The Cancer-Protective Potential of Protocatechuic Acid: A Narrative Review
Abstract
1. Introduction
2. Characteristics and Sources of PCA
3. PCA Absorption and Bioavailability
3.1. Stomach
3.2. Intestines
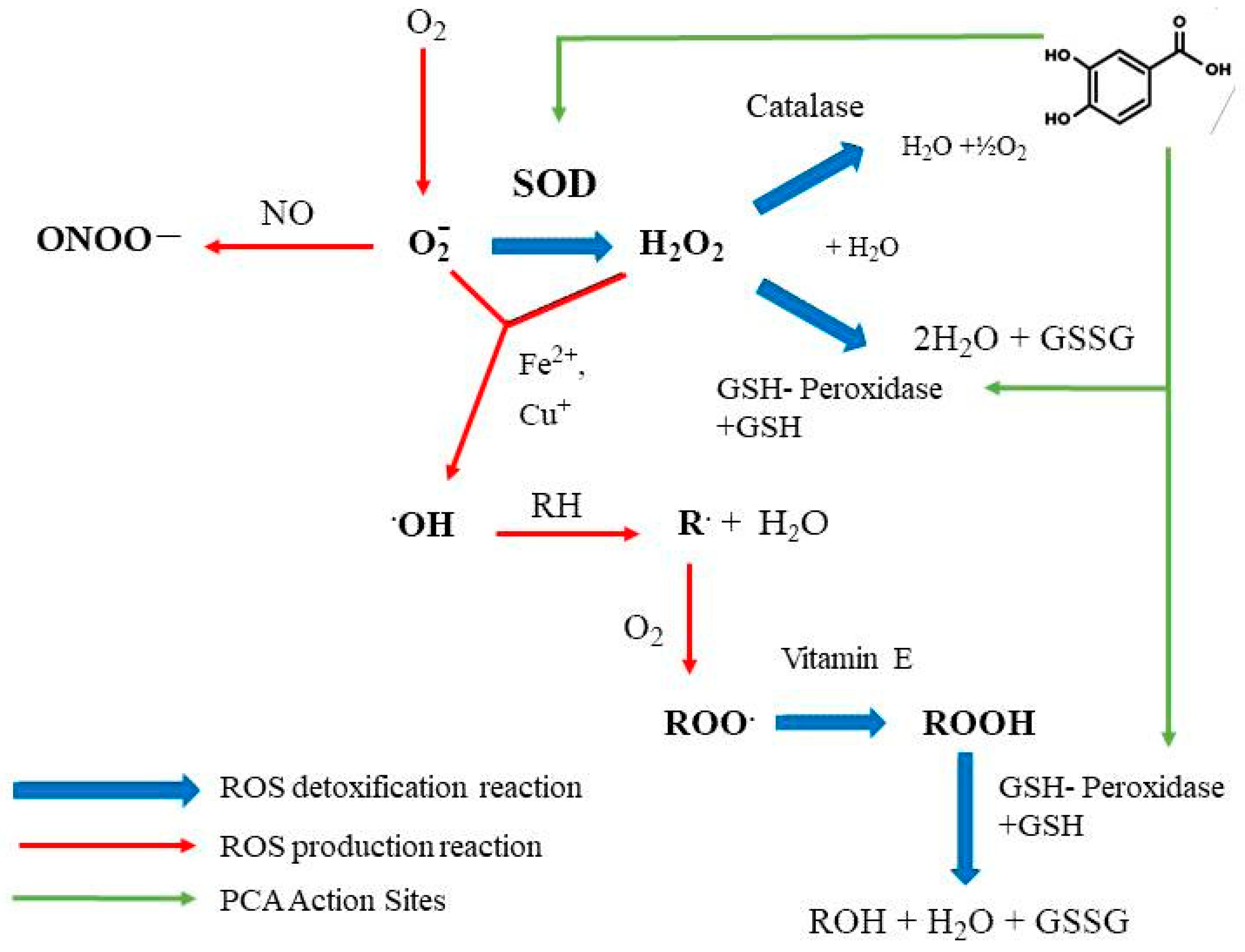
4. Antioxidant Effects of PCA
5. Chemopreventive Capacity of PCA
| Type of Cancer | Cell Line or Animal Used | Activity | Molecular Mechanism | References |
|---|---|---|---|---|
| Colon Cancer | CaCo-2 cells | Prooxidant, Proapoptotic | Modulation of redox balance and inhibition of the HO-1 system leading to p21 activation. | [72] |
| Oral Squamous Cell Carcinoma | BALB/CHSC-3 and CAL-27 mice | Apoptosis, Antioxidant | Inhibition of Sb9, Activation of JNK/p3 signaling pathway. Reduced BMI1 and CD44 expression. Decreased ROS excess. Increased SOD and NRF2 expression. | [73] |
| Esophageal Cancer | Male F-344 rats | Antineoplastic, Antiangiogenic, Anti-inflammatory | Inhibition of tumorigenesis and inflammatory signaling. Induction of PTX3 expression. | [74] |
| Liver Cancer | HepG2 hepatocellular carcinoma cells | Apoptosis | Induction of JNK-dependent hepatocellular carcinoma cell death. | [67] |
| Renal Carcinoma | HK2 cells treated with cisplatin | Cytoprotective, Antitumoral | Suppression of cisplatin-induced cell death by suppressing NAPDH oxidases, including Nox2 and Nox4. ↓ ROS. | [75] |
| Leukemia | HL-60 cells | Apoptosis, Cell cycle arrest | Reduction of Rb phosphorylation. ↓ Bcl-2, ↑ Bax. | [76] |
| Melanoma | B16F10 and SK-MEL-28 cells | Antimelanogenic | Suppression of α-MSH-induced MITF transcription through negative regulation of AMPc-mediated CREB activation. | [77] |
| Colorectal Cancer | Human NK cells, Apc Min/+ mice | Cytoprotective, Chemopreventive, Antitumoral | Decrease in inflammatory markers COX-2 and PGE2. Improved expression of IFN-γ and SMAD4 in cultured primary NK cells. | [78] |
| Human Colon Cancer | WiDr (ATCC CCL-218) and Chang (ATCC CCL-13) cells | Apoptosis, Cell cycle arrest, Pyroptosis | Intrinsic apoptosis by positive regulation of p53, Bax, and caspase-9. Modulation of caspase-8 through the extrinsic pathway. Positive regulation of caspase-1 and -7. | [79] |
| Hepatocellular Carcinoma | Wistar rats | Apoptosis, Cell cycle arrest | Cytochrome P450 reductase activity and glutathione S-transferase induction. ↓ TNF-α and IL-1β. ↓ Cyclin CDK1. ↑ p53 and Bad, ↓ Bcl-xl. | [80] |
| Lung Cancer | A549 and H1299 human lung cancer cells | Anticancer | Suppression of fibronectin, vimentin, N-cadherin, MMP-9, MMP-2, twist, and snail. ↑ Epithelial markers E-cadherin and Occludin levels. ↓ Migratory and invasive potential of tumor cells by reversing epithelial-to-mesenchymal transition (EMT). ↓ PI3K/Akt/mTOR activation. | [81] |
| Lung Cancer | A549, H3255, and Calu-6 cells | Apoptosis, Anticancer | ↑ Caspase-3 and Bax, ↓ Bcl-2. Suppressed FAK, NF-κB, and MAPK pathways. ↓ VEGF, fibronectin, bFGF, MMP-2, and MMP-9. | [82] |
| Gastric Carcinoma | AGS cells | Apoptosis, Repression of Migration, Decreased Matrix Degradation, Inhibition of Metastasis | ↓ Ras/Akt/NF-κB. ↓ PI3K, ↑ p53, and the p38 MAPK/FasL pathway. | [83] |
| Gastric Carcinoma | AGS, MKN45, HepG2, Hep3B | Apoptosis | Activation of JNK/p38 MAPK, both Fas/FasL and p53/Bax apoptotic signaling pathways. | [10] |
6. Inhibition of Tumors and Metastasis
7. Mechanism of Apoptotic Action in PCA
8. Perspectives
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Laskar, Y.B.; Mazumder, P.B. Insight into the molecular evidence supporting the remarkable chemotherapeutic potential of Hibiscus sabdariffa L. Biomed. Pharmacother. 2020, 127, 110153. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Parro, L. Efectos Neuroprotectores de Metabolitos Fenólicos Biodisponibles Derivados de Polifenoles del Vino. Master’s Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2021. Available online: http://hdl.handle.net/10261/263783 (accessed on 14 December 2023).
- Akgeyik, A.U.; Yalçın, E.; Çavuşoğlu, K. Phytochemical fingerprint and biological activity of raw and heat-treated Ornithogalum umbellatum. Sci. Rep. 2023, 13, 13733. [Google Scholar] [CrossRef]
- Graña, T.R.; González, M.P.; Trujillo, N.G.; Lozano, Y.S.; Tamayo, M.H. Estrés Oxidativo: Genética, Dieta y Desarrollo de Enfermedades. Correo Científico Médico 2015, 19, 4. Available online: https://revcocmed.sld.cu/index.php/cocmed/article/view/2151 (accessed on 14 December 2023).
- Fernández Poyatos, M.d.P. Caracterización y Estudio de Compuestos Bioactivos en Especies Vegetales. Ph.D. Thesis, Universidad de Jaén, Jaén, Spain, 2020. [Google Scholar]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid.-Based Complement. Altern. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Lin, H.; Chen, J.; Huang, C.; Wang, C. Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation. Int. J. Cancer 2007, 120, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- González-Vallinas, M.; González-Castejón, M.; Rodríguez-Casado, A.; de Molina, A.R. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Crozier, A. Dietary Flavonoids and Phenolic Compounds. In Plant Phenolics and Human Health, 1st ed.; Fraga, C.G., Ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 1–49. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evid.-Based Complement. Altern. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tanaka, T.; Tanaka, M. Potential Cancer Chemopreventive Activity of Protocatechuic Acid. J. Exp. Clin. Med. 2011, 3, 27–33. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Abonyi, D.O.; Eze, P.M.; Abba, C.C.; Chukwunwejim, C.R.; Ejikeugwu, C.P.; Okoye, F.B.; Esimone, C.O. Metabolites of endophytic Colletotrichum gloeosporioides isolated from leaves of Carica papaya. Am. J. Essent. Oils Nat. Prod. 2019, 7, 39–46. [Google Scholar]
- Xue, H.; Sang, Y.; Gao, Y.; Zeng, Y.; Liao, J.; Tan, J. Research Progress on Absorption, Metabolism, and Biological Activities of Anthocyanins in Berries: A Review. Antioxidants 2022, 12, 3. [Google Scholar] [CrossRef]
- García, P.T. Composición y aporte de sustancias bioactivas de subproductos hortícolas. Rev. Fanus 2022, 3, 1–21. [Google Scholar]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; Alatorre-Cruz, J.M.; Monroy-Torres, R. Importancia nutricional y actividad biológica de los compuestos bioactivos de quelites consumidos en México. Rev. Chil. Nutr. 2019, 46, 593–605. [Google Scholar] [CrossRef]
- Castro Juárez, C.J.; Villa Ruano, N.; Ramírez García, S.A.; Mosso González, C. Uso medicinal de plantas antidiabéticas en el legado etnobotánico oaxaqueño. Rev. Cuba. Plantas Med. 2014, 19, 101–120. [Google Scholar]
- Morales Cabrera, M. Actividad Antimicrobiana y Caracterización Fenólica de Extractos de Cálices de Jamaica (Hibiscus sabdariffa L.). Master’s Thesis, Institución de Enseñanza e Investigación en Ciencias Agrícolas, Estado de México, Mexico, 2011. Available online: http://colposdigital.colpos.mx:8080/xmlui/handle/10521/568 (accessed on 15 December 2023).
- Archivio, M.D.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanità 2007, 43, 348–361. [Google Scholar] [PubMed]
- Hithamani, G.; Srinivasan, K. Bioaccessibility of polyphenols from wheat (Triticum aestivum), sorghum (Sorghum bicolor), green gram (Vigna radiata), and chickpea (Cicer arietinum) as influenced by domestic food processing. J. Agric. Food Chem. 2014, 62, 11170–11179. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Mangos and their bioactive components: Adding variety to the fruit plate for health. Food Funct. 2017, 8, 3010–3032. [Google Scholar] [CrossRef]
- Tian, S.; Nakamura, K.; Cui, T.; Kayahara, H. High-performance liquid chromatographic determination of phenolic compounds in rice. J. Chromatogr. A 2005, 1063, 121–128. [Google Scholar] [CrossRef]
- Sedej, I.; Sakač, M.; Mandić, A.; Mišan, A.; Tumbas, V.; Čanadanović-Brunet, J. Buckwheat (Fagopyrum esculentum Moench) Grain and Fractions: Antioxidant Compounds and Activities. J. Food Sci. 2012, 77, C954–C959. [Google Scholar] [CrossRef]
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and Phytochemical Content of High-Protein Crops. J. Agric. Food Chem. 2016, 64, 7800–7811. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Phenolic substance characterization and chemical and cell-based antioxidant activities of 11 lentils grown in the northern United States. J. Agric. Food Chem. 2010, 58, 1509–1517. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Zhang, X.; Chen, G.-L.; Yu, J.; Yang, L.-Q.; Gao, Y.-Q. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. J. Funct. Foods 2016, 24, 359–372. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and quantification of free and bound phenolic compounds contained in the high-molecular weight melanoidin fractions derived from two different types of cocoa beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.N.; Hazama, C.; Shimoyamada, M.; Ando, H.; Kato, K.; Yamauchi, R. Antioxidative compounds from the outer scales of onion. J. Agric. Food Chem. 2005, 53, 8183–8189. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J. Food Sci. Technol. 2016, 53, 4056–4066. [Google Scholar] [CrossRef]
- Orsavová, J.; Hlaváčová, I.; Mlček, J.; Snopek, L.; Mišurcová, L. Contribution of phenolic compounds, ascorbic acid and vitamin E to antioxidant activity of currant (Ribes L.) and gooseberry (Ribes uva-crispa L.) fruits. Food Chem. 2019, 284, 323–333. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Locatelli, M.; Tartaglia, A.; Ferrone, V.; Juszczak, A.M.; Ozer, M.S.; Tepe, B.; Tomczyk, M. Enzyme and Biological Activities of the Water Extracts from the Plants Aesculus hippocastanum, Olea europaea and Hypericum perforatum That Are Used as Folk Remedies in Turkey. Molecules 2020, 25, 1202. [Google Scholar] [CrossRef]
- Liu, C.-L.; Wang, J.-M.; Chu, C.-Y.; Cheng, M.-T.; Tseng, T.-H. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem. Toxicol. 2002, 40, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Miao, L.; Yu, X.-A.; Orgah, J.O.; Barnabas, O.; Chang, Y.; Liu, E.; Fan, G.; Gao, X. Cynomorium songaricum Rupr demonstrates phytoestrogenic or phytoandrogenic like activities that attenuates benign prostatic hyperplasia via regulating steroid 5-α-reductase. J. Ethnopharmacol. 2019, 235, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lapsley, K.; Rosen, R.T.; Ho, C.T. New Prenylated Benzoic Acid and Other Constituents from Almond Hulls (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Riviello-Flores, L.M. Desarrollo de protocolos para el incremento de metabolitos secundarios de Sechium compositum (Donn. Sm.) C. Jeffrey. Ph.D. Thesis, Colegio de Posgraduados, Texcoco, Estado de México, Mexico.
- Lazcano Peralta, A.R.L. Efecto de los Métodos de Cocinado en la Calidad Nutricional, Contenido de Metabolitos Secundarios y Actividad Antioxidante de las Hojas de Amaranthus hypochondriacus L. Variedad Revancha. Master’s Thesis, Universidad Autónoma de Quéretaro, Quéretaro, Mexico, 2009. Available online: https://ri-ng.uaq.mx/handle/123456789/645 (accessed on 26 December 2023).
- Galicia-Flores, L.A.; Salinas-Moreno, Y.; Espinoza-García, B.M.; Sánchez-Feria, C. Caracterización fisicoquímica y actividad antioxidante de extractos de jamaica (Hibiscus sabdariffa L.) nacional e importada. Rev. Chapingo Ser.Hortic. 2008, 14, 121–129. [Google Scholar]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.; Cassidy, A.; Kay, C. The pharmacokinetics of anthocyanins and their metabolites in humans. J. Cereb. Blood Flow Metab. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic Acid Is the Major Human Metabolite of Cyanidin-Glucosides3. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Lamuela-Raventos, R.M.; Santos-Buelga, C.; Sacanella, E.; Castell, M.; Permanyer, J.; Andres-Lacueva, C. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal. Bioanal. Chem. 2009, 394, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.-Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Martínez Gutiérrez Zetina, S.; Ayuda Durán, B.; González Manzano, S.; González Paramás, A.M.; Santos Buelga, C. Evaluación de la bioactividad del ácido protocatéquico en “Caenorhabditis elegans”. In La ciencia, Ingeniería y Tecnología de los Alimentos Bajo la Perspectiva de los Jóvenes Investigadores: II Congreso Nacional de Jóvenes Investigadores en Ciencia, Ingeniería y Tecnología de los Alimentos; Facultad de Veterinaria, Universidad de León: San Francisco del Rincón, Mexico, 2017; pp. 87–88. ISBN 978-84-9773-900-9. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=6287649 (accessed on 26 December 2023).
- Mallery, S.R.; Budendorf, D.E.; Larsen, M.P.; Pei, P.; Tong, M.; Holpuch, A.S.; Larsen, P.E.; Stoner, G.D.; Fields, H.W.; Chan, K.K.; et al. Effects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev. Res. 2011, 4, 1209–1221. [Google Scholar] [CrossRef]
- Okpara, E.S.; Adedara, I.A.; Guo, X.; Klos, M.L.; Farombi, E.O.; Han, S. Molecular mechanisms associated with the chemoprotective role of protocatechuic acid and its potential benefits in the amelioration of doxorubicin-induced cardiotoxicity: A review. Toxicol. Rep. 2022, 9, 1713–1724. [Google Scholar] [CrossRef]
- Rosales Zárate, V.I. Estabilidad Gastrointestinal y Bioactividad In Vitro de Antocianinas Aisladas de Diferentes Matrices Frutales en Función de la Presencia de Iones Divalentes. Bachelor’s Thesis, Universidad Autónoma de San Luis Potosí, San Luis Potosí, Mexico, 2018. Available online: https://repositorioinstitucional.uaslp.mx/xmlui/bitstream/handle/i/4772/TESIS%20-%20VANESSA%20ITZEL%20ROSALES-ZARATE%20entrega%2022-02-18.pdf?sequence=1&isAllowed=y (accessed on 9 December 2023).
- Martínez Gutiérrez Zetina, S. Metabolitos Fenólicos: Preparación y Evaluación de la Actividad en Sistemas Modelo. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2019. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Hidalgo, M.Á.G. Estrés oxidativo y antioxidantes. Av. Investig. Agropecu. 2018, 22, 29–46. [Google Scholar]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Arista-Ugalde, T.L.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. The Biological Significance of Oxidative Stress, Effects of Fruits as Natural Edible Antioxidants. Curr. Pharm. Des. 2018, 24, 4807–4824. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Yousri, R.; Noaman, E.; El Shawi, O.; Fahmy, N.; Ghazy, M. Evaluation of Anti-Oxidant Status and Radioprotective Activity of a Novel Anti-Cancer Drug in Mice. J. Cancer Ther. 2011, 2, 616–628. [Google Scholar] [CrossRef][Green Version]
- Van Loenhout, J.; Peeters, M.; Bogaerts, A.; Smits, E.; Deben, C. Oxidative Stress-Inducing Anticancer Therapies: Taking a Closer Look at Their Immunomodulating Effects. Antioxidants 2020, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, S.; Hajnos, M.; Skalicka-Woźniak, K.; Matkowski, A. Antioxidant activity of polyphenols from Lycopus lucidus Turcz. Food Chem. 2009, 113, 134–138. [Google Scholar] [CrossRef]
- Vilaplana, M. Antioxidantes presentes en los alimentos. Vitaminas, minerales y suplementos. Offarm 2007, 26, 79–86. [Google Scholar]
- Di Majo, D.; La Guardia, M.; Giammanco, S.; La Neve, L.; Giammanco, M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008, 111, 45–49. [Google Scholar] [CrossRef]
- Zambonin, L.; Caliceti, C.; Sega, F.V.D.; Fiorentini, D.; Hrelia, S.; Landi, L.; Prata, C. Dietary Phenolic Acids Act as Effective Antioxidants in Membrane Models and in Cultured Cells, Exhibiting Proapoptotic Effects in Leukaemia Cells. Oxidative Med. Cell. Longev. 2012, 2012, e839298. [Google Scholar] [CrossRef] [PubMed]
- Varì, R.; D’Archivio, M.; Filesi, C.; Carotenuto, S.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Masella, R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011, 22, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wu, W.-F.; Dong, L.; Ren, G.-L.; Li, H.-D.; Yang, Q.; Li, X.-F.; Xu, T.; Li, Z.; Wu, B.-M.; et al. Protocatechuic Aldehyde Attenuates Cisplatin-Induced Acute Kidney Injury by Suppressing Nox-Mediated Oxidative Stress and Renal Inflammation. Front. Pharmacol. 2016, 7, 479. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Martín, C. Capacidad de la Silibinina de Revertir las Alteraciones Metabólicas y el Estrés Oxidativo en Ratas con Resistencia a la Insulina Inducida por una Dieta Rica en Fructosa. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2011. [Google Scholar]
- Ohnishi, M.; Yoshimi, N.; Kawamori, T.; Ino, N.; Hirose, Y.; Tanaka, T.; Yamahara, J.; Miyata, H.; Mori, H. Inhibitory Effects of Dietary Protocatechuic Acid and Costunolide on 7,12-Dimethylbenz[a]anthracene-induced Hamster Cheek Pouch Carcinogenesis. Jpn. J. Cancer Res. 1997, 88, 111–119. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Szaefer, H.; Krajka-Kuzniak, V. Inhibition of murine hepatic cytochrome P450 activities by natural and synthetic phenolic compounds. Xenobiotica 1998, 28, 735–743. [Google Scholar] [CrossRef]
- Nakamura, H.; Nishikawa, A.; Furukawa, F.; Kasahara, K.; Miyauchi, M.; Son, H.Y.; Hirose, M. Inhibitory effects of protocatechuic acid on the post-initiation phase of hamster pancreatic carcinogenesis induced by N-nitrosobis(2-oxopropyl)amine. Anticancer Res. 2000, 20, 3423–3427. [Google Scholar]
- Acquaviva, R.; Tomasello, B.; Di Giacomo, C.; Santangelo, R.; La Mantia, A.; Naletova, I.; Sarpietro, M.G.; Castelli, F.; Malfa, G.A. Protocatechuic Acid, a Simple Plant Secondary Metabolite, Induced Apoptosis by Promoting Oxidative Stress through HO-1 Downregulation and p21 Upregulation in Colon Cancer Cells. Biomolecules 2021, 11, 1485. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cao, L.; Yang, C.; Liu, T.; Zhao, H.; Luo, X.; Chen, Q. Protocatechuic Acid-Based Supramolecular Hydrogel Targets SerpinB9 to Achieve Local Chemotherapy for OSCC. ACS Appl. Mater. Interfaces 2022, 14, 36379–36394. [Google Scholar] [CrossRef]
- Peiffer, D.S.; Zimmerman, N.P.; Wang, L.-S.; Ransom, B.W.; Carmella, S.G.; Kuo, C.-T.; Siddiqui, J.; Chen, J.-H.; Oshima, K.; Huang, Y.-W.; et al. Chemoprevention of Esophageal Cancer with Black Raspberries, Their Component Anthocyanins, and a Major Anthocyanin Metabolite, Protocatechuic Acid. Cancer Prev. Res. 2014, 7, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Yip, E.C.H.; Chan, A.S.L.; Pang, H.; Tam, Y.K.; Wong, Y.H. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol. Toxicol. 2006, 22, 293–302. [Google Scholar] [CrossRef]
- Tseng, T.-H.; Kao, T.-W.; Chu, C.-Y.; Chou, F.-P.; Lin, W.-L.; Wang, C.-J. Induction of apoptosis by Hibiscus protocatechuic acid in human leukemia cells via reduction of retinoblastoma (RB) phosphorylation and Bcl-2 expression. Biochem. Pharmacol. 2000, 60, 307–315. [Google Scholar] [CrossRef]
- Truong, X.T.; Park, S.-H.; Lee, Y.-G.; Jeong, H.Y.; Moon, J.-H.; Jeon, T.-I. Protocatechuic Acid from Pear Inhibits Melanogenesis in Melanoma Cells. Int. J. Mol. Sci. 2017, 18, 1809. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Lin, C.-W.; Echeveste, C.E.; Huang, Y.-W.; Oshima, K.; Yearsley, M.; Chen, X.; Yu, J.; Wang, L.-S. Protocatechuic Acid, a Gut Bacterial Metabolite of Black Raspberries, Inhibits Adenoma Development and Alters Gut Microbiome Profiles in Apc Min/+ Mice. J. Cancer Prev. 2022, 27, 50–57. [Google Scholar] [CrossRef]
- Yuliana, N.D.; Tuarita, M.Z.; Khatib, A.; Laila, F.; Sukarno, S. GC–MS metabolomics revealed protocatechuic acid as a cytotoxic and apoptosis-inducing compound from black rice brans. Food Sci. Biotechnol. 2020, 29, 825–835. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Luangsuphabool, T.; Wongpoomchai, R. Protocatechuic acid as a potent anticarcinogenic compound in purple rice bran against diethylnitrosamine-initiated rat hepatocarcinogenesis. Sci. Rep. 2022, 12, 10548. [Google Scholar] [CrossRef]
- Yang, M.H.; Baek, S.H.; Chinnathambi, A.; Alharbi, S.A.; Ahn, K.S. Identification of protocatechuic acid as a novel blocker of epithelial-to-mesenchymal transition in lung tumor cells. Phytotherapy Res. 2021, 35, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.-M.; Hsia, T.-C.; Yin, M.-C. Protocatechuic Acid Inhibits Lung Cancer Cells by Modulating FAK, MAPK, and NF-κB Pathways. Nutr. Cancer 2014, 66, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, J.; Chou, F.; Wang, C. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011, 162, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawamori, T.; Ohnishi, M.; Okamoto, K.; Mori, H.; Hara, A. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis by dietary protocatechuic acid during initiation and postinitiation phases. Cancer Res. 1994, 54, 2359–2365. [Google Scholar]
- Wu, Y.-X.; Wu, T.-Y.; Xu, B.-B.; Xu, X.-Y.; Chen, H.-G.; Li, X.-Y.; Wang, G. Protocatechuic acid inhibits osteoclast differentiation and stimulates apoptosis in mature osteoclasts. Biomed. Pharmacother. 2016, 82, 399–405. [Google Scholar] [CrossRef]



| No. | Flower or Fruit | Concentration (mg/kg) | Reference |
|---|---|---|---|
| 1 | Solanum tuberosum L. (potato: peel). | 2560 | [22] |
| 2 | Amaranthus caudatus L. (amaranth: seeds, leaves, and flowers). | 0.0136 | [23] |
| 3 | Cnidoscolus chayamansa (Mill.) I.M.Johnst (chaya: leaves). | 242 ± 0.001 | [24] |
| 4 | Hibiscus sabdariffa L. var. Alma blanca (hibiscus: leaves, root, stem, capsule, and whole and ground seeds). | 86.2 | [25] |
| 5 | Hibiscus sabdariffa L. var. Chiautla (hibiscus: leaves, root, stem, capsule, and whole and ground seeds). | 81 | [25] |
| 6 | Hibiscus sabdariffa L. var. Huajicori (hibiscus: leaves, root, stem, capsule, and whole and ground seeds). | 1397 | [25] |
| 7 | Hibiscus sabdariffa L. var. Tecoanapa (hibiscus: leaves, root, stem, capsule, and whole and ground seeds). | 135.1 | [25] |
| 8 | Rubus idaeus L. (Raspberry). | 100 | [26] |
| 9 | Oleo europaea L. (Olive: olive oil). | 0.22 | [27] |
| 10 | Cicer arietinum L. (chickpea: sprouted, roasted, pressure-cooked, and microwave-heated seeds). | 514.2 | [28] |
| 11 | Mangifera indica L. (mango: mango pulp). | 7.7–68.3 | [29] |
| 12 | Oryza sativa L. (rice: whole and soaked grain). | 23.2–1043 | [30] |
| 13 | Fagopyrum esculentum Moench (buckwheat: whole grain and husk). | 6.61–24.5 | [31] |
| 14 | Pisum sativum L. (green pea: green pea flour). | 1.26–11.38 | [31] |
| 15 | Vicia faba L. (broad bean: broad bean flour). | 0.61–2.42 | [31] |
| 16 | Cannabis sativa L. ((hemp: hemp flour). | 5.63–22.06 | [31] |
| 17 | Lupinus albus L. (lupin: lupin flour). | 0.15 ± 0.02 | [31] |
| 18 | Triticum aestivum L. (common wheat: wheat flour). | 0.07–0.11 | [31] |
| 19 | Lens culinaris Medik (lentils: whole dried seeds). | 20.28–37.72 | [32] |
| 20 | Phaseolus vulgaris L.(common bean: ground whole grain). | 95.34–253.42 | [33] |
| 21 | Theobroma cacao L. (Cocoa: cocoa bean). | 197.9–385.3 | [34] |
| 22 | Allium cepa L. (onion: outer layers of the onion). | 1027 | [35] |
| 23 | Musa × paradisiaca L. (Banana: banana pulp). | 340 | [36] |
| 24 | Ribes rubrum L. (Red currant: whole freeze-dried fruit). | 137.6–464.8 | [37] |
| 25 | Hypericum perforatum L. (St. John’s Wort: aerial parts). | 761.67 | [38] |
| 26 | Olea europaea L. (Olive: olive leaves). | 176.08 | [38] |
| 27 | Hibiscus sabdariffa L. (Hs, Roselle) (Hibiscus: dried flowers). | 94.1 | [39] |
| 28 | Cynomorium songaricum Rupr. (Chinese herb: lyophilized herb). | 148 | [40] |
| 29 | Prunus amygdalus Batsch (almonds: almond shells). | 66.67 | [41] |
| 30 | Sechium edule (Jacq.) Sw. (chayote 30 Gy SC-S. compositum) | 1910 | [42] |
| 31 | Sechium edule (Jacq.) Sw. (chayote 10 Gy H387b) | 1050 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadena-Iñiguez, J.; Santiago-Osorio, E.; Sánchez-Flores, N.; Salazar-Aguilar, S.; Soto-Hernández, R.M.; Riviello-Flores, M.d.l.L.; Macías-Zaragoza, V.M.; Aguiñiga-Sánchez, I. The Cancer-Protective Potential of Protocatechuic Acid: A Narrative Review. Molecules 2024, 29, 1439. https://doi.org/10.3390/molecules29071439
Cadena-Iñiguez J, Santiago-Osorio E, Sánchez-Flores N, Salazar-Aguilar S, Soto-Hernández RM, Riviello-Flores MdlL, Macías-Zaragoza VM, Aguiñiga-Sánchez I. The Cancer-Protective Potential of Protocatechuic Acid: A Narrative Review. Molecules. 2024; 29(7):1439. https://doi.org/10.3390/molecules29071439
Chicago/Turabian StyleCadena-Iñiguez, Jorge, Edelmiro Santiago-Osorio, Nancy Sánchez-Flores, Sandra Salazar-Aguilar, Ramón Marcos Soto-Hernández, María de la Luz Riviello-Flores, Víctor Manuel Macías-Zaragoza, and Itzen Aguiñiga-Sánchez. 2024. "The Cancer-Protective Potential of Protocatechuic Acid: A Narrative Review" Molecules 29, no. 7: 1439. https://doi.org/10.3390/molecules29071439
APA StyleCadena-Iñiguez, J., Santiago-Osorio, E., Sánchez-Flores, N., Salazar-Aguilar, S., Soto-Hernández, R. M., Riviello-Flores, M. d. l. L., Macías-Zaragoza, V. M., & Aguiñiga-Sánchez, I. (2024). The Cancer-Protective Potential of Protocatechuic Acid: A Narrative Review. Molecules, 29(7), 1439. https://doi.org/10.3390/molecules29071439








