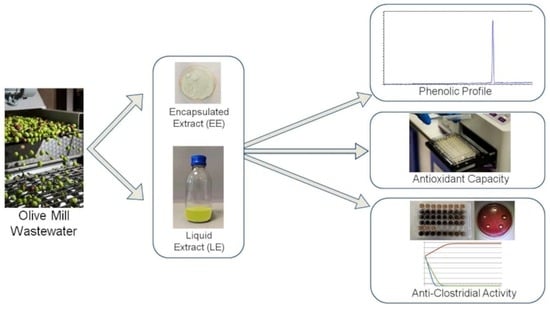
The Effects of Encapsulation on the In Vitro Anti-Clostridial Activity of Olive Mill Wastewater Polyphenolic Extracts: A Promising Strategy to Limit Microbial Growth in Food Systems
Abstract
1. Introduction
2. Results
2.1. Determination of the Phenolic Profile
2.2. Antioxidant Activity
2.3. In Vitro Evaluation of Antibacterial Activity—Agar Well Diffusion and Broth Microdilution
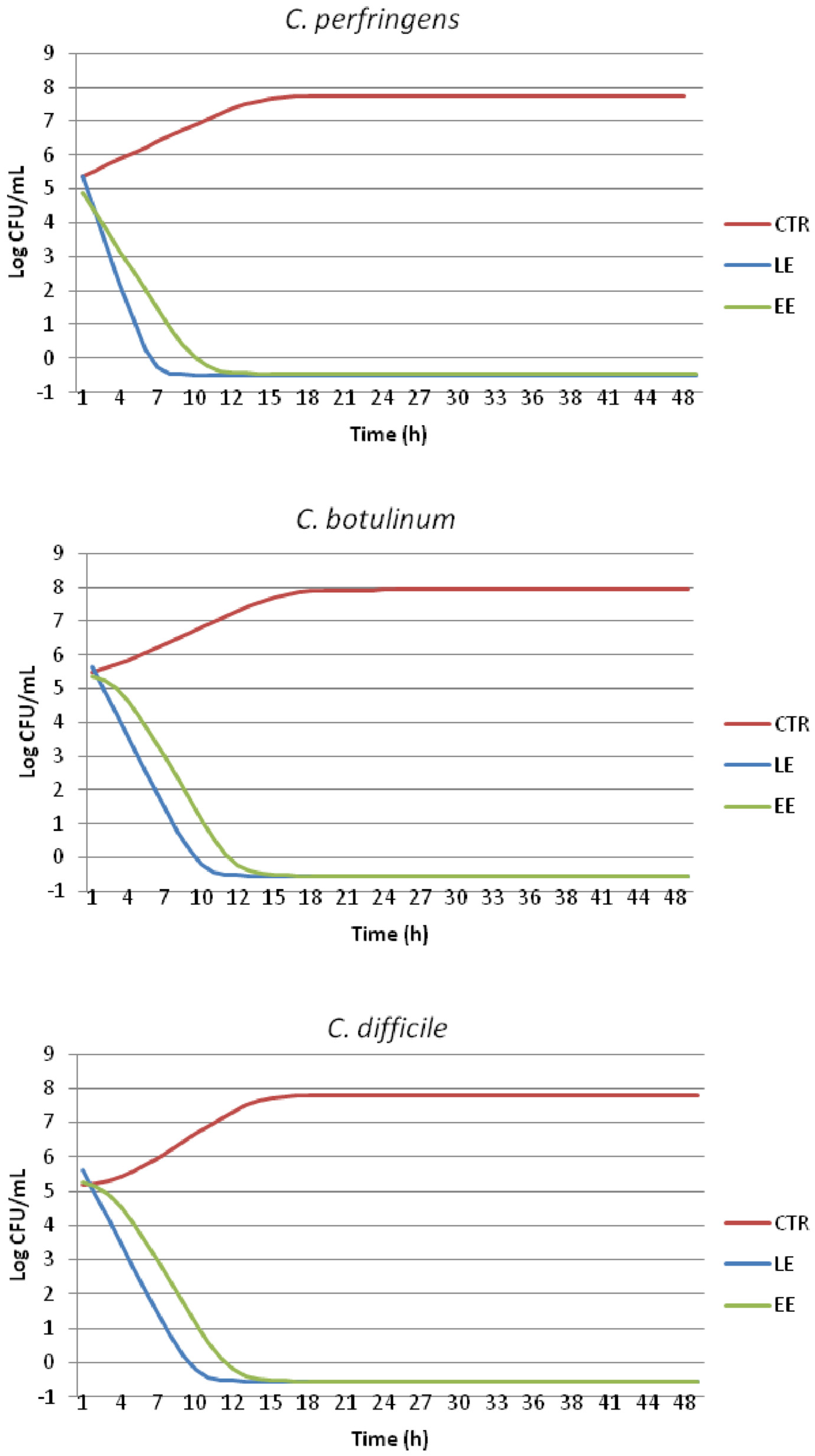
2.4. Time-Kill Test and Evaluation of Growth Dynamics
3. Discussion
4. Materials and Methods
4.1. Test Materials
4.2. Antioxidant Capacity Determination
4.3. In Vitro Evaluation of Antibacterial Activity—Agar Well Diffusion
4.4. MIC/MBC Assay
4.5. Time-Kill Test and Evaluation of Growth Dynamics
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity-A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529. [Google Scholar] [CrossRef]
- Stringer, S.C.; Peck, M.W. Foodborne clostridia and the safety of in-pack preserved foods. In Woodhead Publishing Series in Food Science, Technology and Nutrition, In-Pack Processed Foods; Richardson, P., Ed.; Woodhead Publishing: Shaston, UK, 2008; pp. 251–276. ISBN 9781845692469. [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar]
- Candel-Pérez, C.; Ros-Berruezo, G.; Martínez-Graciá, C. A review of Clostridioides [Clostridium] difficile occurrence through the food chain. Food Microbiol. 2019, 77, 118–129. [Google Scholar] [CrossRef]
- Primavilla, S.; Farneti, S.; Petruzzelli, A.; Drigo, I.; Scuota, S. Contamination of hospital food with Clostridium difficile in Central Italy. Anaerobe 2019, 55, 8–10. [Google Scholar] [CrossRef]
- Singh, S.; Shalini, R. Effect of hurdle technology in food preservation: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Milani, E.; Silva, F.V. Comparing high pressure thermal processing and thermosonication with thermal processing for the inactivation of bacteria, moulds, and yeasts spores in foods. J. Food Eng. 2017, 214, 90–96. [Google Scholar]
- Etienne, J.; Chirico, S.; McEntaggart, K.; Papoutsis, S.; Millstone, E. EU Insights–Consumer perceptions of emerging risks in the food chain. EFSA Support. Publ. 2018, 15, 1394. [Google Scholar]
- Lucera, A.; Costa, C.; Conte, M.; Del Nobile, A. Food applications of natural antimicrobial compounds Front. Microbiol. 2012, 3, 287. [Google Scholar]
- Batiha, G.E.S.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Oulahal, N.; Degraeve, P. Phenolic-rich plant extracts with antimicrobial activity: An alternative to food preservatives and biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef]
- Sanz-Puig, M.; Moreno, P.; Pina-Pérez, M.C.; Rodrigo, D.; Martínez, A. Combined effect of high hydrostatic pressure (HHP) and antimicrobial from agro-industrial by-products against S. Typhimurium. LWT 2017, 77, 126–133. [Google Scholar] [CrossRef]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.; Cabral, L.M. Grape by-product extracts against microbial proliferation and lipid oxidation: A review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef]
- Ibáñez-Peinado, D.; Pina-Pérez, C.; García-Carrión, G.; Martínez, A.; Rodrigo., D. In vivo antimicrobial activity assessment of a cauliflower by-product extract against Salmonella typhimurium. Front. Sustain. Food Syst. 2020, 4, 8. [Google Scholar] [CrossRef]
- Roila, R.; Valiani, A.; Ranucci, D.; Ortenzi, R.; Servili, M.; Veneziani, G.; Branciari, R. Antimicrobial efficacy of a polyphenolic extract from olive oil by-product against “Fior di latte” cheese spoilage bacteria. Int. J. Food Microbiol. 2019, 295, 49–53. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Physicochemical stability and in vitro bioaccessibility of phenolic compounds and anthocyanins from Thai rice bran extracts. Food Chem. 2020, 329, 127157. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Sanchez, V.; Baeza, R.; Galmarini, M.V.; Zamora, M.C.; Chirife, J. Freeze-drying encapsulation of red wine polyphenols in an amorphous matrix of maltodextrin. Food Biopr. Technol. 2013, 6, 1350–1354. [Google Scholar] [CrossRef]
- Ianni, F.; Gagliardi, A.; Taticchi, A.; Servili, M.; Pinna, N.; Schoubben, A.; Sardella, R.; Bruscoli, S. Exploiting food-grade mesoporous silica to preserve the antioxidant properties of fresh olive mill wastewaters phenolic extracts. Antioxidants 2021, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Cui, H.; Gabriel, A.A.; Nakano, H. Antimicrobial efficacies of plant extracts and sodium nitrite against Clostridium botulinum. Food Control 2010, 21, 1030–1036. [Google Scholar] [CrossRef]
- Roila, R.; Sordini, B.; Esposto, S.; Ranucci, D.; Primavilla, S.; Valiani, A.; Taticchi, A.; Branciari, R.; Servili, M. Effect of the Application of a Green Preservative Strategy on Minced Meat Products: Antimicrobial Efficacy of Olive Mill Wastewater Polyphenolic Extract in Improving Beef Burger Shelf-Life. Foods 2022, 11, 2447. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, L.; Gong, X.; Chi, F.; Ma, Z. Fabrication and comparison of active films from chitosan incorporating different spice extracts for shelf life extension of refrigerated pork. LWT 2021, 135, 110181. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Maio, I.D.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef]
- Insani, E.M.; Eyherabide, A.; Grigioni, G.; Sancho, A.M.; Pensel, N.A.; Descalzo, A.M. Oxidative stability and its relationship with natural antioxidants during refrigerated retail display of beef produced in Argentina. Meat Sci. 2008, 79, 444–452. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Ros, G.; Nieto, G. Synthetic vs. Natural Hydroxytyrosol for Clean Label Lamb Burgers. Antioxidants 2020, 9, 851. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Pinna, N.; Ianni, F.; Selvaggini, R.; Urbani, S.; Codini, M.; Grispoldi, L.; Cenci-Goga, B.T.; Cossignani, L.; Blasi, F. Valorization of Pumpkin Byproducts: Antioxidant Activity and Carotenoid Characterization of Extracts from Peel and Filaments. Foods 2023, 12, 4035. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Meneguzzo, F.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Extraction, benefits and valorization of olive polyphenols. Eur. J. Lipid Sci. Technol. 2016, 118, 503–511. [Google Scholar] [CrossRef]
- Primavilla, S.; Pagano, C.; Roila, R.; Branciari, R.; Ranucci, D.; Valiani, A.; Ricci, M.; Perioli, L. Antibacterial Activity of Crocus sativus L. Petals Extracts against Foodborne Pathogenic and Spoilage Microorganisms, with a Special Focus on Clostridia. Life 2022, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Dağdelen, A. Identifying antioxidant and antimicrobial activities of the phenolic extracts and mineral contents of virgin olive oils (Olea europaea L. cv. Edincik Su) from different regions in Turkey. J. Chem. 2016, 9589763. [Google Scholar]
- Medina, E.; de Castro, A.; Romero, C.; Ramírez, E.; Brenes, M. Effect of antimicrobial compounds from olive products on microorganisms related to health, food and agriculture. Microbial Pathogens and Strategies for Combating Them. Sci. Technol. Edu. 2013, 2, 1087–1094. [Google Scholar]
- Ibrahim, H.A.; Soliman, H.S.; Hamed, F.M.; Marrez, D.A.; Othman, S.M. Antibacterial activity of vanillic acid and catechol produced by microbial biotransformation of caffeic acid. J. Pharm. Sci. Res. 2020, 12, 740–743. [Google Scholar]
- Qian, W.; Yang, M.; Wang, T.; Sun, Z.; Liu, M.; Zhang, J.; Zeng, Q.; Li, Y. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant Enterobacter hormaechei. J. Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef]
- Roila, R.; Branciari, R.; Ranucci, D.; Ortenzi, R.; Urbani, S.; Servili, M.; Valiani, A. Antimicrobial activity of olive mill wastewater extract against Pseudomonas fluorescens isolated from mozzarella cheese. Ital. J. Food Saf. 2016, 5, 111–115. [Google Scholar] [CrossRef]
- Fasolato, L.; Cardazzo, B.; Balzan, S.; Carraro, L.; Taticchi, A.; Montemurro, F.; Novelli, E. Minimum bactericidal concentration of phenols extracted from oil vegetation water on spoilers, starters and food-borne bacteria. Ital. J. Food Saf. 2015, 4, 4519. [Google Scholar] [CrossRef][Green Version]
- Mody, D.; Athamneh, A.I.; Seleem, M.N. Curcumin: A natural derivative with antibacterial activity against Clostridium difficile. J. G. Antimicrob. Resist. 2020, 21, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, M.; Ginghina, O.; Raita, S.; Tapaloaga, D.; Ilie, L.; Negrei, C.; Popa, D.E.; Varlas, V.; Multescu, R.; Rosca, A.C.; et al. Natural alternative remedies in the background of updated recommendations for the prophylactic and therapeutic approach of clostridium difficile infections. Farmacia 2018, 66, 563–572. [Google Scholar] [CrossRef]
- Piotrowski, M.; Karpiński, P.; Pituch, H.; Van Belkum, A.; Obuch-Woszczatyński, P. Antimicrobial effects of Manuka honey on in vitro biofilm formation by Clostridium difficile. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Roshan, N.; Riley, T.V.; Knight, D.R.; Steer, J.H.; Hammer, K.A. Natural products show diverse mechanisms of action against Clostridium difficile. J. Appl. Microbiol. 2019, 126, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Balta, I.; Linton, M.; Pinkerton, L.; Kelly, C.; Stef, L.; Pet, I.; Stef, D.; Criste, A.; Gundogdu, O.; Corcionivoschi, N. The effect of natural antimicrobials against Campylobacter spp. and its similarities to Salmonella spp., Listeria spp., Escherichia coli, Vibrio spp., Clostridium spp. and Staphylococcus spp. Food Control 2021, 121, 107745. [Google Scholar] [CrossRef]
- Obied, H.K.; BedgoodJr, D.R.; Prenzler, P.D.; Robards, K. Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Alves, M.J.; de Sousa, M.H.O.; de Moura, N.F.; Cesca, K.; Verruck, S.; Monteiro, A.R.; Valencia, G.A. Starch nanoparticles containing phenolic compounds from green propolis: Characterization and evaluation of antioxidant, antimicrobial and digestibility properties. Int. J. Biol. Macromol. 2023, 255, 128079. [Google Scholar] [CrossRef]
- Salehi, A.; Rezaei, A.; Damavandi, M.S.; Kharazmi, M.S.; Jafari, S.M. Almond gum-sodium caseinate complexes for loading propolis extract: Characterization, antibacterial activity, release, and in-vitro cytotoxicity. Food Chem. 2023, 405, 134801. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, M.J.; Lucini, L. Functional implications of bound phenolic compounds and phenolics–food interaction: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef]
- Elsebaie, E.M.; Essa, R.Y. Microencapsulation of red onion peel polyphenols fractions by freeze drying technicality and its application in cake. J. Food Process. Preserv. 2018, 42, e13654. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L. Natural bioactive compounds from food waste: Toxicity and safety concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; CLSI Document M26-A; CLSI: Wayne, PA, USA, 1998. [Google Scholar]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef] [PubMed]
- SAS. Statistical Software and User’s Guide, Version 8.2; SAS Inst. Inc.: Cary, NC, USA, 2001. [Google Scholar]

| Phenolic Compounds | Polyphenol Sum | |||
|---|---|---|---|---|
| Hydroxytyrosol | Tyrosol | Vanillic Acid | ||
| EE | 14.71 ± 0.27 | 3.95 ± 0.17 | 0.26 ± 0.01 | 18.9 |
| LE | 8.58 ± 0.19 | 2.13 ± 0.05 | 0.21 ± 0.10 | 10.9 |
| EE (g/mL) | LE (g/mL) | PC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 0.25 | 0.125 | Pure | 0.558 | 0.279 | 0.140 | ||
| C. difficile | 10.94 ± 0.10 bB | 8.7 ± 0.45 aA | - | - | 18.58 ± 0.38 bC | 11.38 ± 0.42 bB | 7.88 ± 0.12 aA | - | 31.44 ± 1.12 aD |
| C. botulinum | 8.1 ± 0.20 aA | - | - | - | 17.51 ± 0.27 aC | 10.66 ± 0.47 aB | - | - | 32.24 ± 0.98 bD |
| C. perfringens | 17.01 ± 0.37 cD | 14.37 ± 0.49 bC | 12.19 ± 0.33 B | - | 18.99 ± 0.06 bE | 13.85 ± 0.29 cC | 8.92 ± 0.39 bA | - | 32.91 ± 0.70 bF |
| EE (g/mL) | LE (g/mL) | PC (µg/mL) | ||||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| C. difficile | 0.250 | 0.250 | 0.070 | 0.070 | 6 × 10−8 | 6 × 10−8 |
| C. botulinum | 0.500 | 0.500 | 0.070 | 0.070 | 5 × 10−7 | 5 × 10−7 |
| C. perfringens | 0.125 | 0.125 | 0.070 | 0.070 | 1 × 10−6 | 1 × 10−6 |
| Time (h) | Treatment | C. perfringens | C. botulinum | C. difficile |
|---|---|---|---|---|
| 0 | CTRL | 5.43 ± 0.03 A | 5.52 ± 0.01 A | 5.21 ± 0.04 A |
| LE | 5.36 ± 0.02 C | 5.36 ± 0.04 C | 5.32 ± 0.04 C | |
| EE | 5.36 ± 0.04 D | 5.38 ± 0.02 D | 5.28 ± 0.05 D | |
| 4 | CTRL | 5.41 ± 0.05 cA | 5.87 ± 0.03 cB | 5.46 ± 0.12 cB |
| LE | 1.00 ± 0.00 aB | 3.32 ± 0.00 aB | 3.30 ± 0.06 aB | |
| EE | 1.49 ± 0.20 bC | 3.95 ± 0.17 bC | 3.92 ± 0.06 bC | |
| 8 | CTRL | 6.95 ± 0.01 cB | 6.95 ± 0.01 cC | 6.76 ± 0.03 cC |
| LE | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | |
| EE | 0.94 ± 0.03 bB | 1.60 ± 0.56 bB | 1.65 ± 0.16 bB | |
| 12 | CTRL | 7.25 ± 0.05 bC | 7.25 ± 0.07 bD | 7.27 ± 0.08 bD |
| LE | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | |
| EE | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | |
| 24 | CTRL | 7.71 ± 0.02 bD | 7.90 ± 0.03 bE | 7.79 ± 0.03 bE |
| LE | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | |
| EE | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | |
| 48 | CTRL | 7.82 ± 0.03 bE | 7.96 ± 0.04 bE | 7.83 ± 0.03 bE |
| LE | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | −0.50 ± 0.03 aA | |
| EE | −0.50 ± 0.00 aA | −0.50 ± 0.00 aA | −0.50 ± 0.03 aA |
| EE | LE | CTRL | |
|---|---|---|---|
| C. perfringens | |||
| Initial value | 4.91 ± 0.07 | 5.36 ± 0.02 | 5.27 ± 0.16 |
| Lag phase | - | - | 1.76 ± 2.91 |
| Max growth rate | −0.61 ± 0.01 b | −1.102 ± 0.01 aA | 0.31 ± 0.22 c |
| Final value | −0.45 ± 0.03 aA | −0.50 ± 0.01 a | 7.71 ± 0.09 bA |
| R2 value | 0.891 | 0.983 | 0.892 |
| Standard Error of Fit | 0.750 | 0.020 | 0.357 |
| C. botulinum | |||
| Initial value | 5.51 ± 0.13 | 5.64 ± 0.04 | 5.48 ± 0.03 |
| Lag phase | 2.05 ± 0.07 b | - | 1.31 ± 0.01 a |
| Max growth rate | −0.61 ± 0.04 b | −0.74 ± 0.01 aB | 0.16 ± 0.014 c |
| Final value | −0.56 ± 0.01 aB | −0.56 ± 0.00 a | 7.93 ± 0.03 bC |
| R2 value | 0.989 | 0.962 | 0.956 |
| Standard Error of Fit | 0.262 | 0.507 | 2.215 |
| C. difficile | |||
| Initial value | 5.26 ± 0.04 | 5.60 ± 0.03 | 5.17 ± 0.05 |
| Lag phase | 2.13 ± 0.20 a | - | 2.45 ± 0.33 b |
| Max growth rate | −0.64 ± 0.02 a | −0.74 ± 0.01 aB | 0.24 ± 0.02 b |
| Final value | −0.55 ± 0.01 aB | −0.56 ± 0.01 a | 7.79 ± 0.03 bB |
| R2 value | 0.996 | 0.960 | 0.961 |
| Standard Error of Fit | 0.161 | 0.510 | 0.225 |
| Analyte | RT (min) | Molecular Formula | Precursor Ion (m/z) | Fragment Ion (m/z) | Declustering Potential (V) | Collision Energy (V) |
|---|---|---|---|---|---|---|
| Hydroxytyrosol | 9.2 | C8H10O3 | 153.0557 | 123.0455 | −80 | −14 |
| Tyrosol | 11.9 | C8H10O2 | 137.0608 | 119.0520 | −90 | −18 |
| Vanillic acid | 13.3 | C8H8O4 | 167.0350 | 152.0111 | −70 | −15 |
| Vanillin | 14.9 | C8H8O3 | 151.0401 | 136.0166 | −60 | −14 |
| p-Coumaric acid | 15.4 | C9H8O3 | 163.0401 | 119.0500 | −60 | −14 |
| Verbascoside | 16.4 | C29H36O15 | 623.1981 | 161.0251 | −90 | −38 |
| Oleuropein | 17.3 | C25H32O13 | 539.1770 | 307.0824 | −100 | −27 |
| Pinoresinol | 17.4 | C20H22O6 | 357.1344 | 151.0410 | −80 | −20 |
| Luteolin | 17.5 | C15H10O6 | 285.0405 | 133.0293 | −110 | −36 |
| Oleuropein aglycone | 17.6 | C19H22O8 | 377.1242 | 307.0824 | −80 | −14 |
| Apigenin | 17.7 | C15H10O5 | 269.0456 | 117.0343 | −110 | −35 |
| Microorganisms | Growth Conditions | Positive Controls |
|---|---|---|
| Clostridium perfringens | 37 °C—24–48 h under anaerobic conditions * in MHAB | Penicillin G 10UI/disc |
| Clostridium botulinum ISS CNRB CL 14NT | 37 °C—24–48 h under anaerobic conditions * in MHAB | Penicillin G 10UI/disc |
| Clostridioides difficile | 37 °C—24–48 h under anaerobic conditions * in MHAB | Penicillin G 10UI/disc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roila, R.; Primavilla, S.; Ranucci, D.; Galarini, R.; Paoletti, F.; Altissimi, C.; Valiani, A.; Branciari, R. The Effects of Encapsulation on the In Vitro Anti-Clostridial Activity of Olive Mill Wastewater Polyphenolic Extracts: A Promising Strategy to Limit Microbial Growth in Food Systems. Molecules 2024, 29, 1441. https://doi.org/10.3390/molecules29071441
Roila R, Primavilla S, Ranucci D, Galarini R, Paoletti F, Altissimi C, Valiani A, Branciari R. The Effects of Encapsulation on the In Vitro Anti-Clostridial Activity of Olive Mill Wastewater Polyphenolic Extracts: A Promising Strategy to Limit Microbial Growth in Food Systems. Molecules. 2024; 29(7):1441. https://doi.org/10.3390/molecules29071441
Chicago/Turabian StyleRoila, Rossana, Sara Primavilla, David Ranucci, Roberta Galarini, Fabiola Paoletti, Caterina Altissimi, Andrea Valiani, and Raffaella Branciari. 2024. "The Effects of Encapsulation on the In Vitro Anti-Clostridial Activity of Olive Mill Wastewater Polyphenolic Extracts: A Promising Strategy to Limit Microbial Growth in Food Systems" Molecules 29, no. 7: 1441. https://doi.org/10.3390/molecules29071441
APA StyleRoila, R., Primavilla, S., Ranucci, D., Galarini, R., Paoletti, F., Altissimi, C., Valiani, A., & Branciari, R. (2024). The Effects of Encapsulation on the In Vitro Anti-Clostridial Activity of Olive Mill Wastewater Polyphenolic Extracts: A Promising Strategy to Limit Microbial Growth in Food Systems. Molecules, 29(7), 1441. https://doi.org/10.3390/molecules29071441