Recent Advances in π-Stacking Interaction-Controlled Asymmetric Synthesis
Abstract
1. Introduction
2. Chiral Auxiliary-Induced Asymmetric Synthesis
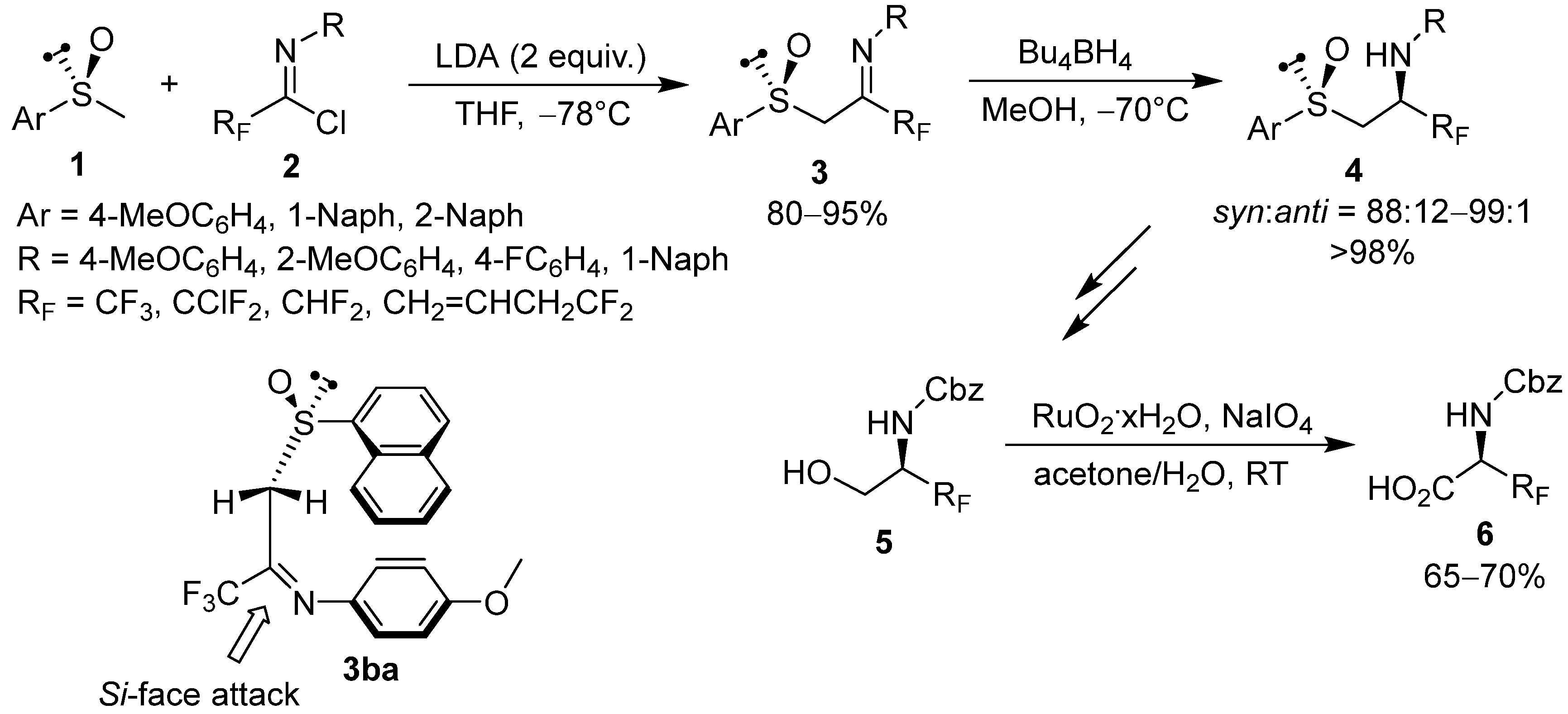
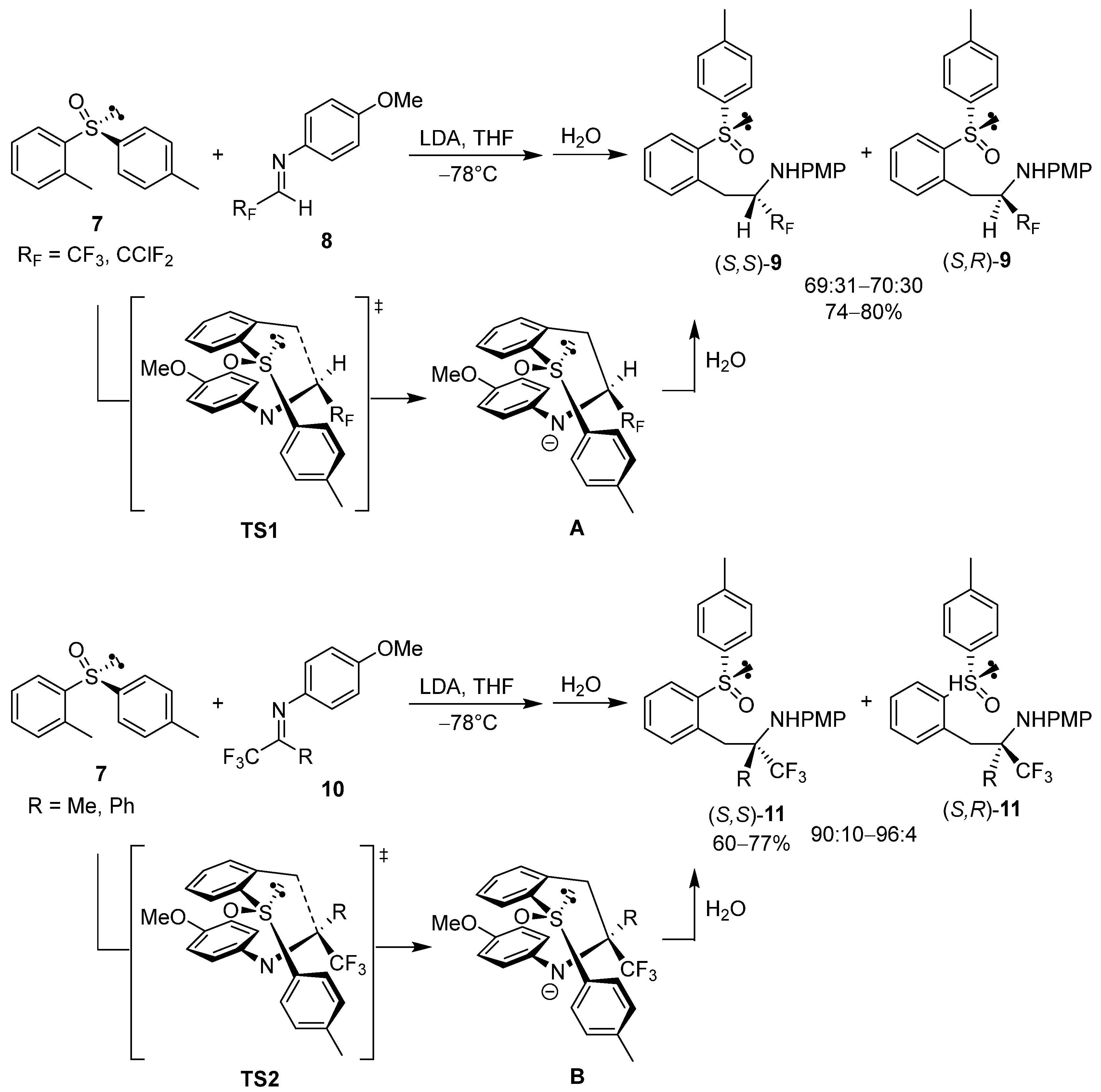
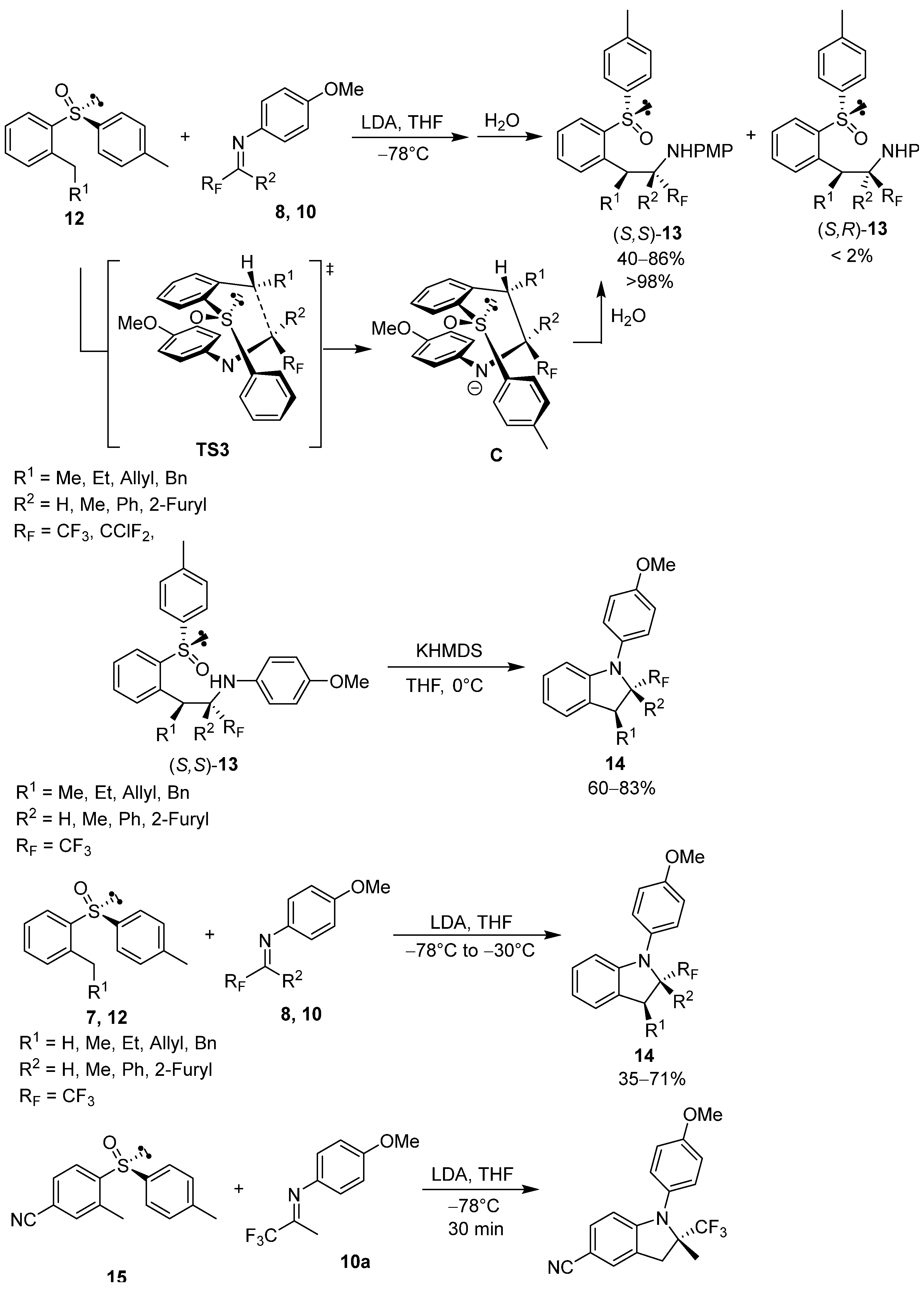
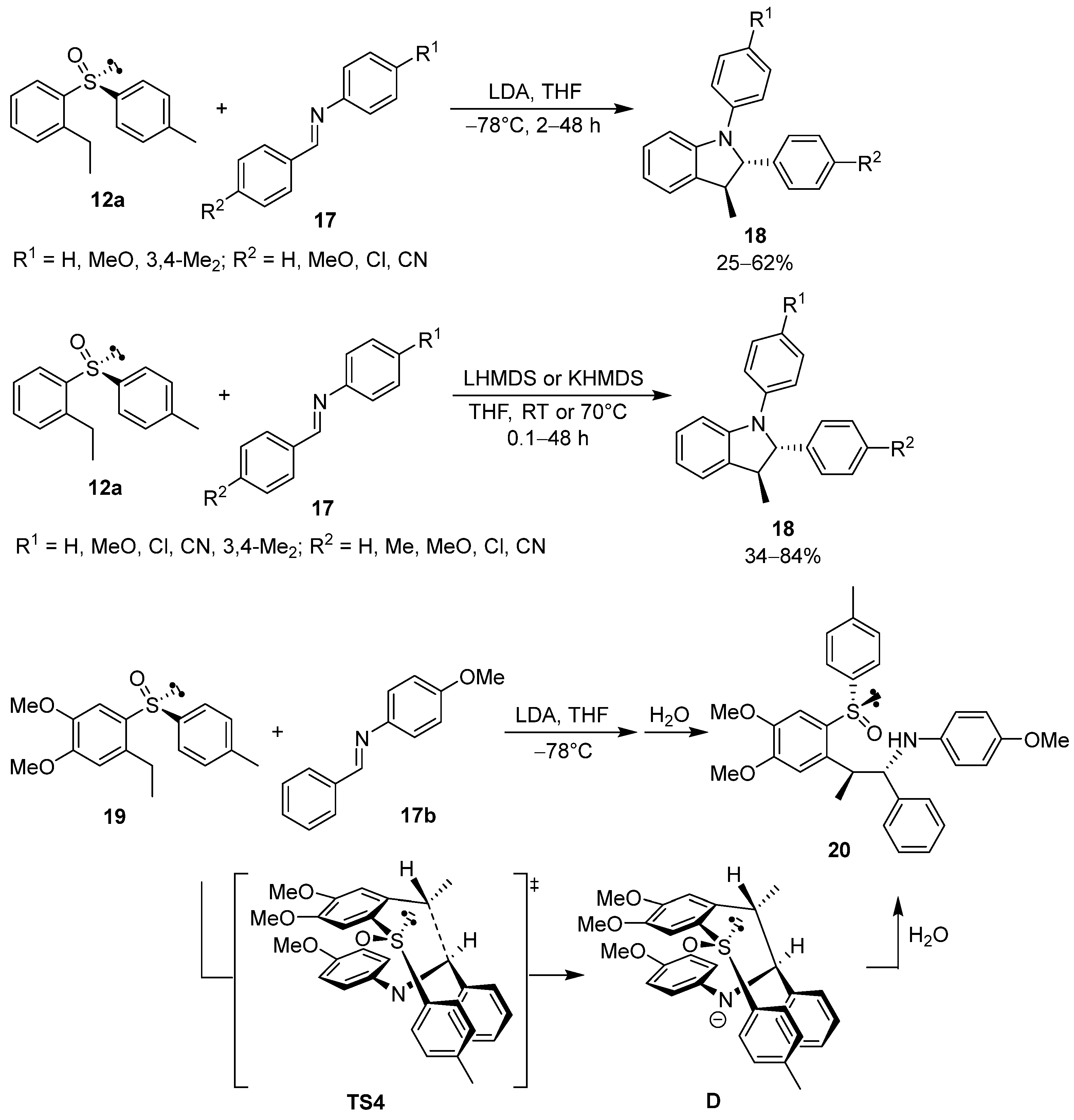
2.1. Chiral Arylsulfinyl-Based Auxiliaries in Asymmetric Synthesis
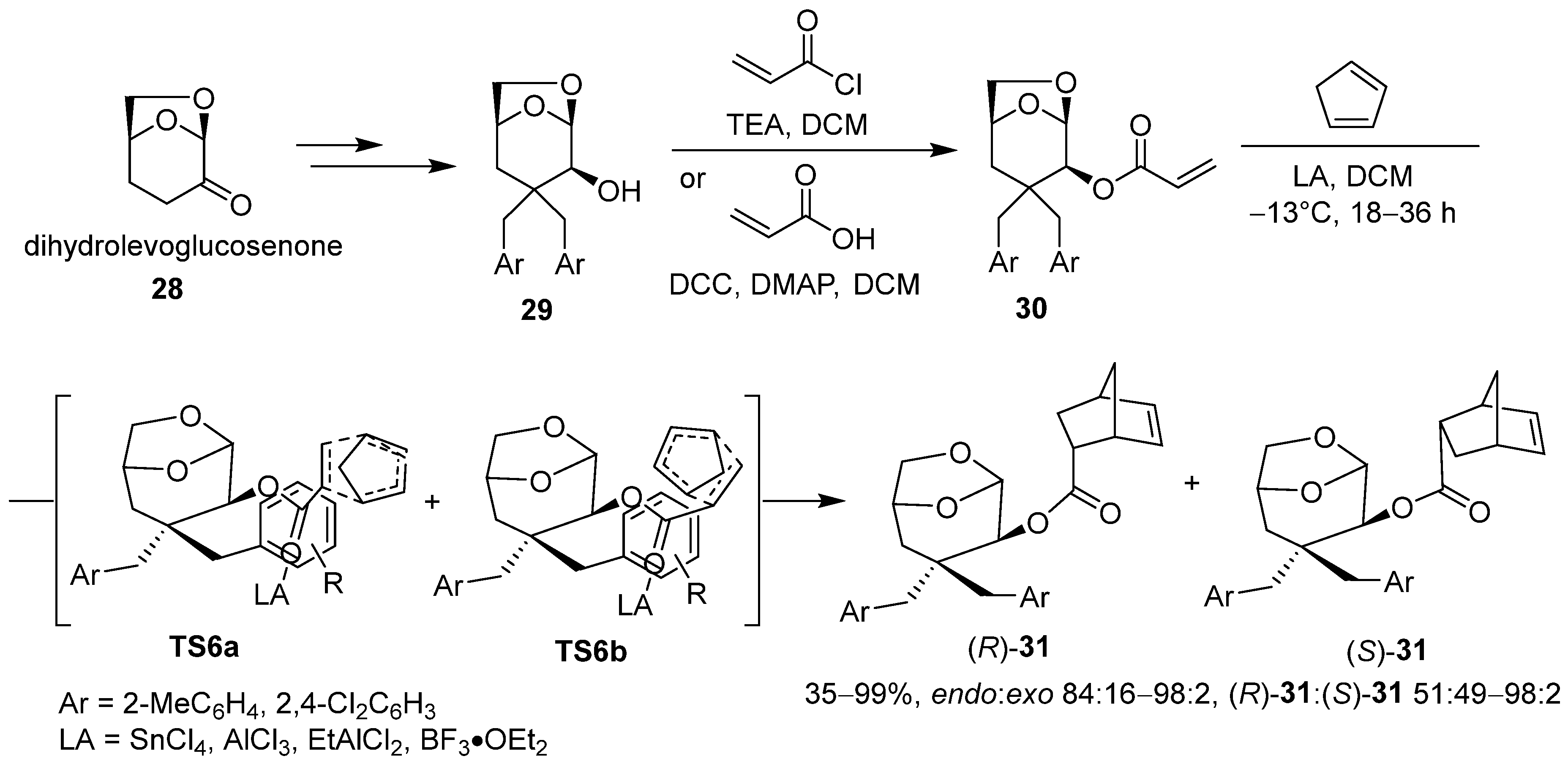
2.2. Adducts of Levoglucosenone and 9-(Aryloxymethyl)arthracenes as Chiral Auxiliaries in Asymmetric Synthesis
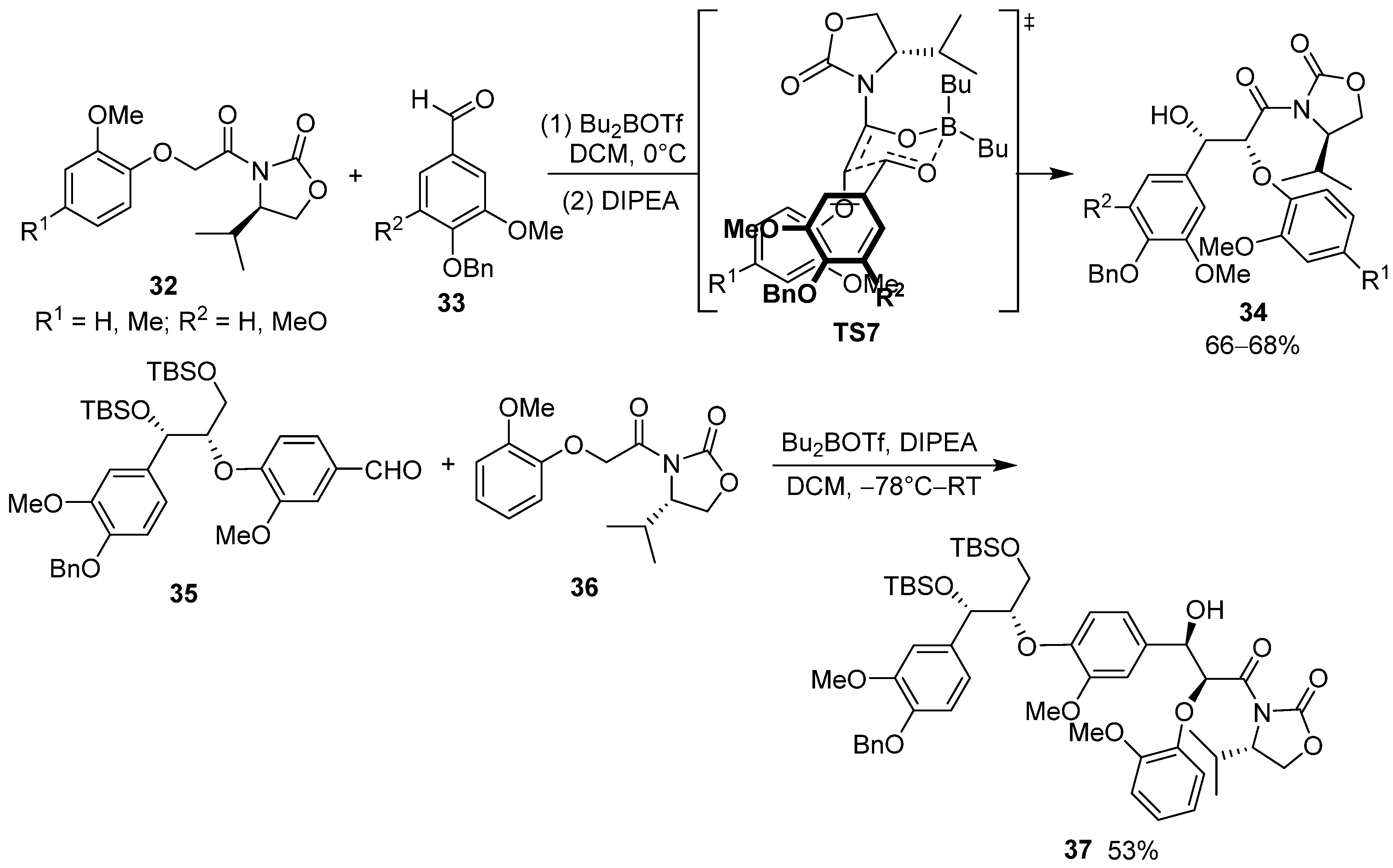
2.3. Chiral Oxazolidinone-Based Auxiliaries in Asymmetric Synthesis
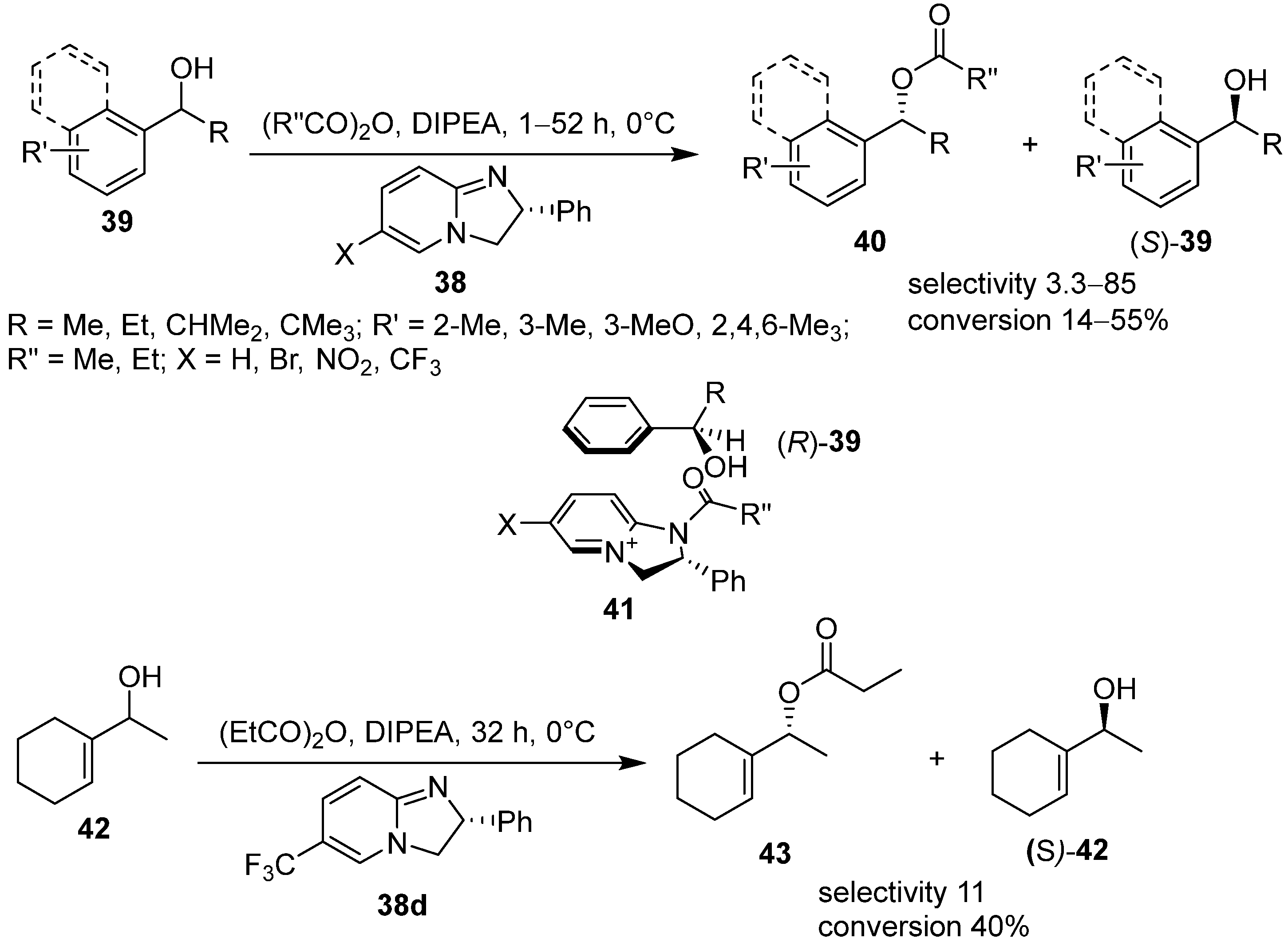
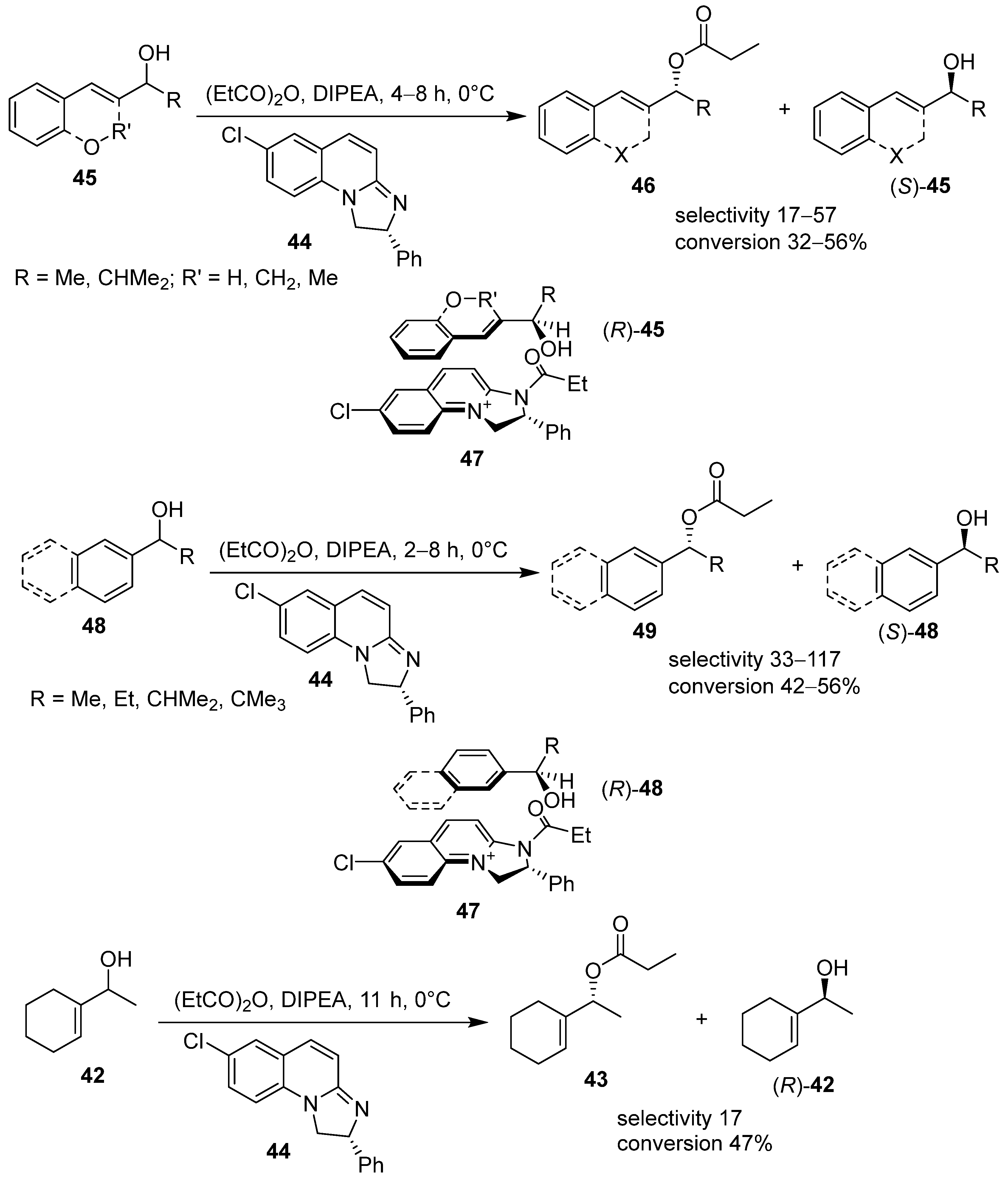
3. Acyl 2,3-Dihydroimidazo[1,2-a]pyridine and 1,2-Dihydroimidazo[1,2-a]quinolines in Kinetic Resolution
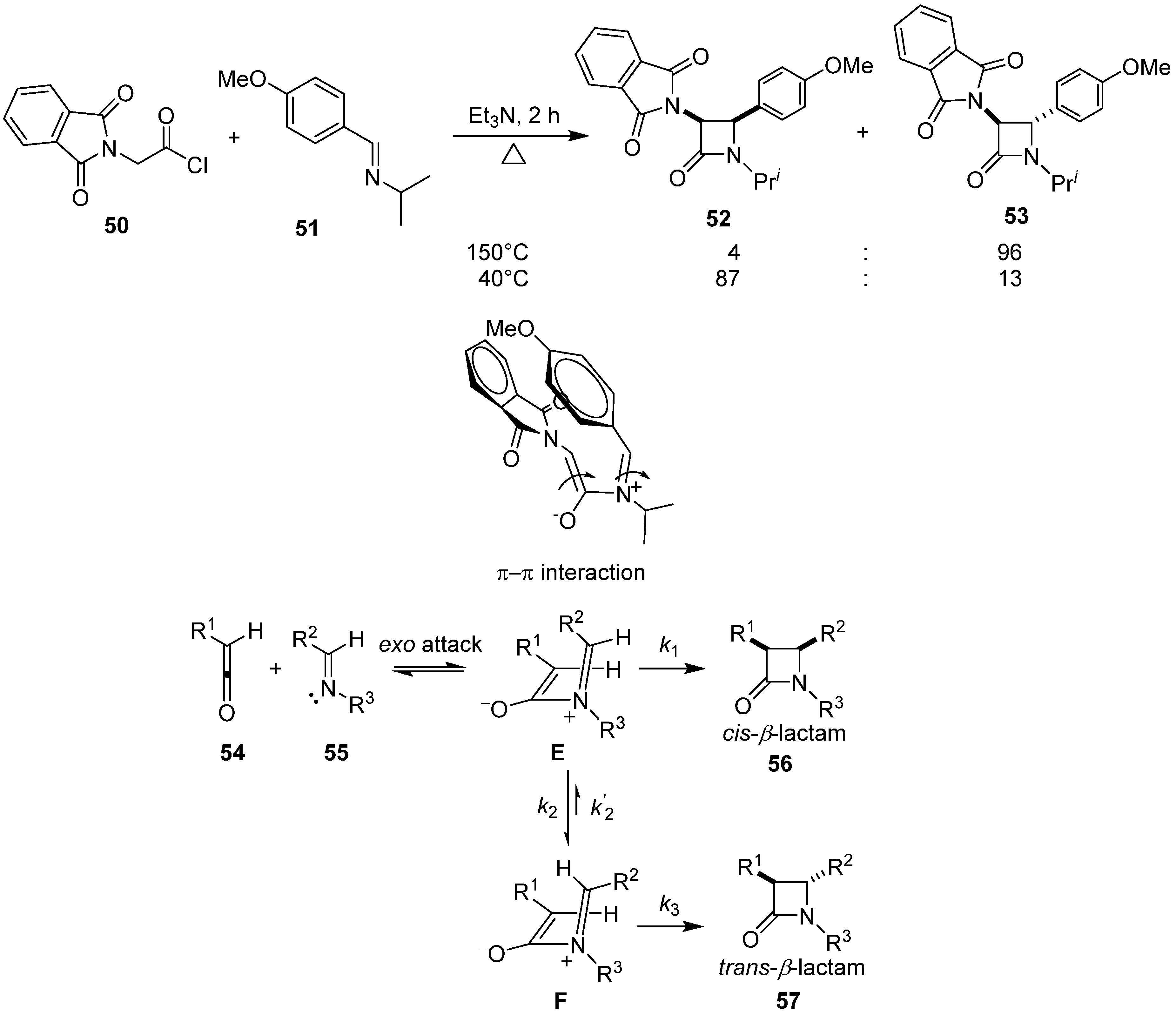
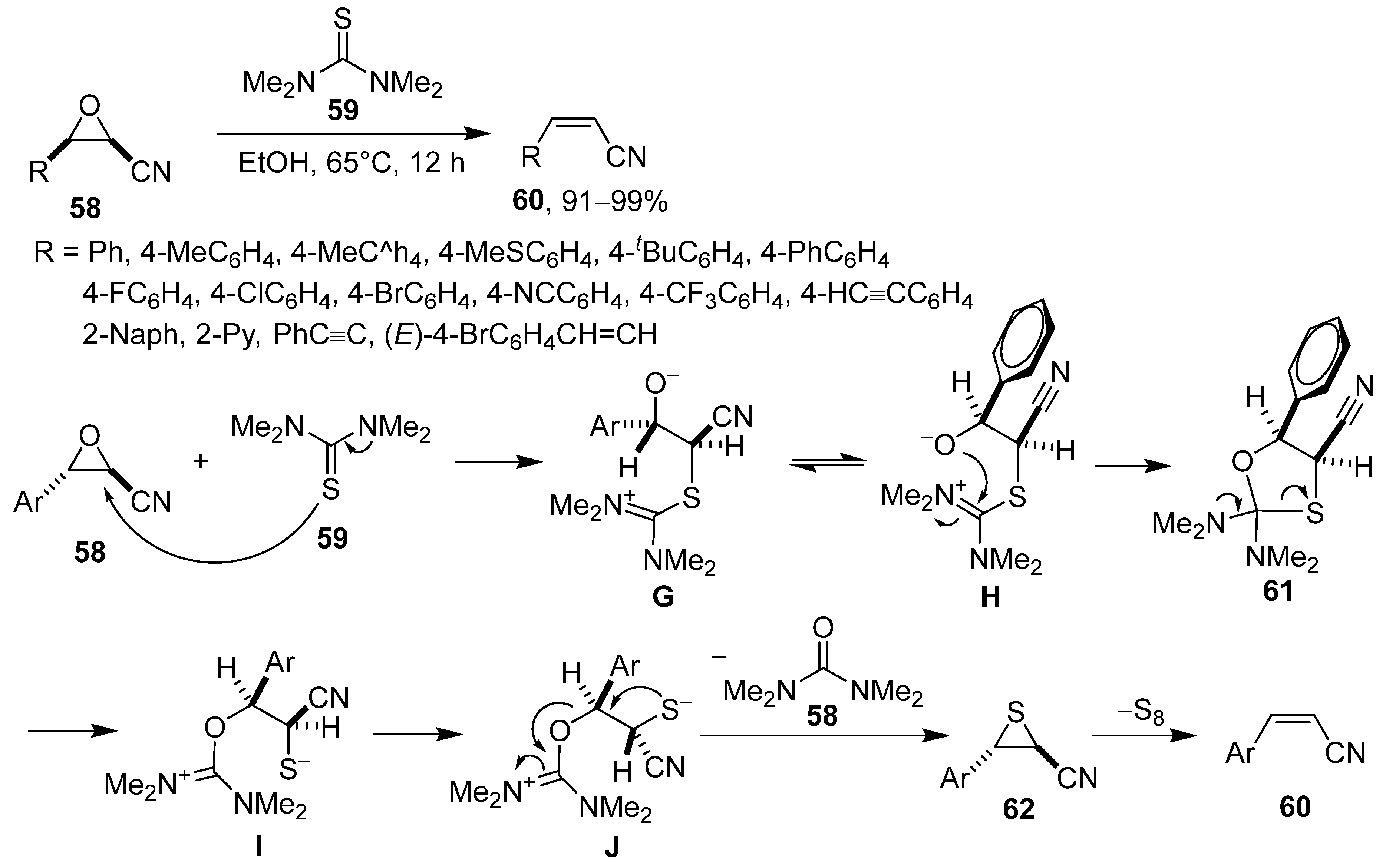
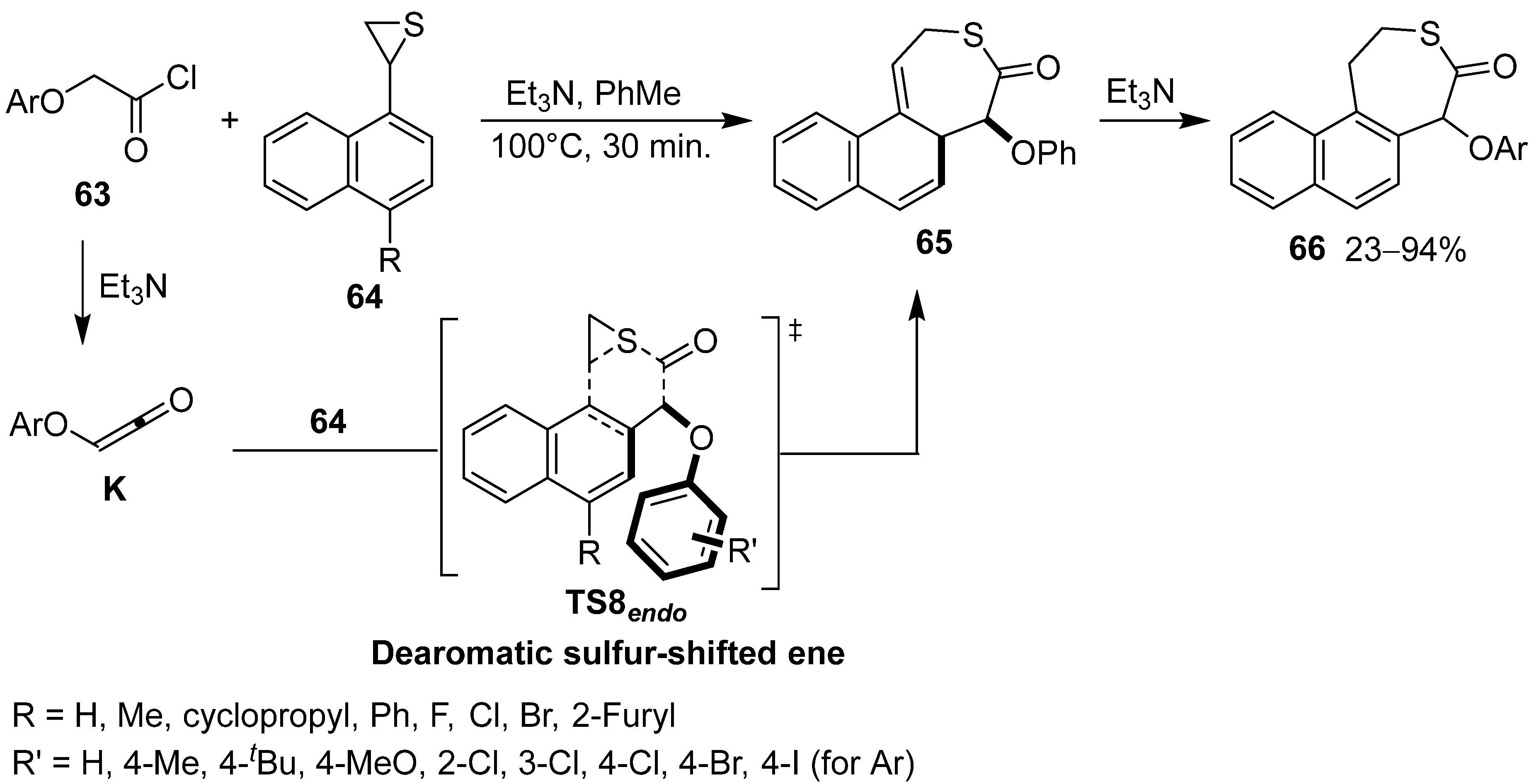
4. Asymmetric Synthesis of β-Lactams and Deoxygenation of Oxiranecarbonitriles via Intramolecular π–π Stacking Interaction
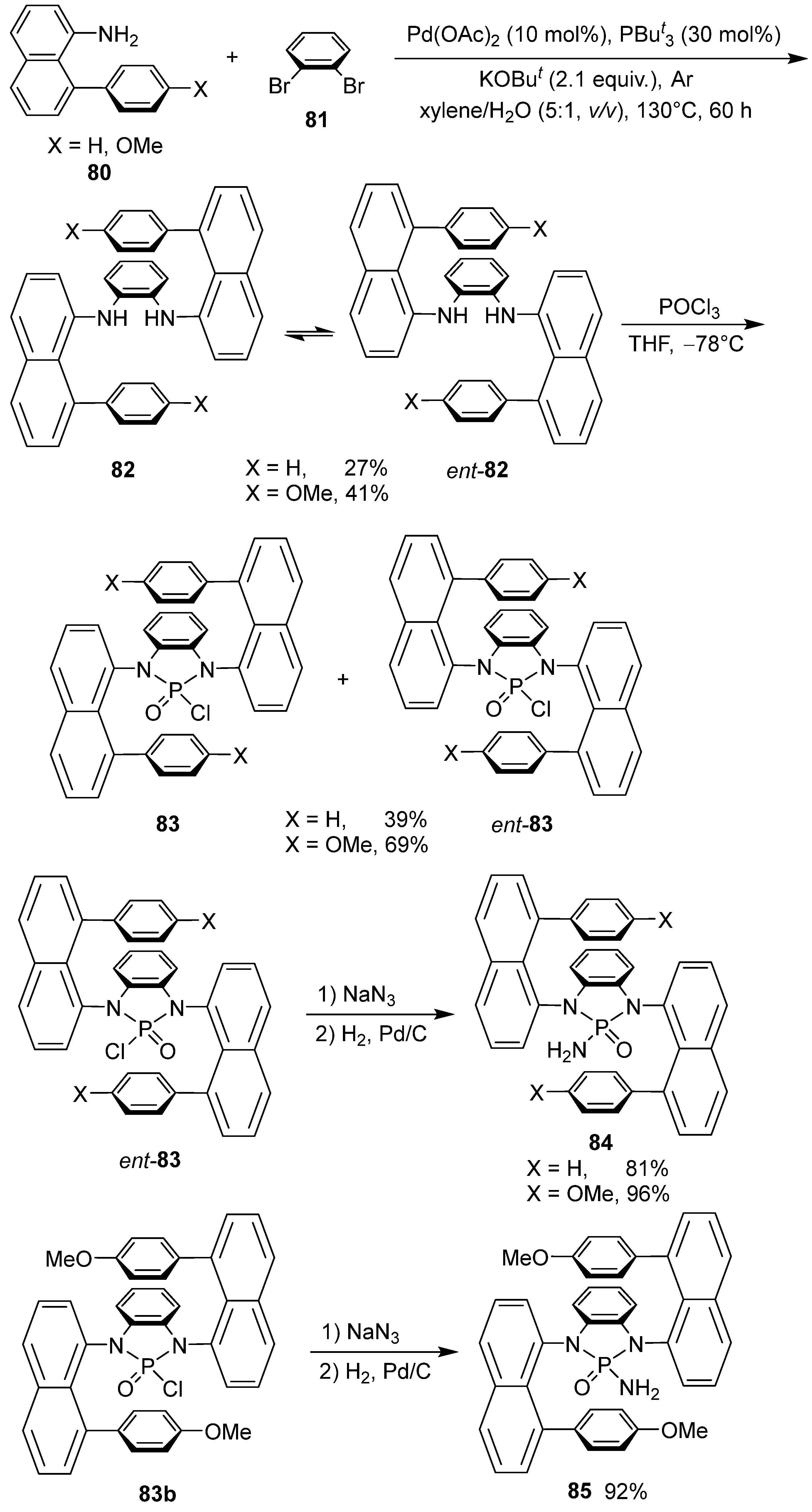
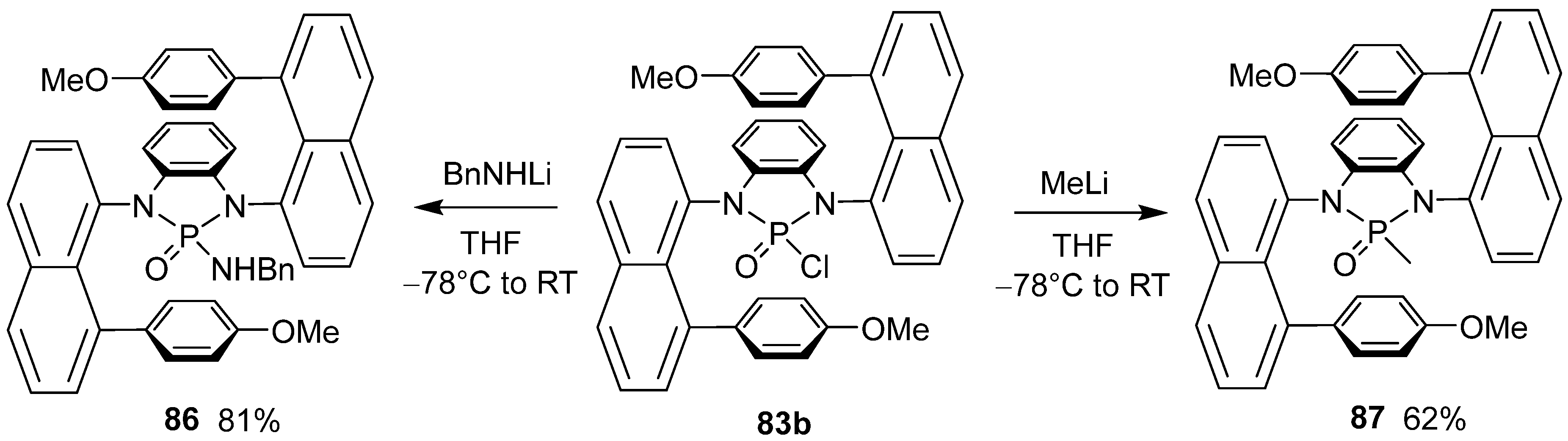
5. Asymmetric Synthesis of Optically Active Helicenes
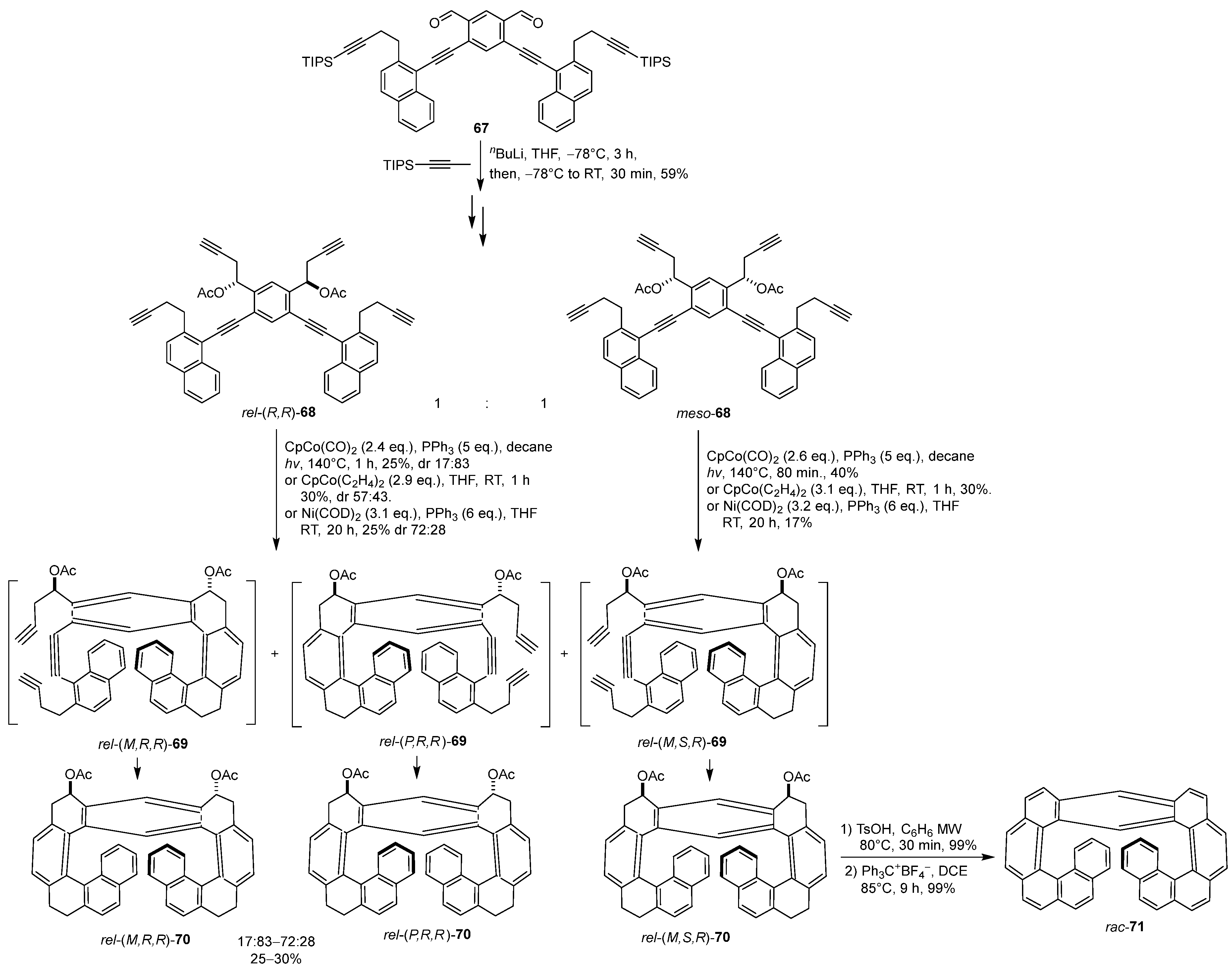
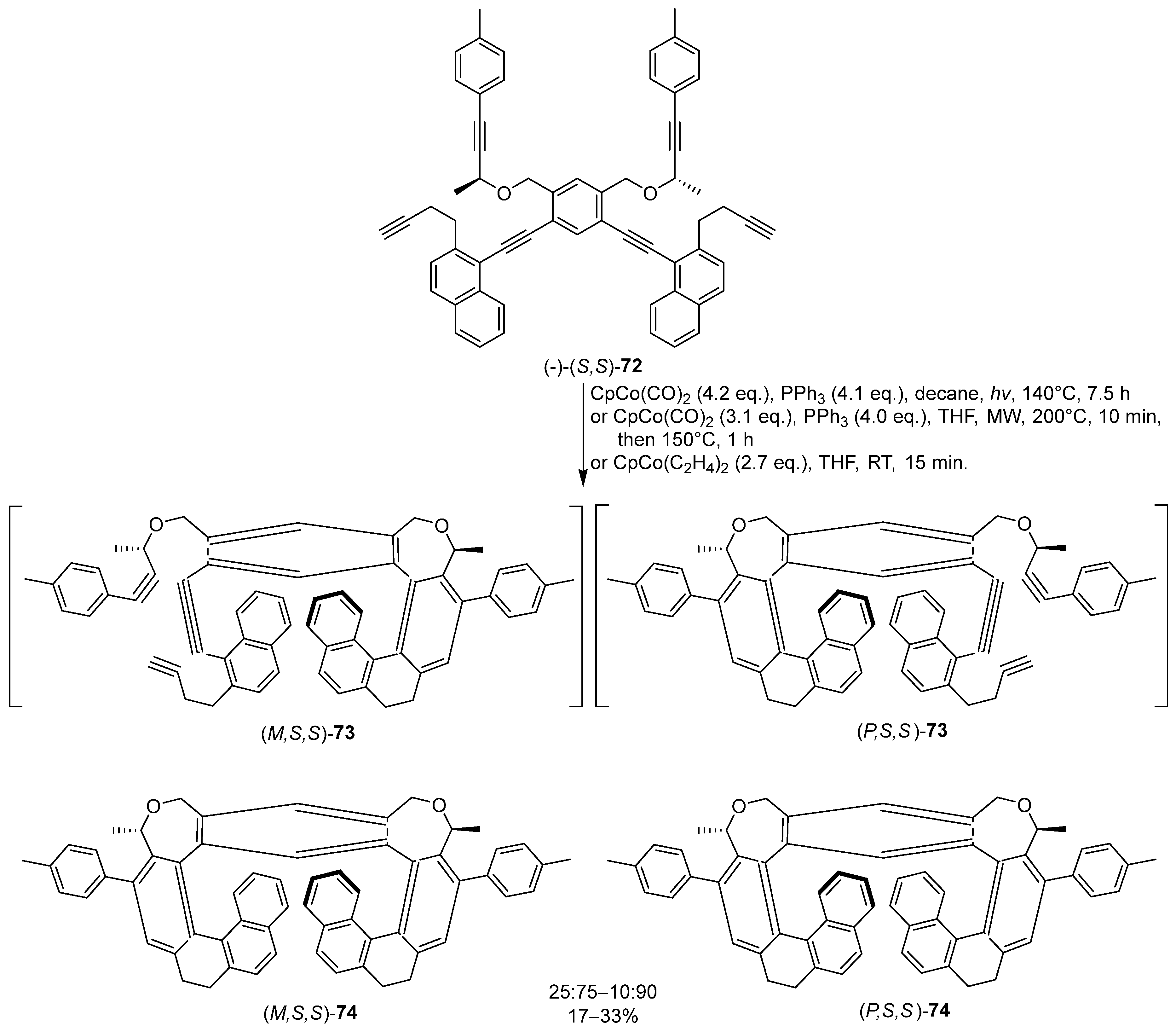
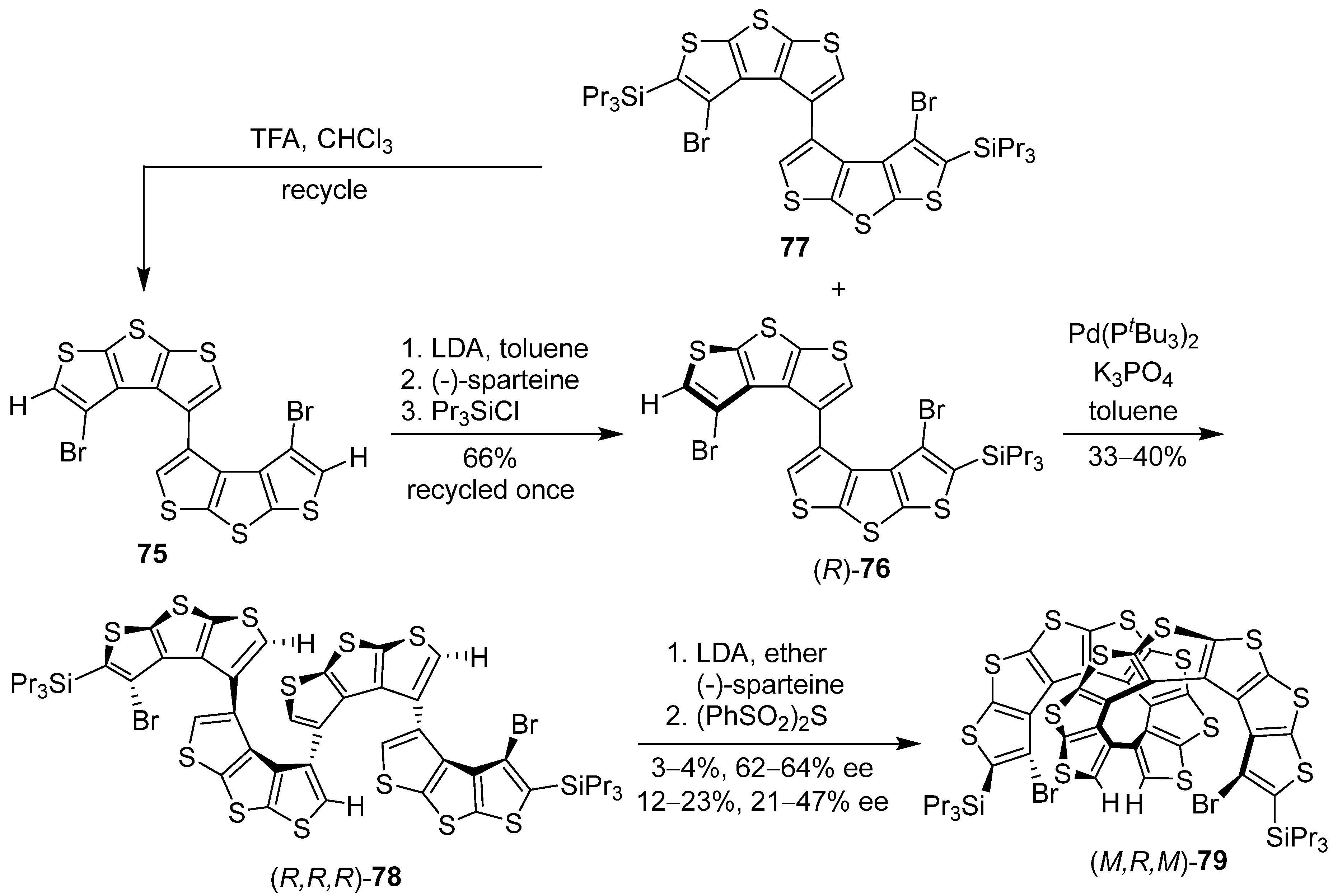
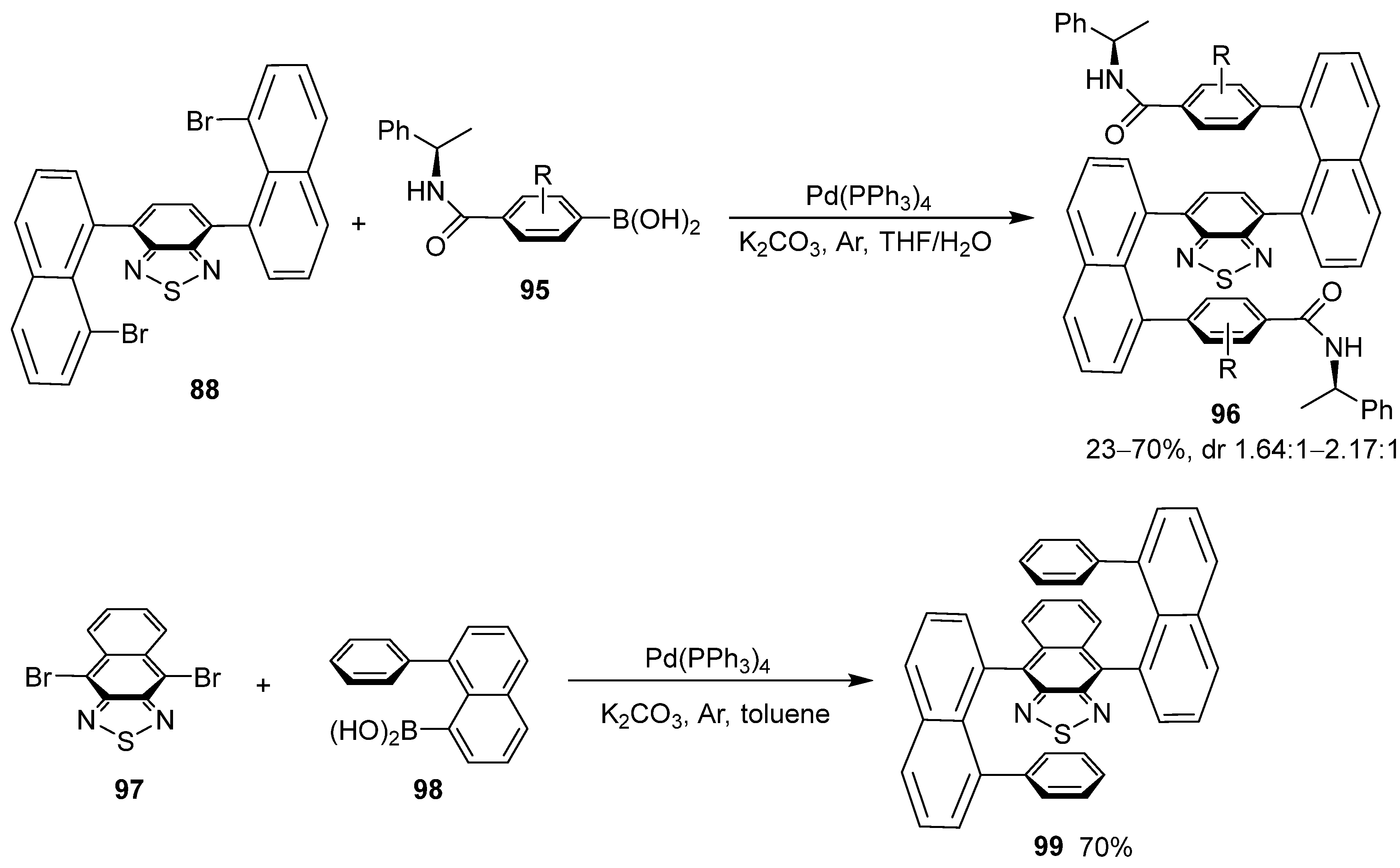
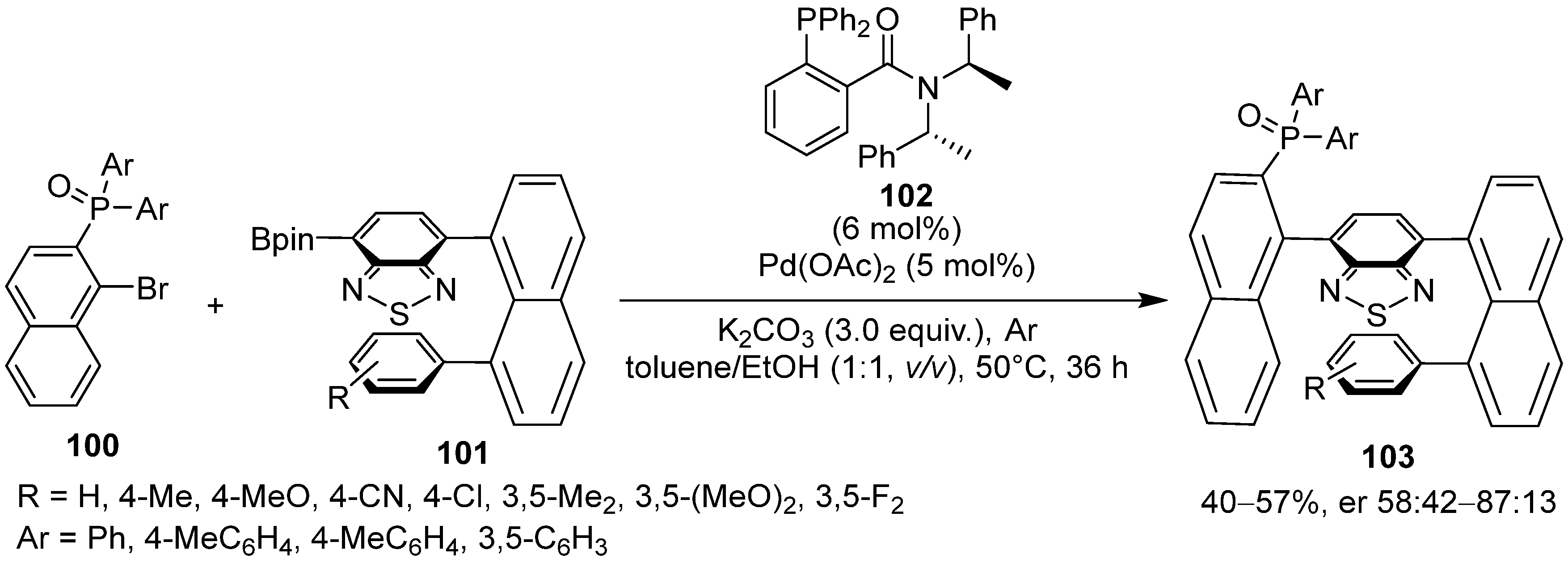
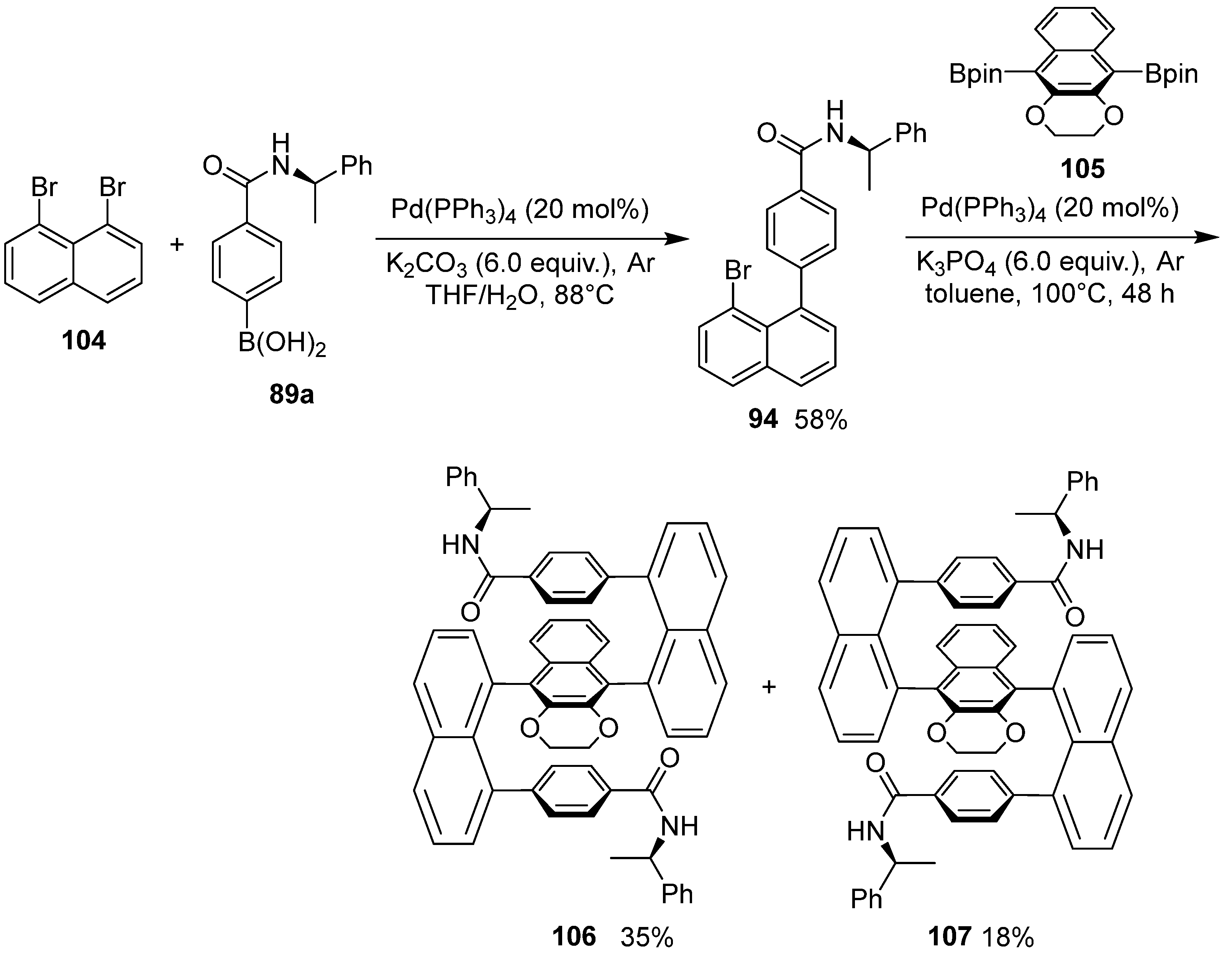
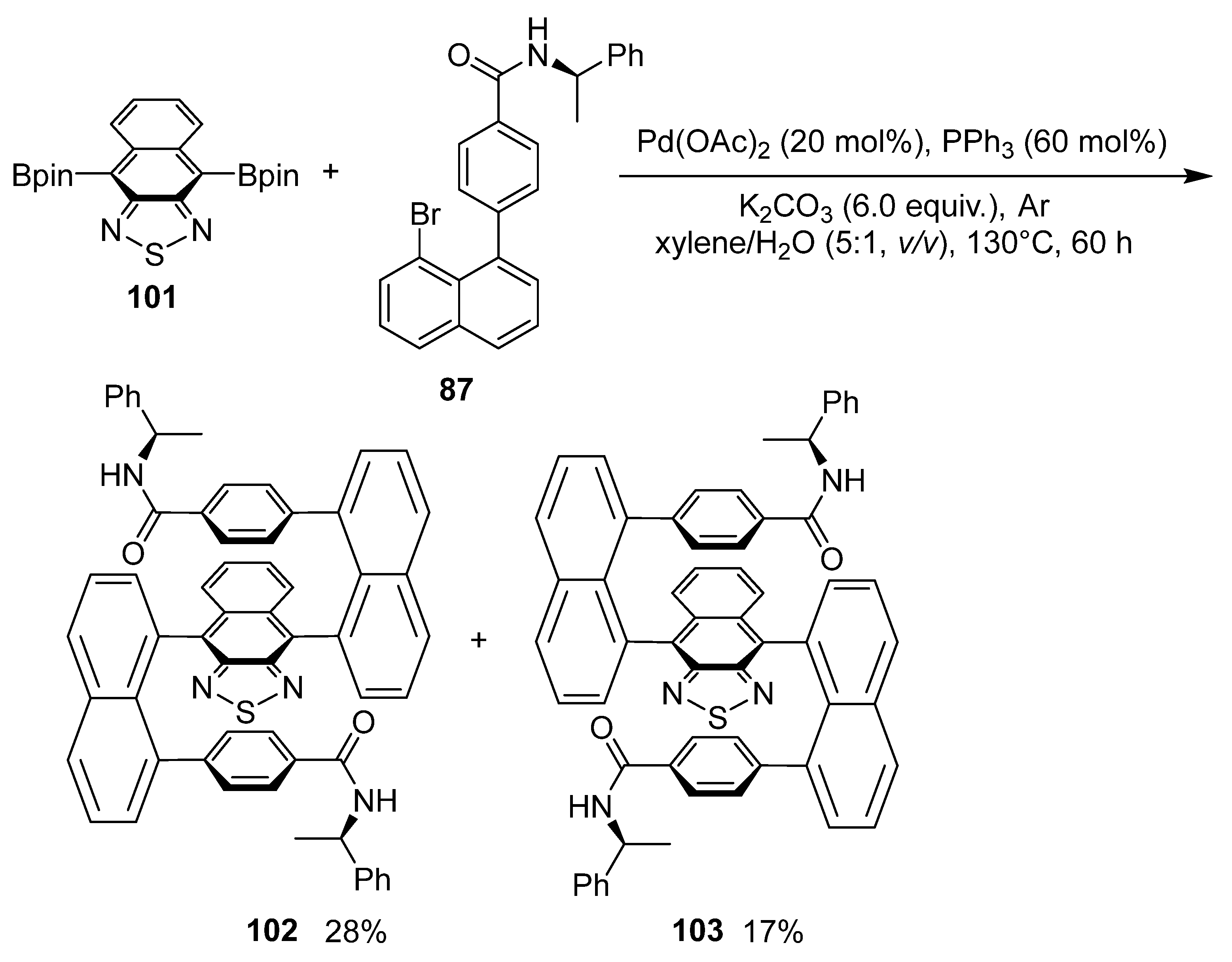
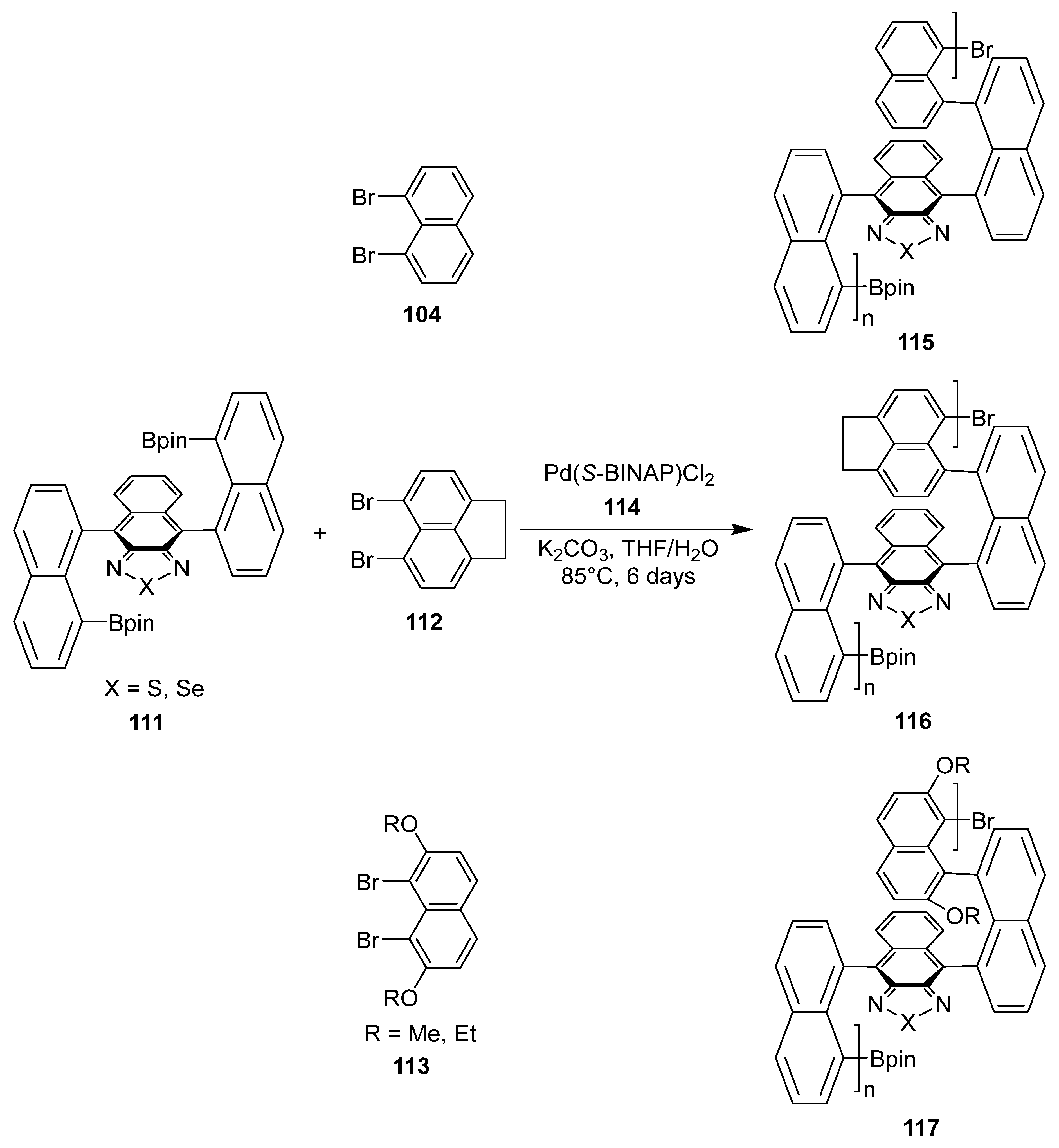
6. Asymmetric Synthesis of Multilayer 3D Chiral Molecules
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, C.J.; Sanders, J.K.M. The nature of π-π interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Sherrill, C.D. Energy component analysis of π interactions. Acc. Chem. Res. 2013, 46, 1020–1028. [Google Scholar] [CrossRef]
- Rahman, M.M.; Muhseen, Z.T.; Junaid, M.; Zhang, H.J. The aromatic stacking interactions between proteins and their macromolecular ligands. Curr. Protein Pept. Sci. 2015, 16, 502–512. [Google Scholar] [CrossRef]
- Davis, J.T.; Spada, G.P. Supramolecular architectures generated by self-assembly of guanosine derivatives. Chem. Soc. Rev. 2007, 36, 296–313. [Google Scholar] [CrossRef]
- Paliwal, S.; Geib, S.; Wilcox, C.S. Molecular torsion balance for weak molecular recognition forces. Effects of “tilted-T” edge-to-face aromatic interactions on conformational selection and solid-state structure. J. Am. Chem. Soc. 1994, 116, 4497–4498. [Google Scholar] [CrossRef]
- Mo, S.Y.; Xu, J.X. Chemospecific intramolecular Büchner reaction catalyzed by copper(II) acetylacetonate. ChemCatChem 2014, 6, 1679–1683. [Google Scholar] [CrossRef]
- Hein, S.J.; Lehnherr, D.; Arslan, H.; Uribe-Romo, F.J.; Dichtel, W.R. Alkyne benzannulation reactions for the synthesis of novel aromatic architectures. Acc. Chem. Res. 2017, 50, 2776–2788. [Google Scholar] [CrossRef]
- Ma, L.G.; Jiao, P.; Zhang, Q.H.; Du, D.-M.; Xu, J.X. Ligand and substrate pi-stacking interaction controlled enantioselectivity in the asymmetric aziridination. Tetrahedron Asymmetry 2007, 18, 878–884. [Google Scholar] [CrossRef]
- Ma, L.G.; Jiao, P.; Zhang, Q.H.; Xu, J.X. Rigid backbone 1,8-anthracene-linked bis-oxazolines (AnBOXes): Design, synthesis, application and characteristics in catalytic asymmetric aziridination. Tetrahedron Asymmetry 2005, 16, 3718–3734. [Google Scholar] [CrossRef]
- Rezazgui, O.; Boëns, B.; Teste, K.; Vergnaud, J.; Trouillas, P.; Zerrouki, R. One-pot and catalyst-free amidation of ester: A matter of non-bonding interactions. Tetrahedron Lett. 2011, 52, 6796–6799. [Google Scholar] [CrossRef]
- Mo, S.Y.; Li, X.H.; Xu, J.X. In situ generated iodonium ylides as safe carbene precursors for the chemoselective intramolecular Buchner reaction. J. Org. Chem. 2014, 79, 9186–9195. [Google Scholar] [CrossRef]
- Langdon, S.M.; Legault, C.Y.; Gravel, M. Origin of chemoselectivity in N-heterocyclic carbene catalyzed cross-benzoin reactions: DFT and experimental insights. J. Org. Chem. 2015, 80, 3597–3610. [Google Scholar] [CrossRef]
- Liu, T.T.; Tang, S.Y.; Hu, B.; Liu, P.; Bi, S.W.; Jiang, Y.Y. Mechanism and origin of Chemoselectivity of Ru-catalyzed cross-coupling of secondary alcohols to β-disubstituted ketones. J. Org. Chem. 2020, 85, 12444–12455. [Google Scholar] [CrossRef]
- Qi, T.; Fu, S.; Zhang, X.; Liu, T.H.; Li, Q.Z.; Gou, C.A.; Li, J.L. Theoretical insight into the origins of chemo- and diastereo-selectivity in the palladium-catalysed (3+2) cyclisation of 5-alkenyl thiazolones. Org. Chem. Front. 2021, 8, 6203–6214. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, J.; Wu, Y. Synthesis and regiochemistry of spiro indane-1,3-dione compounds. Res. Chem. Intermediat. 2012, 38, 413–420. [Google Scholar] [CrossRef]
- Chen, G.; Yang, J.; Gao, S.; Zhang, Y.; Hao, X.J. Theoretical study of the regioselectivity of the Huisgen reaction. Res. Chem. Intermediat. 2013, 39, 1245–1250. [Google Scholar] [CrossRef]
- Yu, H.; Cao, S.L.; Zhang, L.L.; Liu, G.; Xu, J.X. Synthesis of α-aliphatic and β-aromatic substituted taurines via the regioselective ammonia ring-opening of thiiranes. Synthesis 2009, 13, 2205–2209. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Xu, J.X. Regio- and stereoselective synthesis of thiazoline derivatives via the thioketene-induced ring expansion of aziridines. Chem. Commun. 2022, 58, 2714–2717. [Google Scholar] [CrossRef]
- Shen, B.M.; Chen, Y.; Yu, P.Y. Mechanism and origin of regioselectivity in Rh-catalyzed desymmetric [2+2+2] cycloaddition: Charge versus π-π stacking interaction. Org. Chem. Front. 2022, 9, 4625–4632. [Google Scholar] [CrossRef]
- Jones, G.B.; Chapman, B.J. π–Stacking effect in asymmetric synthesis. Synthesis 1995, 1995, 475–497. [Google Scholar] [CrossRef]
- Xu, J.X. Influence of the electronic effect of catalysts on the enantioselectivity: Applicability and complexity. Curr. Org. Synth. 2010, 7, 650–676. [Google Scholar] [CrossRef]
- Yamada, S. Intramolecular cation-π interaction in organic synthesis. Org. Biomol. Chem. 2007, 5, 2903–2912. [Google Scholar] [CrossRef]
- Fanourakis, A.; Docherty, P.J.; Chuentragool, P.; Phipps, R.J. Recent developments in enantioselective transition metal catalysis featuring attractive noncovalent interactions between ligand and substrate. ACS Catal. 2020, 10, 10672–10714. [Google Scholar] [CrossRef]
- Fustero, S.; Navarro, A.; Pina, B.; Soler, J.G.; Bartolome, A.; Asensio, A.; Simon, A.; Bravo, P.; Fronza, G.; Volonterio, A.; et al. Enantioselective synthesis of fluorinated α-amino acids and derivatives in combination with ring-closing methathesis: Intramolecular π–stacking interactions as a source of stereocontrol. Org. Lett. 2001, 3, 2621–2624. [Google Scholar] [CrossRef]
- García Ruano, J.L.; Alemán, J.; Catalán, S.; Marcos, V.; Monteagudo, S.; Parra, A.; del Pozo, C.; Fustero, S. Anionic–anionic asymmetric tandem reactions: One-pot synthesis of optically pure fluorinated indolines from 2-p-tolylsulfinyl alkylbenzenes. Angew. Chem. Int. Ed. 2008, 47, 7941–7944. [Google Scholar] [CrossRef]
- Garcia Ruano, J.L.; Alemán, J.; Soriano, J.F. Facile synthesis of optically pure 1,2-diaryl (and 1-alkyl-2-aryl) ethyl and propylamines. Org. Lett. 2003, 5, 677–680. [Google Scholar] [CrossRef]
- Garcia Ruano, J.L.; Alemán, J.; Parra, A. Highly stereoselective benzylation of N-sulfinylketimines. J. Am. Chem. Soc. 2005, 127, 13048–13054. [Google Scholar] [CrossRef]
- Garcia Ruano, J.L.; Parra, A.; Marcos, V.; del Pozo, C.; Catalan, S.; Monteagudo, S.; Fustero, S.; Poveda, A. Asymmetric synthesis of indolines through intramolecular shifting of aromatic sulfinyl groups. Role of the π-stacking interactions in these unusual SNAr processes. J. Am. Chem. Soc. 2009, 131, 9432–9441. [Google Scholar] [CrossRef]
- Corne, V.; Sarotti, A.M.; de Arellano, C.R.; Spanevello, R.A.; Suarez, A.G. Experimental and theoretical insights in the alkene-arene intramolecular π-stacking interaction. Beilstein J. Org. Chem. 2016, 12, 1616–1623. [Google Scholar] [CrossRef]
- Klepp, J.; Sumby, C.J.; Greatrex, B.W. Synthesis of a chiral auxiliary family from levoglucosenone and evaluation in the Diels–Alder reaction. Synlett 2018, 29, 1441–1446. [Google Scholar]
- Njiojob, C.N.; Bozell, J.J.; Long, B.K.; Elder, T.; Key, R.E.; Hartwig, W.T. Enantioselective synthesis of lignin models: An efficient synthesis of β-O-4 dimers and trimers by using the Evans chiral auxiliary. Chem. Eur. J. 2016, 22, 12506–12517. [Google Scholar] [CrossRef]
- Xu, J.X.; Wei, T.Z.; Zhang, Q.H. Effect of temperature on the enantioselectivity in the oxazaborolidine-catalyzed asymmetric reduction of ketones. non-catalytic borane reduction, a non-neglectable factor in reduction system. J. Org. Chem. 2003, 68, 10146–10151. [Google Scholar] [CrossRef]
- Xu, J.X.; Wei, T.Z.; Zhang, Q.H. Influences of electronic effects and anions on the enantioselectivity in the oxazaborolidine-catalyzed asymmetric borane reduction of ketones. J. Org. Chem. 2004, 69, 6860–6866. [Google Scholar] [CrossRef]
- Xu, J.X.; Wei, T.Z.; Xia, J.K.; Zhang, Q.H.; Wu, H.S. Preparation of highly optically pure homochiral sulfide-containing alcohols via oxazaborolidine-catalyzed asymmetric borane reduction of ketones. Chirality 2004, 16, 341–346. [Google Scholar] [CrossRef]
- Su, X.B.; Zhang, Q.H.; Wu, Y.Q.; Xu, J.X. Preparation of linear secondary alcohols of high optical purity through chemical resolution with chiral α-phenylethylamines. Chin. J. Org. Chem. 2002, 22, 496–500. [Google Scholar]
- Zhang, Q.H.; Lin, C.X.; Zhang, S.S.; Xu, J.X. Preparation of optically active trans-1-(4-alkylcyclohexyl) alcohols. Chin. J. Org. Chem. 2004, 24, 1069–1074. [Google Scholar]
- Birman, V.B.; Uffman, E.W.; Jiang, H.; Li, X.; Kilbane, C.J. 2,3-Dihydroimidazo[1,2-a]pyridines: A new class of enantioselective acyl transfer catalysts and their use in kinetic resolution of alcohols. J. Am. Chem. Soc. 2004, 126, 12226–12227. [Google Scholar] [CrossRef] [PubMed]
- Birman, V.B.; Jiang, H. Kinetic resolution of alcohols using a 1,2-dihydroimidazo[1,2-a]quinolone enantioselective acylation catalyst. Org. Lett. 2005, 7, 3445–3447. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.X. Stereoselectivity in the synthesis of 2-azetidinones from ketenes and imines via the Staudinger reaction. ARKIVOC 2009, 9, 21–44. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, Q.F.; Liang, Y.; Zhang, S.W.; Xu, J.X. A versatile method for the synthesis of 3-alkoxycarbonyl β-lactam derivatives. J. Org. Chem. 2006, 71, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jiao, L.; Zhang, S.W.; Xu, J.X. Microwave and photoirradiation-induced Staudinger reactions of cyclic imines and ketenes generated from α-diazoketones. A further investigation into the Stereochemical process. J. Org. Chem. 2005, 70, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Liang, Y.; Xu, J.X. Origin of the relative stereoselectivity of the β-lactam formation in the Staudinger reaction. J. Am. Chem. Soc. 2006, 128, 6060–6069. [Google Scholar] [CrossRef]
- Wang, Y.K.; Liang, Y.; Jiao, L.; Du, D.-M.; Xu, J.X. Do reaction conditions affect the stereoselectivity in the Staudinger reaction? J. Org. Chem. 2006, 71, 6983–6990. [Google Scholar] [CrossRef]
- Li, B.N.; Wang, Y.K.; Du, D.-M.; Xu, J.X. Notable and obvious ketene substituent-dependent effect of temperature on the stereoselectivity in the Staudinger reaction. J. Org. Chem. 2007, 72, 990–997. [Google Scholar] [CrossRef]
- Liang, Y.; Jiao, L.; Zhang, S.W.; Yu, Z.X.; Xu, J.X. New insights into the torquoselectivity of the Staudinger reaction. J. Am. Chem. Soc. 2009, 131, 1542–1549. [Google Scholar] [CrossRef]
- Xu, C.C.; Xu, J.X. Synthetic applications of oxiranecarbonitriles. Chem. Heterocycl. Compd. 2021, 57, 731–733. [Google Scholar] [CrossRef]
- Xu, C.C.; Xu, J.X. Oxygenophilic Lewis acid-promoted synthesis of 2-arylindoles from anilines and cyanoepoxides in alcohol. J. Org. Chem. 2018, 83, 14733–14742. [Google Scholar] [CrossRef]
- Xu, C.C.; Xu, J.X. BF3·OEt2-promoted tandem Meinwald rearrangement and nucleophilic substitution of oxiranecarbonitriles. Org. Biomol. Chem. 2020, 18, 127–134. [Google Scholar] [CrossRef]
- Xu, C.X.; Lu, Y.; Xu, K.N.; Xu, J.X. BF3·OEt2-Catalyzed synthesis of anti-β-(N-arylamino)-α-hydroxynitriles by regio- and diastereospecific ring-opening of 3-aryloxirane-2-carbonitriles with anilines. Synthesis 2020, 52, 602–608. [Google Scholar] [CrossRef]
- Xu, C.C.; Xie, W.L.; Xu, J.X. Metal-free and regiospecific synthesis of 3-arylindoles. Org. Biomol. Chem. 2020, 18, 2661–2671. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Shi, S.J.; Yang, Z.H. Thiourea-mediated stereospecific deoxygenantion of cyanoepoxides to access highly diastereopure alkenyl nitriles. J. Org. Chem. 2024, 89, 2748–2758. [Google Scholar] [CrossRef] [PubMed]
- D’hooghe, M.; Ha, H.-J. Synthesis of 4-to 7-Membered Heterocycles by Ring Expansion; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Chen, X.P.; Huang, Z.S.; Xu, J.X. Catalyst-free electrophilic ring expansion of N-unprotected aziridines with α-oxoketenes to efficient access 2-alkylidene-1,3-oxazolidines. Adv. Synth. Catal. 2021, 363, 3098–3108. [Google Scholar] [CrossRef]
- Lei, Y.L.; Xu, J.X. Efficient synthesis of ethyl 2-(oxazolin-2-yl)alkanoates via ethoxycarbonylketene-Induced electrophilic ring expansion of aziridines. Beilstein J. Org. Chem. 2022, 18, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Chen, Y.X.; Xu, J.X. π-Stacking-controlled dearomatic sulfur-shifted ene reaction of ketenes and polycyclic arylthiiranes: Access to areno[d]-ε-thiolactones. J. Org. Chem. 2024, 89. [Google Scholar] [CrossRef]
- Sehnal, P.; Stará, I.G.; Šaman, D.; Tichý, M.; Mišek, J.; Cvačka, J.; Rulišek, L.; Chocholoušová, J.; Vacek, J.; Goryl, G.; et al. An organometallic route to long helicenes. Proc. Nat. Acad. Sci. USA 2009, 106, 13169–13174. [Google Scholar] [CrossRef]
- Miyasaka, M.; Pink, M.; Rajca, S.; Rajca, A. Noncovalent interactions in the asymmetric synthesis of rigid, conjugated helical structures. Angew. Chem. Int. Ed. 2009, 48, 5954–5957. [Google Scholar] [CrossRef]
- Rajca, A.; Miyasaka, M.; Pink, M.; Wang, H.; Rajca, S. Helically annelated and cross-conjugated oligothiophenes: Asymmetric synthesis, resolution, and characterization of a carbon-sulfur [7]helicene. J. Am. Chem. Soc. 2004, 126, 15211–15222. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wu, G.Z.; Yang, Z.; Rouh, H.; Katakam, N.; Ahmed, S.; Unruh, D.; Cui, Z.H.; Lischka, H.; Li, G.G. Multi-layer 3D chirality: New synthesis, AIE and computional studies. Sci. China Chem. 2020, 63, 692–698. [Google Scholar] [CrossRef]
- Wu, G.Z.; Liu, Y.X.; Yang, Z.; Jiang, T.; Katakam, N.; Rouh, H.; Ma, L.L.; Tang, Y.; Ahmed, S.; Rahman, A.; et al. Enantioselective assembly of multi-layer 3D chirality. Nat. Sci. Rev. 2020, 7, 588–599. [Google Scholar] [CrossRef]
- Wu, G.Z.; Liu, Y.X.; Rouh, H.; Ma, L.L.; Tang, Y.; Zhang, S.; Zhou, P.; Wang, J.-Y.; Jin, S.Z.; Unruh, D.; et al. Asymmetric catalytic approach to multilayer 3D chirality. Chem. Eur. J. 2021, 27, 8013–8020. [Google Scholar] [CrossRef]
- Liu, H.X.; Rouh, H.; Tang, Y.; Wu, G.Z.; Yuan, Q.K.; Zhang, S.; Wang, J.Y.; Jin, S.Z.; Xu, T.; Wang, Y.; et al. Enantio- and diastereoselective assembly of multi-layer folding chiral targets via asymmetric catalytic single C–C bond formation. Synlett 2023, 34, 153–158. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, G.Z.; Jin, S.W.; Liu, Y.X.; Ma, L.L.; Zhang, S.; Rouh, H.; Ali, A.I.M.; Wang, J.-Y.; Xu, T.; et al. From center-to-multilayer chirality: Asymmetric synthesis of multilayer targets with electron-rich bridges. J. Org. Chem. 2022, 87, 5976–5986. [Google Scholar] [CrossRef]
- Jin, S.W.; Wang, J.-Y.; Tang, Y.; Rouh, H.; Zhang, S.; Xu, T.; Wang, Y.; Yuan, Q.K.; Chen, D.X.; Unruh, D.; et al. Central-to-folding chirality control: Asymmetric synthesis of multilayer 3D targets with electron-deficient bridges. Front. Chem. 2022, 10, 860398. [Google Scholar] [CrossRef]
- Tang, Y.; Jin, S.Z.; Zhang, S.; Wu, G.-Z.; Wang, J.-Y.; Xu, T.; Wang, Y.; Unruh, D.; Surowiec, K.; Ma, Y.Y.; et al. Multilayer 3D chiral folding polymers and their asymmetric catalytic assembly. Research 2022, 2022, 9847949. [Google Scholar] [CrossRef]
- Kanomata, K.; Toda, Y.; Shibata, Y.; Yamanaka, M.; Tsuzuki, S.; Gridneva, I.D.; Terada, M. Secondary stereocontrolling interactions in chiral Brønsted acid catalysis: Study of a Petasis–Ferrier-type rearrangement catalyzed by chiral phosphoric acids. Chem. Sci. 2014, 5, 3515–3523. [Google Scholar] [CrossRef]
- Yang, H.B.; Zhao, Y.Z.; Sang, R.; Wei, Y.; Shi, M. Asymmetric synthesis of bioxindole-substituted hexahydrofuro[2,3-b]furans via hydroquinine anthraquinone-1,4-diyl diether-catalyzed domino annulation of acylidenoxindoles/isatins, acylidenoxindoles and allenoates. Adv. Synth. Catal. 2014, 356, 3799–3808. [Google Scholar] [CrossRef]
- Machuca, E.; Juaristi, E. Organocatalytic activity of α,α-dipeptide derivatives of (S)-proline in the asymmetric aldol reaction in absence of solvent. Evidence for non-covalent π–π interactions in the transition state. Tetrahedron Lett. 2015, 56, 1144–1148. [Google Scholar] [CrossRef]
- Bai, H.-Y.; Tan, F.-X.; Liu, T.-Q.; Zhu, G.-D.; Tian, J.-M.; Ding, T.-M.; Chen, Z.-M.; Zhang, S.-Y. Highly atroposelective synthesis of nonbiaryl naphthalene-1,2-diamine N-C atropisomers through direct enantioselective C-H amination. Nat. Commun. 2019, 10, 3063. [Google Scholar] [CrossRef]
- Zhou, Q.-Y.; Xin Li, X. Atroposelective construction of axially chiral enamides via N-allylic alkylation. Chem. Commun. 2022, 58, 4727–4730. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-Y.; Li, J.; Ling, L.; Liao, S.-H.; Sun, X.-L.; Li, Y.-X.; Wang, L.-J.; Tang, Y. Highly enantioselective [3+3] cycloaddition of aromatic azomethine imines with cyclopropanes directed by π–π stacking interactions. Angew. Chem. Int. Ed. 2013, 52, 1452–1456. [Google Scholar] [CrossRef]
- Jin, M.Y.; Zhen, Q.Q.; Xiao, D.M.F.; Tao, G.Y.; Xing, X.Y.; Yu, P.Y.; Chen Xu, C. Engineered non-covalent π interactions as key elements for chiral recognition. Nat. Commun. 2022, 13, 3276. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Ryu, H.; Hahm, H.; Lee, J.; Hong, S. Palladium catalysis featuring attractive noncovalent interactions enabled highly enantioselective access to β-quaternary δ-lactams. ACS Catal. 2022, 12, 5559–5564. [Google Scholar] [CrossRef]
- Weh, M.; Kroeger, A.A.; Shoyama, K.; Grüne, M.; Karton, A.; Würthner, F. π-π Catalysis made asymmetric—Enantiomerization catalysis mediated by the chiral π-system of a perylene bisimide cyclophane. Angew. Chem. Int. Ed. 2023, 62, e202301301. [Google Scholar] [CrossRef] [PubMed]

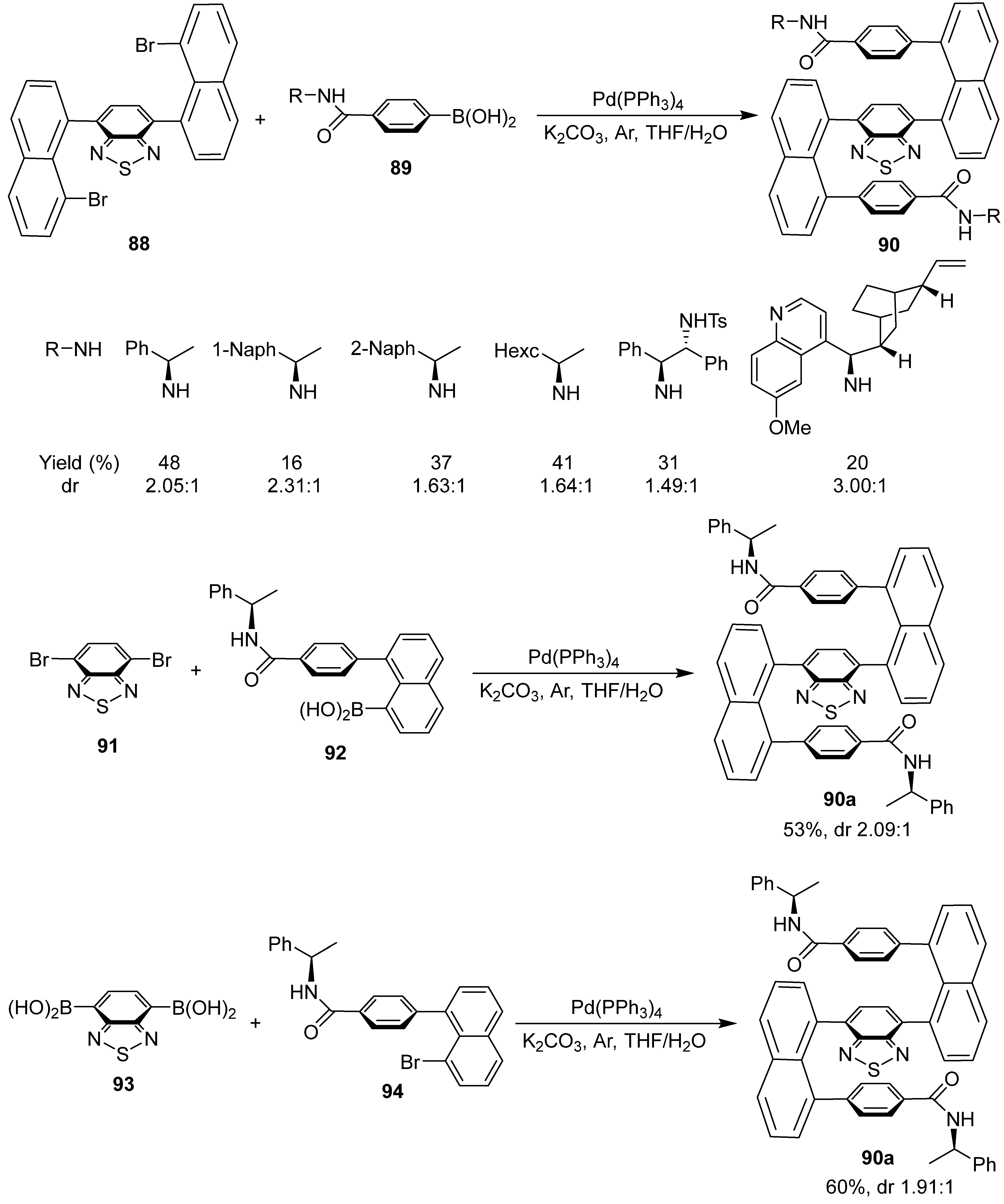
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J. Recent Advances in π-Stacking Interaction-Controlled Asymmetric Synthesis. Molecules 2024, 29, 1454. https://doi.org/10.3390/molecules29071454
Xu J. Recent Advances in π-Stacking Interaction-Controlled Asymmetric Synthesis. Molecules. 2024; 29(7):1454. https://doi.org/10.3390/molecules29071454
Chicago/Turabian StyleXu, Jiaxi. 2024. "Recent Advances in π-Stacking Interaction-Controlled Asymmetric Synthesis" Molecules 29, no. 7: 1454. https://doi.org/10.3390/molecules29071454
APA StyleXu, J. (2024). Recent Advances in π-Stacking Interaction-Controlled Asymmetric Synthesis. Molecules, 29(7), 1454. https://doi.org/10.3390/molecules29071454




