Isolation, Identification and Chemical Modification of Bufadienolides from Bufo melanostictus Schneider and Their Cytotoxic Activities against Prostate Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
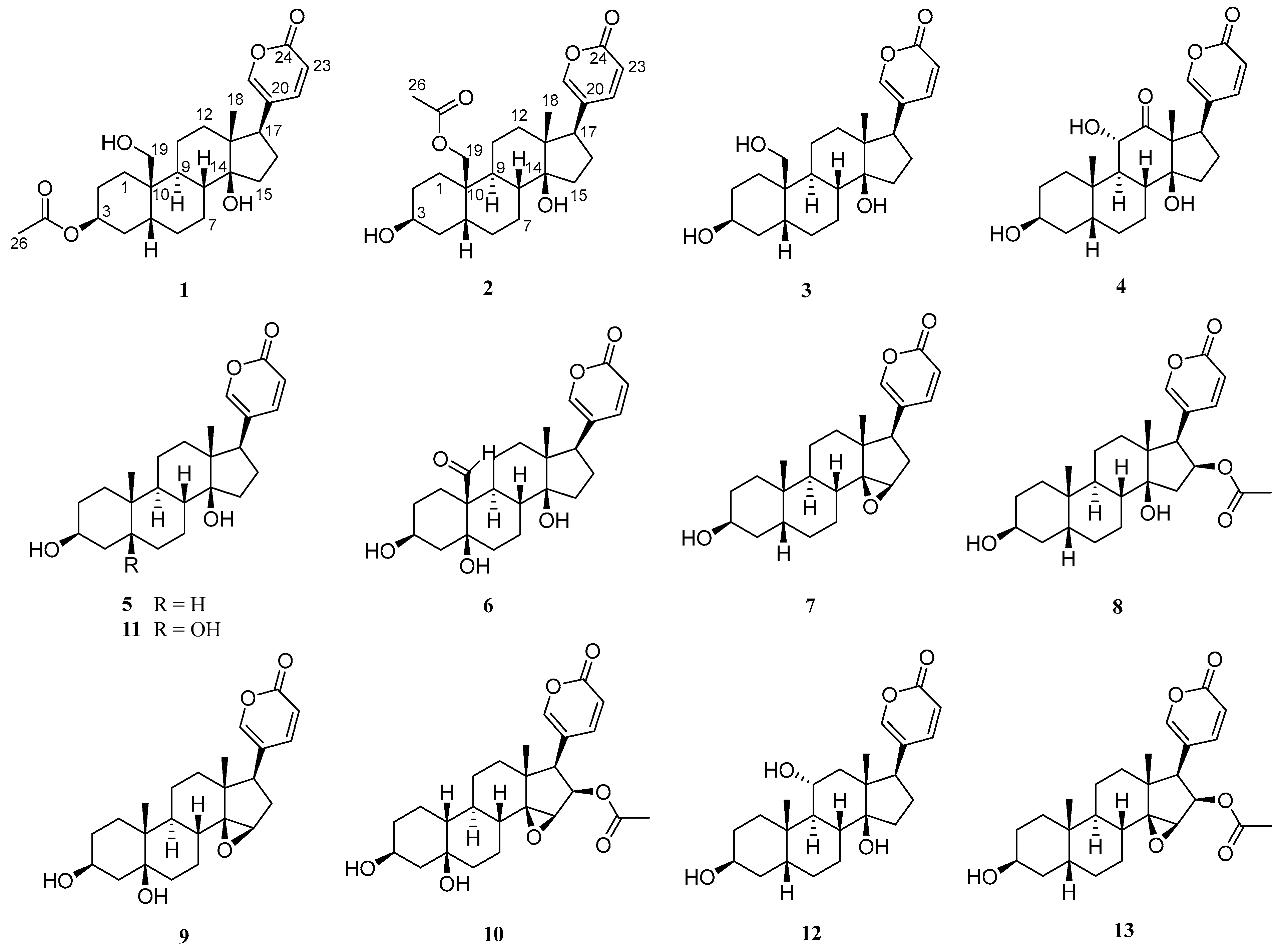
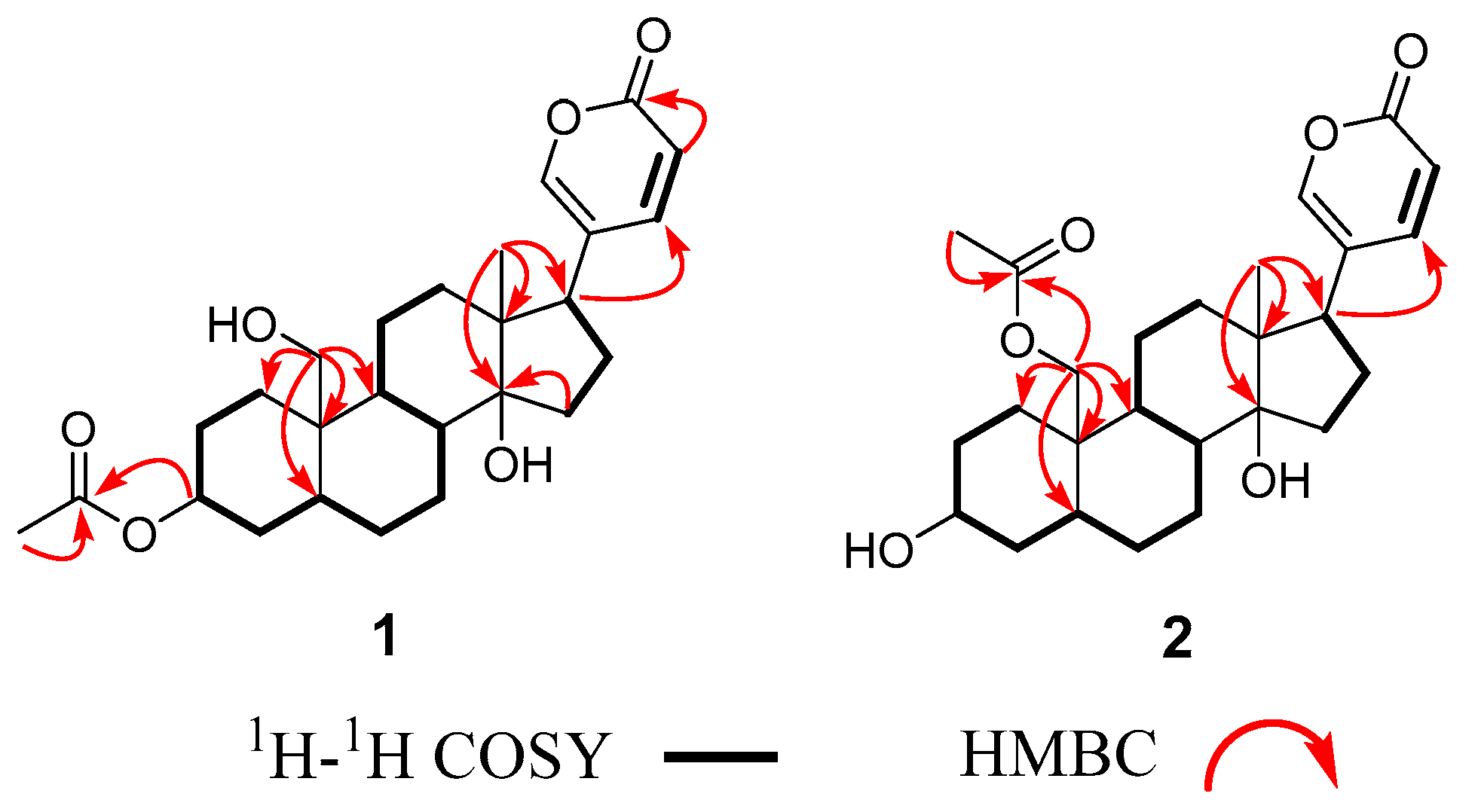
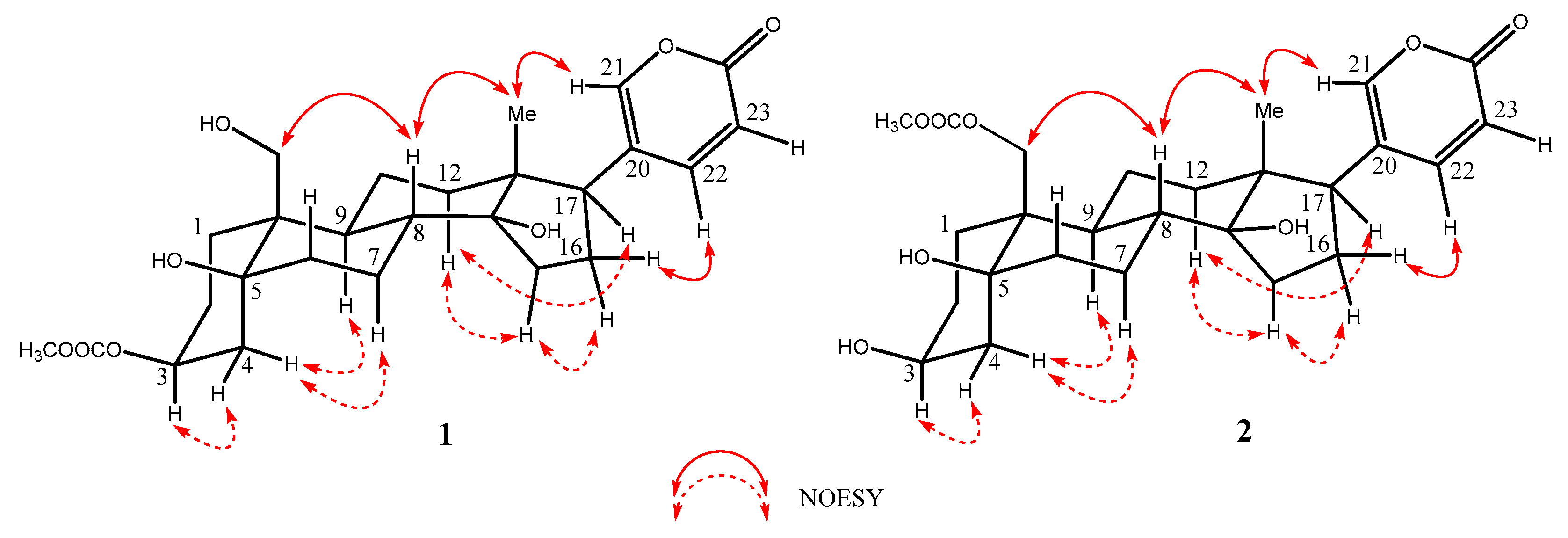
2.1. Structural Elucidation of the Isolated Compounds
2.2. Spectroscopic Data
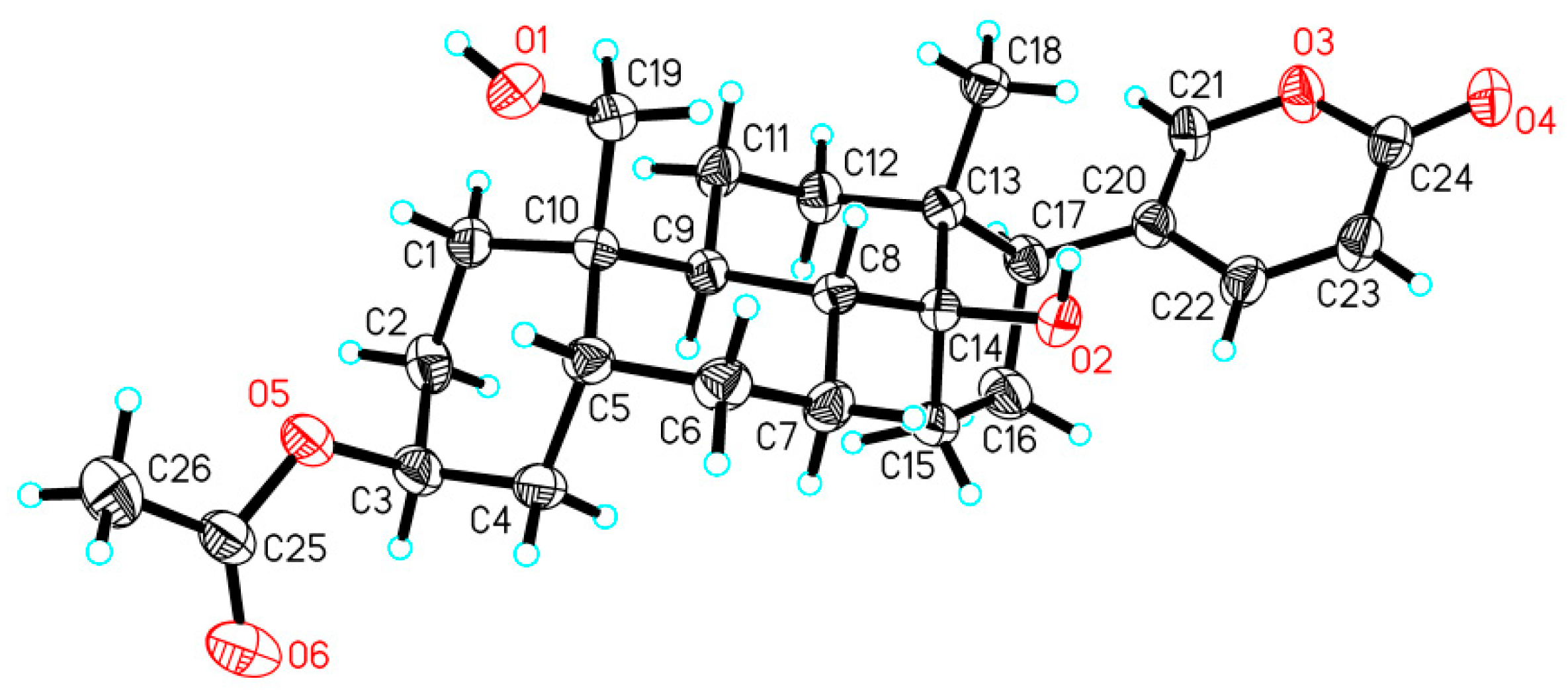
2.3. X-ray Analysis
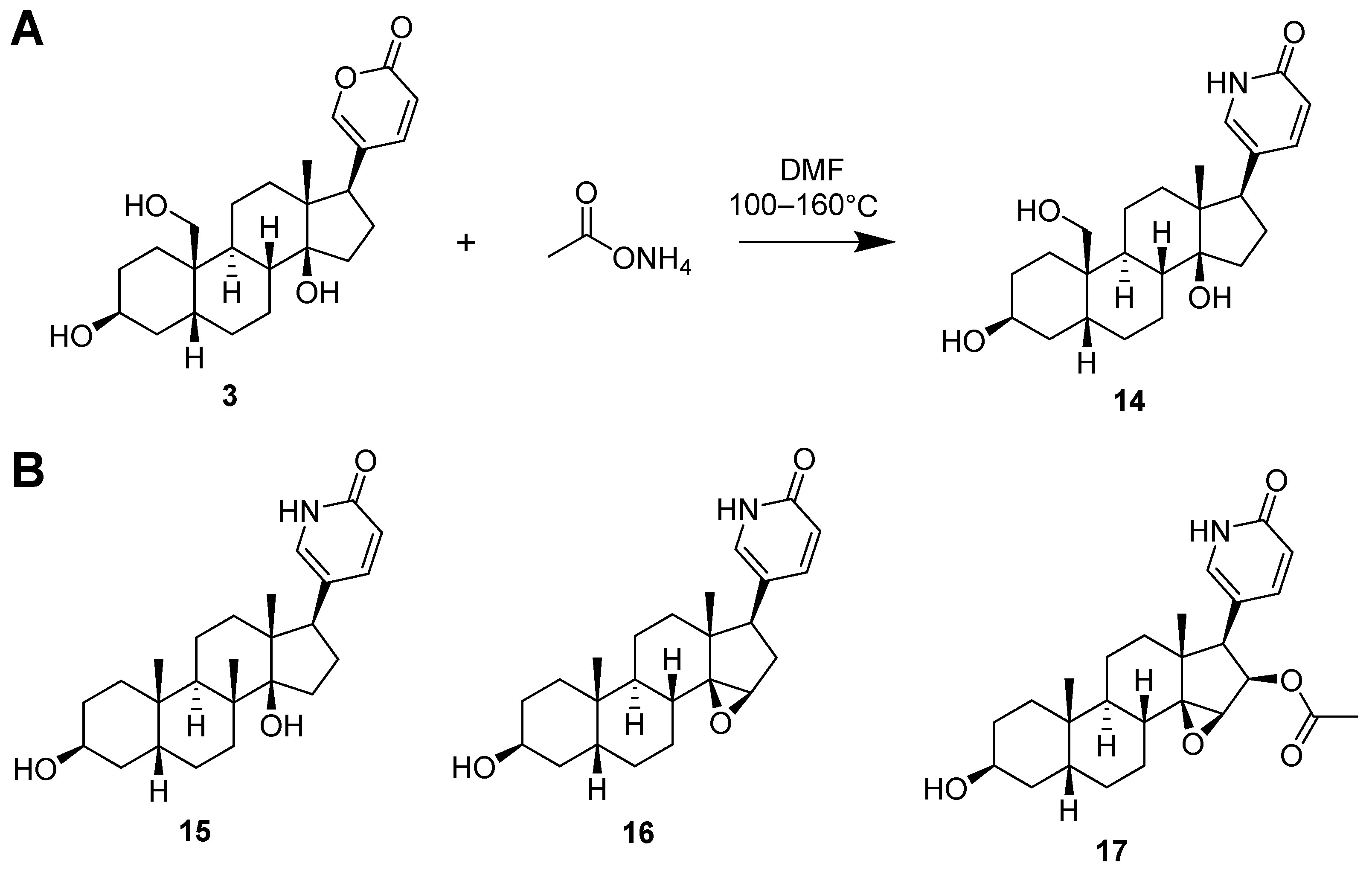
2.4. Synthesis of Lactam Derivatives of BDs
2.5. The Anti-Proliferative Activities of All Isolated Compounds 1–13 and Four Modified Compound in Prostate Cancer Cell Lines
2.6. The Pharmacophore Modeling of BDs against Prostate Cancer Cells
3. Methods and Materials
3.1. General Experimental Procedures
3.2. Materials and Reagents
3.3. Extraction and Isolation
3.4. X-ray Crystallographic Analysis of Compound 1
3.5. The Lactone to Lactam Conversion of the Bufadienolides
3.6. Cell Culture
3.7. Cytotoxicity Assay
3.8. Pharmacophore Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, Q.; Zhou, X.; Han, F.; Zheng, C. Toad Venom-Derived Bufadienolides and Their Therapeutic Application In Prostate Cancers: Current Status and Future Directions. Front. Chem. 2023, 11, 1137547. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, X.; Ren, H.; Han, F.; Lin, R.; Li, J. An Overview of the Past Decade of Bufalin in the Treatment of Refractory and Drug-Resistant Cancers: Current Status, Challenges, and Future Perspectives. Front. Pharmacol. 2023, 14, 1274336. [Google Scholar] [CrossRef]
- Jia, J.; Li, J.; Zheng, Q.; Li, D. A Research Update on the Antitumor Effects of Active Components of Chinese Medicine Chansu. Front. Oncol. 2022, 12, 1014637. [Google Scholar] [CrossRef]
- Kong, L.; Liu, X.; Yu, B.; Yuan, Y.; Zhao, Q.; Chen, Y.; Qu, B.; Du, X.; Tian, X.; Shao, R.; et al. Cinobufacini Injection Inhibits the Proliferation of Triple-Negative Breast Cancer Through the Pin1-Taz Signaling Pathway. Front. Pharmacol. 2022, 13, 797873. [Google Scholar] [CrossRef]
- Li, Q.; Liang, R.L.; Yu, Q.R.; Tian, D.Q.; Zhao, L.N.; Wang, W.W.; Xiao, H.; Yong, X.J.; Peng, X.D. Efficacy and Safety of Cinobufacini Injection Combined with Vinorelbine and Cisplatin Regimen Chemotherapy for Stage Iii/Iv Non-Small Cell Lung Cancer: A Protocol for Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2020, 99, E21539. [Google Scholar] [CrossRef]
- Zheng, Y.; Deng, L.; Cao, H.; Xu, N.; Zhang, D.; Tian, H.; Li, B.; Lu, Z.; Ye, W.; Yu, L.; et al. Screening of Bufadienolides from Toad Venom Identifies Gammabufotalin as a Potential Anti-Inflammatory Agent. Planta Med. 2022, 88, 43–52. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, L.; Yuan, Y.; Turdu, G.; Mirzaakhmedov, S.; Aisa, H.A.; Yili, A. Two New Polyamine Alkaloids from the Bufo Viridis Toad Venom. Nat. Prod. Res. 2022, 37, 3538–3542. [Google Scholar] [CrossRef]
- Li, F.J.; Hu, J.H.; Ren, X.; Zhou, C.M.; Liu, Q.; Zhang, Y.Q. Toad Venom: A Comprehensive Review of Chemical Constituents, Anticancer Activities, and Mechanisms. Arch. Pharm. 2021, 354, E2100060. [Google Scholar] [CrossRef]
- Chen, Y.L.; Dai, Y.H.; Wang, A.D.; Zhou, Z.Y.; Lei, M.; Liu, J.; Lin, B.; Xia, M.Y.; Wang, D. Two New Indole Alkaloids from Toad Venom of Bufo bufo gargarizans. Molecules 2020, 25, 4511. [Google Scholar] [CrossRef]
- Zhou, S.-W.; Quan, J.-Y.; Li, Z.-W.; Ye, G.; Shang, Z.; Chen, Z.-P.; Wang, L.; Li, X.-Y.; Zhang, X.-Q.; Li, J.; et al. Bufadienolides from the eggs of the toad Bufo bufo gargarizans and their antimelanoma activities. J. Nat. Prod. 2021, 84, 1425–1433. [Google Scholar] [CrossRef]
- Liu, J.S.; Deng, L.J.; Tian, H.Y.; Ruan, Z.X.; Cao, H.H.; Ye, W.C.; Zhang, D.M.; Yu, Z.L. Anti-tumor effects and 3D-quantitative structure-activity relationship analysis of bufadienolides from toad venom. Fitoterapia 2019, 134, 362–371. [Google Scholar] [CrossRef]
- Wu, J.H.; Cao, Y.T.; Pan, H.Y.; Wang, L.H. Identification of Antitumor Constituents in Toad Venom by Spectrum-Effect Relationship Analysis and Investigation on Its Pharmacologic Mechanism. Molecules 2020, 25, 4269. [Google Scholar] [CrossRef]
- Dai, Y.H.; Wang, A.D.; Chen, Y.L.; Xia, M.Y.; Shao, X.Y.; Liu, D.C.; Wang, D. A New Indole Alkaloid from the Traditional Chinese Medicine Chansu. J. Asian Nat. Prod. Res. 2018, 20, 581–585. [Google Scholar] [CrossRef]
- Gao, F.; Wang, X.; Li, Z.; Zhou, A.; Tiffany-Castiglioni, E.; Xie, L.; Qian, Y. Identification of Anti-Tumor Components from Toad Venom. Oncol. Lett. 2017, 14, 15–22. [Google Scholar] [CrossRef]
- Meng, L.; Li, S.; Kong, Q.; Wang, M.; Zhang, X.; Zhu, X.; Yu, W.; Jiang, N.; Chun, Z.; Li, N.; et al. Two new 19-hydroxy bufadienolides with cytotoxic activity from the skins of Bufo melanosticus. Nat. Prod. Res. 2021, 35, 4894–4900. [Google Scholar] [CrossRef]
- Rodriguez, C.; Ibanez, R.; Mojica, L.; Ng, M.; Spadafora, C.; Durant-Archibold, A.A.; Gutierrez, M. Bufadienolides from the Skin Secretions of the Neotropical Toad Rhinella Alata (Anura: Bufonidae): Antiprotozoal Activity against Trypanosoma cruzi. Molecules 2021, 26, 4217. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wu, F.K.; Qiu, Y.K.; Wu, Z.; Jiang, Y.T.; Chen, J.Y. Studies On Cytotoxic Constituents from the Skin of the Toad Bufo bufo gargarizans. J. Asian Nat. Prod. Res. 2010, 12, 793–800. [Google Scholar] [CrossRef]
- Gao, H.; Popescu, R.; Kopp, B.; Wang, Z. Bufadienolides And Their Antitumor Activity. Nat. Prod. Rep. 2011, 28, 953–969. [Google Scholar] [CrossRef]
- Wang, D.L.; Qi, F.H.; Tang, W.; Wang, F.S. Chemical Constituents and Bioactivities of the Skin of Bufo bufo gargarizans Cantor. Chem. Biodivers. 2011, 8, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.T.; Deng, S.; Liu, D.; Zhang, B.J.; Sun, H.Z.; Tian, Y.; Wang, C.Y.; Wang, L.; Ma, X.C. Isolation and Identification of Phase I Metabolites of Resibufogenin in Rats. Xenobiotica 2013, 43, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Kamano, Y.; Yamamoto, H.; Hatayama, K.; Tanaka, Y.; Shinohara, M.; Komatsu, M. The Isolation and Structure of New Bufadienolide, Resibufagin and the Isolation of Marinobufagin. Tetrahedron Lett. 1968, 5669–5672. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, M.; Dong, Y.H.; Hu, H.B.; Tao, S.J.; Yin, J.; Guo, D.A. Biotransformation of Bufadienolides by Cell Suspension Cultures of Saussurea involucrata. Phytochemistry 2011, 72, 1779–1785. [Google Scholar] [CrossRef]
- Asrorov, A.M.; Kayumov, M.; Mukhamedov, N.; Yashinov, A.; Mirakhmetova, Z.; Huang, Y.; Yili, A.; Aisa, H.A.; Tashmukhamedov, M.; Salikhov, S.; et al. Toad Venom Bufadienolides and Bufotoxins: An Updated Review. Drug Develop. Res. 2023, 84, 815–838. [Google Scholar] [CrossRef]
- Qiao, L.; Zhou, Y.Z.; Chen, H.; Cao, J.Q.; Pei, Y.H. One New Bufadienolide Biotransformed from Cinobufagin by Cunninghamella elegans. Chin. Chem. Lett. 2008, 19, 299–301. [Google Scholar] [CrossRef]
- Peery, R.; Cui, Q.; Kyei-Baffour, K.; Josephraj, S.; Huang, C.; Dong, Z.; Dai, M.; Zhang, J.T.; Liu, J.Y. A novel survivin dimerization inhibitor without a labile hydrazone linker induces spontaneous apoptosis and synergizes with docetaxel in prostate cancer cells. Bioorgan. Med. Chem. 2022, 65, 116761. [Google Scholar] [CrossRef]
- Yu, W.; Mackerell, A.J. Computer-Aided Drug Design Methods. Methods Mol. Biol. 2017, 1520, 85–106. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, W.; Li, X.; Wang, L.; Yin, W.; Wang, Y.D.; Hou, R.; Chen, W.D. Pharmacophore Modeling and Virtual Screening Studies for Discovery of Novel Farnesoid X Receptor (Fxr) Agonists. RSC Adv. 2021, 11, 2158–2166. [Google Scholar] [CrossRef]

| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 24.9, CH2 | 1.82 a, 1.42 a | 24.7, CH2 | 1.80 a, 1.46 a |
| 2 | 25.7, CH2 | 1.68 a | 27.5, CH2 | 1.82 a, 1.26 a |
| 3 | 72.1, CH | 5.05, br s | 67.1, CH | 4.06, (br. s) |
| 4 | 31.4, CH2 | 2.00 a, 1.48 a | 34.0, CH2 | 1.98 a, 1.39 a |
| 5 | 30.3, CH | 2.16 a | 30.7, CH | 2.16 a |
| 6 | 22.1, CH2 | 1.84 a, 1.34 a | 22.1, CH2 | 1.85 a, 1.36 a |
| 7 | 27.2, CH2 | 1.87 a, 1.24 a | 28.1, CH2 | 1.65 a, 1.58 a |
| 8 | 42.8, CH | 1.68 a | 43.0, CH | 1.68 a |
| 9 | 36.6, CH | 1.80 a | 36.4, CH | 1.87 a |
| 10 | 40.4, C | - | 39.6, C | - |
| 11 | 22.6, CH2 | 1.46 a, 1.22 a | 22.8, CH2 | 1.53 a, 1.22 a |
| 12 | 42.2, CH2 | 1.50 a, 1.42 a | 42.0, CH2 | 1.53 a, 1.44 a |
| 13 | 49.8, C | - | 49.7, C | - |
| 14 | 86.2, C | - | 86.1, C | - |
| 15 | 33.0, CH2 | 2.16 a, 1.73 a | 33.0, CH2 | 2.14 a, 1.73 a |
| 16 | 29.8, CH2 | 2.18 a, 1.73 a | 29.8, CH2 | 2.18 a, 1.74 a |
| 17 | 52.3, CH | 2.56, m | 52.2, CH | 2.56, dd (9.4, 6.0) |
| 18 | 17.3, CH3 | 0.71, s | 17.3, CH3 | 0.72, s |
| 19 | 65.4, CH2 | 3.89 (d, 11.2); 3.38 (d, 11.2) | 68.9, CH2 | 4.38 (d, 11.2); 4.00 (d, 11.2) |
| 20 | 125.0, C | - | 125.0, C | - |
| 21 | 150.5, CH | 7.43 (dd, 2.6, 1.0) | 150.4, CH | 7.44 (dd, 2.6, 1.0) |
| 22 | 149.4, CH | 7.99 (dd, 9.7, 2.6) | 149.3, CH | 8.00 (dd, 9.7, 2.6) |
| 23 | 115.4, CH | 6.28 (dd, 9.7, 1.0) | 115.4, CH | 6.29 (dd, 2.6, 1.0) |
| 24 | 164.8, C | - | 164.7, C | - |
| 25 | 172.7, C | - | 173.3, C | - |
| 26 | 21.3, CH3 | 2.04, s | 20.9, CH3 | 2.07, s |
| Compound | Cancer Cells (μM) | |
|---|---|---|
| PC-3 | DU145 | |
| Paclitaxel | 0.031 ± 0.030 | 0.001 ± 0.001 |
| 1 | 0.162 ± 0.156 | 0.050 ± 0.015 |
| 2 | 0.214 ± 0.286 | 0.211 ± 0.221 |
| 3 | 0.014 ± 0.001 | 0.010 ± 0.007 |
| 4 | 0.091 ± 0.062 | 0.032 ± 0.010 |
| 5 | 0.012 ± 0.003 | 0.017 ± 0.014 |
| 6 | 0.040 ± 0.046 | 0.020 ± 0.016 |
| 7 | 0.259 ± 0.007 | 0.104 ± 0.054 |
| 8 | 6.740 ± 2.763 | 14.947 ± 5.074 |
| 9 | 0.571 ± 0.130 | 2.485 ± 0.772 |
| 10 | 0.035 ± 0.022 | 0.137 ± 0.131 |
| 11 | 0.040 ± 0.026 | 0.037 ± 0.003 |
| 12 | 0.007 ± 0.002 | 0.017 ± 0.007 |
| 13 | 0.050 ± 0.024 | 0.028 ± 0.006 |
| 14 | 6.261 ± 3.901 | 6.667 ± 2.744 |
| 15 | 11.233 ± 0.821 | 13.263 ± 1.506 |
| 16 | >25 | >25 |
| 17 | 56.297 ± 7.763 | 68.563 ± 14.742 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Lin, R.; Chen, Z.; Li, J.; Zheng, C. Isolation, Identification and Chemical Modification of Bufadienolides from Bufo melanostictus Schneider and Their Cytotoxic Activities against Prostate Cancer Cells. Molecules 2024, 29, 1571. https://doi.org/10.3390/molecules29071571
Ye Q, Lin R, Chen Z, Li J, Zheng C. Isolation, Identification and Chemical Modification of Bufadienolides from Bufo melanostictus Schneider and Their Cytotoxic Activities against Prostate Cancer Cells. Molecules. 2024; 29(7):1571. https://doi.org/10.3390/molecules29071571
Chicago/Turabian StyleYe, Qingmei, Rong Lin, Zeping Chen, Juan Li, and Caijuan Zheng. 2024. "Isolation, Identification and Chemical Modification of Bufadienolides from Bufo melanostictus Schneider and Their Cytotoxic Activities against Prostate Cancer Cells" Molecules 29, no. 7: 1571. https://doi.org/10.3390/molecules29071571
APA StyleYe, Q., Lin, R., Chen, Z., Li, J., & Zheng, C. (2024). Isolation, Identification and Chemical Modification of Bufadienolides from Bufo melanostictus Schneider and Their Cytotoxic Activities against Prostate Cancer Cells. Molecules, 29(7), 1571. https://doi.org/10.3390/molecules29071571







