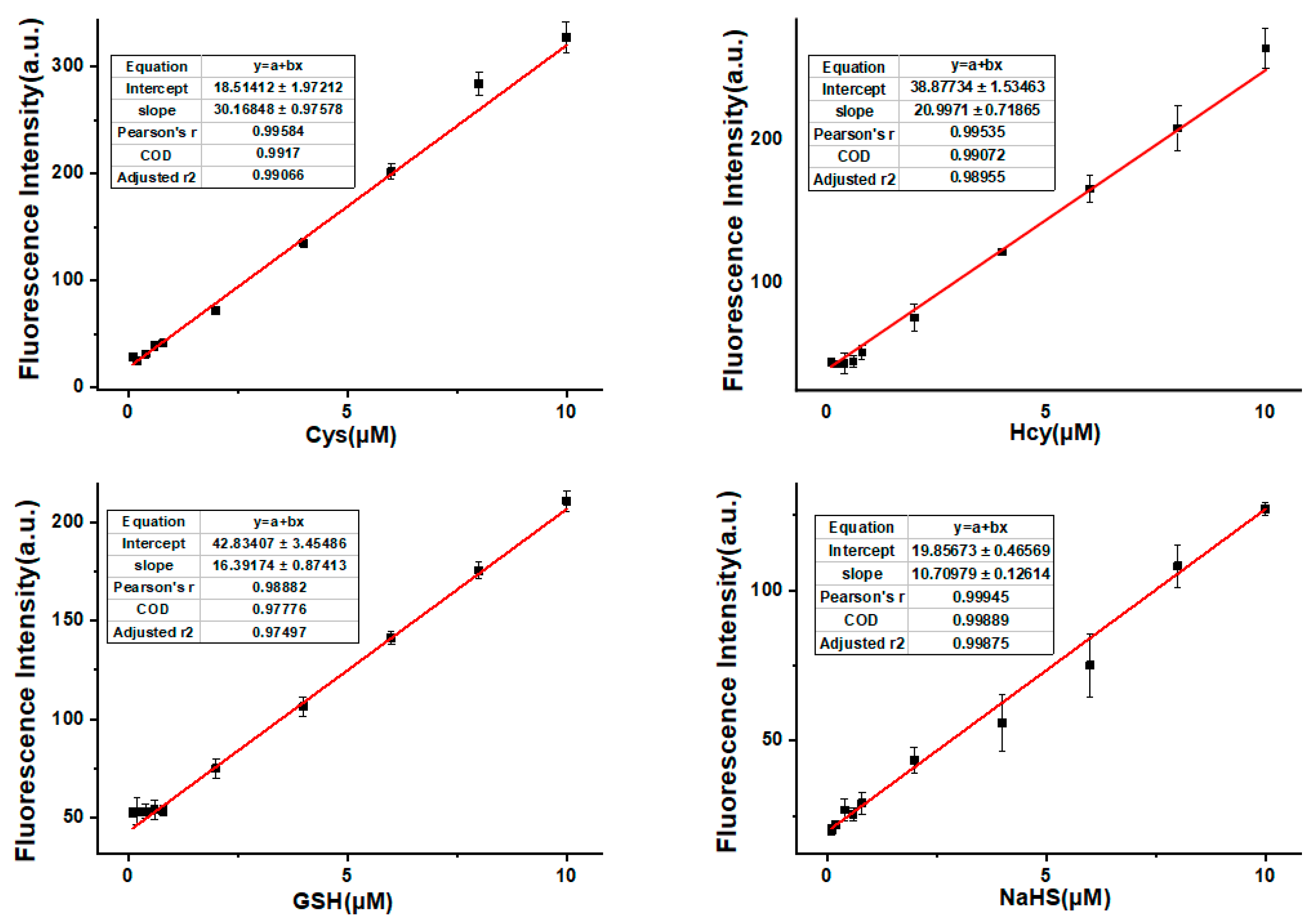
2.5. Detection Limit
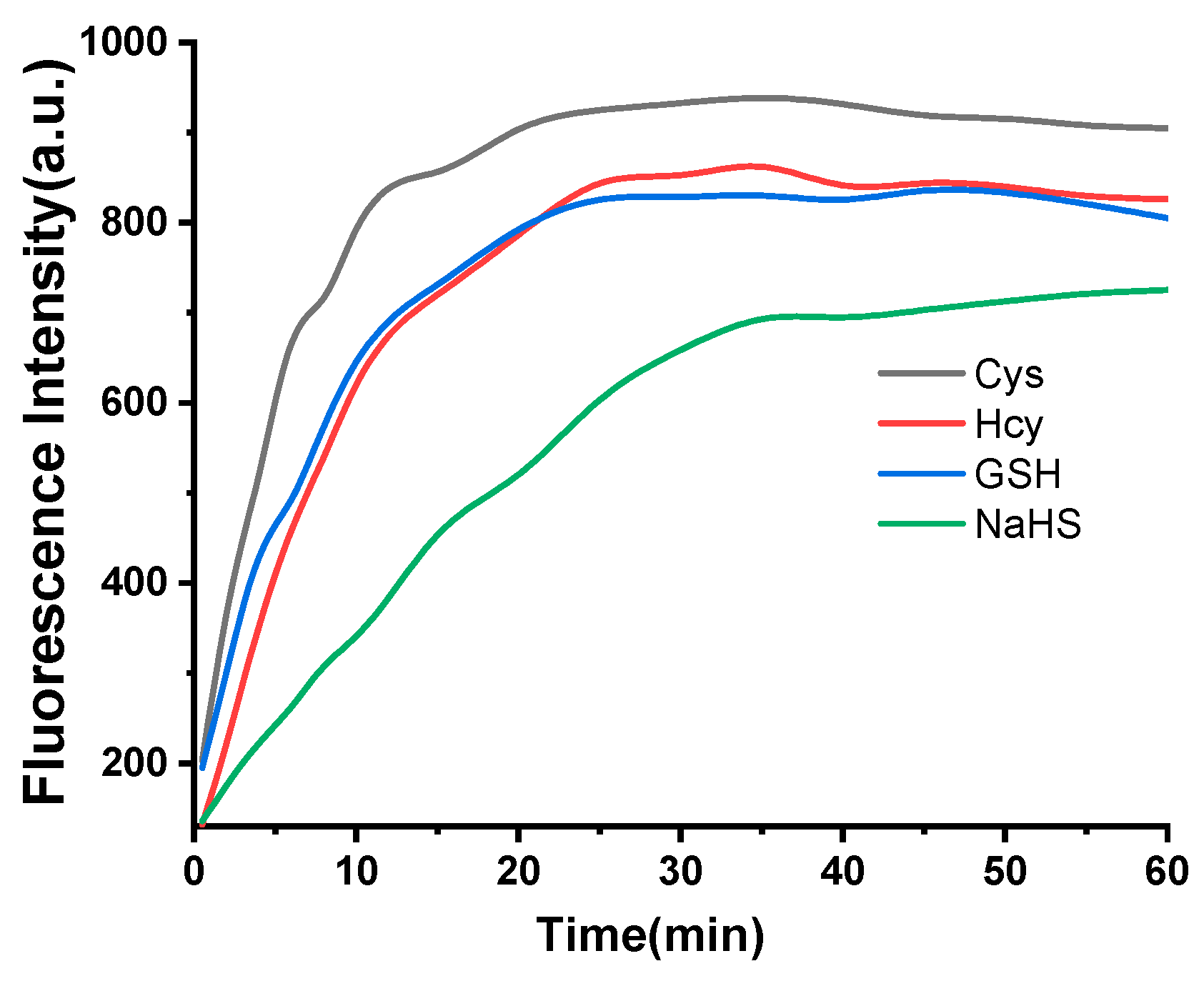
2.6. Time Dependence in Thiol Detection Process
2.7. Response Mechanism
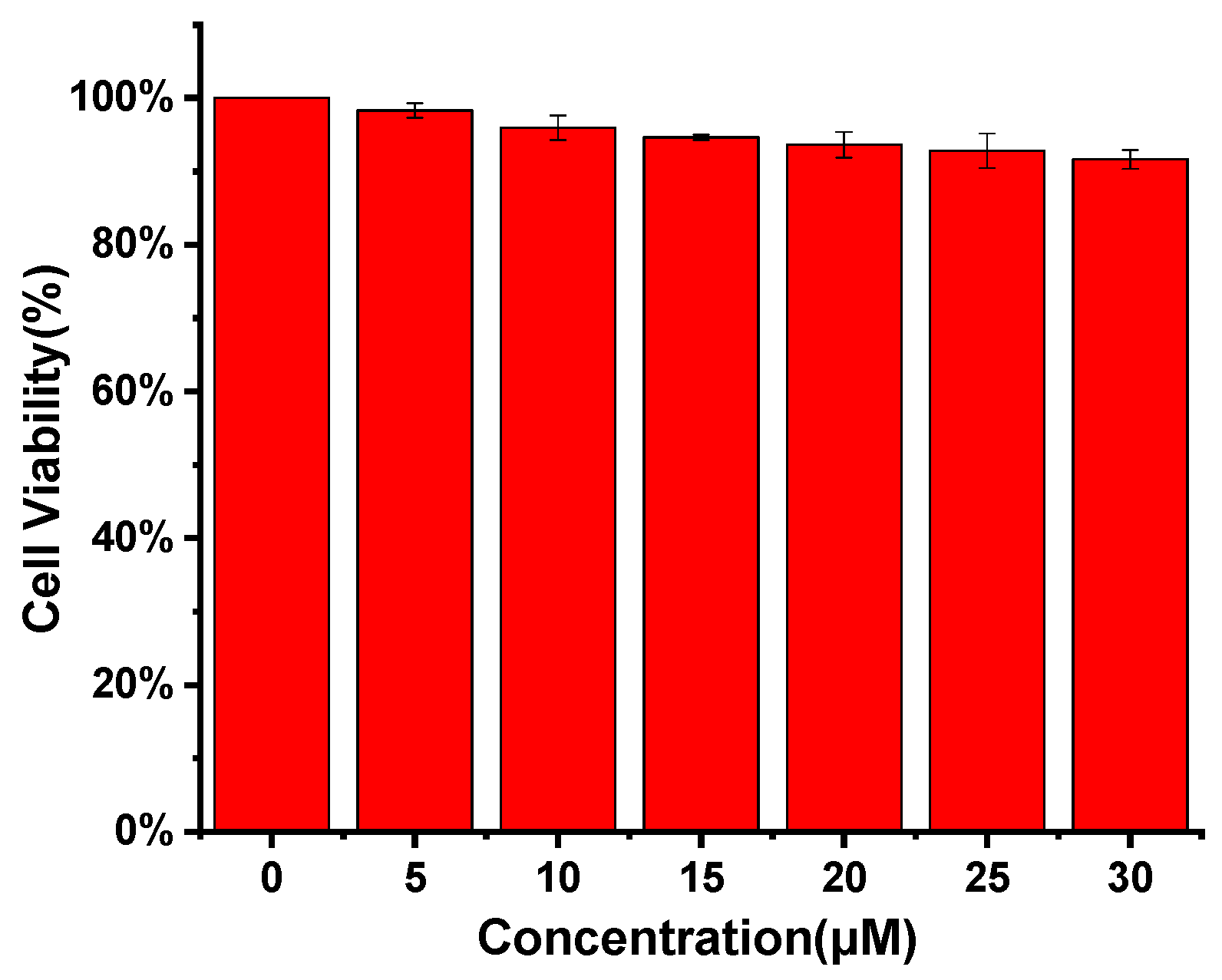
2.8. Cell Culture and Cytotoxicity Test
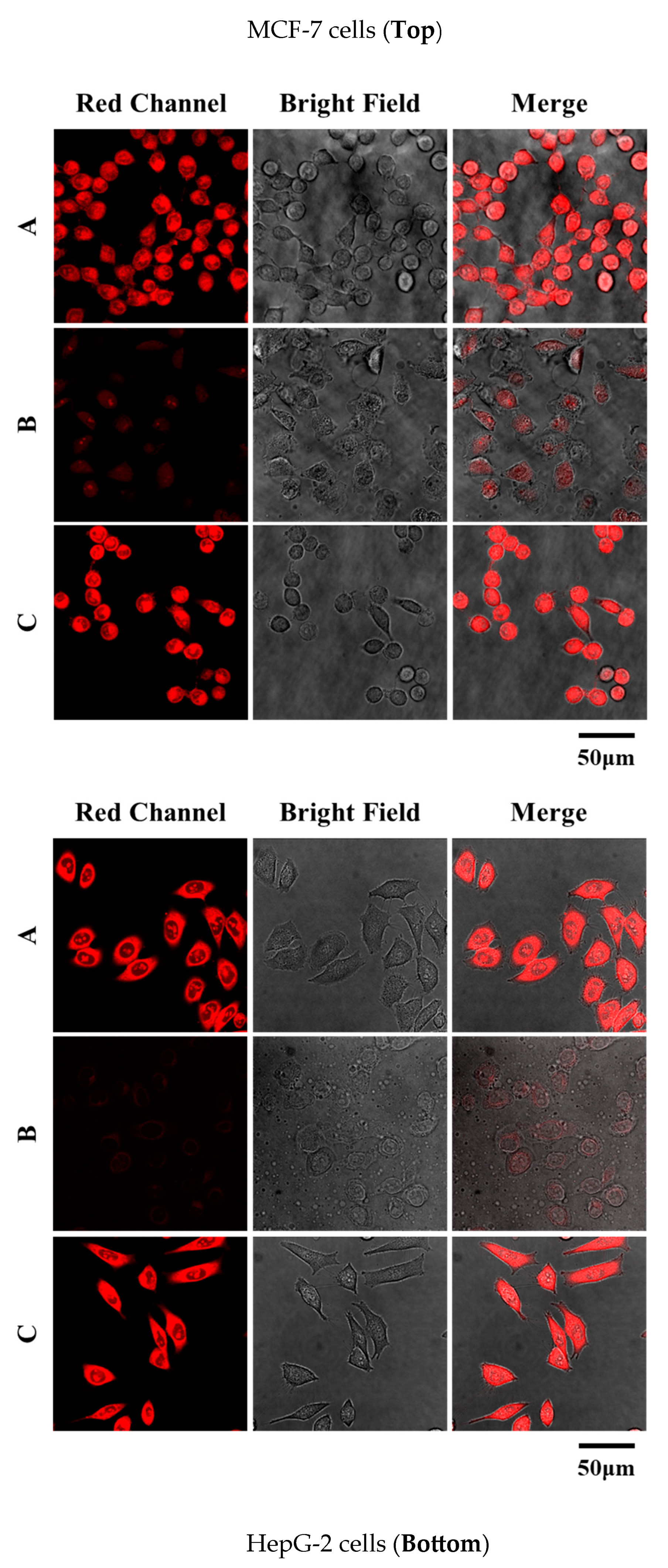
2.9. Fluorescence Imaging in Living Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Instruments
4.2. Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, Q.; Ying, J.; Xiang, L.; Zhang, C. The biologic effect of hydrogen sulfide and its function in various diseases. Medicine 2018, 97, e13065. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.A.; Sakla, R.; Sharma, N.; Gadiyaram, S.; Kaushik, R.; Ghosh, A. Sensing and bioimaging of the gaseous signaling molecule hydrogen sulfide by near-infrared fluorescent probes. ACS Sens. 2020, 5, 3365–3391. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S. Hydrogen sulfide: From physiology to pharmacology. Inflamm. Allergy Drug Targets 2011, 10, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, C.; Huo, F. Chromogenic and fluorogenic chemosensors for hydrogen sulfide: Review of detection mechanisms since the year 2009. RSC Adv. 2015, 5, 2191–2206. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Ong, C.N.; Shen, H.M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004, 208, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Han, J.H.; Kwon, P.S.; Bhuniya, S.; Kim, J.Y.; Sessler, J.L.; Kang, C.; Kim, J.S. Hepatocyte-targeting single galactose-appended naphthalimide: A tool for intracellular thiol imaging in vivo. J. Am. Chem. Soc. 2012, 134, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Weerapana, E.; Wang, C.; Simon, G.M.; Richter, F.; Khare, S.; Dillon, M.B.; Bachovchin, D.A.; Mowen, K.; Baker, D.; Cravatt, B.F. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 2010, 468, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Schoneveld, O.J.; Pappa, A. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Suh, S.W.; Hamby, A.M. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006, 9, 119–126. [Google Scholar] [CrossRef]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.-H.; Tang, K.-T. A review of sensor-based methods for monitoring hydrogen sulfide. TrAC Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoon, S.A.; Cha, Y.; Lee, M.H. Recent advances in fluorescent probes for cellular antioxidants: Detection of NADH, hNQO1, H2S, and other redox biomolecules. Coord. Chem. Rev. 2021, 428, 213613. [Google Scholar] [CrossRef]
- Ibrahim, H.; Serag, A.; Farag, M.A. Emerging analytical tools for the detection of the third gasotransmitter H2S, a comprehensive review. J. Adv. Res. 2021, 27, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xiao, Q.C.; Wang, L.; Yang, Y.Y. A new fluorescent probe for hydrogen sulfide detection in solution and living cells. Molecules 2023, 28, 6195. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Chen, X.; Kim, J.S.; Yoon, J. Recent progress in luminescent and colorimetric chemosensors for detection of thiols. Chem. Soc. Rev. 2013, 42, 6019–6031. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Huo, F.; Zhang, J.; Martinez-Manez, R.; Yang, Y.; Lv, H.; Li, S. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013, 42, 6032–6059. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.Q.; Park, S.; Yoon, J.Y.; Shin, I.J. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef]
- Yin, C.X.; Zhang, W.J.; Liu, T.; Chao, J.B.; Huo, F.J. A near-infrared turn on fluorescent probe for biothiols detection and its application in living cells. Sens. Actuators B Chem. 2017, 246, 988–993. [Google Scholar] [CrossRef]
- Zhu, Z.T.; Liu, W.; Cheng, L.H.; Li, Z.F.; Xi, Z.; Yi, L. New NBD-based fluorescent probes for biological thiols. Tetrahedron Lett. 2015, 56, 3909–3912. [Google Scholar] [CrossRef]
- Ren, A.S.; Zhu, D.J.; Luo, Y.H. A novel Boranil-based turn-on fluorescent probe for imaging of biothiols in living cells. J. Mol. Struct. 2020, 1209, 127914. [Google Scholar] [CrossRef]
- Qin, X.; Yuan, C.L.; Chen, Y.Y.; Wang, Y.L. A fluorescein–gold nanoparticles probe based on inner filter effect and aggregation for sensing of Photobiol. J. Photochem. Photobiol. B 2020, 210, 111986. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.Y.; Huang, H.J.; Kang, X.Y.; Yang, L.; Xi, Z.; Sun, H.Y.; Pluth Michael, D.; Yi, Y. NBD-based synthetic probes for sensing small molecules and proteins: Design, sensing mechanisms and biological applications. Chem. Soc. Rev. 2021, 50, 7436–7495. [Google Scholar] [CrossRef]
- Smith, H.M.; Pluth, M.D. Advances and opportunities in H2S measurement in chemical biology. JACS Au 2023, 3, 2677–2691. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, S.; Liu, T.; Chen, J.; Yuan, B.; Lu, C.; Bo, X.; Xu, Z. A Red-Emission Fluorescent Probe for Intracellular Biothiols and Hydrogen Sulfide Imaging in Living Cells. Molecules 2024, 29, 1572. https://doi.org/10.3390/molecules29071572
Wang Y, Zhang S, Liu T, Chen J, Yuan B, Lu C, Bo X, Xu Z. A Red-Emission Fluorescent Probe for Intracellular Biothiols and Hydrogen Sulfide Imaging in Living Cells. Molecules. 2024; 29(7):1572. https://doi.org/10.3390/molecules29071572
Chicago/Turabian StyleWang, Yuanfan, Shengxiang Zhang, Tianle Liu, Junning Chen, Bingrui Yuan, Cuntao Lu, Xiumei Bo, and Zhou Xu. 2024. "A Red-Emission Fluorescent Probe for Intracellular Biothiols and Hydrogen Sulfide Imaging in Living Cells" Molecules 29, no. 7: 1572. https://doi.org/10.3390/molecules29071572
APA StyleWang, Y., Zhang, S., Liu, T., Chen, J., Yuan, B., Lu, C., Bo, X., & Xu, Z. (2024). A Red-Emission Fluorescent Probe for Intracellular Biothiols and Hydrogen Sulfide Imaging in Living Cells. Molecules, 29(7), 1572. https://doi.org/10.3390/molecules29071572





