Nanomaterial Delivery Vehicles for the Development of Neoantigen Tumor Vaccines for Personalized Treatment
Abstract
1. Introduction
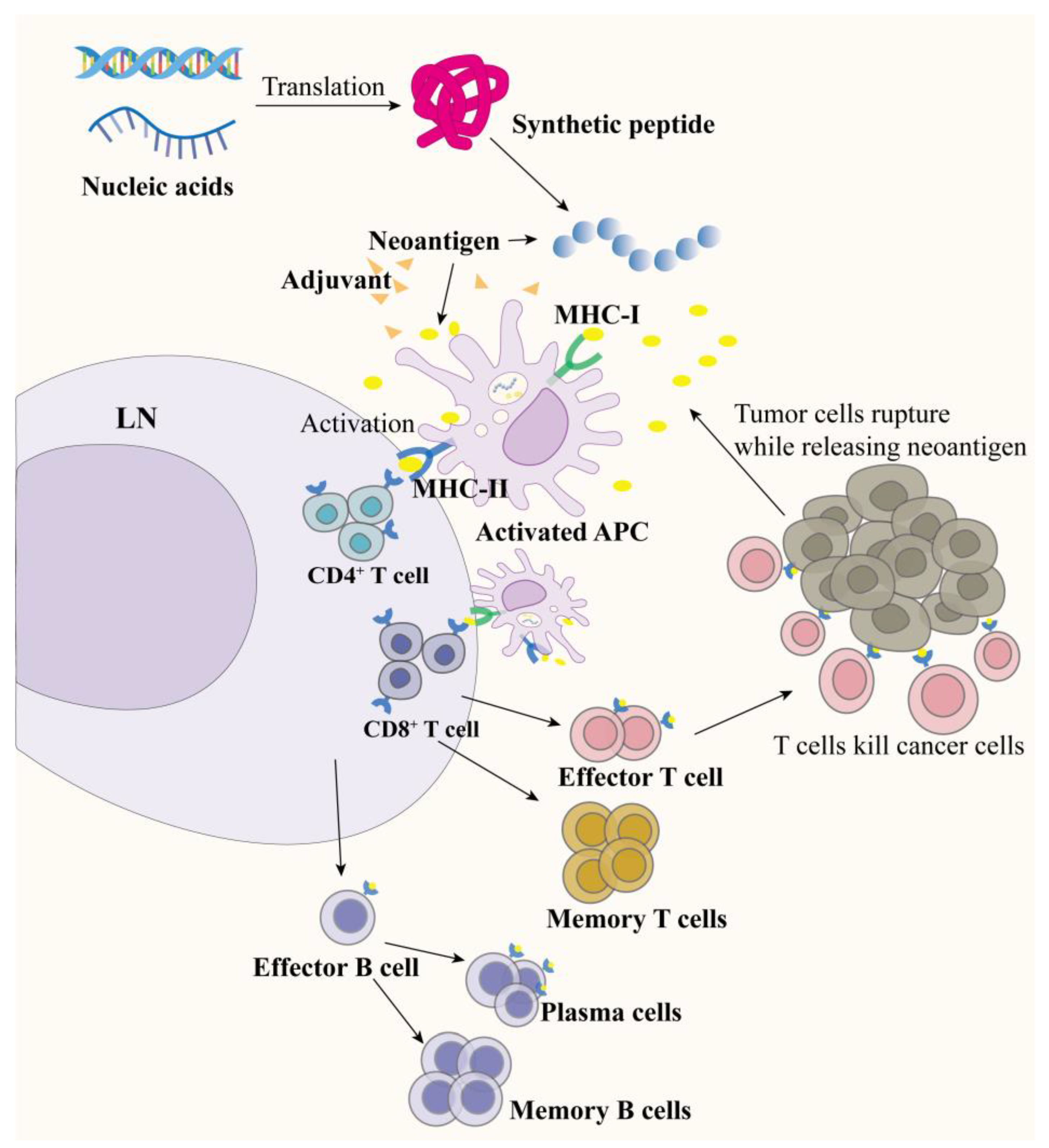
2. Tumor Neoantigen Vaccines
2.1. Dendritic Cell Vaccines
2.2. Peptide-Based Neoantigen Vaccines
2.3. RNA/DNA-Based Neoantigen Vaccines
3. Whole Tumor Cell Vaccine (WTCV)
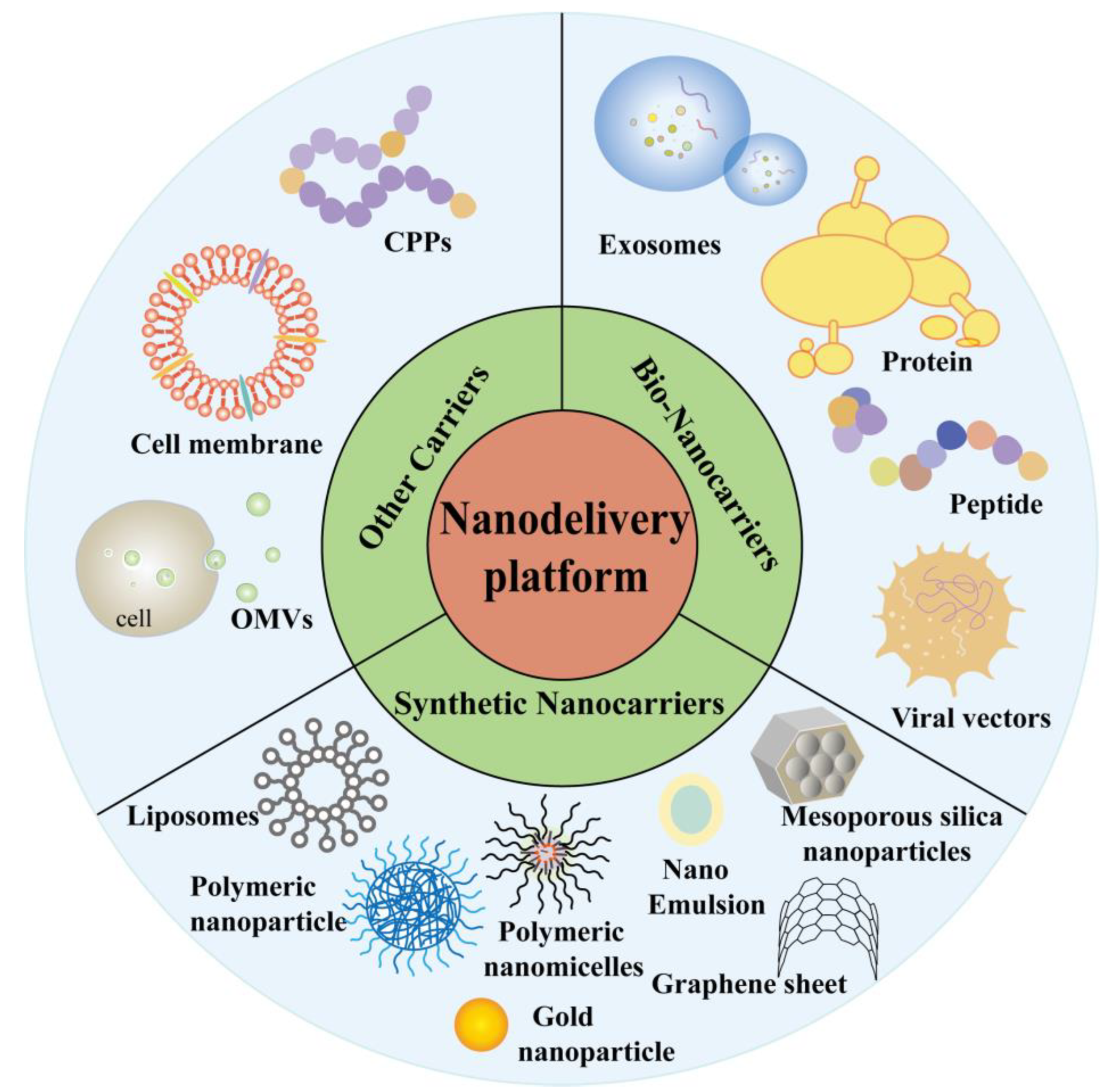
4. Nanodelivery Platform for Vaccine
4.1. Bio-Nanocarriers
4.1.1. Exosomes
4.1.2. Protein/Peptide-Based Nanocarriers
4.1.3. Virus (Virus-like Particle)/Bacterium-Based Nanocarriers
4.2. Lipid Nanoparticles (LNPs)
4.3. Polymeric Nanocarriers
4.4. Inorganic Nanocarriers
4.5. Other Carriers
5. Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Ma, W.; Pham, B.; Li, T. Cancer neoantigens as potential targets for immunotherapy. Clin. Exp. Metastasis 2022, 39, 51–60. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Biswas, N.; Chakrabarti, S.; Padul, V.; Jones, L.D.; Ashili, S. Designing neoantigen cancer vaccines, trials, and outcomes. Front. Immunol. 2023, 14, 1105420. [Google Scholar] [CrossRef]
- Sharpnack, M.F.; Johnson, T.S.; Chalkley, R.; Han, Z.; Carbone, D.; Huang, K.; He, K. TSAFinder: Exhaustive tumor-specific antigen detection with RNAseq. Bioinformatics 2022, 38, 2422–2427. [Google Scholar] [CrossRef]
- Holtsträter, C.; Schrörs, B.; Bukur, T.; Löwer, M. Bioinformatics for Cancer Immunotherapy. In Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2120, pp. 1–9. [Google Scholar]
- Supabphol, S.; Li, L.; Goedegebuure, S.P.; Gillanders, W.E. Neoantigen vaccine platforms in clinical development: Understanding the future of personalized immunotherapy. Expert Opin. Investig. Drugs 2021, 30, 529–541. [Google Scholar] [CrossRef]
- Davodabadi, F.; Sarhadi, M.; Arabpour, J.; Sargazi, S.; Rahdar, A.; Díez-Pascual, A.M. Breast cancer vaccines: New insights into immunomodulatory and nano-therapeutic approaches. J. Control. Release Off. J. Control. Release Soc. 2022, 349, 844–875. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness-A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef]
- Diao, L.; Liu, M. Rethinking Antigen Source: Cancer Vaccines Based on Whole Tumor Cell/tissue Lysate or Whole Tumor Cell. Adv. Sci. 2023, 10, e2300121. [Google Scholar] [CrossRef]
- Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza, M.I.N. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. Int. J. Mol. Sci. 2021, 22, 8044. [Google Scholar] [CrossRef]
- Duarte, A.; Zangirolami, A.B.; Santos, I.; Niemann, F.S.; Honma, H.N.; Amaro, E.C.; Perroud, M.W., Jr.; Pericole, F.V.; Gilli, S.C.O.; Benites, B.D.; et al. Production of dendritic cell vaccines using different methods with equivalent results: Implications for emerging centers. Hematol. Transfus. Cell Ther. 2024, 46, 30–35. [Google Scholar] [CrossRef]
- Maruoka, S.; Ojima, T.; Iwamoto, H.; Kitadani, J.; Tabata, H.; Tominaga, S.; Katsuda, M.; Hayata, K.; Takeuchi, A.; Yamaue, H. Tumor RNA transfected DCs derived from iPS cells elicit cytotoxicity against cancer cells induced from colorectal cancer patients in vitro. Sci. Rep. 2022, 12, 3295. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Q.; Zhang, R.; Xie, L.; Shu, Y.; Gao, S.; Wang, P.; Su, X.; Qin, Y.; Wang, Y.; et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct. Target. Ther. 2021, 6, 26. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Deng, C.; Huang, X.; Huang, S.; Liu, Z.; Yang, J.; Mo, J.; Chen, H.J.; Wang, J.; et al. Nanochannel Electro-Injection as a Versatile Platform for Efficient RNA/DNA Programming on Dendritic Cells. Small 2023, 19, e2303088. [Google Scholar] [CrossRef]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef]
- Faiena, I.; Comin-Anduix, B.; Berent-Maoz, B.; Bot, A.; Zomorodian, N.; Sachdeva, A.; Said, J.; Cheung-Lau, G.; Pang, J.; Macabali, M.; et al. A Phase I, Open-label, Dose-escalation, and Cohort Expansion Study to Evaluate the Safety and Immune Response to Autologous Dendritic Cells Transduced With AdGMCA9 (DC-AdGMCAIX) in Patients With Metastatic Renal Cell Carcinoma. J. Immunother. 2020, 43, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell. Mol. Immunol. 2023, 20, 432–447. [Google Scholar] [CrossRef]
- Lee, K.W.; Yam, J.W.P.; Mao, X. Dendritic Cell Vaccines: A Shift from Conventional Approach to New Generations. Cells 2023, 12, 2147. [Google Scholar] [CrossRef]
- Wang, T.; Han, M.; Han, Y.; Jiang, Z.; Zheng, Q.; Zhang, H.; Li, Z. Antigen Self-Presented Personalized Nanovaccines Boost the Immunotherapy of Highly Invasive and Metastatic Tumors. ACS Nano 2024, 18, 6333–6347. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Nan, L.; Xiao, C.; Su, F.; Li, K.; Ji, Q.A.; Wei, Q.; Liu, Y.; Bao, G. PEGylated nano-Rehmannia glutinosa polysaccharide induces potent adaptive immunity against Bordetella bronchiseptica. Int. J. Biol. Macromol. 2021, 168, 507–517. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Wang, L.; Liu, B. Personalized neoantigen vaccination with synthetic long peptides: Recent advances and future perspectives. Theranostics 2020, 10, 6011–6023. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Schumacher, T.; Bunse, L.; Pusch, S.; Sahm, F.; Wiestler, B.; Quandt, J.; Menn, O.; Osswald, M.; Oezen, I.; Ott, M.; et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 2014, 512, 324–327. [Google Scholar] [CrossRef]
- Thakur, S.; Jain, M.; Zhang, C.; Major, C.; Bielamowicz, K.J.; Lacayo, N.J.; Vaske, O.; Lewis, V.; Murguia-Favela, L.; Narendran, A. Identification and in vitro validation of neoantigens for immune activation against high-risk pediatric leukemia cells. Hum. Vaccines Immunother. 2021, 17, 5558–5562. [Google Scholar] [CrossRef]
- van den Ende, T.C.; Heuts, J.M.M.; Gential, G.P.P.; Visser, M.; van de Graaff, M.J.; Ho, N.I.; Jiskoot, W.; Valentijn, A.; Meeuwenoord, N.J.; Overkleeft, H.S.; et al. Simplified Monopalmitoyl Toll-like Receptor 2 Ligand Mini-UPam for Self-Adjuvanting Neoantigen-Based Synthetic Cancer Vaccines. Chembiochem A Eur. J. Chem. Biol. 2021, 22, 1215–1222. [Google Scholar] [CrossRef]
- Abd-Aziz, N.; Poh, C.L. Development of Peptide-Based Vaccines for Cancer. J. Oncol. 2022, 2022, 9749363. [Google Scholar] [CrossRef]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef]
- Dolgin, E. The tangled history of mRNA vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef]
- Fritah, H.; Rovelli, R.; Chiang, C.L.; Kandalaft, L.E. The current clinical landscape of personalized cancer vaccines. Cancer Treat. Rev. 2022, 106, 102383. [Google Scholar] [CrossRef]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 2020, 5, 11. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Doener, F.; Zanzinger, K.; Noth, J.; Baumhof, P.; Fotin-Mleczek, M.; Heidenreich, R. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine 2016, 34, 3882–3893. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J.; Jafferji, M.S.; et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Investig. 2020, 130, 5976–5988. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, X.; Zhang, S.; Yi, X.; Zhang, T.; Wei, Q.; Li, H.; Ai, J. Tumor antigens and immune subtypes guided mRNA vaccine development for kidney renal clear cell carcinoma. Mol. Cancer 2021, 20, 159. [Google Scholar] [CrossRef]
- Li, Y.F.; Hou, Q.Q.; Zhao, S.; Chen, X.; Tang, M.; Li, L. Identification of tumor-specific neoantigens and immune clusters of hepatocellular carcinoma for mRNA vaccine development. J. Cancer Res. Clin. Oncol. 2023, 149, 623–637. [Google Scholar] [CrossRef]
- Sittplangkoon, C.; Alameh, M.G.; Weissman, D.; Lin, P.J.C.; Tam, Y.K.; Prompetchara, E.; Palaga, T. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front. Immunol. 2022, 13, 983000. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Hu, H.; Qin, H.; Liao, X.; Wang, F.; Zhang, W.; Yin, Q.; Su, X.; He, Y.; et al. Anti-tumour effect of neo-antigen-reactive T cells induced by RNA mutanome vaccine in mouse lung cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 3255–3268. [Google Scholar] [CrossRef]
- Xia, X. Detailed Dissection and Critical Evaluation of the Pfizer/BioNTech and Moderna mRNA Vaccines. Vaccines 2021, 9, 734. [Google Scholar] [CrossRef]
- Willis, E.; Pardi, N.; Parkhouse, K.; Mui, B.L.; Tam, Y.K.; Weissman, D.; Hensley, S.E. Nucleoside-modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice. Sci. Transl. Med. 2020, 12, eaav5701. [Google Scholar] [CrossRef]
- Orlandini von Niessen, A.G.; Poleganov, M.A.; Rechner, C.; Plaschke, A.; Kranz, L.M.; Fesser, S.; Diken, M.; Löwer, M.; Vallazza, B.; Beissert, T.; et al. Improving mRNA-Based Therapeutic Gene Delivery by Expression-Augmenting 3’ UTRs Identified by Cellular Library Screening. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 824–836. [Google Scholar] [CrossRef]
- Salomon, N.; Vascotto, F.; Selmi, A.; Vormehr, M.; Quinkhardt, J.; Bukur, T.; Schrörs, B.; Löewer, M.; Diken, M.; Türeci, Ö.; et al. A liposomal RNA vaccine inducing neoantigen-specific CD4+ T cells augments the antitumor activity of local radiotherapy in mice. Oncoimmunology 2020, 9, 1771925. [Google Scholar] [CrossRef]
- Yang, L.; Wang, T.; Zhang, D.; Huang, X.; Dong, Y.; Gao, W.; Ye, Y.; Ren, K.; Zhao, W.; Qiao, H.; et al. Black Phosphorus Nanosheets Assist Nanoerythrosomes for Efficient mRNA Vaccine Delivery and Immune Activation. Adv. Healthc. Mater. 2023, 12, e2300935. [Google Scholar] [CrossRef]
- Duperret, E.K.; Perales-Puchalt, A.; Stoltz, R.; Hiranjith, G.H.; Mandloi, N.; Barlow, J.; Chaudhuri, A.; Sardesai, N.Y.; Weiner, D.B. A Synthetic DNA, Multi-Neoantigen Vaccine Drives Predominately MHC Class I CD8+ T-cell Responses, Impacting Tumor Challenge. Cancer Immunol. Res. 2019, 7, 174–182. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Z.; Cai, Z.; Mao, Q.; Li, Z.; Li, H.; Zhang, C.; Zhang, Y.; Zhong, A.; Wu, L.; et al. Spleen-targeted neoantigen DNA vaccine for personalized immunotherapy of hepatocellular carcinoma. EMBO Mol. Med. 2023, 15, e16836. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Wang, X.; Kim, S.W.; Herndon, J.M.; Becker-Hapak, M.K.; Carreno, B.M.; Myers, N.B.; Sturmoski, M.A.; McLellan, M.D.; et al. Optimized polyepitope neoantigen DNA vaccines elicit neoantigen-specific immune responses in preclinical models and in clinical translation. Genome Med. 2021, 13, 56. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Ye, T.; Li, F.; Ma, G.; Wei, W. Enhancing therapeutic performance of personalized cancer vaccine via delivery vectors. Adv. Drug Deliv. Rev. 2021, 177, 113927. [Google Scholar] [CrossRef]
- Chiang, C.L.; Benencia, F.; Coukos, G. Whole tumor antigen vaccines. Semin. Immunol. 2010, 22, 132–143. [Google Scholar] [CrossRef]
- Parmiani, G.; Pilla, L.; Maccalli, C.; Russo, V. Autologous versus allogeneic cell-based vaccines? Cancer J. 2011, 17, 331–336. [Google Scholar] [CrossRef]
- Ye, J.; Wang, H.; Medina, R.; Chakraborty, S.; Sun, M.; Valenzuela, A.; Sang, X.; Zhang, Y.; Uher, O.; Zenka, J.; et al. rWTC-MBTA: Autologous vaccine prevents metastases via antitumor immune responses. J. Exp. Clin. Cancer Res. CR 2023, 42, 163. [Google Scholar] [CrossRef]
- Leaf, R.K.; Stroopinsky, D.; Pyzer, A.R.; Kruisbeek, A.M.; van Wetering, S.; Washington, A.; Ephraim, A.; Cole, L.; Morin, A.; Jain, S.; et al. DCOne as an Allogeneic Cell-based Vaccine for Multiple Myeloma. J. Immunother. 2017, 40, 315–322. [Google Scholar] [CrossRef]
- Dawood, S.; Austin, L.; Cristofanilli, M. Cancer stem cells: Implications for cancer therapy. Oncology 2014, 28, 1101–1107, 1110. [Google Scholar]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Xue, T.; Cheng, Q.; Ye, X.; Wang, C.; Yu, Y.; Ji, X.; Wu, M.; Zhang, X.; et al. Liposomes Encapsulating Neoantigens and Black Phosphorus Quantum Dots for Enhancing Photothermal Immunotherapy. J. Biomed. Nanotechnol. 2020, 16, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Zhou, X.; Guo, Z.; Zhang, J.; Zhu, P.; Yao, S.; Zhu, M. Ferritin nanoparticle-based SpyTag/SpyCatcher-enabled click vaccine for tumor immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 69–78. [Google Scholar] [CrossRef]
- Xu, C.; Nam, J.; Hong, H.; Xu, Y.; Moon, J.J. Positron Emission Tomography-Guided Photodynamic Therapy with Biodegradable Mesoporous Silica Nanoparticles for Personalized Cancer Immunotherapy. ACS Nano 2019, 13, 12148–12161. [Google Scholar] [CrossRef]
- Xu, C.; Dobson, H.E.; Yu, M.; Gong, W.; Sun, X.; Park, K.S.; Kennedy, A.; Zhou, X.; Xu, J.; Xu, Y.; et al. STING agonist-loaded mesoporous manganese-silica nanoparticles for vaccine applications. J. Control. Release Off. J. Control. Release Soc. 2023, 357, 84–93. [Google Scholar] [CrossRef]
- Xu, C.; Hong, H.; Lee, Y.; Park, K.S.; Sun, M.; Wang, T.; Aikins, M.E.; Xu, Y.; Moon, J.J. Efficient Lymph Node-Targeted Delivery of Personalized Cancer Vaccines with Reactive Oxygen Species-Inducing Reduced Graphene Oxide Nanosheets. ACS Nano 2020, 14, 13268–13278. [Google Scholar] [CrossRef]
- Park, K.S.; Nam, J.; Son, S.; Moon, J.J. Personalized combination nano-immunotherapy for robust induction and tumor infiltration of CD8+ T cells. Biomaterials 2021, 274, 120844. [Google Scholar] [CrossRef]
- Shae, D.; Baljon, J.J.; Wehbe, M.; Christov, P.P.; Becker, K.W.; Kumar, A.; Suryadevara, N.; Carson, C.S.; Palmer, C.R.; Knight, F.C.; et al. Co-delivery of Peptide Neoantigens and Stimulator of Interferon Genes Agonists Enhances Response to Cancer Vaccines. ACS Nano 2020, 14, 9904–9916. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; He, X.; Cui, X.; Liang, Z.; Wang, L.; Deng, X.; Zhang, Z.; Sheng, W.; Han, X.D. CD16 CAR-T cells enhance antitumor activity of CpG ODN-loaded nanoparticle-adjuvanted tumor antigen-derived vaccinevia ADCC approach. J. Nanobiotechnology 2023, 21, 159. [Google Scholar] [CrossRef]
- Garrido, G.; Schrand, B.; Rabasa, A.; Levay, A.; D’Eramo, F.; Berezhnoy, A.; Modi, S.; Gefen, T.; Marijt, K.; Doorduijn, E.; et al. Tumor-targeted silencing of the peptide transporter TAP induces potent antitumor immunity. Nat. Commun. 2019, 10, 3773. [Google Scholar] [CrossRef]
- Cheng, K.; Zhao, R.; Li, Y.; Qi, Y.; Wang, Y.; Zhang, Y.; Qin, H.; Qin, Y.; Chen, L.; Li, C.; et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat. Commun. 2021, 12, 2041. [Google Scholar] [CrossRef]
- Gong, N.; Zhang, Y.; Teng, X.; Wang, Y.; Huo, S.; Qing, G.; Ni, Q.; Li, X.; Wang, J.; Ye, X.; et al. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1053–1064. [Google Scholar] [CrossRef]
- Jing, Z.; Wang, S.; Xu, K.; Tang, Q.; Li, W.; Zheng, W.; Shi, H.; Su, K.; Liu, Y.; Hong, Z. A Potent Micron Neoantigen Tumor Vaccine GP-Neoantigen Induces Robust Antitumor Activity in Multiple Tumor Models. Adv. Sci. 2022, 9, e2201496. [Google Scholar] [CrossRef]
- Li, A.W.; Sobral, M.C.; Badrinath, S.; Choi, Y.; Graveline, A.; Stafford, A.G.; Weaver, J.C.; Dellacherie, M.O.; Shih, T.Y.; Ali, O.A.; et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat. Mater. 2018, 17, 528–534. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, T.; Qin, X.; Qiao, Q.; Shang, L.; Song, Q.; Yang, C.; Zhang, Z. Intracellularly Generated Immunological Gold Nanoparticles for Combinatorial Photothermal Therapy and Immunotherapy against Tumor. Nano Lett. 2019, 19, 6635–6646. [Google Scholar] [CrossRef]
- Feola, S.; Russo, S.; Martins, B.; Lopes, A.; Vandermeulen, G.; Fluhler, V.; De Giorgi, C.; Fusciello, M.; Pesonen, S.; Ylösmäki, E.; et al. Peptides-Coated Oncolytic Vaccines for Cancer Personalized Medicine. Front. Immunol. 2022, 13, 826164. [Google Scholar] [CrossRef]
- Mørk, S.K.; Kadivar, M.; Bol, K.F.; Draghi, A.; Westergaard, M.C.W.; Skadborg, S.K.; Overgaard, N.; Sørensen, A.B.; Rasmussen, I.S.; Andreasen, L.V.; et al. Personalized therapy with peptide-based neoantigen vaccine (EVX-01) including a novel adjuvant, CAF®09b, in patients with metastatic melanoma. Oncoimmunology 2022, 11, 2023255. [Google Scholar] [CrossRef]
- Chu, Y.; Qian, L.; Ke, Y.; Feng, X.; Chen, X.; Liu, F.; Yu, L.; Zhang, L.; Tao, Y.; Xu, R.; et al. Lymph node-targeted neoantigen nanovaccines potentiate anti-tumor immune responses of post-surgical melanoma. J. Nanobiotechnology 2022, 20, 190. [Google Scholar] [CrossRef]
- Qiu, F.; Becker, K.W.; Knight, F.C.; Baljon, J.J.; Sevimli, S.; Shae, D.; Gilchuk, P.; Joyce, S.; Wilson, J.T. Poly(propylacrylic acid)-peptide nanoplexes as a platform for enhancing the immunogenicity of neoantigen cancer vaccines. Biomaterials 2018, 182, 82–91. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Vogel, M.; Riether, C.; Muller, J.; Salatino, S.; Ternette, N.; Gomes, A.C.; Cabral-Miranda, G.; El-Turabi, A.; Ruedl, C.; et al. Targeting Mutated Plus Germline Epitopes Confers Pre-clinical Efficacy of an Instantly Formulated Cancer Nano-Vaccine. Front. Immunol. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Xiao, P.; Wang, J.; Fang, L.; Zhao, Z.; Sun, X.; Liu, X.; Cao, H.; Zhang, P.; Wang, D.; Li, Y. Nanovaccine-Mediated Cell Selective Delivery of Neoantigens Potentiating Adoptive Dendritic Cell Transfer for Personalized Immunization. Adv. Funct. Mater. 2021, 31, 2104068. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, X.; Kong, Y.; Zhang, L.; Song, Q.; Zhao, H.; Han, L.; Jiao, J.; Feng, Q.; Wang, L. Hyperthermia based individual in situ recombinant vaccine enhances lymph nodes drainage for de novo antitumor immunity. Acta Pharm. Sin. B 2022, 12, 3398–3409. [Google Scholar] [CrossRef]
- Li, Q.; Ren, J.; Liu, W.; Jiang, G.; Hu, R. CpG Oligodeoxynucleotide Developed to Activate Primate Immune Responses Promotes Antitumoral Effects in Combination with a Neoantigen-Based mRNA Cancer Vaccine. Drug Des. Dev. Ther. 2021, 15, 3953–3963. [Google Scholar] [CrossRef]
- Yang, X.; Fan, J.; Wu, Y.; Ma, Z.; Huang, J.; Zhang, Y.; Zhou, Z.; Mo, F.; Liu, X.; Yuan, H.; et al. Synthetic multiepitope neoantigen DNA vaccine for personalized cancer immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102443. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, Z.; Wang, J.; Zhang, P.; Ding, Y.; Jiang, Y.; Wang, D.; Li, Y. Engineering autologous tumor cell vaccine to locally mobilize antitumor immunity in tumor surgical bed. Sci. Adv. 2020, 6, eaba4024. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Y.; Guo, F.; Han, Y.; Qiu, Q.; Li, Y.; Dong, H.; Ren, T.; Li, Y. A vaccine for photodynamic immunogenic cell death: Tumor cell caged by cellular disulfide-thiol exchange for immunotherapy. Biomater. Sci. 2021, 9, 973–984. [Google Scholar] [CrossRef]
- Ye, X.; Liang, X.; Chen, Q.; Miao, Q.; Chen, X.; Zhang, X.; Mei, L. Surgical Tumor-Derived Personalized Photothermal Vaccine Formulation for Cancer Immunotherapy. ACS Nano 2019, 13, 2956–2968. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, L.; Tian, Y.; Ji, X.; Hu, Q.; Zhou, B.; Ding, Z.; Xu, H.; Yang, L. DP7-C-modified liposomes enhance immune responses and the antitumor effect of a neoantigen-based mRNA vaccine. J. Control. Release Off. J. Control. Release Soc. 2020, 328, 210–221. [Google Scholar] [CrossRef]
- Chandra, J.; Teoh, S.M.; Kuo, P.; Tolley, L.; Bashaw, A.A.; Tuong, Z.K.; Liu, Y.; Chen, Z.; Wells, J.W.; Yu, C.; et al. Manganese-Doped Silica-Based Nanoparticles Promote the Efficacy of Antigen-Specific Immunotherapy. J. Immunol. 2021, 206, 987–998. [Google Scholar] [CrossRef]
- Su, T.; Cheng, F.; Qi, J.; Zhang, Y.; Zhou, S.; Mei, L.; Fu, S.; Zhang, F.; Lin, S.; Zhu, G. Responsive Multivesicular Polymeric Nanovaccines that Codeliver STING Agonists and Neoantigens for Combination Tumor Immunotherapy. Adv. Sci. 2022, 9, e2201895. [Google Scholar] [CrossRef]
- Sun, W.; Ji, P.; Zhou, T.; Li, Z.; Xing, C.; Zhang, L.; Wei, M.; Yang, G.; Yuan, L. Ultrasound Responsive Nanovaccine Armed with Engineered Cancer Cell Membrane and RNA to Prevent Foreseeable Metastasis. Adv. Sci. 2023, 10, e2301107. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, T.; Zheng, Y.; Yang, S.L.; Yu, Z.; Duo, Y.; Zhang, Y.; Adah, D.; Shi, L.; Sun, Z.; et al. Immunogenic exosome-encapsulated black phosphorus nanoparticles as an effective anticancer photo-nanovaccine. Nanoscale 2020, 12, 19939–19952. [Google Scholar] [CrossRef]
- Sha, H.; Liu, Q.; Xie, L.; Shao, J.; Yu, L.; Cen, L.; Li, L.; Liu, F.; Qian, H.; Wei, J.; et al. Case Report: Pathological Complete Response in a Lung Metastasis of Phyllodes Tumor Patient Following Treatment Containing Peptide Neoantigen Nano-Vaccine. Front. Oncol. 2022, 12, 800484. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, L.; Tian, Y.; Ji, X.; Hu, Q.; Zhou, B.; Zhenyu, D.; Heng, X.; Yang, L. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials 2020, 241, 119852. [Google Scholar] [CrossRef]
- Zuo, B.; Zhang, Y.; Zhao, K.; Wu, L.; Qi, H.; Yang, R.; Gao, X.; Geng, M.; Wu, Y.; Jing, R.; et al. Universal immunotherapeutic strategy for hepatocellular carcinoma with exosome vaccines that engage adaptive and innate immune responses. J. Hematol. Oncol. 2022, 15, 46. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Zhao, B.; Chen, H.; Cai, Z.; Zheng, Y.; Zeng, Y.; Zhang, D.; Liu, X. Neoantigen Immunotherapeutic-Gel Combined with TIM-3 Blockade Effectively Restrains Orthotopic Hepatocellular Carcinoma Progression. Nano Lett. 2022, 22, 2048–2058. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, Z.; Wu, M.; Cai, Z.; Zheng, Y.; He, L.; Li, Z.; Zhou, J.; Sun, L.; Chen, G.; et al. Cytosolic Delivery of Thiolated Neoantigen Nano-Vaccine Combined with Immune Checkpoint Blockade to Boost Anti-Cancer T Cell Immunity. Adv. Sci. 2021, 8, 2003504. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Zhao, B.; Zheng, Y.; Zhuang, Q.; Liao, N.; Wang, P.; Cai, Z.; Zhang, D.; Zeng, Y.; et al. Remodeling Tumor-Associated Neutrophils to Enhance Dendritic Cell-Based HCC Neoantigen Nano-Vaccine Efficiency. Adv. Sci. 2022, 9, e2105631. [Google Scholar] [CrossRef]
- Schmitt, S.; Tahk, S.; Lohner, A.; Hänel, G.; Maiser, A.; Hauke, M.; Patel, L.; Rothe, M.; Josenhans, C.; Leonhardt, H.; et al. Fusion of Bacterial Flagellin to a Dendritic Cell-Targeting αCD40 Antibody Construct Coupled With Viral or Leukemia-Specific Antigens Enhances Dendritic Cell Maturation and Activates Peptide-Responsive T Cells. Front. Immunol. 2020, 11, 602802. [Google Scholar] [CrossRef]
- Li, W.; Jing, Z.; Wang, S.; Li, Q.; Xing, Y.; Shi, H.; Li, S.; Hong, Z. P22 virus-like particles as an effective antigen delivery nanoplatform for cancer immunotherapy. Biomaterials 2021, 271, 120726. [Google Scholar] [CrossRef]
- Scheetz, L.; Kadiyala, P.; Sun, X.; Son, S.; Hassani Najafabadi, A.; Aikins, M.; Lowenstein, P.R.; Schwendeman, A.; Castro, M.G.; Moon, J.J. Synthetic High-density Lipoprotein Nanodiscs for Personalized Immunotherapy against Gliomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4369–4380. [Google Scholar] [CrossRef]
- Reuven, E.M.; Leviatan Ben-Arye, S.; Yu, H.; Duchi, R.; Perota, A.; Conchon, S.; Bachar Abramovitch, S.; Soulillou, J.P.; Galli, C.; Chen, X.; et al. Biomimetic Glyconanoparticle Vaccine for Cancer Immunotherapy. ACS Nano 2019, 13, 2936–2947. [Google Scholar] [CrossRef]
- Delitto, D.; Zabransky, D.J.; Chen, F.; Thompson, E.D.; Zimmerman, J.W.; Armstrong, T.D.; Leatherman, J.M.; Suri, R.; Lopez-Vidal, T.Y.; Huff, A.L.; et al. Implantation of a neoantigen-targeted hydrogel vaccine prevents recurrence of pancreatic adenocarcinoma after incomplete resection. Oncoimmunology 2021, 10, 2001159. [Google Scholar] [CrossRef]
- Yang, X.X.; Sun, C.; Wang, L.; Guo, X.L. New insight into isolation, identification techniques and medical applications of exosomes. J. Control. Release Off. J. Control. Release Soc. 2019, 308, 119–129. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Caballero-Baños, M.; Benitez-Ribas, D.; Tabera, J.; Varea, S.; Vilana, R.; Bianchi, L.; Ayuso, J.R.; Pagés, M.; Carrera, G.; Cuatrecasas, M.; et al. Phase II randomised trial of autologous tumour lysate dendritic cell plus best supportive care compared with best supportive care in pre-treated advanced colorectal cancer patients. Eur. J. Cancer 2016, 64, 167–174. [Google Scholar] [CrossRef]
- Almeida, J.P.; Drezek, R.A.; Foster, A.E. Controlling melanoma at local and systemic levels: Is a combination of ablative therapy and immunotherapy the way forward? Immunotherapy 2014, 6, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, X.; Zhou, Y.; Shi, S.; Liang, C.; Yu, X.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; et al. Exosomes derived from immunogenically dying tumor cells as a versatile tool for vaccination against pancreatic cancer. Biomaterials 2022, 280, 121306. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Zucchetti, A.E.; Enserink, L.; Jouve, M.; Lankar, D.; Saitakis, M.; Martin-Jaular, L.; Théry, C. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017, 36, 3012–3028. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Peng, Y.; Du, Y.; Yang, Z.; Qi, X. Dendritic cell derived exosomes loaded neoantigens for personalized cancer immunotherapies. J. Control. Release Off. J. Control. Release Soc. 2023, 353, 423–433. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, B.; Yu, Z.; Zhao, K.; Zhang, Y.; He, K.; Seow, Y.; Yin, H. Complete remission of tumors in mice with neoantigen-painted exosomes and anti-PD1 therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2023, 31, 3579–3593. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, H.; Wang, Z.; Yang, H.; Zhao, H.; Zhang, Y.; Ge, K.; Wang, X.; Luo, L.; Zhou, X.; et al. Exosome transportation-mediated immunosuppression relief through cascade amplification for enhanced apoptotic body vaccination. Acta Biomater. 2022, 153, 529–539. [Google Scholar] [CrossRef]
- Koyama, Y.; Ito, T.; Hasegawa, A.; Eriguchi, M.; Inaba, T.; Ushigusa, T.; Sugiura, K. Exosomes derived from tumor cells genetically modified to express Mycobacterium tuberculosis antigen: A novel vaccine for cancer therapy. Biotechnol. Lett. 2016, 38, 1857–1866. [Google Scholar] [CrossRef]
- Herrera Estrada, L.P.; Champion, J.A. Protein nanoparticles for therapeutic protein delivery. Biomater. Sci. 2015, 3, 787–799. [Google Scholar] [CrossRef]
- Mehta, N.K.; Pradhan, R.V.; Soleimany, A.P.; Moynihan, K.D.; Rothschilds, A.M.; Momin, N.; Rakhra, K.; Mata-Fink, J.; Bhatia, S.N.; Wittrup, K.D.; et al. Pharmacokinetic tuning of protein-antigen fusions enhances the immunogenicity of T-cell vaccines. Nat. Biomed. Eng. 2020, 4, 636–648. [Google Scholar] [CrossRef]
- Taylor, D.N.; Treanor, J.J.; Strout, C.; Johnson, C.; Fitzgerald, T.; Kavita, U.; Ozer, K.; Tussey, L.; Shaw, A. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI). Vaccine 2011, 29, 4897–4902. [Google Scholar] [CrossRef]
- Zhu, G.; Lynn, G.M.; Jacobson, O.; Chen, K.; Liu, Y.; Zhang, H.; Ma, Y.; Zhang, F.; Tian, R.; Ni, Q.; et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat. Commun. 2017, 8, 1954. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, L.; Zhao, B.; Tian, Y.; Zhou, B.; Mu, Y.; Yang, L. A Peptide-Based Small RNA Delivery System to Suppress Tumor Growth by Remodeling the Tumor Microenvironment. Mol. Pharm. 2021, 18, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, L.; Wang, Y.; Tian, Y.; Wu, S.; Zhou, B.; Dong, C.; Zhao, B.; Yang, Y.; Xie, D.; et al. A Dendrimer Peptide (KK2DP7) Delivery System with Dual Functions of Lymph Node Targeting and Immune Adjuvants as a General Strategy for Cancer Immunotherapy. Adv. Sci. 2023, 10, e2300116. [Google Scholar] [CrossRef]
- Qiu, Y.; Man, R.C.H.; Liao, Q.; Kung, K.L.K.; Chow, M.Y.T.; Lam, J.K.W. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J. Control. Release Off. J. Control. Release Soc. 2019, 314, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Sato-Dahlman, M.; LaRocca, C.J.; Yanagiba, C.; Yamamoto, M. Adenovirus and Immunotherapy: Advancing Cancer Treatment by Combination. Cancers 2020, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- D’Alise, A.M.; Brasu, N.; De Intinis, C.; Leoni, G.; Russo, V.; Langone, F.; Baev, D.; Micarelli, E.; Petiti, L.; Picelli, S.; et al. Adenoviral-based vaccine promotes neoantigen-specific CD8+ T cell stemness and tumor rejection. Sci. Transl. Med. 2022, 14, eabo7604. [Google Scholar] [CrossRef] [PubMed]
- Saeed, U.; Insaf, R.A.; Piracha, Z.Z.; Tariq, M.N.; Sohail, A.; Abbasi, U.A.; Fida Rana, M.S.; Gilani, S.S.; Noor, S.; Noor, E.; et al. Crisis averted: A world united against the menace of multiple drug-resistant superbugs -pioneering anti-AMR vaccines, RNA interference, nanomedicine, CRISPR-based antimicrobials, bacteriophage therapies, and clinical artificial intelligence strategies to safeguard global antimicrobial arsenal. Front. Microbiol. 2023, 14, 1270018. [Google Scholar]
- Lim, D.; Kim, K.; Duysak, T.; So, E.; Jeong, J.H.; Choy, H.E. Bacterial cancer therapy using the attenuated fowl-adapted Salmonella enterica serovar Gallinarum. Mol. Ther. Oncolytics 2023, 31, 100745. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Jun, S.; Lim, H.; Cho, H.; You, S.H.; Ha, S.J.; Min, J.J.; Bang, D. Engineered Attenuated Salmonella typhimurium Expressing Neoantigen Has Anticancer Effects. ACS Synth. Biol. 2021, 10, 2478–2487. [Google Scholar] [CrossRef]
- Yau, A.; Lee, J.; Chen, Y. Nanomaterials for Protein Delivery in Anticancer Applications. Pharmaceutics 2021, 13, 155. [Google Scholar] [CrossRef]
- Russell, L.M.; Hultz, M.; Searson, P.C. Leakage kinetics of the liposomal chemotherapeutic agent Doxil: The role of dissolution, protonation, and passive transport, and implications for mechanism of action. J. Control. Release Off. J. Control. Release Soc. 2018, 269, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yuba, E.; Akazawa, T.; Wijewardana, V.; Kakihara, Y.; Azuma, A.; Hagimori, K.; Kanegi, R.; Hatoya, S.; Inoue, N.; et al. Potent adjuvant effect elicited for tumor immunotherapy by a liposome conjugated pH-sensitive polymer and dendritic cell-targeting Toll-like-receptor ligand. Vaccine 2022, 40, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, P.; Chen, B.; Zhu, Y.; Chen, X.; Min, Y. Stimuli-Responsive Nanoadjuvant Rejuvenates Robust Immune Responses to Sensitize Cancer Immunotherapy. ACS Nano 2023, 17, 21455–21469. [Google Scholar] [CrossRef] [PubMed]
- Thaxton, C.S.; Rink, J.S.; Naha, P.C.; Cormode, D.P. Lipoproteins and lipoprotein mimetics for imaging and drug delivery. Adv. Drug Deliv. Rev. 2016, 106 Pt A, 116–131. [Google Scholar] [CrossRef]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Dai, Y.; Zhao, Y.; Qi, S.; Liu, L.; Lu, L.; Luo, Q.; Zhang, Z. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat. Commun. 2020, 11, 1110. [Google Scholar] [CrossRef]
- Guevara, M.L.; Persano, S.; Persano, F. Lipid-Based Vectors for Therapeutic mRNA-Based Anti-Cancer Vaccines. Curr. Pharm. Des. 2019, 25, 1443–1454. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Gómez-Aguado, I.; Rodríguez-Castejón, J.; Vicente-Pascual, M.; Rodríguez-Gascón, A.; Pozo-Rodríguez, A.D.; Solinís Aspiazu, M. Nucleic Acid Delivery by Solid Lipid Nanoparticles Containing Switchable Lipids: Plasmid DNA vs. Messenger RNA. Molecules 2020, 25, 5995. [Google Scholar] [CrossRef]
- Mohammadian Haftcheshmeh, S.; Zamani, P.; Mashreghi, M.; Nikpoor, A.R.; Tavakkol-Afshari, J.; Jaafari, M.R. Immunoliposomes bearing lymphocyte activation gene 3 fusion protein and P5 peptide: A novel vaccine for breast cancer. Biotechnol. Prog. 2021, 37, e3095. [Google Scholar] [CrossRef]
- Gerhardt, A.; Voigt, E.; Archer, M.; Reed, S.; Larson, E.; Van Hoeven, N.; Kramer, R.; Fox, C.; Casper, C. A flexible, thermostable nanostructured lipid carrier platform for RNA vaccine delivery. Mol. Ther. Methods Clin. Dev. 2022, 25, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Li, Y.M. Chemical Strategies to Boost Cancer Vaccines. Chem. Rev. 2020, 120, 11420–11478. [Google Scholar] [CrossRef]
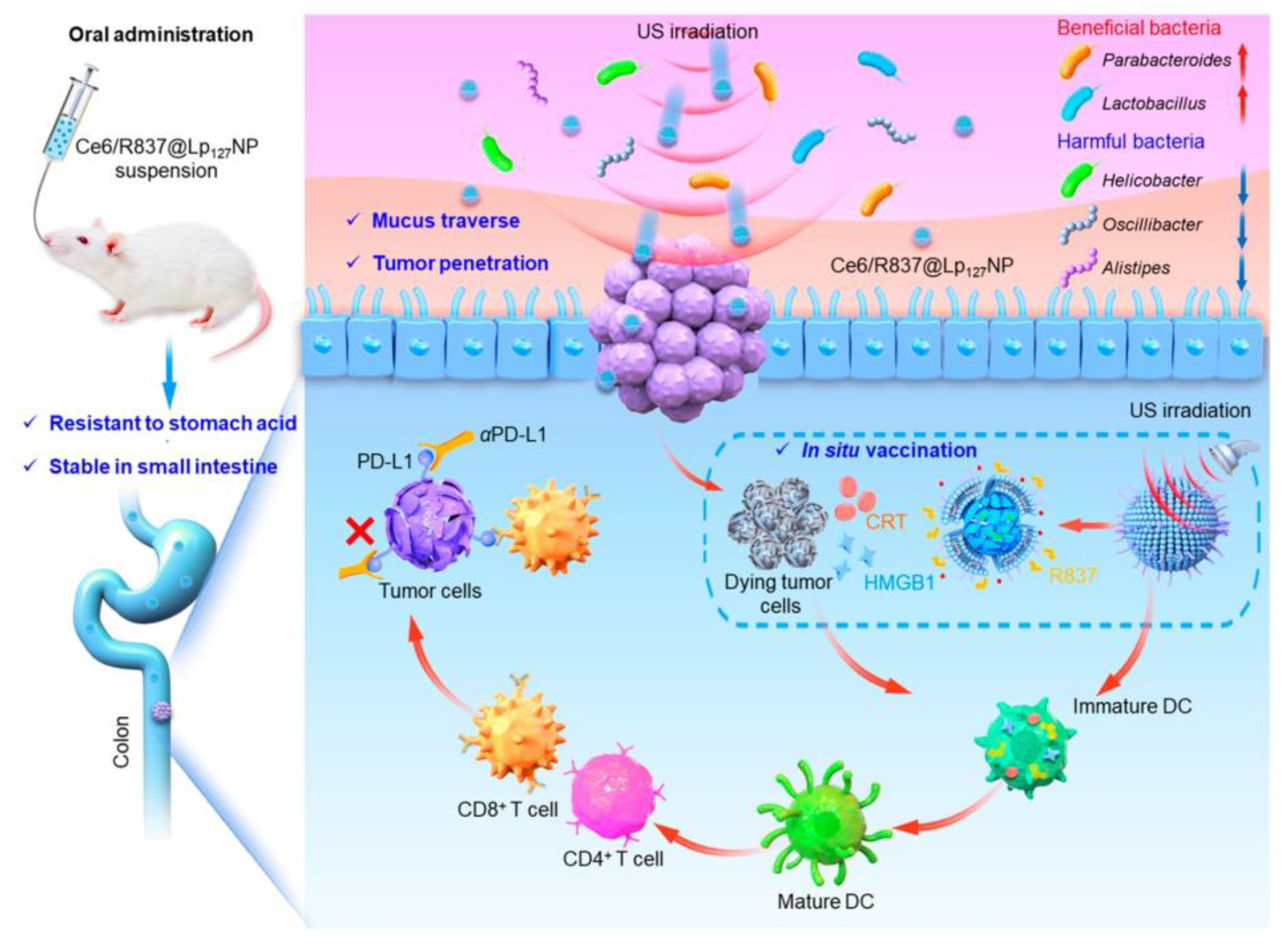
- Zu, M.; Ma, Y.; Zhang, J.; Sun, J.; Shahbazi, M.A.; Pan, G.; Reis, R.L.; Kundu, S.C.; Liu, J.; Xiao, B. An Oral Nanomedicine Elicits In Situ Vaccination Effect against Colorectal Cancer. ACS Nano 2024, 18, 3651–3668. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Park, K.S.; Moon, J.J. Modularly Programmable Nanoparticle Vaccine Based on Polyethyleneimine for Personalized Cancer Immunotherapy. Adv. Sci. 2021, 8, 2002577. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Huang, H.; Luo, Z.; Zhang, Z.; Sun, R.; Wan, Z.; Sun, J.; Lu, B.; Li, S. Farnesylthiosalicylic acid-derivatized PEI-based nanocomplex for improved tumor vaccination. Mol. Ther. Nucleic Acids 2021, 26, 594–602. [Google Scholar] [CrossRef]
- Moldovan, R.P.; Gündel, D.; Teodoro, R.; Ludwig, F.A.; Fischer, S.; Toussaint, M.; Schepmann, D.; Wünsch, B.; Brust, P.; Deuther-Conrad, W. Design, Radiosynthesis and Preliminary Biological Evaluation in Mice of a Brain-Penetrant 18F-Labelled σ2 Receptor Ligand. Int. J. Mol. Sci. 2021, 22, 5447. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Xiang, J.; Wang, H.; Zhuang, Q.; Wei, T.; Cao, Z.; Gu, Q.; Liu, Z.; Peng, R. Fluoroalkane modified cationic polymers for personalized mRNA cancer vaccines. Chem. Eng. J. 2023, 456, 140930. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.N.; Zhang, C.N.; Xu, R.; Niu, J.F.; Song, H.J.; Zhang, X.Y.; Wang, W.W.; Wang, Y.M.; Li, C.; Wei, X.Q.; et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef]
- Zhou, X.; He, X.; Shi, K.; Yuan, L.; Yang, Y.; Liu, Q.; Ming, Y.; Yi, C.; Qian, Z. Injectable Thermosensitive Hydrogel Containing Erlotinib-Loaded Hollow Mesoporous Silica Nanoparticles as a Localized Drug Delivery System for NSCLC Therapy. Adv. Sci. 2020, 7, 2001442. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Li, L.; Zhang, Z.; Lin, Z.; Zhang, J.; Song, X.; Xue, T.; Xing, C.; Liang, X.; Zhang, X. Biosynthetic neoantigen displayed on bacteria derived vesicles elicit systemic antitumour immunity. J. Extracell. Vesicles 2022, 11, e12289. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Ye, T.; Wang, S.; Na, X.; Wang, J.; Qing, S.; Gao, X.; Wang, C.; Li, F.; Wei, W.; et al. Self-healing microcapsules synergetically modulate immunization microenvironments for potent cancer vaccination. Sci. Adv. 2020, 6, eaay7735. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.T.T.; Yin, Y.; Le, T.M.D.; Jeong, J.H.; Lee, D.S. Highly Prolonged Release of the Cancer Vaccine and Immunomodulator via a Two-Layer Biodegradable Microneedle for Prophylactic Treatment of Metastatic Cancer. Biomacromolecules 2023, 24, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, C.; Zhang, X.; Hu, Q.; Zhang, Y.; Liu, Q.; Wen, D.; Milligan, J.; Bellotti, A.; Huang, L.; et al. A melanin-mediated cancer immunotherapy patch. Sci. Immunol. 2017, 2, eaan5692. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, P.; Qin, X.; Cheng, L.; Wang, F.; Xiong, X.; Huang, C.; Zhang, Z. HSA-templated self-generation of gold nanoparticles for tumor vaccine delivery and combinational therapy. J. Mater. Chem. B 2022, 10, 8750–8759. [Google Scholar] [CrossRef]
- Li, F.; Nie, W.; Zhang, F.; Lu, G.; Lv, C.; Lv, Y.; Bao, W.; Zhang, L.; Wang, S.; Gao, X.; et al. Engineering Magnetosomes for High-Performance Cancer Vaccination. ACS Cent. Sci. 2019, 5, 796–807. [Google Scholar] [CrossRef]
- Amin, M.U.; Ali, S.; Ali, M.Y.; Tariq, I.; Nasrullah, U.; Pinnapreddy, S.R.; Wölk, C.; Bakowsky, U.; Brüßler, J. Enhanced efficacy and drug delivery with lipid coated mesoporous silica nanoparticles in cancer therapy. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik E.V 2021, 165, 31–40. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Cha, B.G.; Choi, Y.; Im, J.; Kim, J. Injectable dual-scale mesoporous silica cancer vaccine enabling efficient delivery of antigen/adjuvant-loaded nanoparticles to dendritic cells recruited in local macroporous scaffold. Biomaterials 2020, 239, 119859. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Yin, Y.; Choi, Y.; Jeong, J.H.; Kim, J. Enhanced Cancer DNA Vaccine via Direct Transfection to Host Dendritic Cells Recruited in Injectable Scaffolds. ACS Nano 2020, 14, 11623–11636. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Li, M.; Zhao, L.; Ren, J.; Li, D.; Tan, Z.; Wang, K.; Li, H.; Hussain, M.; et al. Polyethylenimine Hybrid Thin-Shell Hollow Mesoporous Silica Nanoparticles as Vaccine Self-Adjuvants for Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2019, 11, 47798–47809. [Google Scholar] [CrossRef]
- Yue, H.; Wei, W.; Gu, Z.; Ni, D.; Luo, N.; Yang, Z.; Zhao, L.; Garate, J.A.; Zhou, R.; Su, Z.; et al. Exploration of graphene oxide as an intelligent platform for cancer vaccines. Nanoscale 2015, 7, 19949–19957. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Wu, H.; Li, J.; Xie, J.; Zang, F.; Ma, M.; Gu, N.; Zhang, Y. Magnetic targeting combined with active targeting of dual-ligand iron oxide nanoprobes to promote the penetration depth in tumors for effective magnetic resonance imaging and hyperthermia. Acta Biomater. 2019, 96, 491–504. [Google Scholar] [CrossRef]
- Mateu Ferrando, R.; Lay, L.; Polito, L. Gold nanoparticle-based platforms for vaccine development. Drug Discov. Today Technol. 2020, 38, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Q.; Guo, Z.; Li, M.; Yang, X.; Wan, G.; Chen, H.; Zhang, Q.; Wang, Y. An Intelligent Biomimetic Nanoplatform for Holistic Treatment of Metastatic Triple-Negative Breast Cancer via Photothermal Ablation and Immune Remodeling. ACS Nano 2020, 14, 15161–15181. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Jiang, H.; Song, Y.; Zhu, Y.; Qin, M.; He, C.; Du, G.; Sun, X. Aluminum nanoparticles deliver a dual-epitope peptide for enhanced anti-tumor immunotherapy. J. Control. Release Off. J. Control. Release Soc. 2022, 344, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Q.; Huang, Q.; Liu, Z.; Zeng, L.; Zhang, L.; Chen, X.; Song, H.; Zhang, J. Photothermal MnO2 nanoparticles boost chemo-photothermal therapy-induced immunogenic cell death in tumor immunotherapy. Int. J. Pharm. 2022, 617, 121578. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Ding, L.; Jin, H.J.; Li, X. RNA Hydrogel Combined with MnO2 Nanoparticles as a Nano-Vaccine to Treat Triple Negative Breast Cancer. Front. Chem. 2021, 9, 797094. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Dash, S.K.; Mandal, D.; Das, B.; Tripathy, S.; Dey, A.; Pramanik, P.; Roy, S. Metal based nanoparticles as cancer antigen delivery vehicles for macrophage based antitumor vaccine. Vaccine 2016, 34, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhang, Y.; Tan, L.; Zheng, T.; Hou, Y.; Hong, X.; Du, G.; Chen, X.; Zhang, Y.; Sun, X. An aluminum adjuvant-integrated nano-MOF as antigen delivery system to induce strong humoral and cellular immune responses. J. Control. Release Off. J. Control. Release Soc. 2019, 300, 81–92. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Q.; Wu, J.P.; Kirk, T.B.; Xu, J.; Liu, Z.; Xue, W. Reduction-Responsive Codelivery System Based on a Metal-Organic Framework for Eliciting Potent Cellular Immune Response. ACS Appl. Mater. Interfaces 2018, 10, 12463–12473. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Y.; Zou, J.; Yang, Y.; Yao, Y.; Cheng, G.; Yang, Y. Advanced Oxidation Nanoprocessing Boosts Immunogenicity of Whole Tumor Cells. Adv. Sci. 2023, 10, e2302250. [Google Scholar] [CrossRef]
- Zuo, Q.; Li, T.; Huang, L.; Liu, Z.; Xue, W. Macro-microporous ZIF-8 MOF complexed with lysosomal pH-adjusting hexadecylsulfonylfluoride as tumor vaccine delivery systems for improving anti-tumor cellular immunity. Biomater. Sci. 2023, 11, 5025–5045. [Google Scholar] [CrossRef]
- Zhu, J.; Chang, R.; Wei, B.; Fu, Y.; Chen, X.; Liu, H.; Zhou, W. Photothermal Nano-Vaccine Promoting Antigen Presentation and Dendritic Cells Infiltration for Enhanced Immunotherapy of Melanoma via Transdermal Microneedles Delivery. Research 2022, 2022, 9816272. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, Q.; Xiao, J.; Zhang, X.; Han, X.; Shi, X.; Hu, J.; Li, L.; Qian, X. Cancer Cell Membrane-Coated Gambogic Acid Nanoparticles for Effective Anticancer Vaccination by Activating Dendritic Cells. Int. J. Nanomed. 2023, 18, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Ren, Y.; Li, H.; Tang, Y.; Yan, J.; Shen, Z.; Zhang, H.; Chen, F. Cancer Cell-Membrane Biomimetic Boron Nitride Nanospheres for Targeted Cancer Therapy. Int. J. Nanomed. 2021, 16, 2123–2136. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, R.; Nie, G. Nanocarriers based on bacterial membrane materials for cancer vaccine delivery. Nat. Protoc. 2022, 17, 2240–2274. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, L.; Chen, P. Membrane Internalization Mechanisms and Design Strategies of Arginine-Rich Cell-Penetrating Peptides. Int. J. Mol. Sci. 2022, 23, 9038. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.M.; Trabulo, S.; Cardoso, A.L.; Maia, S.; Gomes, P.; Jurado, A.S.; Pedroso de Lima, M.C. Comparison of the efficiency of complexes based on S4(13)-PV cell-penetrating peptides in plasmid DNA and siRNA delivery. Mol. Pharm. 2013, 10, 2653–2666. [Google Scholar] [CrossRef] [PubMed]

| Types of Cancer | Vaccine Types | Vaccine Name | Composition | Combination with | Ref. |
|---|---|---|---|---|---|
| Colon cancer | peptides | Adpgk-BPQDs-liposome | liposome | PTT | [57] |
| ferritin-E7 (43–62) NP/ferritin-Reps1/Adpgk/Dpagt1 NP | SC-ferritin NP | ICIs | [58] | ||
| bMSN (CpG/Ce6)-Adpgk | bMSN | PDT | [59] | ||
| MnOx@HMSN (CDA + Adpgk) | MnOx@HMSN | — | [60] | ||
| RGO(CpG)-PEG-Adpgk | RGO-PEG | ICIs | [61] | ||
| NP Vacc | PEI-PEG | STING agonist | [62] | ||
| nanoSTING-peptide antigens-cGAMP | nanoSTING-vax | ICIs | [63] | ||
| CNPs-TCL/neoantigen | CNPs | — | [64] | ||
| RNA | RNA-LPX | liposome | CPI | [44] | |
| Nucl-TAP siRNA | nucleolin aptamer | ICIs | [65] | ||
| protein | CC-SpT-Adpgk OMVs | OMV | — | [66] | |
| Melanoma | peptides | NP Vacc | PEI-PEG | STING agonist | [62] |
| nanoSTING-peptide antigens-cGAMP | nanoSTING-vax | ICIs | [63] | ||
| NTV | NT2 | ICIs | [67] | ||
| GP-M30 | GPs | — | [68] | ||
| MSR-PEI E7 | PEI | — | [69] | ||
| AuNP@B16F10 | AuNP | PTT | [70] | ||
| PeptiCRAd-SIINFEKL | OVs | — | [71] | ||
| EVX-01 | cationic liposome (CAF@09b) | CPI | [72] | ||
| DSPE-PEG2000-peptide-NIR797 nanoparticles | DSPE-PEG2000 | ICIs | [73] | ||
| peptide/pPAA nanoplexes | polyanion pPAA | — | [74] | ||
| multi-target VLP-based vaccine (MTV) | Qβ-VLP/CpG | — | [75] | ||
| M-NP-Ag | PLGA nanoparticles | DCV | [76] | ||
| protein | FA-TSL/AuNCs/SV | AuNCs | PTT | [77] | |
| RNA | LNP-mRNA | LNPs | CpG2018B | [78] | |
| DNA | DD-TMG-IL12/CpG | liposome | — | [79] | |
| WTCV | PC-Cell@gel | FK-PBA hydrogel | PDT | [80] | |
| CpG@LL-B16F10 | CpG@eBSA NPs | PDT | [81] | ||
| tumor cell membrane | Gel-BPQD-CCNVs | BPQD-CCNVs | ICB, PTT | [82] | |
| Kidney cancer | RNA | DOTAP/DP7-C/mRNA | DOTAP liposome | — | [83] |
| Cervical cancer | peptide | ferritin-E7 (43–62) NP/ferritin-Reps1/Adpgk/Dpagt1 NP | SC-ferritin NP | ICIs | [58] |
| Mn4+-SNPs + GF001 | Mn4+-SNPs | — | [84] | ||
| cGAMP/antigen-codelivering NVs | NP | ICB | [85] | ||
| Breast cancer | peptide | GP-M25 | GPs | — | [68] |
| M-NP-Ag | PLGA nanoparticles | DCV | [76] | ||
| RNA | CM-RNA@Ce6/PLGA | Ce6/PLGA | USI | [86] | |
| Lung cancer | — | hEX@BP | hEX | PTT | [87] |
| peptide | neoantigen peptide DSPE-PEG2000-NHS | DSPE-PEG2000-NHS | — | [88] | |
| DC | DP7-C/neoantigen-pulsed DCs vaccine | DP7-C | — | [89] | |
| Hepatocellular carcinoma | peptide | DEXP&A2&N | DEX | — | [90] |
| NGC-gels vaccines | silk gels | TIM-3 blockade | [91] | ||
| thiolated nanovaccine | CpG-ODN NPs | ICB | [92] | ||
| mD@cSMN | cSMN | PDT | [93] | ||
| AML | DC | αCD40-DC | bacterial flagellin | — | [94] |
| Lymphoma | peptide | VLP-neoantigen peptide | P22 VLPs | — | [95] |
| Glioma | peptide | NeoAgs-CpG-nanodisc | sHDL nanodiscs | ICIs | [96] |
| Neu5Gc-positive tumor cells | Neu5Gc-neoantigens | biomimetic glyconanoparticle vaccine | NGs | — | [97] |
| Pancreatic adenocarcinoma | peptide | PancVax gel | hydrogel | operation | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhu, X.; Yang, H.; Li, Q.; Gai, L.; Sui, X.; Lu, H.; Feng, J. Nanomaterial Delivery Vehicles for the Development of Neoantigen Tumor Vaccines for Personalized Treatment. Molecules 2024, 29, 1462. https://doi.org/10.3390/molecules29071462
Huang X, Zhu X, Yang H, Li Q, Gai L, Sui X, Lu H, Feng J. Nanomaterial Delivery Vehicles for the Development of Neoantigen Tumor Vaccines for Personalized Treatment. Molecules. 2024; 29(7):1462. https://doi.org/10.3390/molecules29071462
Chicago/Turabian StyleHuang, Xiaoyu, Xiaolong Zhu, Huan Yang, Qinyi Li, Lizhi Gai, Xinbing Sui, Hua Lu, and Jiao Feng. 2024. "Nanomaterial Delivery Vehicles for the Development of Neoantigen Tumor Vaccines for Personalized Treatment" Molecules 29, no. 7: 1462. https://doi.org/10.3390/molecules29071462
APA StyleHuang, X., Zhu, X., Yang, H., Li, Q., Gai, L., Sui, X., Lu, H., & Feng, J. (2024). Nanomaterial Delivery Vehicles for the Development of Neoantigen Tumor Vaccines for Personalized Treatment. Molecules, 29(7), 1462. https://doi.org/10.3390/molecules29071462





