Catalytic Oxidation of Benzene over Atomic Active Site AgNi/BCN Catalysts at Room Temperature
Abstract
1. Introduction
2. Results and Discussion
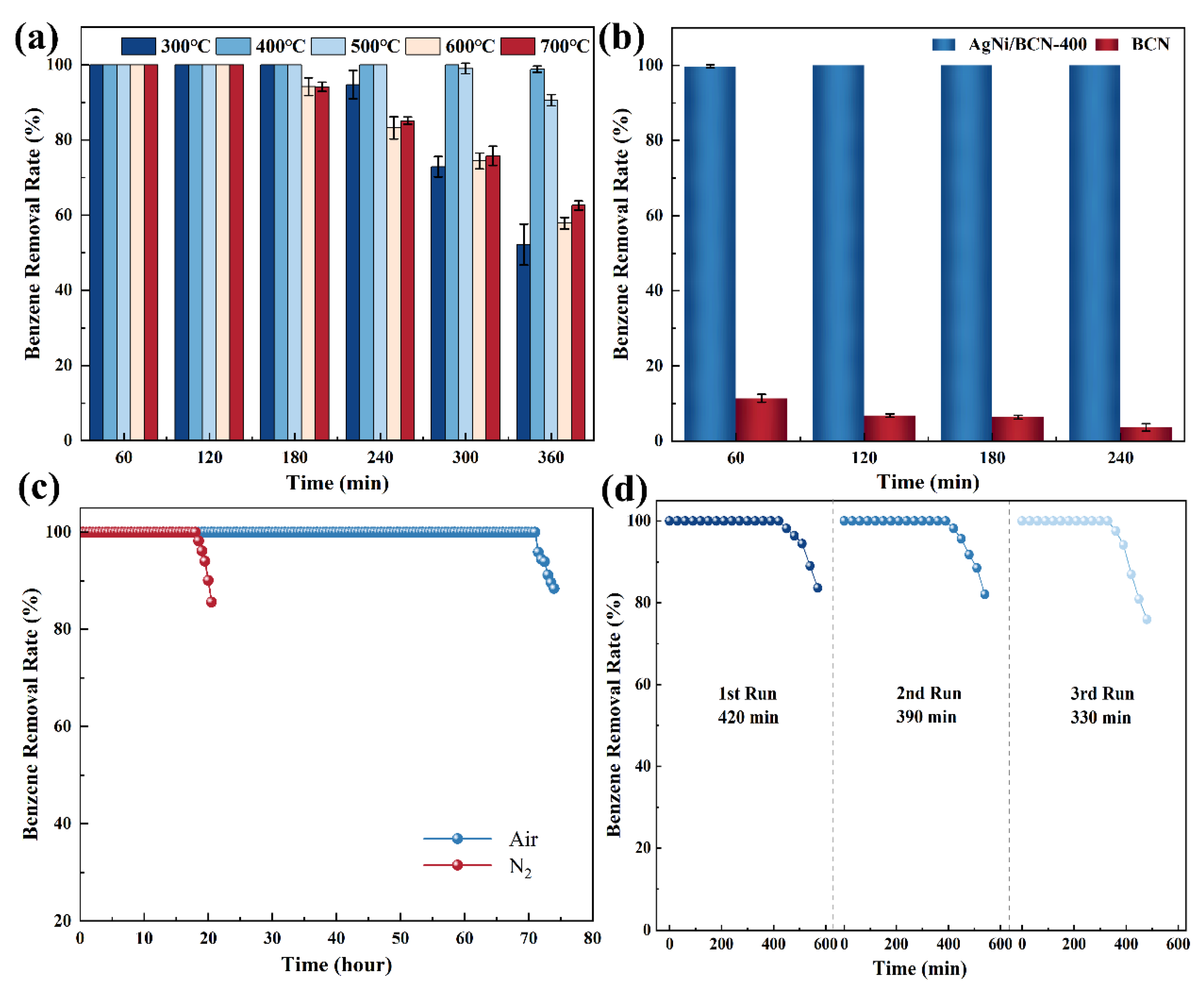
2.1. Catalytic Oxidation of Benzene at Room Temperature
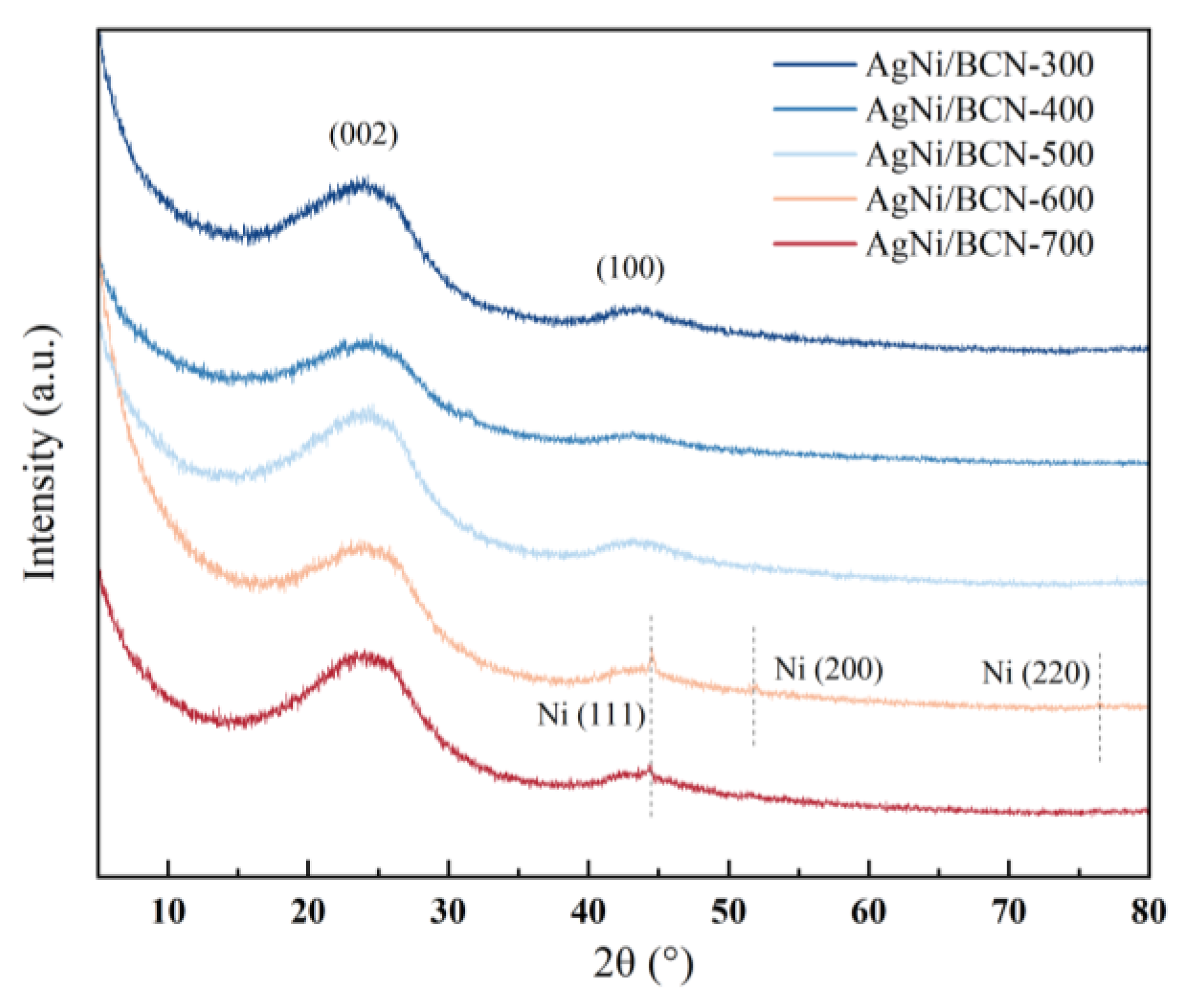
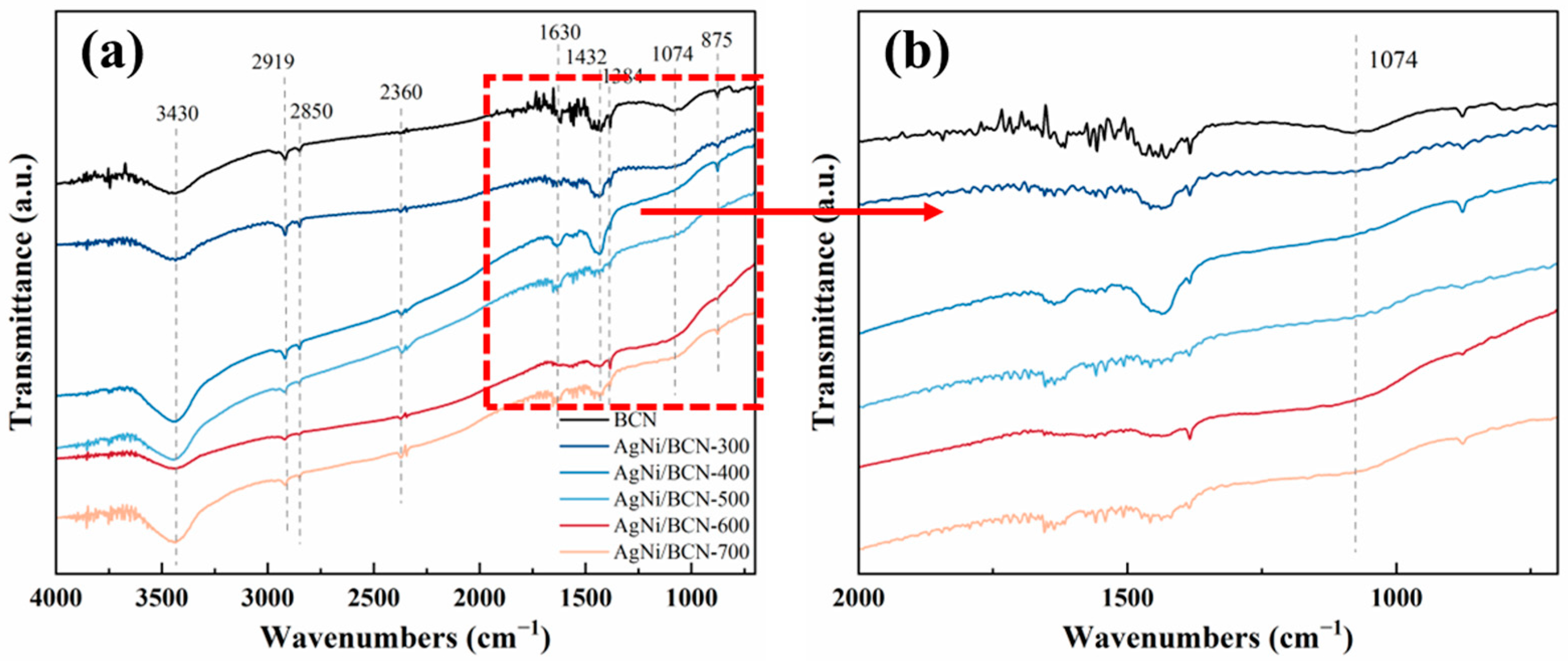
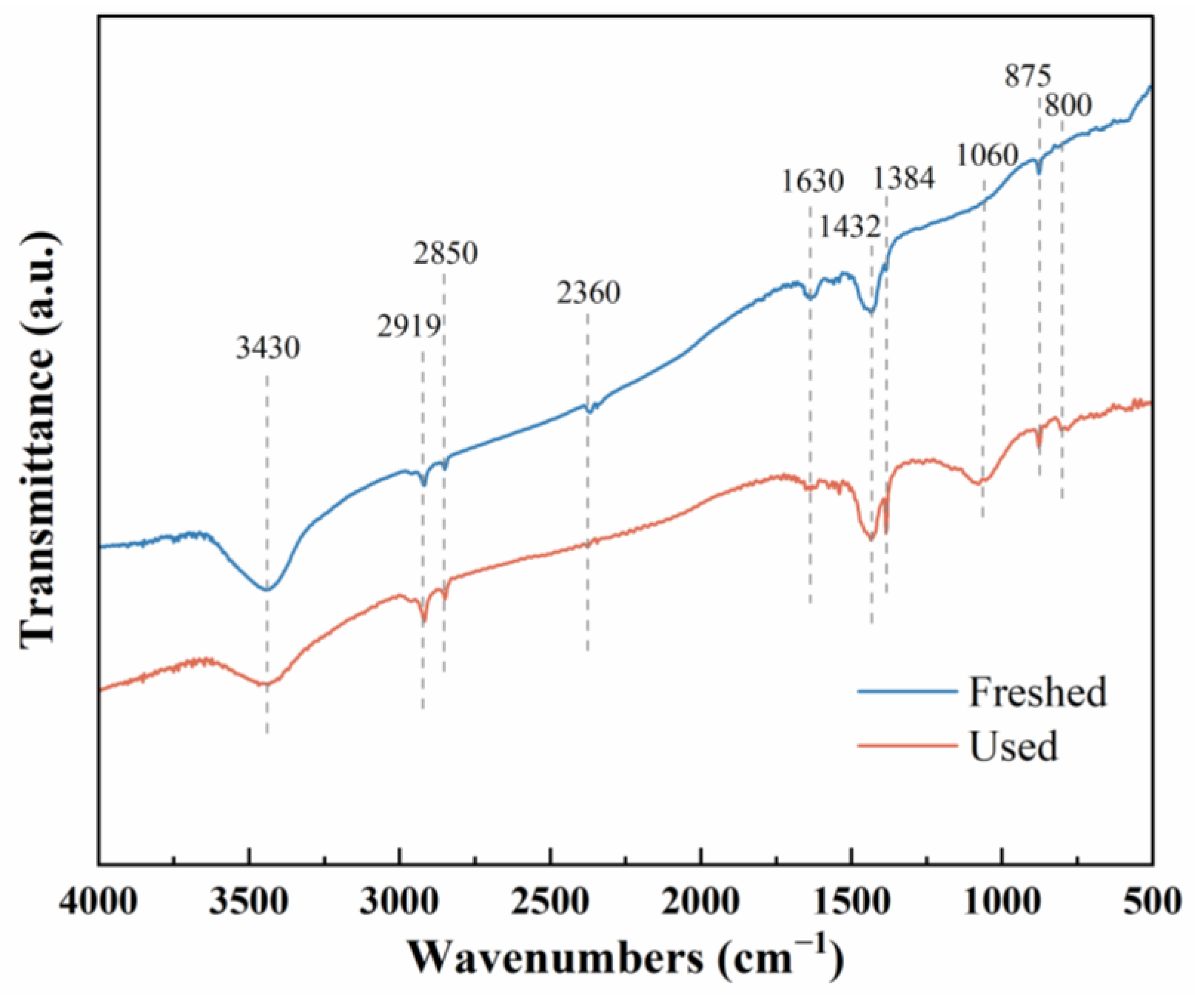
2.2. AgNi/BCN Catalyst Bulk Structure
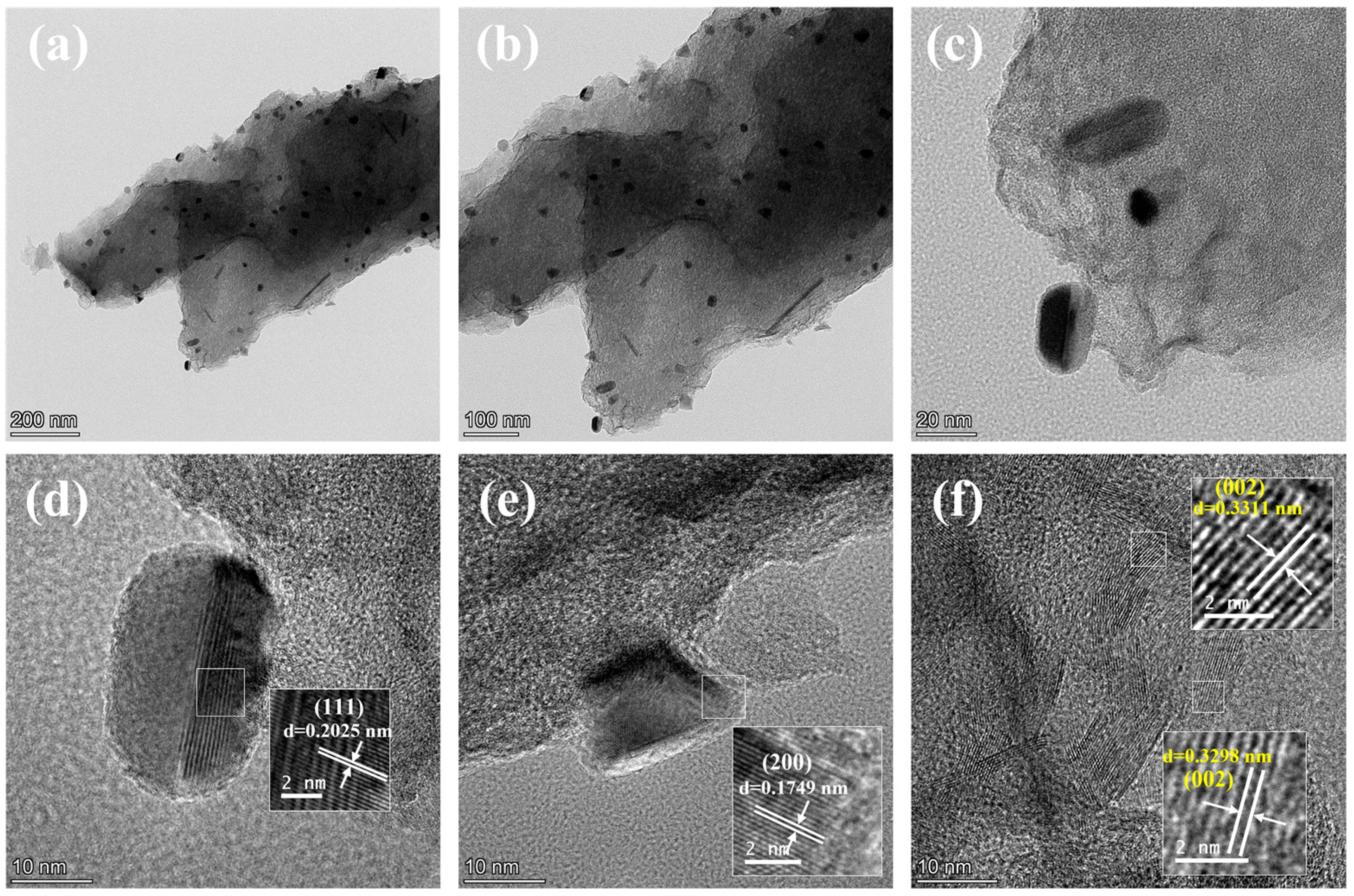
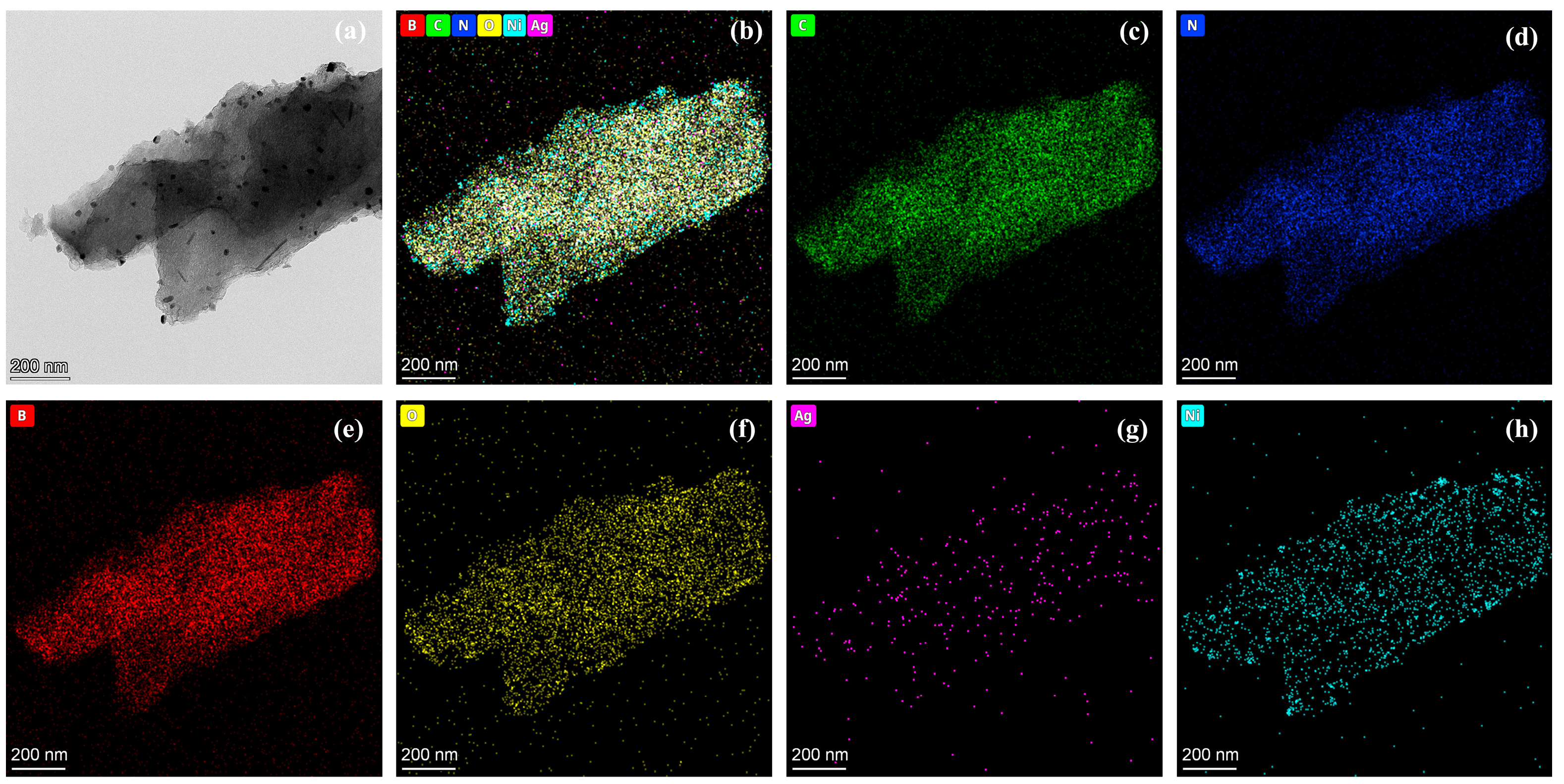
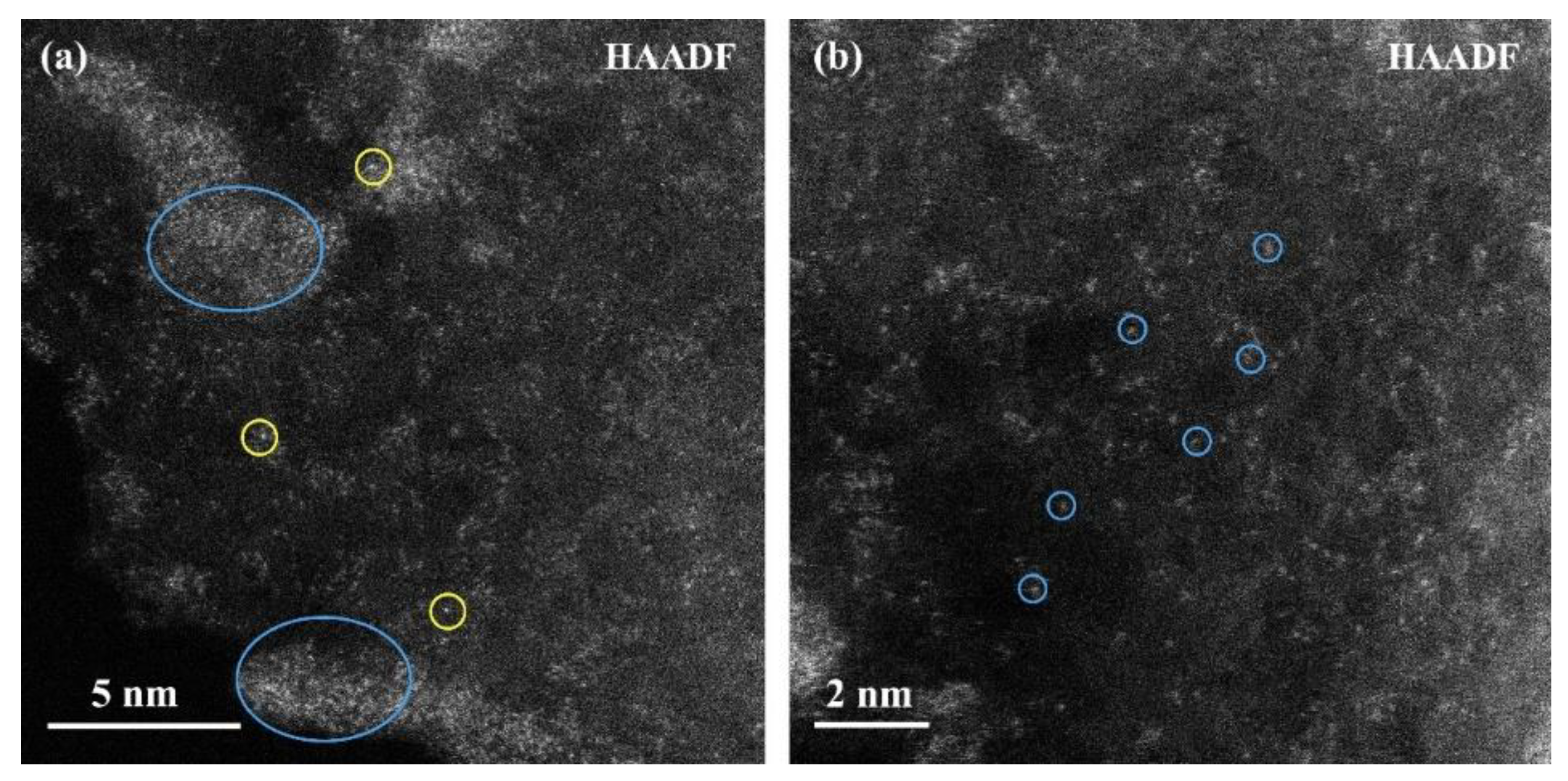
2.3. Morphology of AgNi/BCN Catalyst
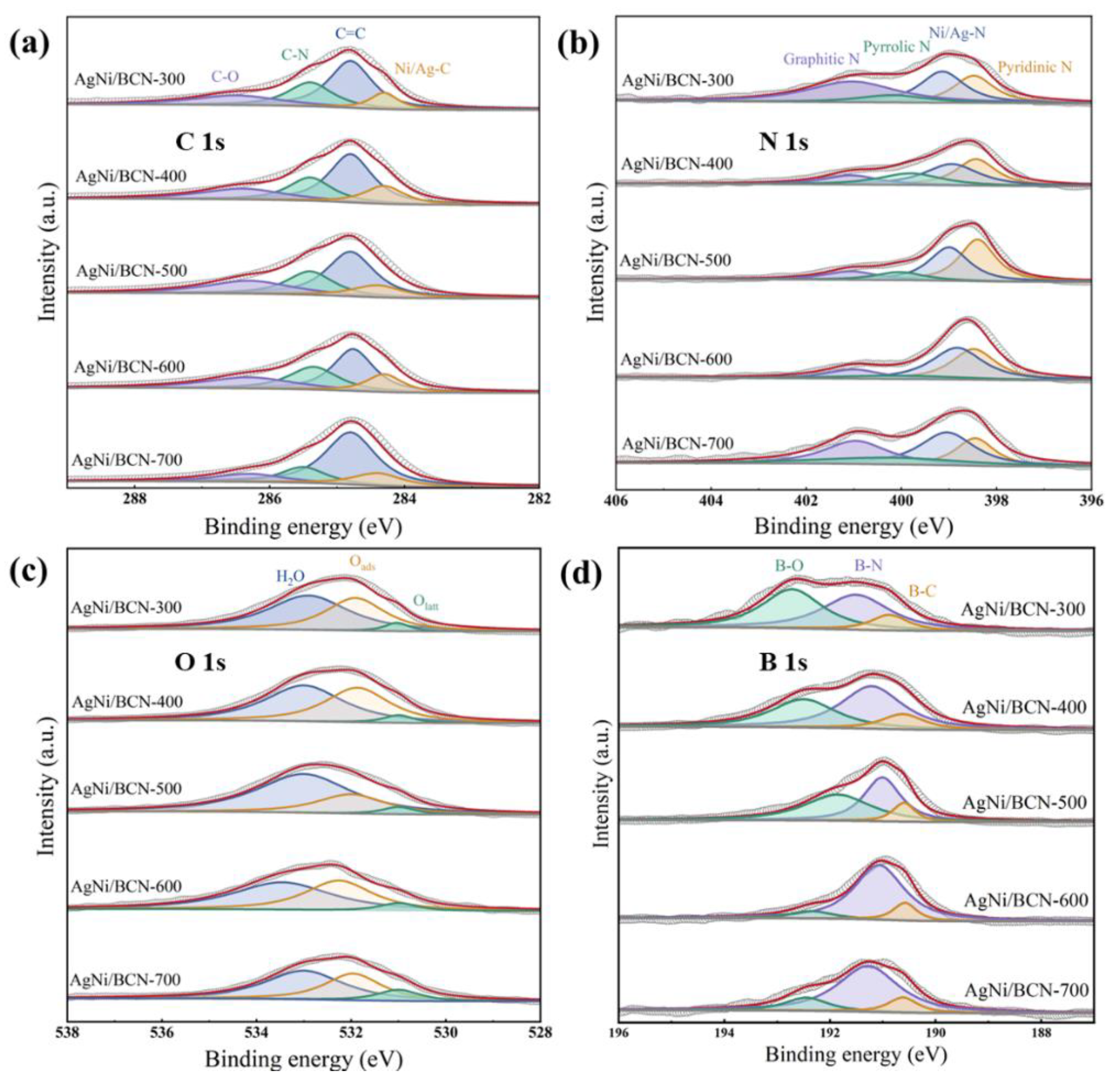
2.4. Coordination Environment and Valence State of AgNi/BCN Catalyst
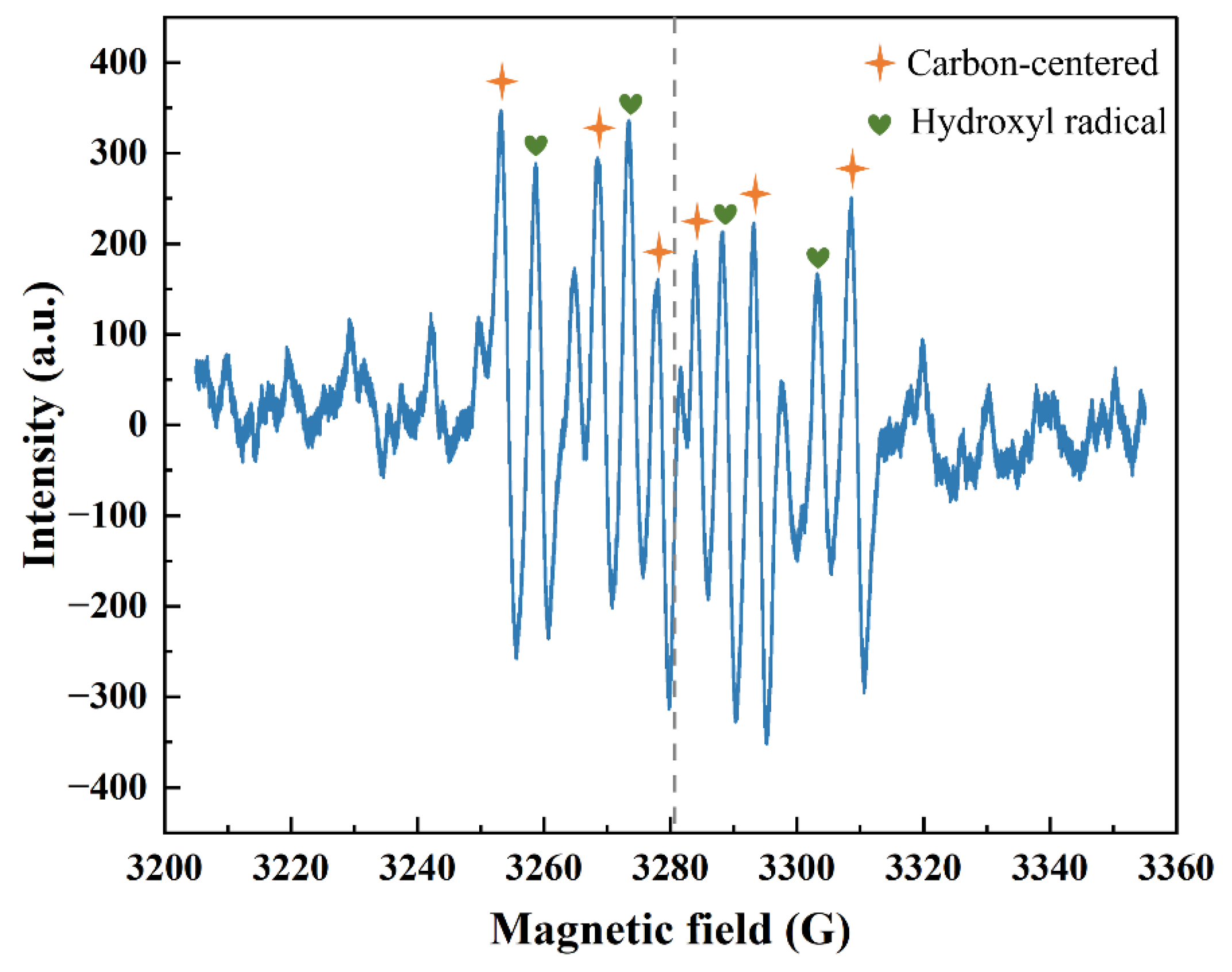
2.5. The Catalytic Mechanism of AgNi/BCN-400
3. Experimental
3.1. Chemicals and Materials
3.2. Catalyst Preparation
3.3. Catalyst Evaluation
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mu, Y.; Williams, P.T. Recent advances in the abatement of volatile organic compounds (VOCs) and chlorinated-VOCs by non-thermal plasma technology: A review. Chemosphere 2022, 308, 136481. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Wang, Y.; Wang, Y.; Zhao, Z.; Shen, Z.; Huang, Y.; Veerapandian, S.K.P.; De Geyter, N.; Wang, C.; Chen, Q.; et al. A critical review on plasma-catalytic removal of VOCs: Catalyst development, process parameters and synergetic reaction mechanism. Sci. Total Environ. 2022, 828, 154290. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.; Wang, J.; Lv, H.; Zhu, T. Catalytic oxidation of benzene over MnOx/TiO2 catalysts and the mechanism study. J. Mol. Catal. A Chem. 2015, 408, 221–227. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, D.; Zheng, Y.; Feng, X.; Chen, Q.; Zhang, K.; Wang, X.; Jiang, L. MnO2 nanoparticles encapsuled in spheres of Ce-Mn solid solution: Efficient catalyst and good water tolerance for low-temperature toluene oxidation. Appl. Surf. Sci. 2020, 504, 144481. [Google Scholar] [CrossRef]
- Qin, C.; Dang, X.; Huang, J.; Teng, J.; Huang, X. Plasma-catalytic oxidation of adsorbed toluene on Ag-Mn/gamma-Al2O3: Comparison of gas flow-through and gas circulation treatment. Chem. Eng. J. 2016, 299, 85–92. [Google Scholar] [CrossRef]
- Ding, H.; Xue, L.; Cui, J.; Wang, Y.; Zhao, D.; Zhi, X.; Liu, R.; Fu, J.; Liu, S.; Fu, B.; et al. Catalytic degradation of benzene at room temperature over FeN4O2 sites embedded in porous carbon. J. Hazard. Mater. 2023, 460, 132520. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Hüsing, N. Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects. Appl. Catal. B Environ. 2018, 221, 530–555. [Google Scholar] [CrossRef]
- Kim, S.-I.; Im, M.; Cho, E.; Jang, H.; Jang, S.Y.; Kim, D.W.; Kim, K.W.; Heo, I.; Kim, Y.J.; Lee, J.H. Effects of thermal aging on the electronic and structural properties of Pt-Pd and toluene oxidation activity. Sci. Total Environ. 2022, 847, 157482. [Google Scholar] [CrossRef]
- Peng, R.; Li, S.; Sun, X.; Ren, Q.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Size effect of Pt nanoparticles on the catalytic oxidation of toluene over Pt/CeO2 catalysts. Appl. Catal. B Environ. 2018, 220, 462–470. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, J.; Zhou, R. Preparation of size-controlled Pt supported on Al2O3 nanocatalysts for deep catalytic oxidation of benzene at lower temperature. Appl. Surf. Sci. 2019, 465, 15–22. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, J.; Liu, N.; Wu, Y.; Huang, J.; Zheng, Y.; Li, Q. Oxygen-enriched biomass-activated carbon supported platinum nanoparticles as an efficient and durable catalyst for oxidation in benzene. ACS Sustain. Chem. Eng. 2021, 9, 7255–7266. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, F.; Wang, Y.; Jia, M.; Tao, X.; Jin, Y.; Zhang, X. MOF-derived CeO2 supported Ag catalysts for toluene oxidation: The effect of synthesis method. Mol. Catal. 2021, 515, 111922. [Google Scholar] [CrossRef]
- Ma, X.; Yu, X.; Ge, M. Highly efficient catalytic oxidation of benzene over Ag assisted Co3O4 catalysts. Catal. Today 2021, 376, 262–268. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, C.; Liu, Z.; Chen, C.; Li, Y. Structural regulation with atomic-level precision: From single-atomic site to diatomic and atomic interface catalysis. Matter 2020, 2, 78–110. [Google Scholar] [CrossRef]
- Li, X.; Bi, W.; Zhang, L.; Tao, S.; Chu, W.; Zhang, Q.; Luo, Y.; Wu, C.; Xie, Y. Single-atom Pt as Co-catalyst for enhanced photocatalytic H2 Evolution. Adv. Mater. 2016, 28, 2427–2431. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, H.; Wang, Y.; Chuang, C.-H.; Xing, L.; Dong, M.; Lu, Y.-R.; Casillas-Garcia, G.; Zheng, Y.; Chen, S.; et al. Coexisting single-atomic Fe and Ni sites on hierarchically ordered porous carbon as a highly efficient ORR electrocatalyst. Adv. Mater. 2020, 32, 2004670. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, R.; Sun, Y.; Liu, Q.; Xia, M.; Li, Y.; Gao, F.; Zhao, Y.; Tse, J.S. Identifying the Zn–Co binary as a robust bifunctional electrocatalyst in oxygen reduction and evolution reactions via shifting the apexes of the volcano plot. J. Energy Chem. 2021, 55, 162–168. [Google Scholar] [CrossRef]
- Tian, S.; Wang, B.; Gong, W.; He, Z.; Xu, Q.; Chen, W.; Zhang, Q.; Zhu, Y.; Yang, J.; Fu, Q.; et al. Dual-atom Pt heterogeneous catalyst with excellent catalytic performances for the selective hydrogenation and epoxidation. Nat. Commun. 2021, 12, 3181. [Google Scholar] [CrossRef]
- Tong, M.; Sun, F.; Xie, Y.; Wang, Y.; Yang, Y.; Tian, C.; Wang, L.; Fu, H. Operando Cooperated Catalytic Mechanism of Atomically Dispersed Cu-N-4 and Zn-N-4 for Promoting Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2021, 60, 14005–14012. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Zhao, Y.; Wei, S.; Triana, C.A.; Li, J.; Arcifa, A.; Allen, C.S.; Cao, R.; Patzke, G.R. Mechanistic insight into the active centers of single/dual-atom Ni/Fe-based oxygen electrocatalysts. Nat. Commun. 2021, 12, 5589. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-C.; Wang, H.-F.; Li, C.; Xu, Q. From metal-organic frameworks to single/dual-atom and cluster metal catalysts for energy applications. Energy Environ. Sci. 2020, 13, 1658–1693. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Y.; Zhang, Y.-X.; Niu, Z. Dual-atom catalysts: Controlled synthesis and electrocatalytic applications. Sci. China-Chem. 2021, 64, 1908–1922. [Google Scholar] [CrossRef]
- Wang, C.; Ying, J.; Zhang, X.; Zhang, B.; Tian, A.-X.; Wang, X.-L. Multifunctional photoelectric sensors and catalysts for CO2RR and Cr(VI) solution based on a series of POM-based materials. CrystEngComm 2021, 23, 2424–2431. [Google Scholar] [CrossRef]
- Yin, F.; Lin, X.; He, X.; Chen, B.; Li, G.; Yin, H. High faraday efficiency for electrochemical nitrogen reduction reaction on Co@N-doped carbon derived from a metal-organic framework under ambient conditions. Mater. Lett. 2019, 248, 109–113. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; An, M.; Gao, Y.; Cao, Z.; Liu, J. W–N/C@Co9S8@WS2-hollow carbon nanocage as multifunctional electrocatalysts for DSSCS, ORR and OER. Electrochim. Acta 2020, 351, 136249. [Google Scholar] [CrossRef]
- Zhong, X.; Ye, S.; Tang, J.; Zhu, Y.; Wu, D.; Gu, M.; Pan, H.; Xu, B. Engineering Pt and Fe dual-metal single atoms anchored on nitrogen-doped carbon with high activity and durability towards oxygen reduction reaction for zinc-air battery. Appl. Catal. B Environ. 2021, 286, 119891. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Zheng, L.; Zhang, C.; Wang, Y.; Shan, W.; Liu, F.; He, H. A nonoxide catalyst system study: Alkali metal-promoted Pt/AC catalyst for formaldehyde oxidation at ambient temperature. ACS Catal. 2021, 11, 456–465. [Google Scholar] [CrossRef]
- Tian, M.Z.; Liu, S.J.; Wang, L.L.; Ding, H.; Zhao, D.; Wang, Y.Q.; Cui, J.H.; Fu, J.F.; Shang, J.; Li, G.K. Complete degradation of gaseous methanol over Pt/FeOx catalysts by normal temperature catalytic ozonation. Environ. Sci. Technol. 2020, 54, 1938–1945. [Google Scholar] [CrossRef]
- Pei, J.J.; Wang, T.; Sui, R.; Zhang, X.J.; Zhou, D.N.; Qin, F.J.; Zhao, X.; Liu, Q.H.; Yan, W.S.; Dong, J.C.; et al. N-Bridged Co-N-Ni: New bimetallic sites for promoting electrochemical CO2 reduction. Energy Environ. Sci. 2021, 14, 3019–3028. [Google Scholar] [CrossRef]
- Feng, S.Q.; Lin, X.S.; Song, X.G.; Mei, B.B.; Mu, J.L.; Li, J.W.; Liu, Y.; Jiang, Z.; Ding, Y.J. Constructing efficient single Rh sites on activated carbon via surface carbonyl groups for methanol carbonylation. ACS Catal. 2021, 11, 682–690. [Google Scholar] [CrossRef]
- Wang, F.; Miao, Z.; Mu, J.; Zhao, Y.; Liang, M.; Meng, J.; Wu, X.; Zhou, P.; Zhao, J.; Zhuo, S.; et al. A Ni nanoparticles encapsulated in N-doped carbon catalyst for efficient electroreduction CO2: Identification of active sites for adsorption and activation of CO2 molecules. Chem. Eng. J. 2022, 428, 131323. [Google Scholar] [CrossRef]
- Gao, J.; Hou, Z.Y.; Liu, X.S.; Zeng, Y.W.; Luo, M.F.; Zheng, X.M. Methane autothermal reforming with CO2 and O2 to synthesis gas at the boundary between Ni and ZrO2. Int. J. Hydrogen Energy 2009, 34, 3734–3742. [Google Scholar] [CrossRef]
- Yao, P.; Zhang, J.; Qiu, Y.; Zheng, Q.; Zhang, H.; Yan, J.; Li, X. Atomic-dispersed coordinated unsaturated nickel-nitrogen sites in hollow carbon spheres for the efficient electrochemical CO2 reduction. ACS Sustain. Chem. Eng. 2021, 9, 5437–5444. [Google Scholar] [CrossRef]
- Feng, Y.; Long, S.; Chen, B.; Jia, W.; Xie, S.; Sun, Y.; Tang, X.; Yang, S.; Zeng, X.; Lin, L. Inducing electron dissipation of pyridinic N enabled by single Ni–N4 sites for the reduction of aldehydes/ketones with ethanol. ACS Catal. 2021, 11, 6398–6405. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Xu, C.-Y.; Liu, Q.; Liu, J.; Chen, R.; Zhu, J.; Li, R.; Wang, J. Atomically dispersed Ni–N4 species and Ni nanoparticles constructing N-doped porous carbon fibers for accelerating hydrogen evolution. Carbon 2021, 185, 96–104. [Google Scholar] [CrossRef]
- Wei, B.; Wu, W.; Xie, D.; Nastasi, M.; Wang, J. Strength, plasticity, thermal stability and strain rate sensitivity of nanograined nickel with amorphous ceramic grain boundaries. Acta Mater. 2021, 212, 116918. [Google Scholar] [CrossRef]
- Mao, F.; Liu, P.F.; Yang, P.; Gu, J.; Yang, H.G. One-step coating of commercial Ni nanoparticles with a Ni, N-codoped carbon shell towards efficient electrocatalysts for CO2 reduction. Chem. Commun. 2020, 56, 7495–7498. [Google Scholar] [CrossRef]
- Yu, P.; Luo, Z.; Wang, Q.; Fang, M.; Zhou, J.; Wang, W.; Liang, X.; Cai, W. Activated carbon-based CO2 uptake evaluation at different temperatures: The correlation analysis and coupling effects of the preparation conditions. J. CO2 Util. 2020, 40, 101214. [Google Scholar] [CrossRef]
- Dong, N.; Chen, M.; Ye, Q.; Zhang, D.; Dai, H. Catalytic elimination of carbon monoxide, ethyl acetate, and toluene over the Ni/OMS-2 Catalysts. Catalysts 2021, 11, 581. [Google Scholar] [CrossRef]
- Hashem, A.; Fletcher, A.J.; Younis, H.; Mauof, H.; Abou-Okeil, A. Adsorption of Pb(II) ions from contaminated water by 1,2,3,4-butanetetracarboxylic acid-modified microcrystalline cellulose: Isotherms, kinetics, and thermodynamic studies. Int. J. Biol. Macromol. 2020, 164, 3193–3203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, D.; Liu, J.; He, D.; Cao, X.; Han, C.; Lu, J.; Luo, Y. Tuning the metal-support interaction on chromium-based catalysts for catalytically eliminate methyl mercaptan: Anchored active chromium species through surface hydroxyl groups. Chem. Eng. J. 2020, 389, 124384. [Google Scholar] [CrossRef]
- Nabais, J.; Carrott, P.; Carrott, M.; Menéndez, J. Preparation and modification of activated carbon fibres by microwave heating. Carbon 2004, 42, 1315–1320. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Y.; Xu, Y.; Jiang, T.; Zhao, M.; Deng, M.; Wu, R.; Wang, Y. Carbon nanotube-bridged N-doped mesoporous carbon nanosphere with atomic and nanoscaled M (M = Fe, Co) species for synergistically enhanced oxygen reduction reaction. Chem. Eng. J. 2021, 421, 129689. [Google Scholar] [CrossRef]
- Xu, D.; Pan, Y.; Zhu, L.; Yusran, Y.; Zhang, D.; Fang, Q.; Xue, M.; Qiu, S. Simple coordination complex-derived Ni NP anchored N-doped porous carbons with high performance for reduction of nitroarenes. Crystengcomm 2017, 19, 6612–6619. [Google Scholar] [CrossRef]
- Liang, S.; Jiang, Q.; Wang, Q.; Liu, Y. Revealing the real role of nickel decorated nitrogen-doped carbon catalysts for electrochemical reduction of CO2 to CO. Adv. Energy Mater. 2021, 11, 2101477. [Google Scholar] [CrossRef]
- Wang, X.; Sang, X.; Dong, C.-L.; Yao, S.; Shuai, L.; Lu, J.; Yang, B.; Li, Z.; Lei, L.; Qiu, M.; et al. Proton capture strategy for enhancing electrochemical CO2 reduction on atomically dispersed metal-nitrogen active sites. Angew. Chem. Int. Ed. 2021, 60, 11959–11965. [Google Scholar] [CrossRef]
- Mamtani, K.; Jain, D.; Zemlyanov, D.; Celik, G.; Luthman, J.; Renkes, G.; Co, A.C.; Ozkan, U.S. Probing the Oxygen Reduction Reaction Active Sites over Nitrogen-Doped Carbon Nanostructures (CN) in Acidic Media Using Phosphate Anion. ACS Catal. 2016, 6, 7249–7259. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, D.; Zhang, Q.; Cui, R.; Hassan, M.; Dong, L.; Li, J.; He, Y. Single Mn atom anchored on N-doped porous carbon as highly efficient fenton-like catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2020, 279, 119363. [Google Scholar] [CrossRef]
- Xiang, N.; Tian, J.; Li, Q.; Hou, Y.; Huang, Z. Promotional mechanism of nitrogen-doping in activated carbon for formaldehyde removal: Enhanced attractive noncovalent interactions coupled with Cannizzaro-type disproportionation reaction. Sep. Purif. Technol. 2024, 332, 125761. [Google Scholar] [CrossRef]
- Bai, P.; Tian, F.; Wang, H.; Yang, T.; Bi, X.; Chai, Z.; Wang, X. Electrocatalytic enhancement of 0D/1D/2D multidimensional PtCo alloy@cobalt benzoate/graphene composite catalyst for alcohol electro-oxidation. Adv. Mater. Interfaces 2019, 6, 1900946. [Google Scholar] [CrossRef]
- Ma, M.; Yang, R.; Jiang, Z.; Chen, C.; Liu, Q.; Albilali, R.; He, C. Fabricating M/Al2O3/cordierite (M = Cr, Mn, Fe, Co, Ni and Cu) monolithic catalysts for ethyl acetate efficient oxidation: Unveiling the role of water vapor and reaction mechanism. Fuel 2021, 303, 121244. [Google Scholar] [CrossRef]
- Si, W.; Wang, Y.; Zhao, S.; Hu, F.; Li, J. A facile method for in situ preparation of the MnO2/LaMnO3 catalyst for the removal of toluene. Environ. Sci. Technol. 2016, 50, 4572–4578. [Google Scholar] [CrossRef]
- Luo, Z.; Fang, Y.; Zhou, M.; Wang, X. A borocarbonitride ceramic aerogel for photoredox catalysis. Angew. Chem. Int. Ed. 2019, 58, 6033–6037. [Google Scholar] [CrossRef]
- Li, X.; Lin, B.; Li, H.; Yu, Q.; Ge, Y.; Jin, X.; Liu, X.; Zhou, Y.; Xiao, J. Carbon doped hexagonal BN as a highly efficient metal-free base catalyst for Knoevenagel condensation reaction. Appl. Catal. B Environ. 2018, 239, 254–259. [Google Scholar] [CrossRef]
- Lv, L.; Tang, B.; Ji, Q.; Li, N.; Wang, Y.; Feng, S.; Duan, H.; Wang, C.; Tan, H.; Yan, W. Highly exposed NiFeOx nanoclusters supported on boron doped carbon nanotubes for electrocatalytic oxygen evolution reaction. Chin. Chem. Lett. 2023, 34, 107524. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Li, Y.; Lu, P.; Wu, Z.-S. A general bimetal-ion adsorption strategy to prepare nickel single atom catalysts anchored on graphene for efficient oxygen evolution reaction. J. Energy Chem. 2020, 43, 52–57. [Google Scholar] [CrossRef]
- Ma, L.; Wang, D.; Li, J.; Bai, B.; Fu, L.; Li, Y. Ag/CeO2 nanospheres: Efficient catalysts for formaldehyde oxidation. Appl. Catal. B Environ. 2014, 148–149, 36–43. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Tao, L.; Jiang, P.; Ye, C.; Lin, R.; Huang, Z.; Li, A.; Pang, D.; Yan, H.; et al. Silver single-atom catalyst for efficient electrochemical CO2 reduction synthesized from thermal transformation and surface reconstruction. Angew. Chem. Int. Ed. 2021, 60, 6170–6176. [Google Scholar] [CrossRef]
- Romand, M.; Roubin, M.; Deloume, J.P. ESCA studies of some copper and silver selenides. J. Electron. Spectrosc. Relat. Phenom. 1978, 13, 229–242. [Google Scholar] [CrossRef]
- Schön, G.; Tummavuori, J.; Lindström, B.; Enzell, C.; Swahn, C.-G. ESCA studies of Ag, Ag2O and AgO. Acta Chem. Scand. 1973, 27, 2623–2633. [Google Scholar] [CrossRef]
- Liu, R.; Wu, H.; Shi, J.; Xu, X.; Zhao, D.; Ng, Y.H.; Zhang, M.; Liu, S.; Ding, H. Recent progress on catalysts for catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2022, 12, 6945–6991. [Google Scholar] [CrossRef]
- Chen, W.R.; Sharpless, C.M.; Linden, K.G.; Suffet, I.H. Treatment of volatile organic chemicals on the EPA contaminant candidate list using ozonation and the O3/H2O2 advanced oxidation process. Environ. Sci. Technol. 2006, 40, 2734–2739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xie, M.; Zhao, J.; Wei, S.; Zheng, H.; Zhang, S. Key structural features that determine the selectivity of UV/acetylacetone for the degradation of aromatic pollutants when compared to UV/H2O2. Water Res. 2021, 196, 117046. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.S.; Bhagde, T.; Moore, K.B.; Moberg, D.R.; Jasper, A.W.; Georgievskii, Y.; Vansco, M.F.; Klippenstein, S.J.; Lester, M.I. Watching a hydroperoxyalkyl radical (QOOH) dissociate. Science 2021, 373, 679–682. [Google Scholar] [CrossRef]
- Lao, Y.; Jiang, X.; Huang, J.; Zhang, Z.; Wang, X. Catalytic oxidation of ethyl acetate on Ce-Mn-O catalysts modified by La. Rare Met. 2020, 40, 547–554. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Zhang, Q.; Zeng, M.; Wu, S.; Lan, L.; Zhao, X. Novel photoactivation and solar-light-driven thermocatalysis on ε-MnO2 nanosheets lead to highly efficient catalytic abatement of ethyl acetate without acetaldehyde as unfavorable by-product. J. Mater. Chem. A 2018, 6, 14195–14206. [Google Scholar] [CrossRef]

| Sample | Specific Surface Area (m2/g) | Average Pore Size (nm) | Pore Volume (mL/g) |
|---|---|---|---|
| BCN | 61.8 | 2.96 | 0.0457 |
| AgNi/BCN-300 | 545 | 2.75 | 0.375 |
| AgNi/BCN-400 | 503 | 2.70 | 0.339 |
| AgNi/BCN-500 | 405 | 2.44 | 0.246 |
| AgNi/BCN-600 | 555 | 2.61 | 0.363 |
| AgNi/BCN-700 | 498 | 2.66 | 0.331 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.; Zhang, L.; Gao, G.; Xin, C.; Fu, B.; Liu, S.; Ding, H. Catalytic Oxidation of Benzene over Atomic Active Site AgNi/BCN Catalysts at Room Temperature. Molecules 2024, 29, 1463. https://doi.org/10.3390/molecules29071463
Zuo X, Zhang L, Gao G, Xin C, Fu B, Liu S, Ding H. Catalytic Oxidation of Benzene over Atomic Active Site AgNi/BCN Catalysts at Room Temperature. Molecules. 2024; 29(7):1463. https://doi.org/10.3390/molecules29071463
Chicago/Turabian StyleZuo, Xin, Lisheng Zhang, Ge Gao, Changchun Xin, Bingfeng Fu, Shejiang Liu, and Hui Ding. 2024. "Catalytic Oxidation of Benzene over Atomic Active Site AgNi/BCN Catalysts at Room Temperature" Molecules 29, no. 7: 1463. https://doi.org/10.3390/molecules29071463
APA StyleZuo, X., Zhang, L., Gao, G., Xin, C., Fu, B., Liu, S., & Ding, H. (2024). Catalytic Oxidation of Benzene over Atomic Active Site AgNi/BCN Catalysts at Room Temperature. Molecules, 29(7), 1463. https://doi.org/10.3390/molecules29071463








