Aerobic Oxidative Desulfurization by Supported Polyoxometalate Ionic Liquid Hybrid Materials via Facile Ball Milling
Abstract
1. Introduction
2. Results and Discussion
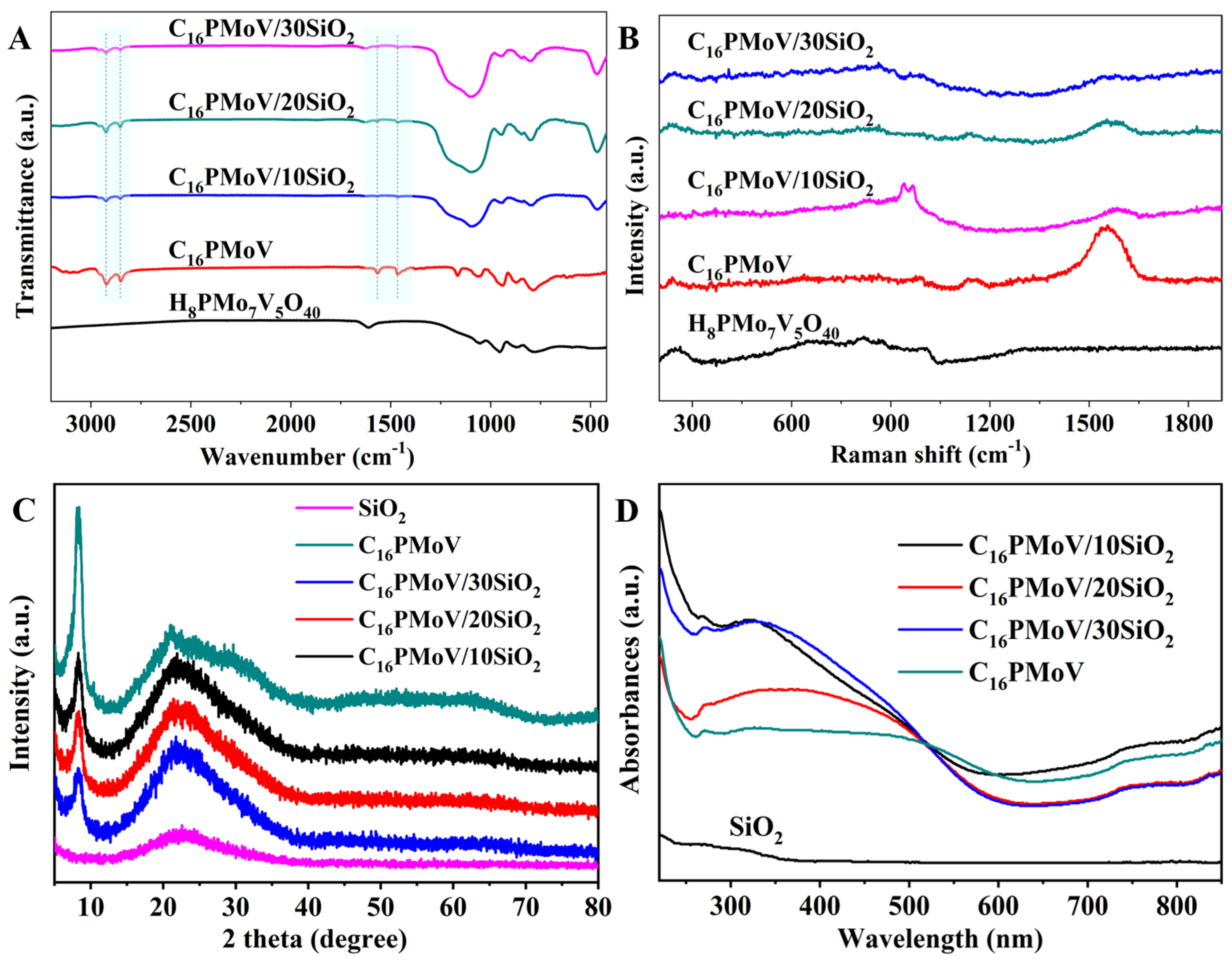
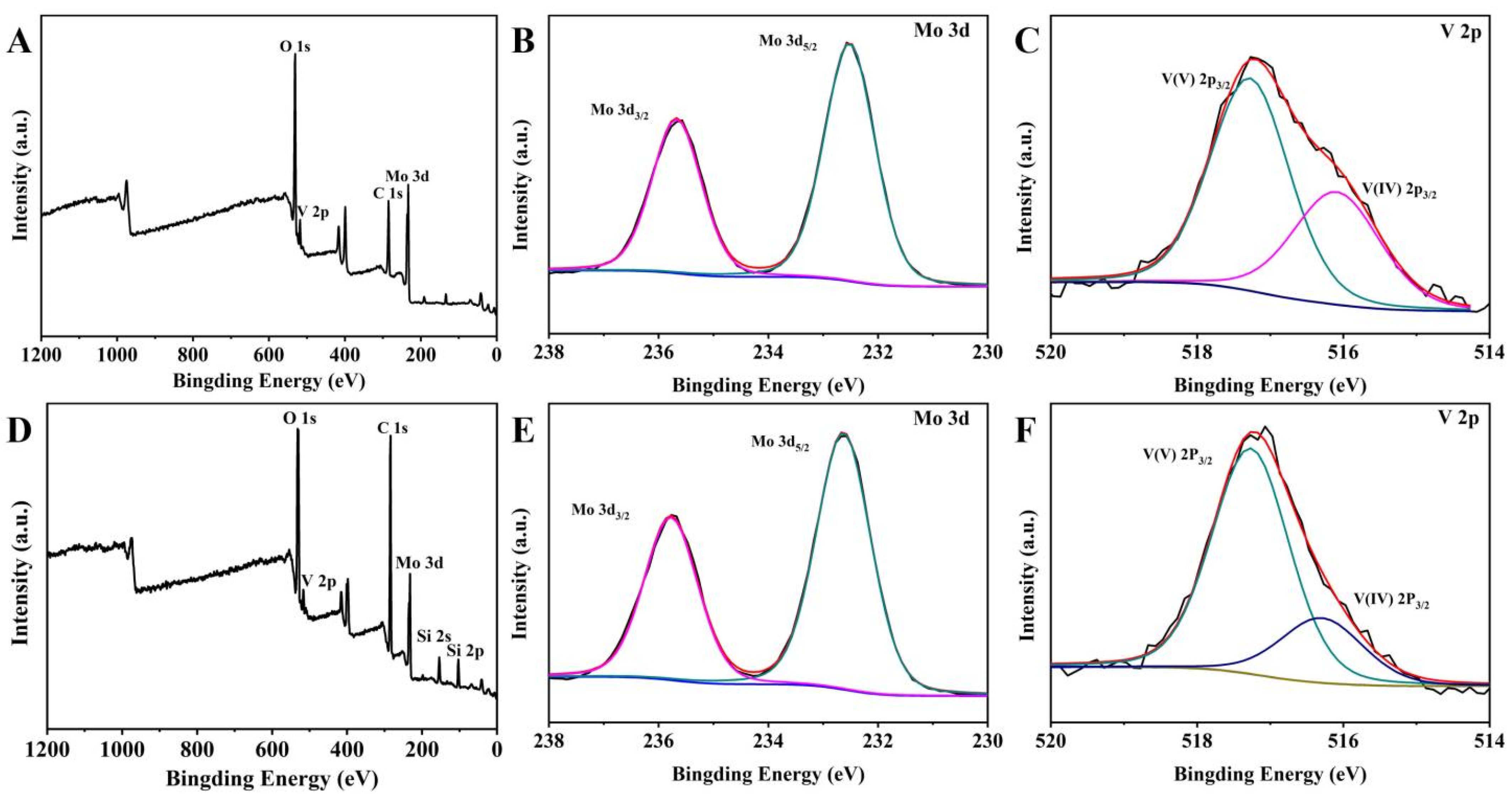
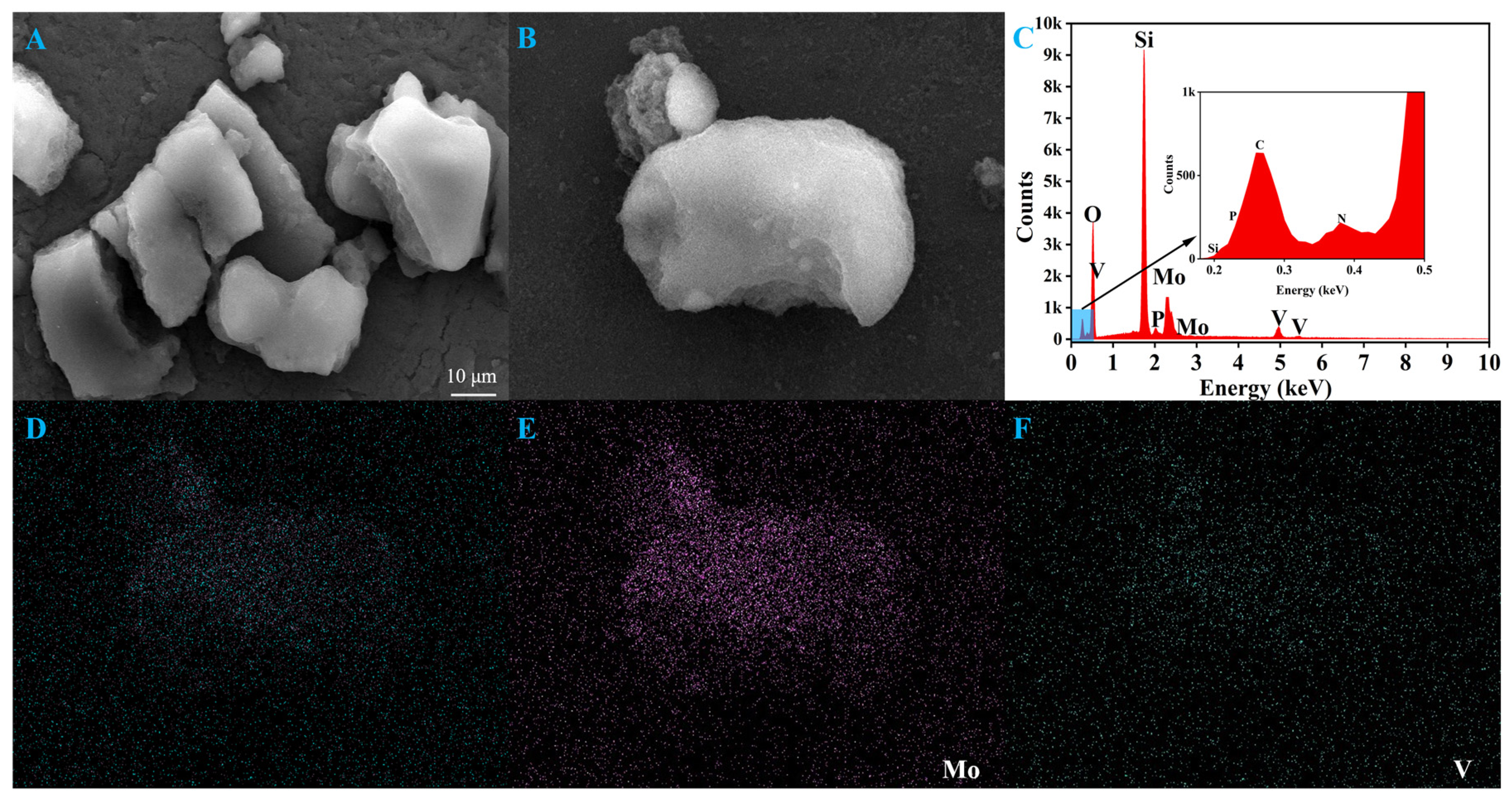
2.1. Characterization of the Catalyst
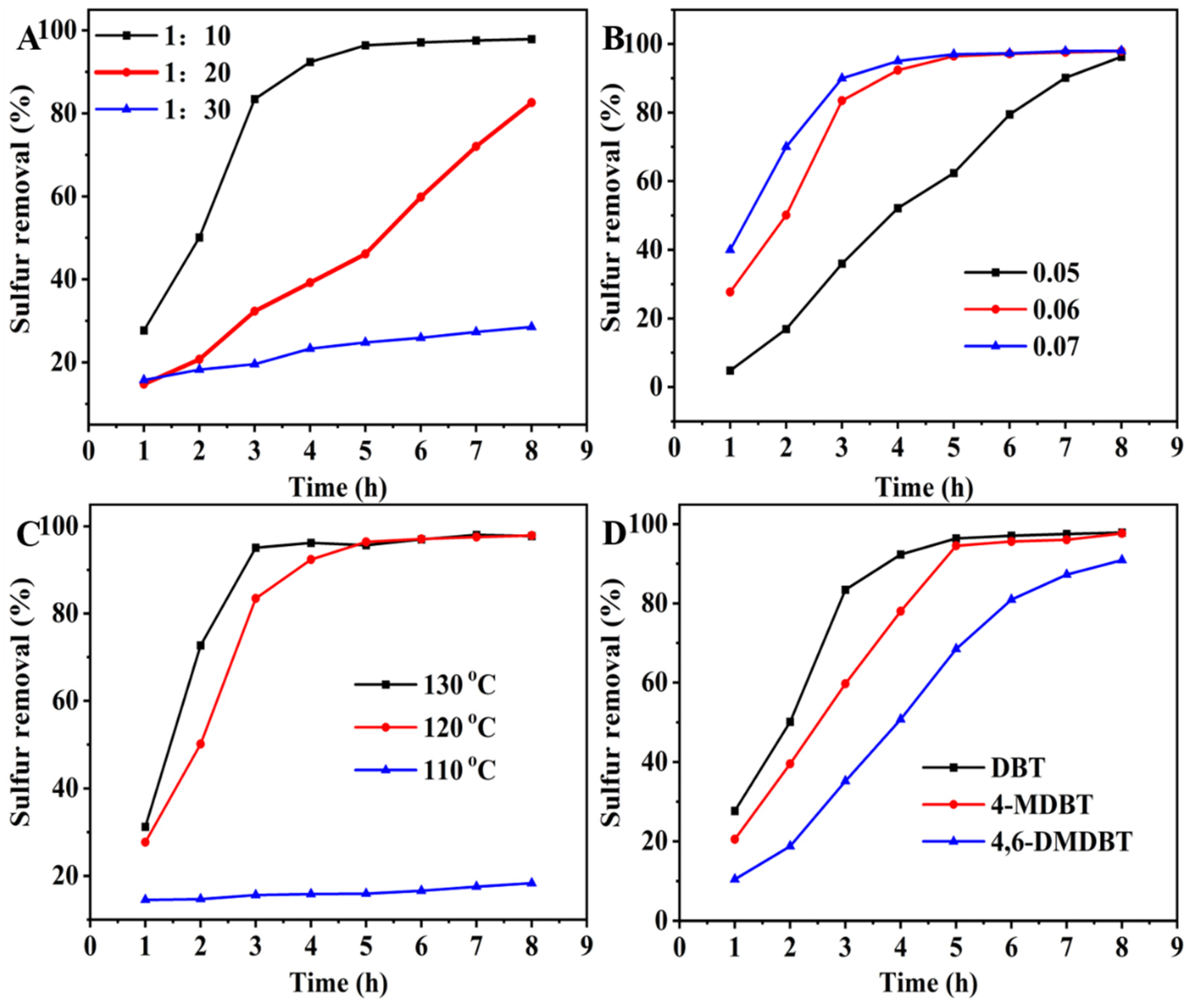
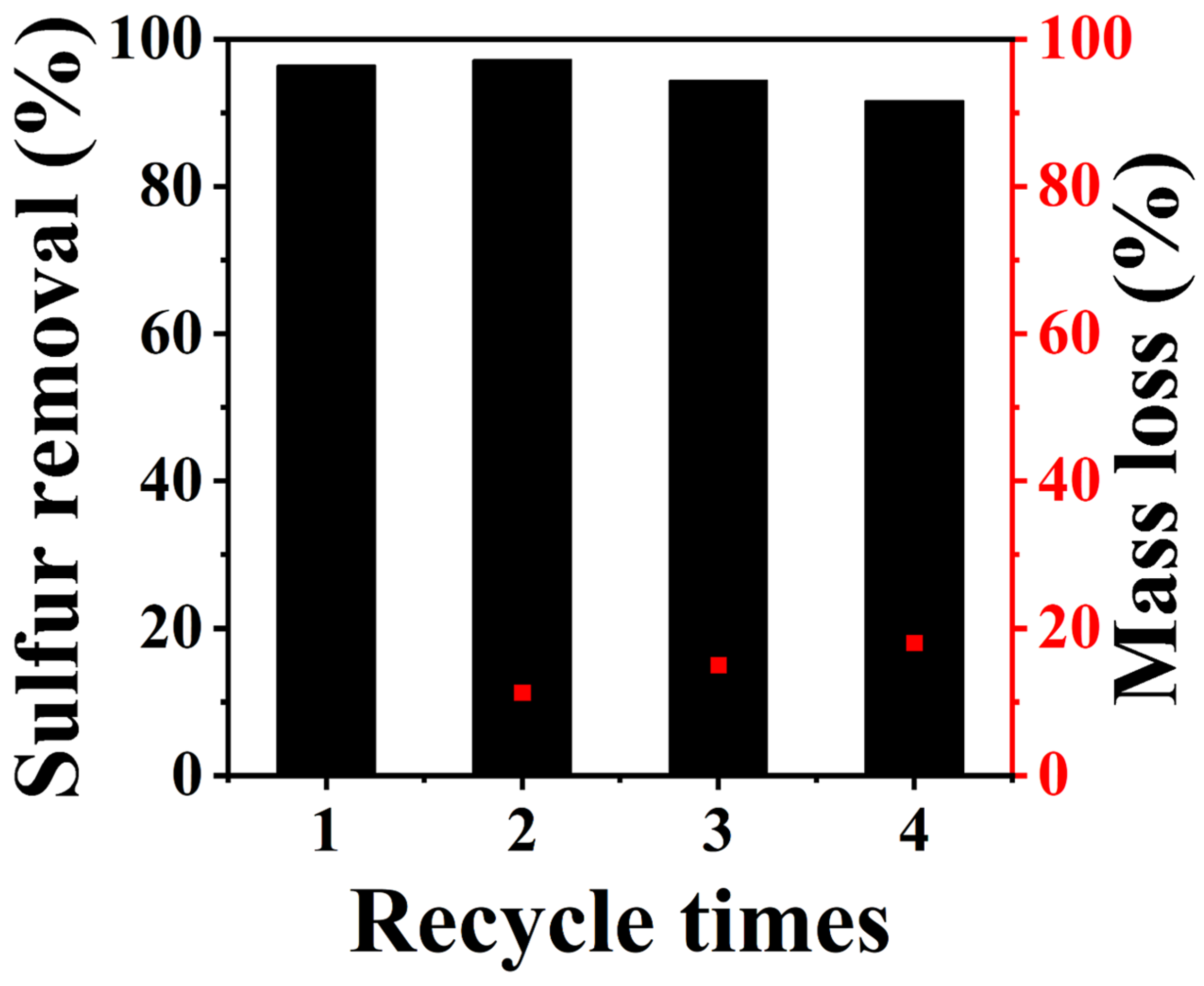
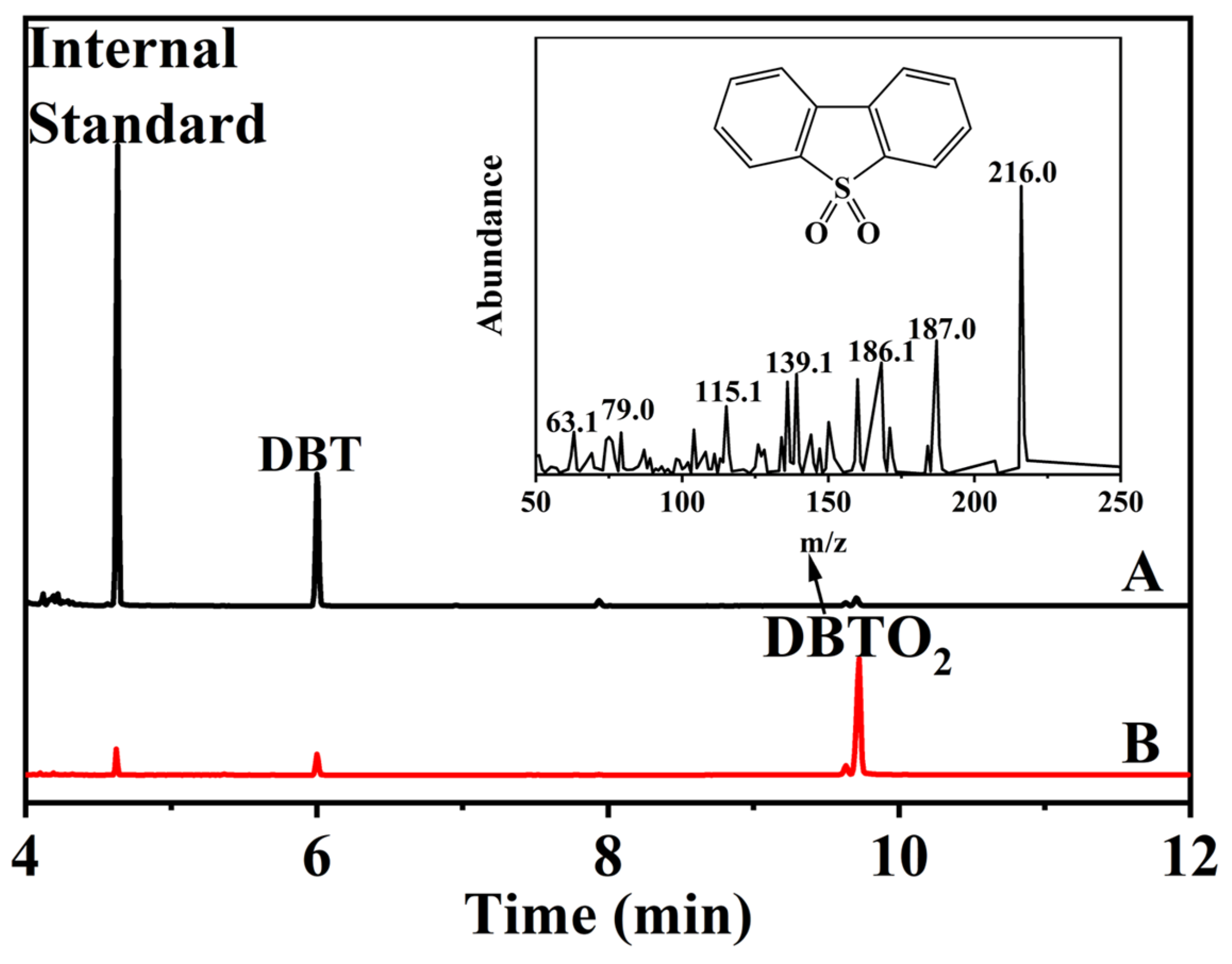
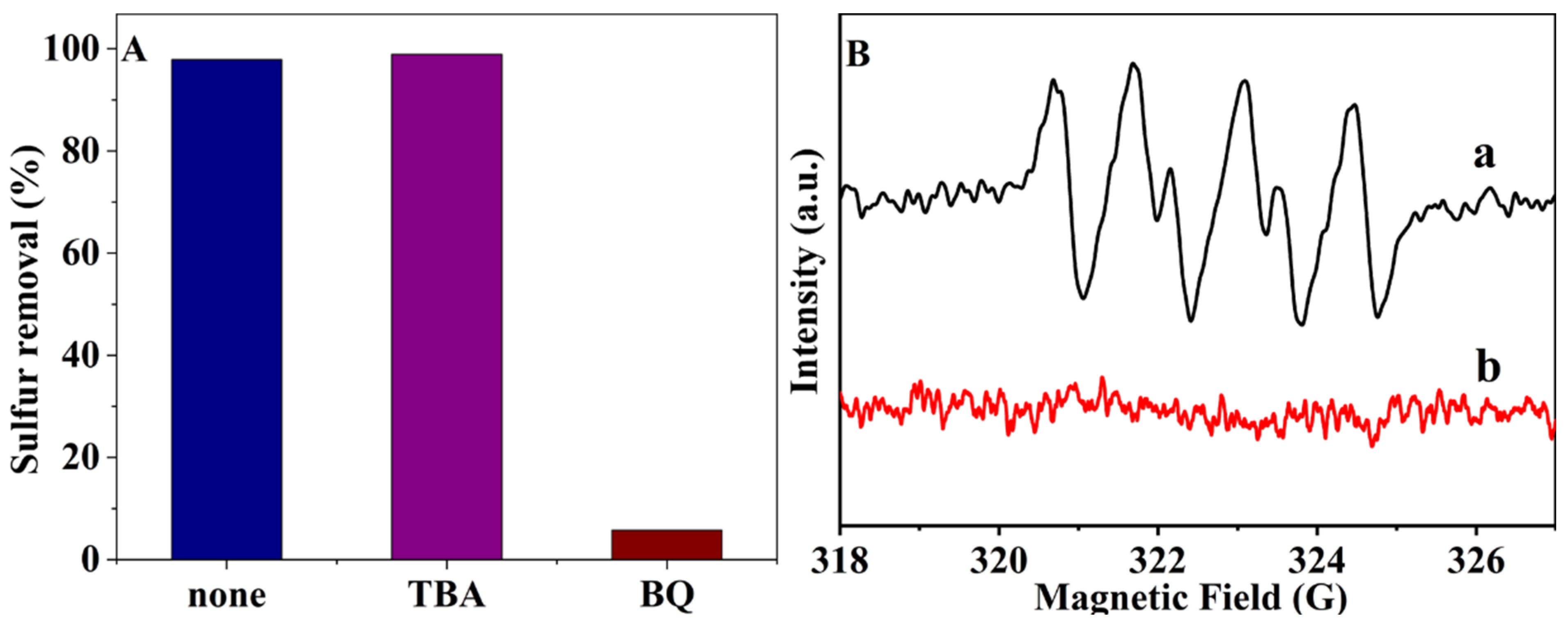
2.2. Catalytic Activity
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Polyoxometalate H8PMo7V5O40
3.3. Synthesis of Polyoxometalate Ionic Liquid
3.4. Synthesis of Polyoxometalate Ionic Liquid Supported Material
3.5. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janzwood, A.; Harrison, K. The political economy of fossil fuel production in the Post-Paris Era: Critically evaluating Nationally Determined Contributions. Energy Res. Soc. Sci. 2023, 102, 103095. [Google Scholar] [CrossRef]
- Skovgaard, J.; van Asselt, H. The politics of fossil fuel subsidies and their reform: Implications for climate change mitigation. Wiley Interdiscip. Rev. Clim. Chang. 2019, 10, e581. [Google Scholar] [CrossRef]
- Zhu, B.; Hu, X.; Deng, Y.Y.; Zhang, B.K.; Li, X.R. The differential effects of climate risks on non-fossil and fossil fuel stock markets: Evidence from China. Financ. Res. Lett. 2023, 55, 103962. [Google Scholar] [CrossRef]
- Dehghan, R.; Anbia, M. Zeolites for adsorptive desulfurization from fuels: A review. Fuel Process. Technol. 2017, 167, 99–116. [Google Scholar] [CrossRef]
- Piccinino, D.; Abdalghani, I.; Botta, G.; Crucianelli, M.; Passacantando, M.; Di Vacri, M.L.; Saladino, R. Preparation of wrapped carbon nanotubes poly(4-vinylpyridine)/MTO based heterogeneous catalysts for the oxidative desulfurization (ODS) of model and synthetic diesel fuel. Appl. Catal. B 2017, 200, 392–401. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Yang, W.S.; Hussain, M. Recent developments in alumina supported hydrodesulfurization catalysts for the production of sulfur-free refinery products: A technical review. Catal. Rev. Sci. Eng. 2022, 64, 1–86. [Google Scholar] [CrossRef]
- Yang, H.W.; Jiang, B.; Sun, Y.L.; Zhang, L.H.; Huang, Z.H.; Sun, Z.N.; Yang, N. Heterogeneous oxidative desulfurization of diesel fuel catalyzed by mesoporous polyoxometallate-based polymeric hybrid. J. Hazard. Mater. 2017, 333, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.S.; Afonso, C.A.M. Deep desulfurization of diesel fuel using ionic liquids: Current status and future challenges. Green Chem. 2010, 12, 1139–1149. [Google Scholar] [CrossRef]
- Li, S.W.; Wang, W.; Zhao, J.S. Magnetic-heteropolyacid mesoporous catalysts for deep oxidative desulfurization of fuel: The influence on the amount of APES used. J. Colloid Interface Sci. 2020, 571, 337–347. [Google Scholar] [CrossRef]
- Xiao, J.; Wu, L.M.; Wu, Y.; Liu, B.; Dai, L.; Li, Z.; Xia, Q.B.; Xi, H.X. Effect of gasoline composition on oxidative desulfurization using a phosphotungstic acid/activated carbon catalyst with hydrogen peroxide. Appl. Energy 2014, 113, 78–85. [Google Scholar] [CrossRef]
- Yang, C.P.; Zhao, K.; Cheng, Y.; Zeng, G.M.; Zhang, M.M.; Shao, J.J.; Lu, L. Catalytic oxidative desulfurization of BT and DBT from n-octane using cyclohexanone peroxide and catalyst of molybdenum supported on 4A molecular sieve. Sep. Purif. Technol. 2016, 163, 153–161. [Google Scholar] [CrossRef]
- Gu, Q.Q.; Wen, G.D.; Ding, Y.X.; Wu, K.H.; Chen, C.M.; Su, D.S. Reduced graphene oxide: A metal-free catalyst for aerobic oxidative desulfurization. Green Chem. 2017, 19, 1175–1181. [Google Scholar] [CrossRef]
- Hussain, M.; Song, S.K.; Ihm, S.K. Synthesis of hydrothermally stable MCM-41 by the seed crystallization and its application as a catalyst support for hydrodesulfurization. Fuel 2013, 106, 787–792. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, W.S.; Xun, S.H.; Li, H.M.; Gu, Q.Q.; Zhao, Z.; Wang, Q. Deep oxidative desulfurization of dibenzothiophene with POM-based hybrid materials in ionic liquids. Chem. Eng. J. 2013, 220, 328–336. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.Q.; Li, H.P.; Wei, Y.C.; Fu, Y.J.; Liao, W.Y.; Zhu, L.H.; Chen, G.Y.; Zhu, W.S.; Li, H.M. Tuning the electrophilicity of vanadium-substituted polyoxometalate based ionic liquids for high-efficiency aerobic oxidative desulfurization. Appl. Catal. B 2020, 271, 118936. [Google Scholar] [CrossRef]
- Abazari, R.; Esrafili, L.; Morsali, A.; Wu, Y.H.; Gao, J.K. PMo12@ UiO-67 nanocomposite as a novel non-leaching catalyst with enhanced performance durability for sulfur removal from liquid fuels with exceptionally diluted oxidant. Appl. Catal. B 2021, 283, 119582. [Google Scholar] [CrossRef]
- Tsui, T.H.; Zhang, L.; Zhang, J.X.; Dai, Y.J.; Tong, Y.W. Engineering interface between bioenergy recovery and biogas desulfurization: Sustainability interplays of biochar application. Renew. Sustain. Energy Rev. 2022, 157, 112053. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Leclercq, L.; Clacens, J.M.; De Campo, F.; Nardello-Rataj, V. Pickering Interfacial Catalysis for Biphasic Systems: From Emulsion Design to Green Reactions. Angew. Chem. Int. Ed. 2015, 54, 2006–2021. [Google Scholar] [CrossRef]
- Dai, C.N.; Zhang, J.; Huang, C.P.; Lei, Z.G. Ionic Liquids in Selective Oxidation: Catalysts and Solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef] [PubMed]
- Samaniyan, M.; Mirzaei, M.; Khajavian, R.; Eshtiagh-Hosseini, H.; Streb, C. Heterogeneous Catalysis by Polyoxometalates in Metal-Organic Frameworks. ACS Catal. 2019, 9, 10174–10191. [Google Scholar] [CrossRef]
- Li, A.; Song, H.Y.; Meng, H.; Lu, Y.Z.; Li, C.X. Peroxovanadic based core-shell bifunctional poly(ionic liquid)s catalyst CuO/SiO2@V-PIL: Its in-situ free radical initiation mechanism for air oxidative desulfurization. Fuel 2022, 310, 122430. [Google Scholar] [CrossRef]
- Li, S.W.; Li, J.R.; Gao, Y.; Liang, L.L.; Zhang, R.L.; Zhao, J.S. Metal modified heteropolyacid incorporated into porous materials for a highly oxidative desulfurization of DBT under molecular oxygen. Fuel 2017, 197, 551–561. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Chang, X.; Zong, M.-Y.; Fan, C.-Z.; Guo, D.-X.; Xu, J.; Wang, D.-H.; Bu, X.-H. Rational design of ionic V-MOF with confined Mo species for highly efficient oxidative desulfurization. Appl. Catal. B 2021, 298, 120594. [Google Scholar] [CrossRef]
- Yin, J.; Fu, W.D.; Zhang, J.R.; Liu, X.Y.; Zhang, X.M.; Wang, C.; He, J.; Jiang, W.; Li, H.P.; Li, H.M. UiO-66(Zr)-based porous ionic liquids for highly efficient extraction coupled catalytic oxidative desulfurization. Chem. Eng. J. 2023, 470, 144290. [Google Scholar] [CrossRef]
- Zou, J.C.; Lin, Y.; Wu, S.H.; Zhong, Y.Y.; Yang, C.P. Molybdenum Dioxide Nanoparticles Anchored on Nitrogen-Doped Carbon Nanotubes as Oxidative Desulfurization Catalysts: Role of Electron Transfer in Activity and Reusability. Adv. Funct. Mater. 2021, 31, 2100442. [Google Scholar] [CrossRef]
- Huang, T.Q.; Qiu, X.F.; Zhu, L.H.; Wang, C.; Li, H.P.; Fan, Y.Q.; Zhang, M.; Li, H.M.; Zhu, W.S. Engineering high specific surface area over poly(ionic liquids)-derived molybdenum-silica hybrid materials for enhanced oxidative desulfurization. J. Environ. Chem. Eng. 2023, 11, 111487. [Google Scholar] [CrossRef]
- Chen, H.X.; Hou, S.T.; Cui, H.Y.; Wang, C.; Zhang, M.; Li, H.P.; Xu, H.; Wu, J.Q.; Zhu, W.S. Construction of amphiphilic and polyoxometalate poly(ionic liquids) for enhanced oxidative desulfurization in fuel. J. Mol. Liq. 2023, 379, 121650. [Google Scholar] [CrossRef]
- Zhu, D.A.; Xu, L.X.; Zhang, B.B.; Zhu, L.H.; He, J.; Li, H.P.; Li, H.M.; Jiang, W. Designing Inorganic-Organic Dual-Acid Deep Eutectic Solvents for Synergistically Enhanced Extractive and Oxidative Desulfurization. Molecules 2023, 28, 7743. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Wang, H.; Chen, W.; Chu, H.; Wei, J.; Wang, D.; Wang, J.; Li, Y. In Situ Implanting of Single Tungsten Sites into Defective UiO-66(Zr) by Solvent-Free Route for Efficient Oxidative Desulfurization at Room Temperature. Angew. Chem. 2021, 60, 20318–20324. [Google Scholar] [CrossRef]
- Mahboob, I.; Shafiq, I.; Shafique, S.; Akhter, P.; Amjad, U.E.S.; Hussain, M.; Park, Y.K. Effect of active species scavengers in photocatalytic desulfurization of hydrocracker diesel using mesoporous Ag3VO4. Chem. Eng. J. 2022, 441, 136063. [Google Scholar] [CrossRef]
- Wan, Z.X.; Zhang, T.K.; Liu, Y.Z.; Liu, P.; Zhang, J.W.; Fang, L.; Sun, D.S. Enhancement of desulfurization by hydroxyl ammonium ionic liquid supported on active carbon. Environ. Res. 2022, 213, 113637. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549. [Google Scholar] [CrossRef]
- Fan, Y.H.; Du, M.; Li, Y.X.; Zhu, W.J.; Pang, J.Y.; Bai, Y.; Dang, D.B. Construction of Water-Stable Rare-Earth Organic Frameworks with Ambient High Proton Conductivity Based on Zirconium Sandwiched Heteropolytungstate. Inorg. Chem. 2022, 61, 13829–13835. [Google Scholar] [CrossRef]
- Skoda-Foldes, R. The Use of Supported Acidic Ionic Liquids in Organic Synthesis. Molecules 2014, 19, 8840–8884. [Google Scholar] [CrossRef]
- Xun, S.H.; Zhu, W.S.; Zheng, D.; Zhang, L.; Liu, H.; Yin, S.; Zhang, M.; Li, H.M. Synthesis of metal-based ionic liquid supported catalyst and its application in catalytic oxidative desulfurization of fuels. Fuel 2014, 136, 358–365. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.; Yang, J.P.; Li, H.P.; Liu, J.Q.; Chen, X.; Zhu, W.S.; Li, H.M. Polyoxometalate-based silica-supported ionic liquids for heterogeneous oxidative desulfurization in fuels. Pet. Sci. 2018, 15, 882–889. [Google Scholar] [CrossRef]
- Mao, S.X.; Zhou, Q.H.; Guo, H.L.; Du, M.; Zhu, W.S.; Li, H.M.; Pang, J.Y.; Dang, D.B.; Bai, Y. Porous phosphomolybdate-based poly(ionic liquid) hybrids with reversible water absorption for enhancement of oxidative desulfurization. Fuel 2023, 333, 126392. [Google Scholar] [CrossRef]
- Guo, H.L.; Xing, X.X.; Mao, S.X.; Feng, T.; Fan, Y.H.; Qin, Z.J.; Pang, J.Y.; Bai, Y.; Dang, D.B. Two three-dimensional Fe(II) frameworks based on {P4Mo6} tetrameric clusters exhibiting efficient visible-light photocatalytic properties for the degradation of Cr(VI) and methylene blue. Dalton Trans. 2022, 51, 18090–18098. [Google Scholar] [CrossRef]
- Xing, X.X.; Guo, H.L.; He, T.M.; An, X.; Li, H.P.; Zhu, W.S.; Li, H.M.; Pang, J.Y.; Dang, D.B.; Bai, Y. Tungstovanadate-Based Ionic Liquid Catalyst [C2(MIM)2]2VW12O40 Used in Deep Desulfurization for Ultraclean Fuel with Simultaneous Recovery of the Sulfone Product. ACS Sustain. Chem. Eng. 2022, 10, 11533–11543. [Google Scholar] [CrossRef]
- Li, J.Y.; Peng, J.J.; Bai, Y.; Hu, Y.Q.; Qiu, H.Y.; Lai, G.Q.; Li, X.N. Hydrosilylation Catalyzed with Rh(PPh3)3Cl/Ionic-Liquid-Functionalized SiO2. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 484–490. [Google Scholar] [CrossRef]
- Lei, Q.; Zheng, C.; He, F.; Zhao, J.; Liu, Y.; Zhao, X.P.; Yin, J.B. Enhancing Electroresponsive Electrorheological Effect and Temperature Dependence of Poly(ionic liquid) Particles by Hard Core Confinement. Langmuir 2018, 34, 15827–15838. [Google Scholar] [CrossRef]
- Long, Z.Y.; Zhou, Y.; Chen, G.J.; Zhao, P.P.; Wang, J. 4,4′-Bipyridine-modified molybdovanadophosphoric acid: A reusable heterogeneous catalyst for direct hydroxylation of benzene with O2. Chem. Eng. J. 2014, 239, 19–25. [Google Scholar] [CrossRef]
- Aliyari, E.; Alvand, M.; Shemirani, F. Modified surface-active ionic liquid-coated magnetic graphene oxide as a new magnetic solid phase extraction sorbent for preconcentration of trace nickel. RSC Adv. 2016, 6, 64193–64202. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Haeri, Z.; Ramezanzadeh, M. A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance. Chem. Eng. J. 2016, 303, 511–528. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packag. Shelf Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Liu, D.P.; Quek, X.Y.; Hu, S.Q.; Li, L.S.; Lim, H.M.; Yang, Y.H. Mesostructured TUD-1 supported molybdophosphoric acid (HPMo/TUD-1) catalysts for n-heptane hydroisomerization. Catal. Today 2009, 147, S51–S57. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions. Chem. Rev. 2002, 98, 171–198. [Google Scholar] [CrossRef]
- Li, J.L.; Hu, B.; Hu, C.Q. Deep Desulfurization of Fuels by Heteropolyanion-Based Ionic Liquid. Bull. Korean Chem. Soc. 2013, 34, 225–230. [Google Scholar] [CrossRef][Green Version]
- Lefebvre, F. 31P MAS NMR study of H3PW12O40 supported on silica: Formation of (SiOH2+)(H2PW12O40−). J. Chem. Soc., Chem. Commun. 1992, 10, 756–757. [Google Scholar] [CrossRef]
- Cai, Y.J.; Song, H.Y.; An, Z.; Xiang, X.; Shu, X.; He, J. The confined space electron transfer in phosphotungstate intercalated ZnAl-LDHs enhances its photocatalytic performance for oxidation/extraction desulfurization of model oil in air. Green Chem. 2018, 20, 5509–5519. [Google Scholar] [CrossRef]
- Liang, X.; Gao, G.H.; Liu, Y.D.; Zhang, T.Q.; Wu, G.M. Synthesis and characterization of Fe-doped vanadium oxide nanorods and their electrochemical performance. J. Alloys Compd. 2017, 715, 374–383. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, X.Y.; Lu, Y.M.; Sun, H.Y.; Meng, Q.L.; Liu, S.L.; Feng, W.; Han, X.K. The influence of SiO2 doping on the microstructure and photochromic behavior of phosphomolybdic acid/polyvinyl pyrrolidone hybrid films. J. Mol. Struct. 2014, 1075, 154–158. [Google Scholar] [CrossRef]
- Fu, J.S.; Zhang, M.J.; Jin, L.; Liu, L.; Li, N.; Shang, L.; Li, M.; Xiao, L.H.; Ao, Y.H. Enhancing interfacial properties of carbon fibers reinforced epoxy composites via Layer-by-Layer self assembly GO/SiO2 multilayers films on carbon fibers surface. Appl. Surf. Sci. 2019, 470, 543–554. [Google Scholar] [CrossRef]
- Gunjakar, J.L.; Kim, T.W.; Kim, H.N.; Kim, I.Y.; Hwang, S.J. Mesoporous Layer-by-Layer Ordered Nanohybrids of Layered Double Hydroxide and Layered Metal Oxide: Highly Active Visible Light Photocatalysts with Improved Chemical Stability. J. Am. Chem. Soc. 2011, 133, 14998–15007. [Google Scholar] [CrossRef]

| Sample | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| C16PMoV/10SiO2 | 256.7 | 0.67 | 1.51 |
| C16PMoV/20SiO2 | 263.6 | 0.63 | 0.89 |
| C16PMoV/30SiO2 | 339.7 | 1.02 | 0.88 |
| SiO2 | 447.9 | 1.13 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Huang, T.; Tong, S.; Wang, C.; Li, H.; Zhang, M. Aerobic Oxidative Desulfurization by Supported Polyoxometalate Ionic Liquid Hybrid Materials via Facile Ball Milling. Molecules 2024, 29, 1548. https://doi.org/10.3390/molecules29071548
Wang Q, Huang T, Tong S, Wang C, Li H, Zhang M. Aerobic Oxidative Desulfurization by Supported Polyoxometalate Ionic Liquid Hybrid Materials via Facile Ball Milling. Molecules. 2024; 29(7):1548. https://doi.org/10.3390/molecules29071548
Chicago/Turabian StyleWang, Qian, Tianqi Huang, Shuang Tong, Chao Wang, Hongping Li, and Ming Zhang. 2024. "Aerobic Oxidative Desulfurization by Supported Polyoxometalate Ionic Liquid Hybrid Materials via Facile Ball Milling" Molecules 29, no. 7: 1548. https://doi.org/10.3390/molecules29071548
APA StyleWang, Q., Huang, T., Tong, S., Wang, C., Li, H., & Zhang, M. (2024). Aerobic Oxidative Desulfurization by Supported Polyoxometalate Ionic Liquid Hybrid Materials via Facile Ball Milling. Molecules, 29(7), 1548. https://doi.org/10.3390/molecules29071548







