Correlation between Flow Temperature and Average Molar Ionic Potential of Ash during Gasification of Coal and Phosphorus-Rich Biomass
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of CaO/P2O5 Ratios on the Ia of Synthetic Ashes
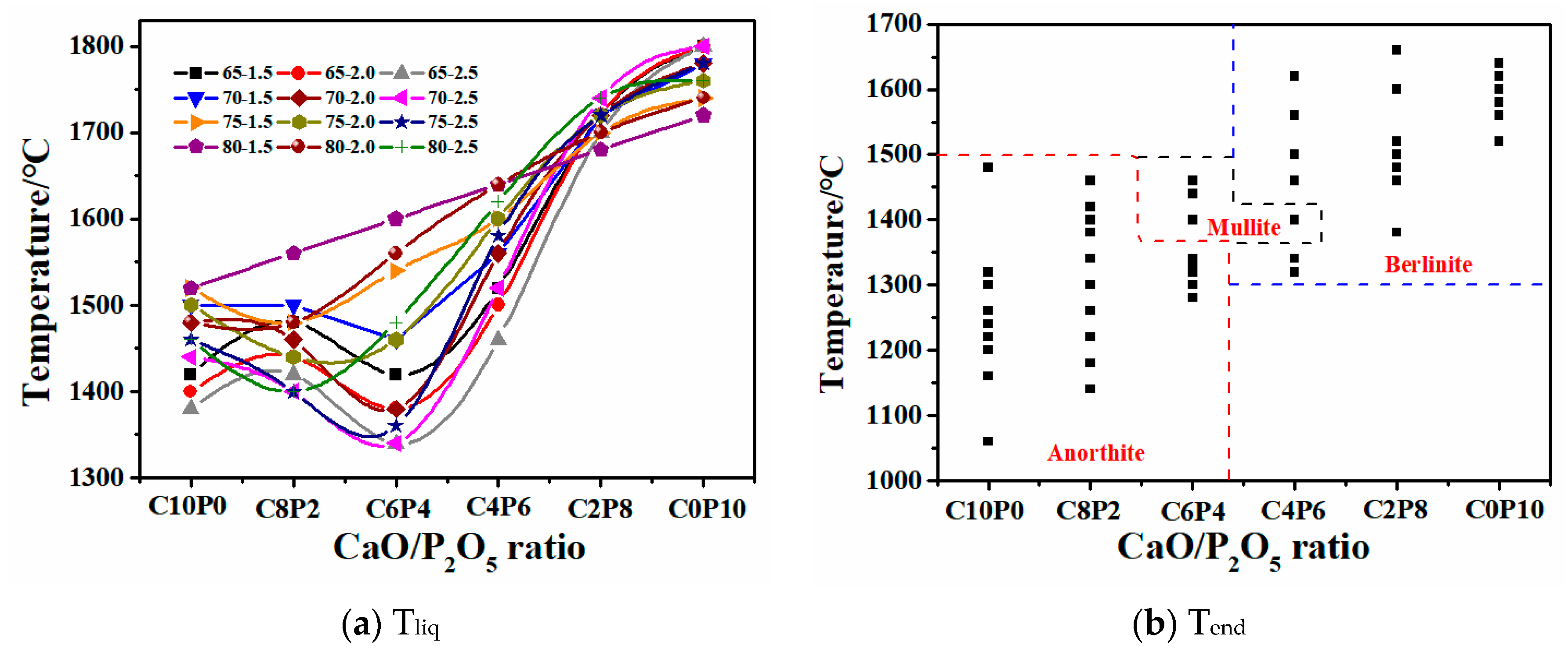
2.2. Effects of CaO/P2O5 Ratios on the FT of Synthetic Ash
2.2.1. Variation of FT
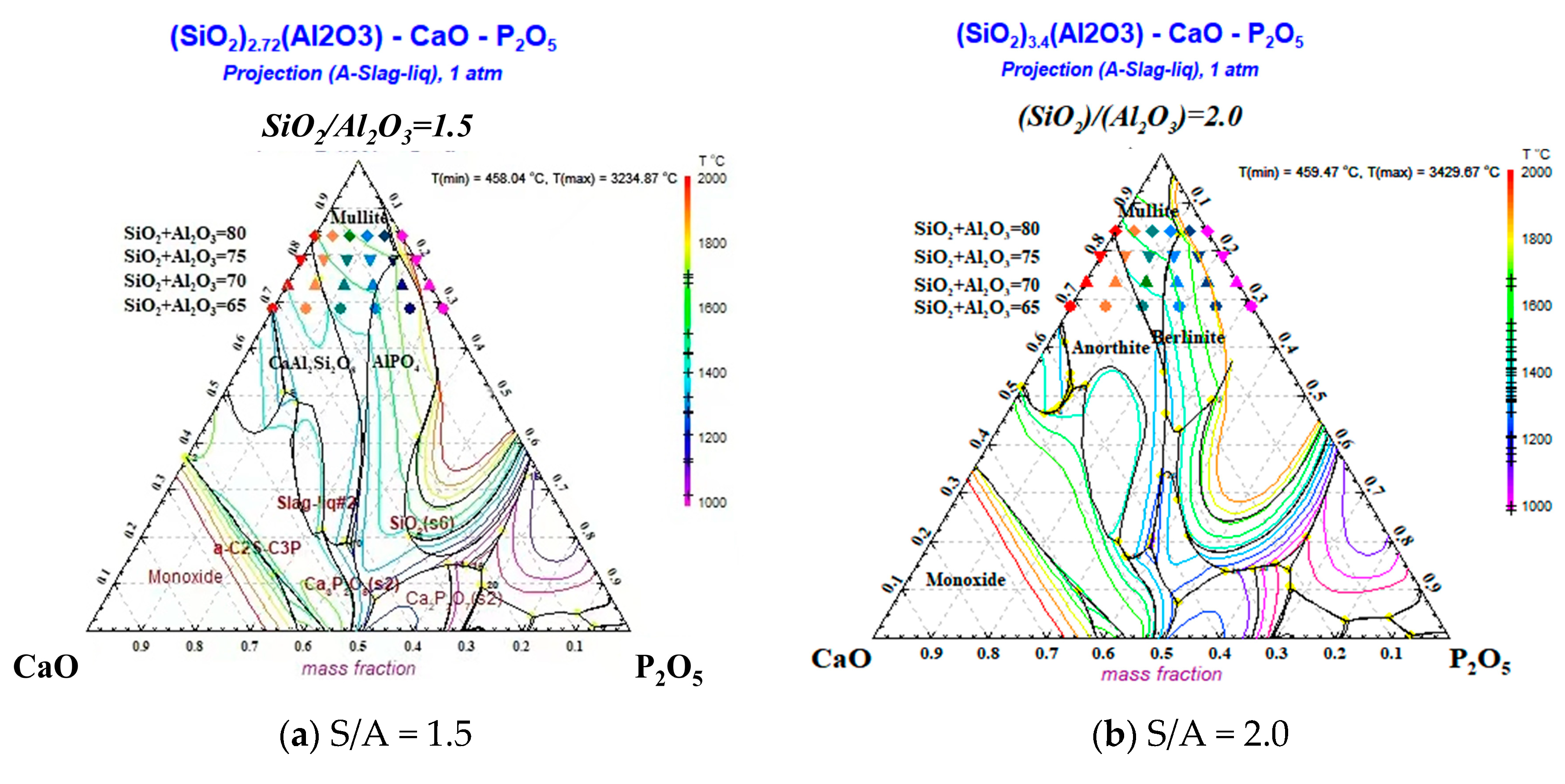
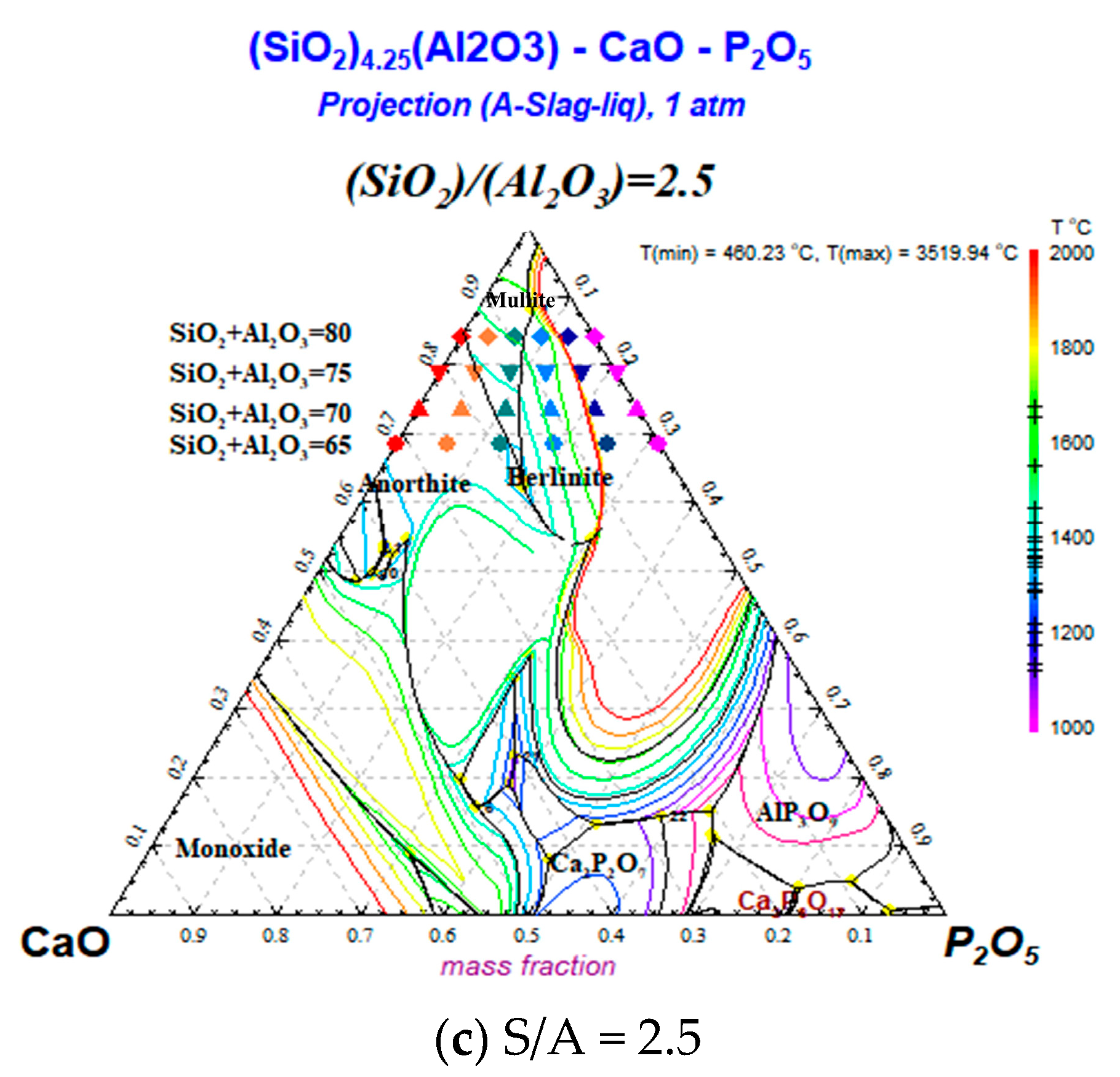
2.2.2. Mechanism of the Effect of CaO/P2O5 Ratios on the FT of Synthetic Ash
2.3. Construction and Verification of the Model
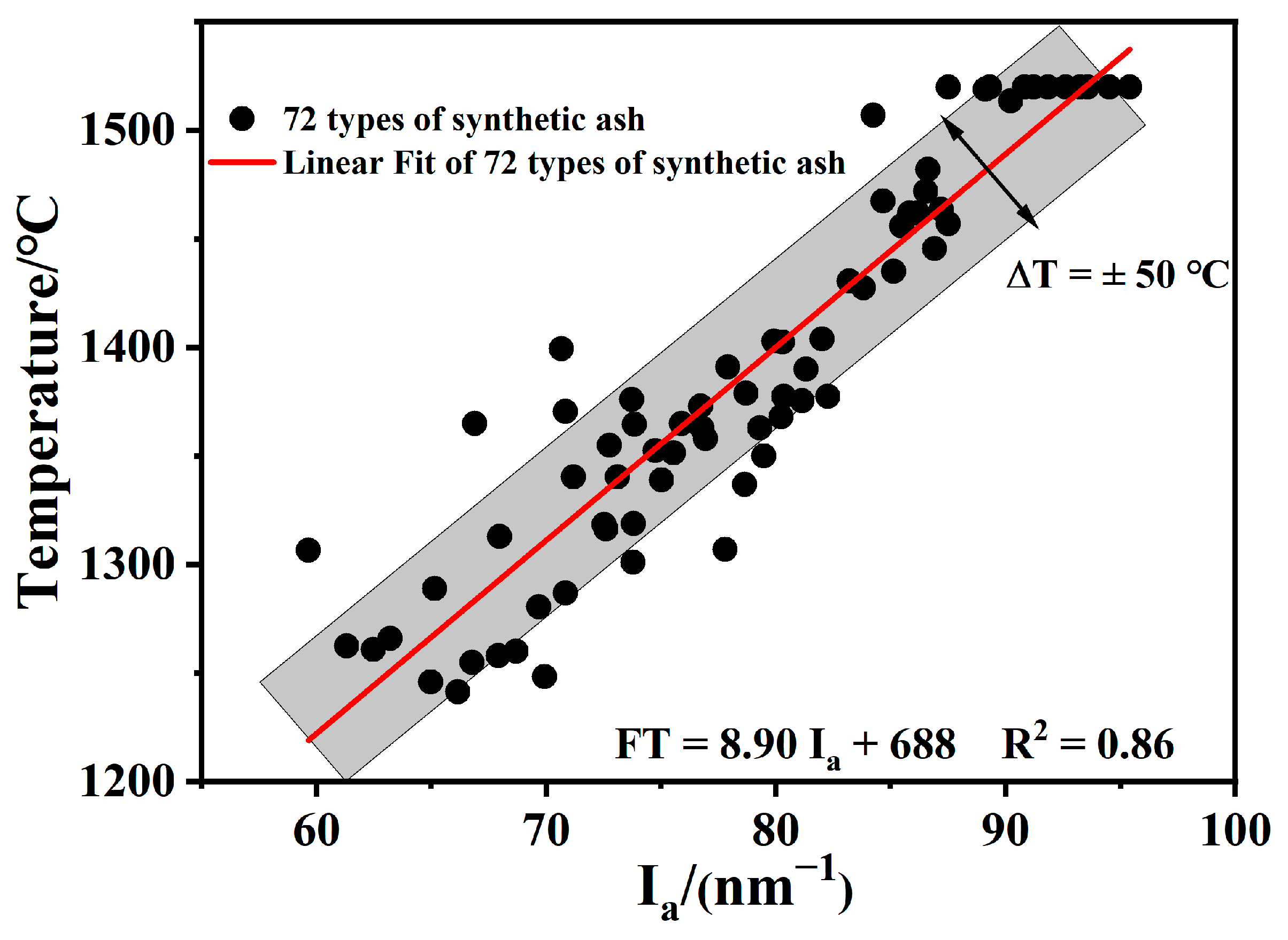
2.3.1. Modeling the FT-Ia of Synthetic Ash
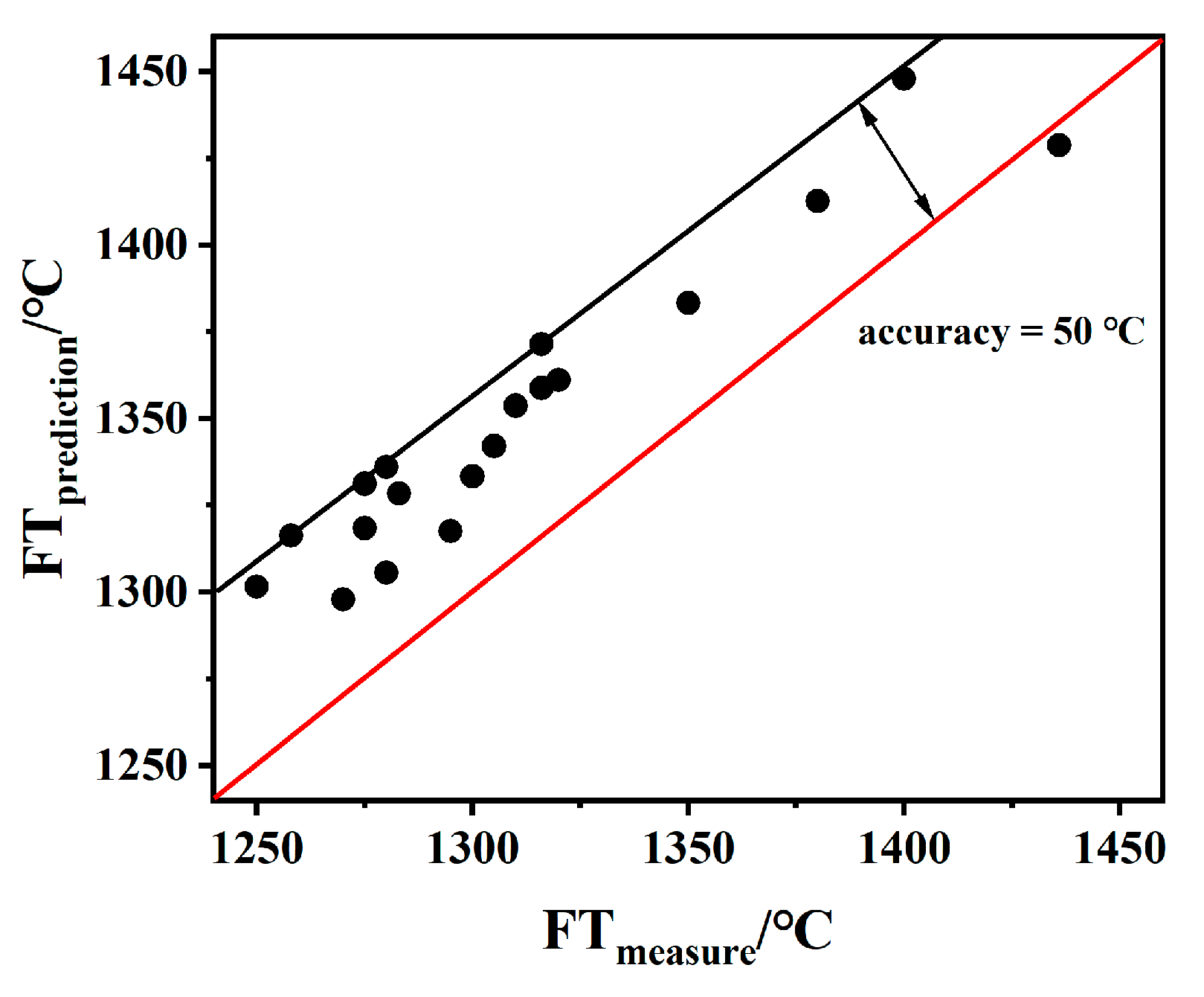
2.3.2. Validation
3. Materials and Methods
3.1. Preparation of Synthetic Ashes
3.2. Methods
3.2.1. AFT Test
3.2.2. Method for Calculating the Ia
3.2.3. Modeling of the FT-Ia
3.2.4. FactSage Thermodynamic Simulation
4. Conclusions
- (1)
- With a decreasing CaO/P2O5 ratio, Ia gradually increased, and FT showed a stepwise upward trend; the mineral types in the ash changed in the direction of anorthite → mullite → berlinite, and the Tend temperature for the different types of minerals was TBerlinite > TMullite > TAnorthite, resulting in an increased slag FT.
- (2)
- When S + A was 65–80% and the S/A ratio is in the range of 1.5–2.5, FT and Ia are linearly correlated; the formula of this model is FT = 8.90 Ia + 688, which has a correlation coefficient of more than 0.86 and a deviation in the range of ±50 °C. Using the model proposed in this work, 19 samples were predicted within a deviation range of ±50 °C.
- (3)
- The proposed correlation between FT and Ia can be used to predict the low-rank coal and phosphorus-rich biomass and their mixed ash, which provides a theoretical guide for the co-gasification of phosphorus-rich biomass and coal.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Tang, Y.; Guo, X.; Xie, Q.; Finkelman, R.B.; Li, P.; Chen, P. Fate of potentially hazardous trace elements during the entrained-flow coal gasification processes in China. Sci. Total Environ. 2019, 668, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, W.; Li, J.; Fan, H.; Xu, M.; Han, G.; Guo, M.; Wang, Z.; Huang, J.; Fang, Y. Investigation on influencing mechanisms of phosphogypsum (PG) on the ash fusion behaviors of coal. Energy 2023, 268, 126699. [Google Scholar] [CrossRef]
- Zhang, X.; Dou, H.; Bai, J.; Kong, L.; Feng, W.; Li, H.; Guo, Z.; Bai, Z.; Li, P.; Li, W. Effect of phosphorus on viscosity-temperature behavior of high-sodium coal ash slag. Fuel 2023, 343, 127930. [Google Scholar] [CrossRef]
- Ge, Z.; Cao, X.; Zha, Z.; Ma, Y.; Zeng, M.; Wu, Y.; Li, F.; Zhang, H. The sintering analysis of biomass waste ash based on the in-situ exploration and thermal chemical calculation in the gasification process. Combust. Flame 2022, 245, 112381. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, J.; Vassilev, S.V.; Kong, L.; Huang, L.; Li, H.; Bai, Z.; Li, P.; Li, W. Effect of phosphorus-based additives on ash fusion characteristics of high-sodium coal under gasification condition. Fuel 2022, 317, 123472. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Wang, L.; Yang, W.; Yang, H.; Jiang, L.; Du, Z.; Shao, J.; Chen, H. Effect of phosphorus-based additives on the sintering characteristics of cornstalk ash. J. Energy Inst. 2021, 97, 37–47. [Google Scholar] [CrossRef]
- He, C.; Du, Y.; Cai, X.; Wang, J.; Qin, Y.; Zhao, Z.; Li, H.; Vassilev, S.V.; Vassileva, C.G. In-situ analysis of the sintering behavior of coal ash and a phosphorus-rich biomass ash under gasification condition. Biomass Bioenergy 2023, 168, 106671. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Qian, Y.; Xu, M.; Li, W.; Xu, J. The influence of phosphorus on ash fusion temperature of sludge and coal. Fuel Process. Technol. 2013, 110, 218–226. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Zhao, C.; Fan, H.; Xu, M.; Guo, Q.; Guo, M.; Wang, Z.; Huang, J.; Fang, Y. Investigation on ash-fusion characteristics of livestock manure and low-rank coals. Energy Fuels 2020, 34, 5804–5812. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, J.; Hou, F.; Zhang, Y.; Wang, G.; Liu, B.; Zhai, Z. Understanding effect of phosphorus-based additive on ash deposition characteristics during high-sodium and high-calcium Zhundong coal combustion in drop-tube furnace. Fuel 2021, 287, 119462. [Google Scholar] [CrossRef]
- Li, F.; Fan, H.; Wang, X.; Wang, T.; Fang, Y. Influences of phosphorus on ash fusion characteristics of coal and its regulation mechanism. Fuel 2019, 239, 1338–1350. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Liu, X.; Cao, X.; Guo, Q.; Yu, G. Effects of phosphorous-based additive on flow properties of high silicon-aluminum coal ash. Fuel 2022, 328, 125238. [Google Scholar] [CrossRef]
- Wang, Q.; Han, K.; Wang, J.; Gao, J.; Lu, C. Influence of phosphorous based additives on ash melting characteristics during combustion of biomass briquette fuel. Renew. Energy 2017, 113, 428–437. [Google Scholar] [CrossRef]
- Li, H.; Kong, L.; Bai, J.; Bai, Z.; Guo, Z.; Li, W. Modification of ash flow properties of coal rich in calcium and iron by coal gangue addition. Chin. J. Chem. Eng. 2021, 35, 239–246. [Google Scholar] [CrossRef]
- Ninomiya, Y.; Sato, A. Ash melting behavior under an entrained bed coal gasification: Comparison between experimental results and thermodynamic calculations. J. Earth Syst. Sci. 2014, 123, 1439–1449. [Google Scholar] [CrossRef]
- Shi, W.; Dai, X.; Bai, J.; Kong, L.; Xu, J.; Li, X.; Bai, Z.; Li, W. A new method of estimating the liquidus temperature of coal ash slag using ash composition. Chem. Eng. Sci. 2018, 175, 278–285. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, Y.; Li, J.; Bao, Z.; Mu, L.; Yu, G. Prediction of coal ash fusibility based on metal ionic potential concentration. J. Energy Inst. 2021, 98, 29–34. [Google Scholar] [CrossRef]
- Li, F.; Xiao, H.; Fang, Y. Correlation between ash flow temperature and its ionic potentials under reducing atmosphere. Appl. Therm. Eng. 2017, 110, 1007–1010. [Google Scholar] [CrossRef]
- Song, T.; Cheng, P.; Wang, X.; Xu, J. Inorganic Chemistry, 4th ed.; Higher Education Press: Beijing, China, 2012; pp. 376–384. [Google Scholar]
- Hsieh, P.Y.; Kwong, K.S.; Bennett, J. Correlation between the critical viscosity and ash fusion temperatures of coal gasifier ashes. Fuel Process. Technol. 2016, 142, 13–26. [Google Scholar] [CrossRef]
- Meng, Y.; Jiang, P.; Yan, Y.; Pan, Y.; Wu, X.; Zhao, H.; Sharmin, N.; Lester, E.; Wu, T.; Pang, C. An advanced ash fusion study on the melting behaviour of coal, oil shale and blends under gasification conditions using picture analysis and graphing method. Chin. J. Chem. Eng. 2021, 32, 393–407. [Google Scholar] [CrossRef]
- Xuan, W.; Wang, H.; Xia, D. Depolymerization mechanism of CaO on network structure of synthetic coal slags. Fuel Process. Technol. 2019, 187, 21–27. [Google Scholar] [CrossRef]
- Bi, H.; Ni, Z.; Tian, J.; Wang, C.; Jiang, C.; Zhou, W.; Bao, L.; Sun, H.; Lin, Q. Influence of biomass on multi-component reaction model and combustion products of coal gangue. Combust. Flame 2022, 240, 111999. [Google Scholar] [CrossRef]
- Reinmöller, M.; Klinger, M.; Schreiner, M.; Gutte, H. Relationship between ash fusion temperature of ashes from hard coal, brown coal, and biomass and mineral phases under different atmospheres: A combined FactSageTM computational and network theoretical approach. Fuel 2015, 151, 118–123. [Google Scholar] [CrossRef]
- Sasi, T.; Mighani, M.; Örs, E.; Tawani, R.; Gräbner, M. Prediction of ash fusion behavior from coal ash composition for entrained fow gasifcation. Fuel Process. Technol. 2018, 176, 64–75. [Google Scholar] [CrossRef]
- Dai, X.; Bai, J.; Yuan, P.; Du, S.; Li, D.; Wen, X.; Li, W. The application of molecular simulation in ash chemistry of coal. Chin. J. Chem. Eng. 2020, 28, 2723–2732. [Google Scholar] [CrossRef]
- Li, X.; Zhi, L.; Shi, W.; Kong, L.; Bai, J.; Yu, J.; Reinmöller, M.; Guhl, S.; Meyer, B.; Li, W. Effect of K2O/Na2O on fusion behavior of coal ash with high silicon and aluminum level. Fuel 2020, 265, 116964. [Google Scholar] [CrossRef]
- Chakravarty, S.; Mohanty, A.; Banerjee, A.; Tripathy, R.; Mandal, G.K.; Basariya, M.R.; Sharma, M. Composition, mineral matter characteristics and ash fusion behavior of some Indian coals. Fuel 2015, 150, 96–101. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Ma, M.; Yu, B.; Zhao, C.; Fang, Y. Prediction of ash flow temperature based on liquid phase mass fraction by FactSage. J. Energy Inst. 2020, 93, 2228–2231. [Google Scholar] [CrossRef]
- Li, W.; Bai, J. Chenmistry of Ash from Coal; Science Press: Beijing, China, 2013; pp. 123–124. [Google Scholar]
- Yan, T.; Bai, J.; Kong, L.; Li, H.; Wang, Z.; Bai, Z.; Zhao, H.; Li, W. Improved prediction of critical-viscosity temperature by fusion behavior of coal ash. Fuel 2019, 253, 1521–1530. [Google Scholar] [CrossRef]
- ASTM 1857-04; Standard Test Method for Fusibility of Coal and Coke Ash. ASTM: West Conshohocken, PA, USA, 2003. Available online: http://www.doc88.com/p-9475259405290.html (accessed on 1 May 2023).
- ISO 540-2008; Hard Coal and Coke e Determination of Ash Fusibility. ISO: Geneva, Switzerland, 2008. Available online: http://www.doc88.com/p-9032738008485.html (accessed on 1 May 2023).
- GBT212-2008; Proximate Analysis of Coal. Standardization Administration of China: Beijing, China, 2008. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781119037699.ch5 (accessed on 27 November 2023).
- Yan, T.; Bai, J.; Kong, L.; Bai, Z.; Li, W.; Xu, J. Effect of SiO2/Al2O3 on fusion behavior of coal ash at high temperature. Fuel 2017, 193, 275–283. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Wei, J.; Bai, Y.; Song, X.; Xu, G.; Pan, Y.; Yu, G. Synergistic effects of CaO and MgO on ash fusion characteristics in entrained-flow gasifier. Energy Fuels 2021, 35, 425–432. [Google Scholar] [CrossRef]
- He, C.; Bai, J.; Kong, L.; Guhl, S.; Schwitalla, D.H.; Xu, J.; Bai, Z.; Li, W. The precipitation of metallic iron from coal ash slag in the entrained flow coal gasifier: By thermodynamic calculation. Fuel Process. Technol. 2017, 162, 98–104. [Google Scholar] [CrossRef]
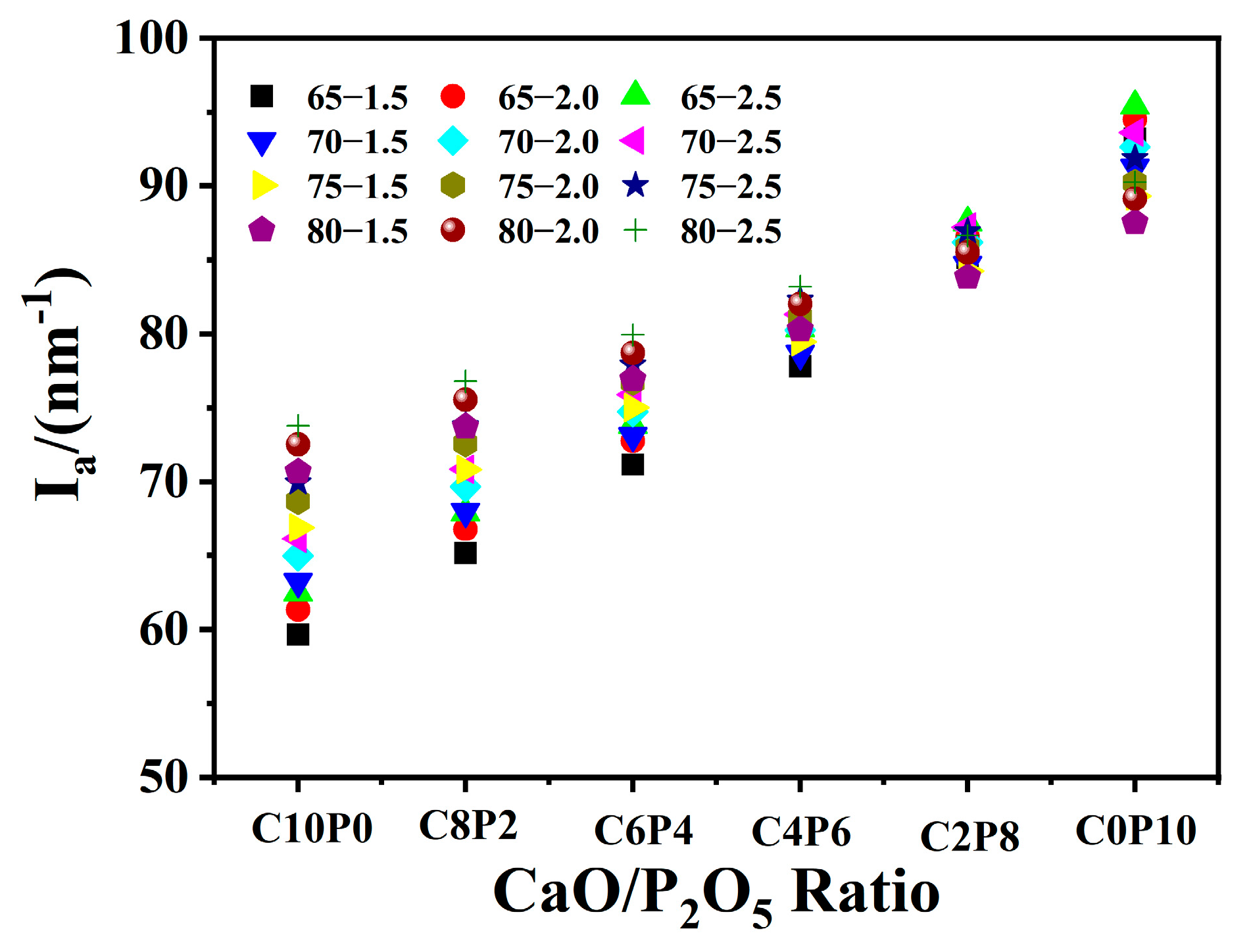

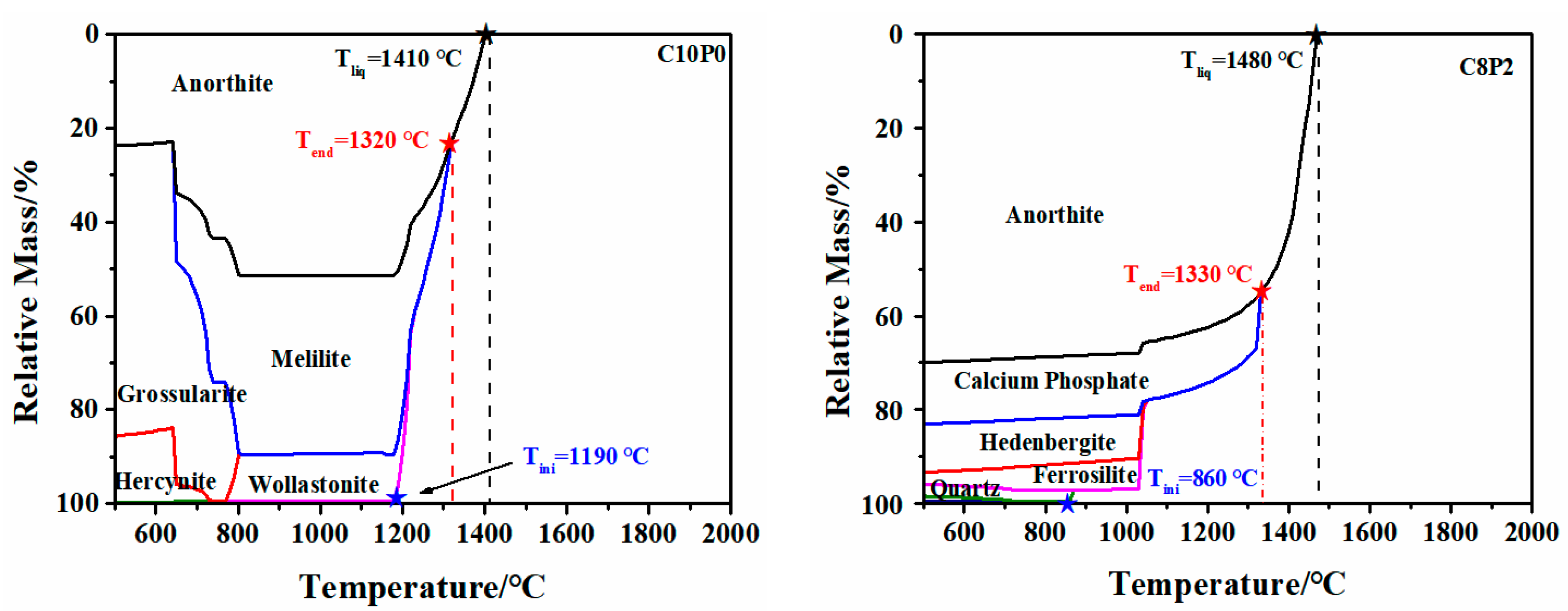
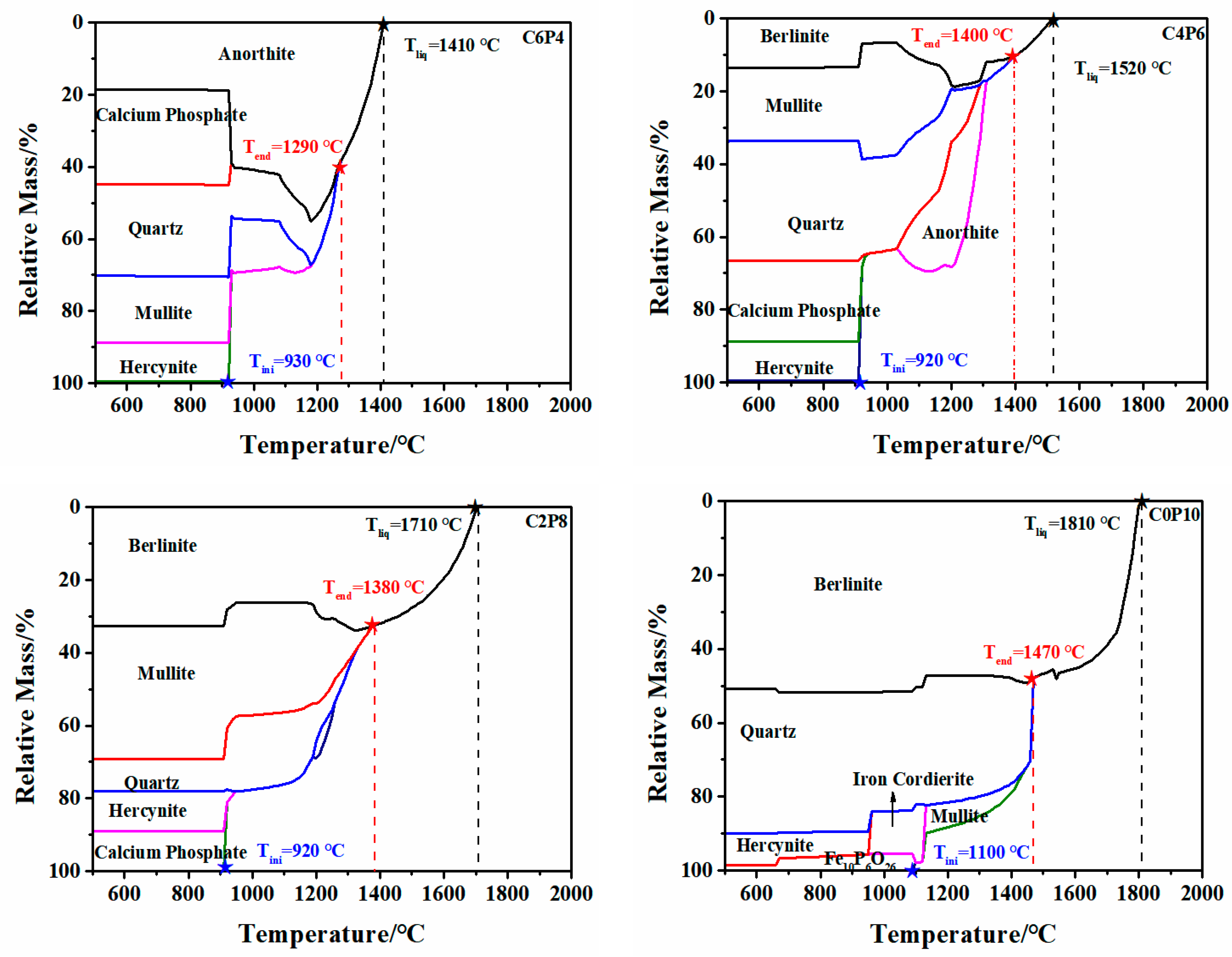
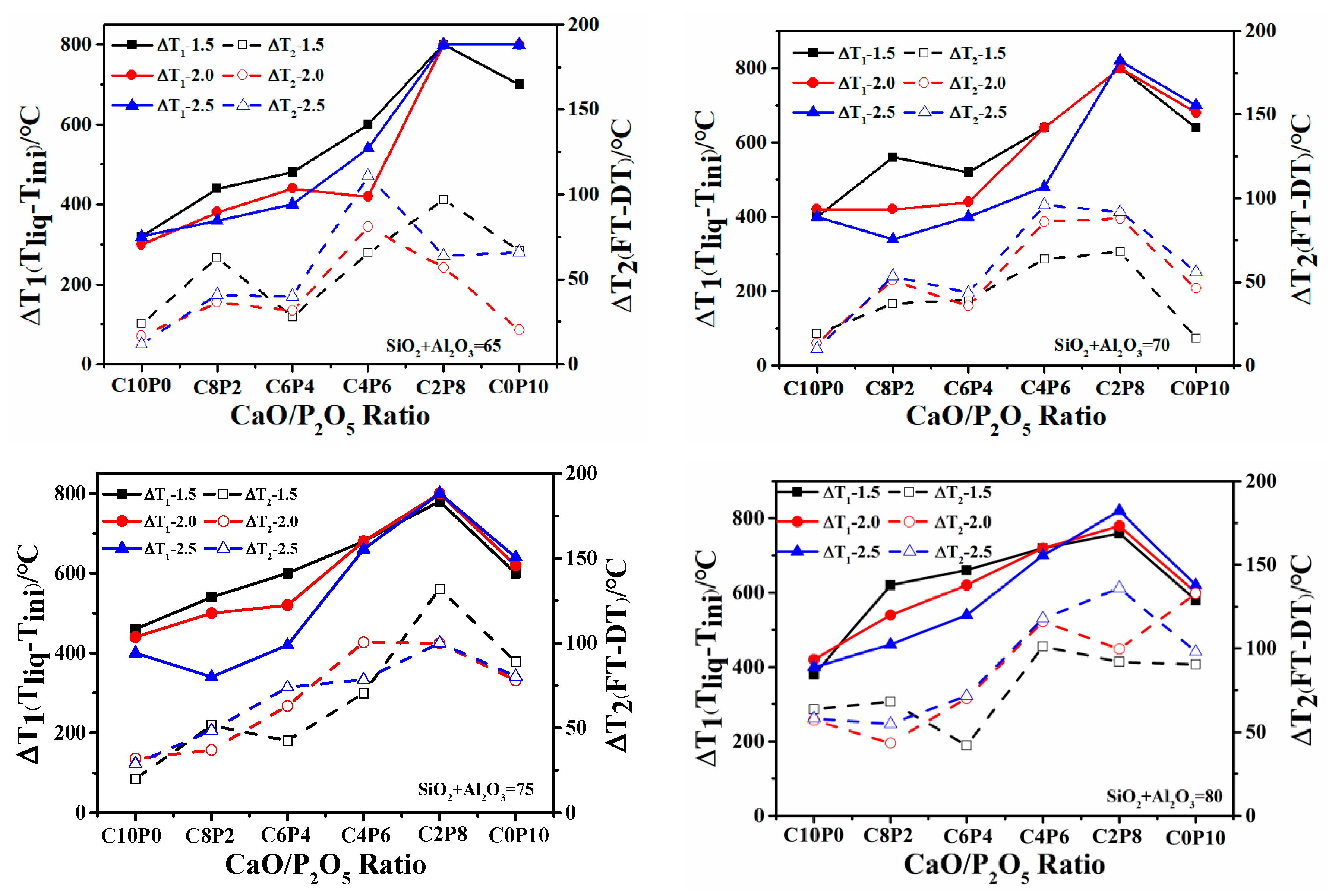
| Samples | Ash Composition (wt/%) | S/A | S + A (wt/%) a | CaO/ P2O5 | Ia (nm−1) | FTmeasure (°C) | FTpredication (°C) | ΔT b (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | FeO | CaO | P2O5 | ||||||||
| 1 | 56.82 | 23.89 | 7.12 | 10.39 | 0.99 | 2.38 | 80.71 | 9.13:0.87 | 75.63 | 1320 | 1361.11 | 41.11 |
| 2 | 54.58 | 22.32 | 6.92 | 13.18 | 2.23 | 2.45 | 76.90 | 8.55:1.45 | 70.74 | 1295 | 1317.59 | 22.59 |
| 3 | 52.33 | 20.75 | 6.71 | 15.98 | 3.48 | 2.52 | 73.08 | 8.21:1.79 | 72.27 | 1275 | 1331.20 | 56.20 |
| 4 | 50.09 | 19.17 | 6.51 | 18.78 | 4.72 | 2.61 | 69.26 | 7.99:2.01 | 70.60 | 1258 | 1316.34 | 58.34 |
| 5 | 47.85 | 17.60 | 6.31 | 21.57 | 5.97 | 2.72 | 65.45 | 7.83:2.17 | 68.94 | 1250 | 1301.57 | 51.57 |
| 6 | 52.38 | 22.04 | 6.81 | 15.07 | 2.94 | 2.38 | 74.42 | 8.37:1.63 | 72.51 | 1300 | 1333.34 | 33.34 |
| 7 | 47.95 | 20.18 | 6.51 | 19.75 | 4.88 | 2.38 | 68.13 | 8.02:1.98 | 69.40 | 1280 | 1305.66 | 25.66 |
| 8 | 58.36 | 22.43 | 6.21 | 10.43 | 1.69 | 2.61 | 80.99 | 8.61:1.39 | 76.79 | 1316 | 1371.43 | 55.43 |
| 9 | 55.87 | 20.84 | 6.08 | 13.53 | 3.00 | 2.68 | 76.71 | 8.19:1.81 | 74.80 | 1310 | 1353.72 | 43.72 |
| 10 | 53.19 | 19.26 | 5.96 | 16.63 | 4.30 | 2.76 | 72.45 | 7.95:2.05 | 72.82 | 1280 | 1336.10 | 56.10 |
| 11 | 50.50 | 17.67 | 5.84 | 19.74 | 5.61 | 2.86 | 68.17 | 7.79:2.21 | 70.84 | 1275 | 1318.48 | 43.48 |
| 12 | 56.36 | 22.14 | 6.11 | 12.31 | 2.40 | 2.55 | 78.50 | 8.37:1.63 | 75.37 | 1316 | 1358.79 | 42.79 |
| 13 | 51.49 | 20.28 | 5.88 | 17.30 | 4.40 | 2.54 | 71.76 | 7.97:2.03 | 71.95 | 1283 | 1328.36 | 45.36 |
| 14 | 46.61 | 18.41 | 5.65 | 22.29 | 6.40 | 2.53 | 65.02 | 7.77:2.23 | 68.53 | 1270 | 1297.92 | 27.92 |
| 15 | 41.66 | 35.47 | 3.50 | 3.70 | 15.28 | 1.17 | 77.13 | 1.95:8.05 | 83.24 | 1436 | 1428.79 | −7.21 |
| 16 | 39.21 | 33.38 | 3.30 | 3.48 | 20.26 | 1.17 | 72.59 | 1.47:8.53 | 85.38 | 1400 | 1447.91 | 47.91 |
| 17 | 45.12 | 28.68 | 15.57 | 4.59 | 4.31 | 1.57 | 73.8 | 5.16:4.84 | 73.49 | 1305 | 1342.06 | 37.06 |
| 18 | 46.92 | 33.73 | 4.54 | 7.15 | 7.16 | 1.39 | 80.65 | 5.00:5.00 | 78.12 | 1350 | 1383.27 | 33.27 |
| 19 | 43.18 | 29.02 | 8.50 | 13.92 | 4.43 | 1.49 | 72.2 | 7.59:2.41 | 81.42 | 1380 | 1412.68 | 32.68 |
| S + A a | S/A | SiO2 | Al2O3 | Fe2O3 | CaO/P2O5 Mass Ratio (wt. %) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C10P0 b | C8P2 c | C6P4 | C4P6 | C2P8 | C0P10 | |||||||||||
| CaO | P2O5 | CaO | P2O5 | CaO | P2O5 | CaO | P2O5 | CaO | P2O5 | CaO | P2O5 | |||||
| 65 | 1.5 | 39 | 26 | 5 | 30 | 0 | 24 | 6 | 18 | 12 | 12 | 18 | 6 | 24 | 0 | 30 |
| 2.0 | 43.33 | 21.67 | ||||||||||||||
| 2.5 | 46.43 | 18.57 | ||||||||||||||
| 70 | 1.5 | 42 | 28 | 25 | 0 | 20 | 5 | 15 | 10 | 10 | 15 | 5 | 20 | 0 | 25 | |
| 2.0 | 46.67 | 23.33 | ||||||||||||||
| 2.5 | 50 | 20 | ||||||||||||||
| 75 | 1.5 | 45 | 30 | 20 | 0 | 16 | 4 | 12 | 8 | 8 | 12 | 4 | 16 | 0 | 20 | |
| 2.0 | 50 | 25 | ||||||||||||||
| 2.5 | 53.57 | 21.43 | ||||||||||||||
| 80 | 1.5 | 48 | 32 | 15 | 0 | 12 | 3 | 9 | 6 | 6 | 9 | 3 | 12 | 0 | 15 | |
| 2.0 | 53.33 | 26.67 | ||||||||||||||
| 2.5 | 57.14 | 22.86 | ||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Wang, Q.; Men, X.; Li, Y.; Zhang, L.; Bai, Y.; Song, X.; Wang, J.; Yao, M.; Yu, G. Correlation between Flow Temperature and Average Molar Ionic Potential of Ash during Gasification of Coal and Phosphorus-Rich Biomass. Molecules 2023, 28, 7858. https://doi.org/10.3390/molecules28237858
Zhao C, Wang Q, Men X, Li Y, Zhang L, Bai Y, Song X, Wang J, Yao M, Yu G. Correlation between Flow Temperature and Average Molar Ionic Potential of Ash during Gasification of Coal and Phosphorus-Rich Biomass. Molecules. 2023; 28(23):7858. https://doi.org/10.3390/molecules28237858
Chicago/Turabian StyleZhao, Chaoyue, Qingyun Wang, Xiaoyong Men, Yuchen Li, Linmin Zhang, Yonghui Bai, Xudong Song, Jiaofei Wang, Min Yao, and Guangsuo Yu. 2023. "Correlation between Flow Temperature and Average Molar Ionic Potential of Ash during Gasification of Coal and Phosphorus-Rich Biomass" Molecules 28, no. 23: 7858. https://doi.org/10.3390/molecules28237858
APA StyleZhao, C., Wang, Q., Men, X., Li, Y., Zhang, L., Bai, Y., Song, X., Wang, J., Yao, M., & Yu, G. (2023). Correlation between Flow Temperature and Average Molar Ionic Potential of Ash during Gasification of Coal and Phosphorus-Rich Biomass. Molecules, 28(23), 7858. https://doi.org/10.3390/molecules28237858








