Abstract
Solid-phase peptide synthesis (SPPS) is the preferred strategy for synthesizing most peptides for research purposes and on a multi-kilogram scale. One key to the success of SPPS is the continual evolution and improvement of the original method proposed by Merrifield. Over the years, this approach has been enhanced with the introduction of new solid supports, protecting groups for amino acids, coupling reagents, and other tools. One of these improvements is the use of the so-called “safety-catch” linkers/resins. The linker is understood as the moiety that links the peptide to the solid support and protects the C-terminal carboxylic group. The “safety-catch” concept relies on linkers that are totally stable under the conditions needed for both α-amino and side-chain deprotection that, at the end of synthesis, can be made labile to one of those conditions by a simple chemical reaction (e.g., an alkylation). This unique characteristic enables the simultaneous use of two primary protecting strategies: tert-butoxycarbonyl (Boc) and fluorenylmethoxycarbonyl (Fmoc). Ultimately, at the end of synthesis, either acids (which are incompatible with Boc) or bases (which are incompatible with Fmoc) can be employed to cleave the peptide from the resin. This review focuses on the most significant “safety-catch” linkers.
1. Introduction
Solid-phase peptide synthesis (SPPS) is an innovative methodology that has revolutionized the field of peptide chemistry and peptide-based drug discovery. Developed by R.B. Merrifield in early 1963 [1], SPPS has since become a necessary tool for the effective synthesis of peptides. It was developed to overcome the limitations of traditional solution peptide synthesis [2]. This synthesis approach is much less time consuming, keeping suitable yields and purities of the final peptides. The need for SPPS arises from the difficulties of the manipulation of the intermediates in solution peptide synthesis. This traditional strategy involved repetitive isolation and, very often, purification of the intermediates, leading to a tedious workup, which very often translated into low yields [3]. Merrifield’s vision was to attach the peptide chain onto a solid support; in other words, to use a polymeric and solid C-terminal protecting group and avoid the isolation and purification of the intermediates. Furthermore, as the growing peptide was anchored to the solid support, excess reagents could be used because the unreacted reagents and the soluble side-products can easily be removed by simple washings [1]. As the growing peptide stays during all the synthetic processes anchored to the solid support, this can be considered a reactor and, therefore, is amenable to automation. SPPS has shown an important impact in multiple scientific disciplines, providing insights into a large number of biological processes [4,5,6]. However, SPPS has been pivotal in the drug discovery arena, allowing the speeding up of the first steps of the process (hit detection, hit to lead to candidate) and, at the end of the process, facilitates the production of peptides with more than 40 amino acids on a multi-kilogram scale with the sufficient purity to fulfill the requirements of the regulatory agencies and, therefore, to be used in humans [7,8]. SPPS can be defined as a proper combination of the protecting groups (PGs) and coupling reagents in a proper solvent. As mentioned earlier, the linkers are the protecting group of the C-carboxylic group. The linker should be totally stable during the elongation of the peptide and then should liberate the peptide with the best yield possible. Very often, the final cleavage will also remove the protecting groups of the side-chain of the amino acids. However, for other applications, the release of the peptide from the linker resin should keep those protecting groups rendering a protected peptide [9]. Considering the linker (or linker resins) versus the other protecting groups for α-amino and for the side-chain protecting groups, linkers can be classified into four categories: (i) Kinetic Fine-Tuning-Based; (ii) Bis-Orthogonal; (iii) Three-Orthogonal; and (iv) Safety-Catch. The best example of the first class is the Merrifield strategy, benzyl (Bzl) groups for the linker and the side-chain protecting groups, and tert-butyloxycarbonyl (Boc) for the α-amino (Figure 1a). The Boc and the Bzl groups are removed by the same mechanism, acidolysis, but through different kinetics. The Boc group is removed with trifluoroacetic acid (TFA) and the Bzl groups by anh. HF or trifluoromethanesulfonic acid (TFMSA) [10]. The best example of a bis-orthogonal category is the combination of p-methoxybenzyl (Wang) linker for SPPS, tert-butyl (tBu) for side-chain protecting groups, and fluorenylmethoxycarbonyl (Fmoc) for the α-amino group [11]. There are two different chemical mechanisms: acidolysis with TFA for the first and base through a β-elimination for the Fmoc (Figure 1b). Thus, we can remove ones in the presence of others and vice versa. Very important for this category is that the conditions can be forced; for instance, the Fmoc can be removed at high temperatures, keeping in place the rest of the groups. This is not possible in the kinetic fine-tuning-based systems, because removing the Boc at high temperatures or for long durations will detach the peptide from the resin. A variation of this class is when three different classes of protecting groups are present; one is independently orthogonal to the other two, which in turn are removed by the same chemical method but with different kinetics. The best example of this category is Cl-tritrylchloride (CTC) resin together with tBu side-chain protecting groups and Fmoc (Figure 1c). The latter is orthogonal to the remaining two, but the peptide is released from the CTC resin with dilute TFA (2%) solutions and the side-chain protecting groups with concentrated TFA. This means that the side-chain cannot be deprotected while the peptide is still anchored to the solid support [11]. A three-orthogonal scheme is then formed; for instance, a photolabile linker such as the nitroBzl together with tBu side-chain protecting groups and Fmoc (Figure 1d). The three different mechanisms are totally independent among them, and, therefore, it is possible to remove the protecting groups of the side-chain on the resin or cleave the protected peptide from the resin [12]. This ideal scheme allows for forcing the deprotection condition to increase the yields. The last category and object of this study is the safety-catch one.
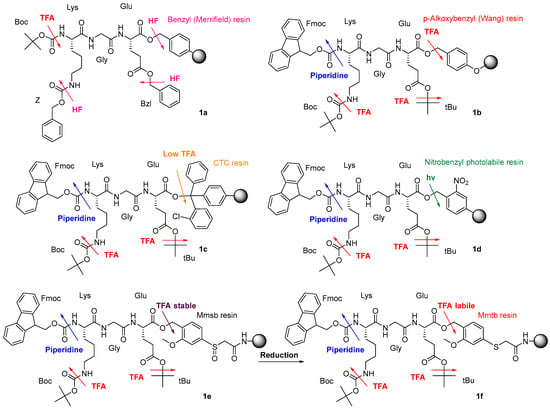
Figure 1.
Different classes of protecting group schemes including the linker.
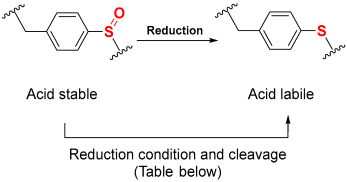
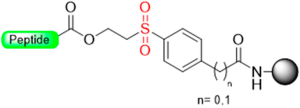
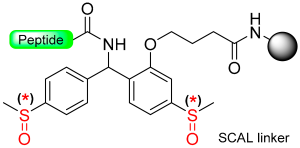
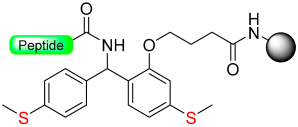
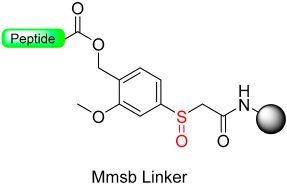
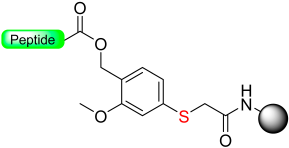
The “safety-catch” concept relies on linkers totally stable under the conditions needed for both α-amino and side-chain deprotection that, at the end of synthesis, can be made labile to one of those conditions by a simple chemical reaction. For example, Figure 1e,f) show the uses of the pair 2-methoxy-4-methylsulfinylbenzyl alcohol (Mmsb) and 2-methoxy-4-methylthiobenzyl alcohol (Mmtb) [13]. Mmsb (Figure 1e) has a sulfinyl/sulfoxide electron-withdrawing group in the para position, which makes the ester bond that links the peptide to the resin stable under TFA conditions and, therefore, is compatible with Fmoc and Boc chemistry. However, at the end of the synthesis, a reductive treatment converts the sulfinyl/sulfoxide into a thio (Figure 1f), which is an electron-donating group, and, therefore, makes the ester bond cleavable with TFA.
1.1. Safety-Catch Linkers
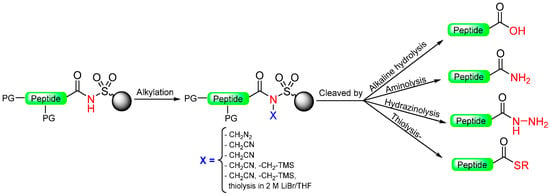
As mentioned above, safety-catch linkers (SCLs) are stable during the elongation of the peptide chain usually to an acid and/or base, ensuring the attached peptides are securely held until intended release from the solid support [14,15]. This can take place after the modification of the linker, which makes it more labile under those conditions (acid or base) than before it was stable, etc. The fundamental principle of SCLs involves their inertness throughout the synthesis process, requiring conversion from a stable form to an activated, labile state before cleavage. An excellent application of SCLs is the synthesis of peptides with diverse functional groups at the C-terminal, including carboxylics, amides, thioesters, and hydrazides. These functional groups are valuable for a variety of downstream applications, such as native chemical ligations [16] and chemo-selective conjugations [17]. There are a number of SCLs that have been developed for years, each with its own application.
1.2. Kenner and Sulfonamide Safety-Catch Linker
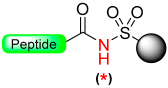
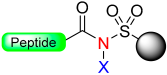
The initial carboxy anchor employed to illustrate the safety-catch principle was the sulfonamide resin (1) described by Kenner and co-workers (Scheme 1). [18] Acyl sulfonamides remain stable even in the presence of strong anhydrous acids, HBr/AcOH, as well as highly nucleophilic reagents and aqueous alkali. In this case, the stability persists because any basic attack ionizes the acidic SO2NH group (with a pKa~2.5). Their final cleavage involves activating a sulfonylacylamide group through the negative inductive effect of a nearby substituent, the sulfonyl. Thus, the N-alkylation turns the stable linker into its labile form in front of nucleophiles [18].
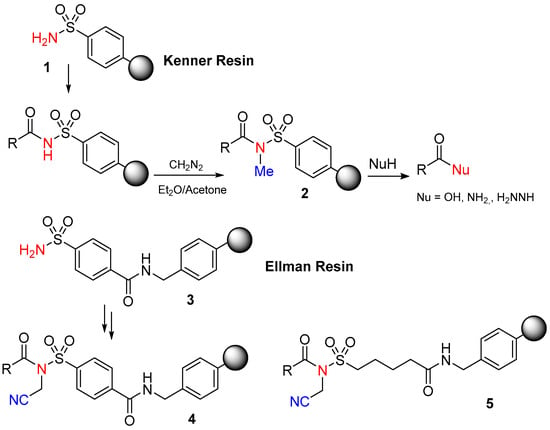
Scheme 1.
Kenner and Ellman safety-catch resins.
The initial safety-catch activation process of acyl-Kenner resin involved N-methylation with diazomethane (CH2N2) in diethylether (Et2O)/acetone, resulting in the formation of the labile N-methyl acyl sulfonamide 2. This labile N-methyl acyl sulfonamide 2 can be cleaved in the presence of nucleophiles using alkaline hydrolysis (0.5 M NaOH), aminolysis (0.5 M NH3/dioxane), hydrazinolysis (methanolic hydrazine), and thiolysis.
The initial N-methyl acyl sulfonamide from Kenner’s resin has low reactivity, leading to poor cleavage yields with less nucleophilic amines like anilines and requiring excess reagent for successful cleavage with more nucleophilic alkylamines. To address this issue, Ellman and co-workers [19] have slightly modified the Kenner strategy (Ellman resin) (Scheme 1). First of all, they used a sulfamoylbenzamide 3, which makes the NH more acidic, and then the activation step is carried with ICH2CN, enhancing the reactivity of linker 4 with various amines and nucleophiles [20].
This method allows the use of limited amounts of even sterically hindered and non-basic amines, diversifying the final amide products. The linker is also compatible with common peptide-coupling reagents, enolate alkylation [19], and Suzuki reaction conditions [21]. However, there are limitations, such as incomplete alkylation of the acyl sulfonamide when carboxylic acids with an α-electron-withdrawing group are used (Scheme 1). This limitation has been overcome by using aliphatic sulfonamide linker 5 [22]. Comparative experiments demonstrated improved cleavage yields compared to the original aryl sulfonamide linker 1. Nevertheless, challenges, such as incomplete and racemization-free acylation with protected amino acids due to the low nucleophilicity of the sulfonamide and the strong basic conditions required for the alkylation, still need to be addressed. A different method to activate N-acyl-sulfonamide nitrogen by using palladium (0) catalysis and allylation was proposed [23]. This generated a highly reactive intermediate that enables activation under gentle and neutral conditions, reducing the risk of racemization in the first protected amino acid [24] (Scheme 2). Although Kenner’s linker was first used with tert-butoxycarbonyl (Boc)-benzyl (Bzl) chemistry, it is perfectly compatible with fluorenyl methoxycarbonyl (Fmoc)-tert-butyl (tBu) chemistry.
Scheme 2.
General acyl sulfonamide safety-catch linker, activation, and cleavage.
1.3. Isonitrile/Benzamide Safety-Catch Linker
A variant of the Kenner idea is the isonitrile/benzamide linker. The resin-bound isonitrile allows for construction of the peptide on the resin using a Ugi multicomponent reaction (MCR) [25,26]. At the end of the synthetic process, the benzamide resin is activated by Boc protection of the benzamide, which becomes susceptible to hydrolytic cleavage (aq. LiOH/H2O2) to produce peptide carboxylic acids, or alcoholic cleavage (MeONa/MeOH) to yield methyl ester peptides, respectively (Scheme 3) [26].
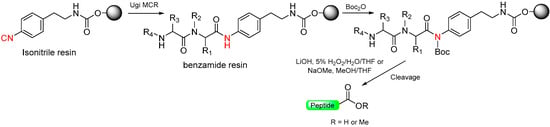
Scheme 3.
N-Protection with Boc safety-catch linkers.
1.4. The Oxidative Safety-Catch Linkers
Marshall et al. introduced the oxidative safety-catch linker, initially designed for protecting peptide fragments using Boc/Bzl chemistry [27]. They proposed a phenolsulfide resin (Scheme 4) conveniently prepared from chloromethylated polystyrene resin and p-mercaptophenol carbonate. The peptide chain was extended using the Boc/Bzl strategy. The oxidative activation of the linker was carried out using H2O2 to produce the sulfone linker. This facilitated cleavage through alkali hydrolysis and ammonolysis, leading to the release of the fully protected peptide [28]. A similar application of this phenolsulfide linker was reported, where m-CPBA was used to activate the linker to sulfone, and an internal aminolysis cleavage released the cyclic peptide [28]. Yager et al. [29] determined that oxidation of the linker is not necessary for the efficient cleavage of peptides because the phenol group is a suitable enough leaving group [30]. A significant challenge hindering the practical use of this linker is its own lability in front of the bases, which makes it not compatible with a Fmoc/tBu strategy. Furthermore, the oxidation process can also exclude delicate amino acids like Met and Cys [31].
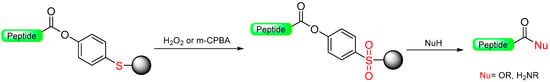
Scheme 4.
Marshall’s oxidative safety-catch linker.
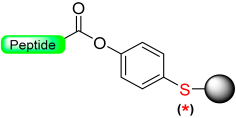
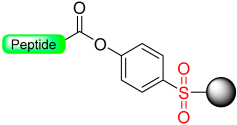
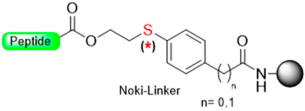
Our group [32] recently developed a base-catalyzed β-elimination safety-catch linker, 2-hydroxyethylthio acid derivatives (Scheme 5) [32]. The first protected amino acid forms an ester bond stable to bases such as secondary amines and acids such as trifluoroacetic acid (TFA) and, therefore, compatible with Fmoc and Boc chemistry. After the elongation of the peptide chain has taken place, the oxidation to the sulfone form will convert the ester bond labile secondary amines through a β-elimination reaction. The linker activation was conducted in SPPS using a multidetachable (double) linker strategy.
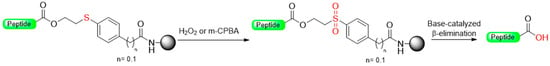
Scheme 5.
Base-catalyzed β-elimination safety-catch linker.
Schwyzer et al. developed a similar oxidative safety-catch linker that can be used for the synthesis of oligonucleotides [33]. Furthermore, Garcia-Echeverria [34] and Sowing and co-workers [35] independently reported similar strategies for the solid phase of small organic molecules in a combinatorial approach concept.
1.5. Aryl Hydrazine
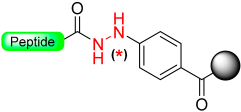
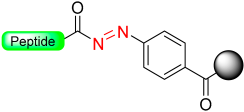
Wieland et al. [36], based on a Patchornik work [37], developed a safety-catch linker using benzyl hydrazide functionality. An unreactive N-acyl hydrazine linker was transformed into a reactive diazene intermediate through oxidation with N-bromosuccinimide (NBS) (Scheme 6). The acyl diazo intermediate reacts with nucleophiles, amines, or alcohols to produce functionalized acyl derivatives, releasing nitrogen in the process.
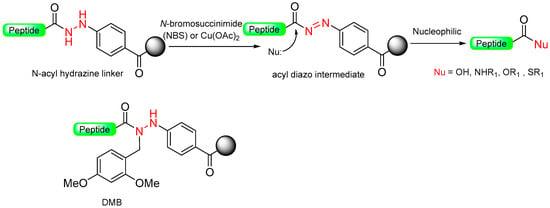
Scheme 6.
The latent arylhydrazine safety-catch linker.
Berst et al. developed a better safety-catch linker containing latent aryl hydrazine, designed to be compatible with both Mitsunobu and N-alkylation and stable toward nucleophiles [38]. However, during their investigations, they discovered that the aryl hydrazine safety-catch linker was incompatible with Mitsunobu N-alkylation conditions, leading to the formation of unwanted byproducts through linker alkylation [38]. To address this issue, they employed the 2,4-dimethoxybenzyl (DMB) protecting group to block the reactive hydrazine functionality (Scheme 6). The cleavage of the 2,4-dimethoxybenzyl arylhydrazine (DMBAH) linker from the solid support was achieved in two steps: first, acidic treatment with TFA-DCM was used to remove the DMB protecting group, and then oxidation of the resulting acyl arylhydrazine was carried out in the presence of copper (II) acetate in methanol, serving as an external nucleophile to render the methyl ester [39]. Later, this arylhydrazine linker strategy was used for the synthesis of esters, thioesters for Natural Chemical Ligation (NCL), and p-nitroanilides [40].
Numerous proteins that play essential roles in cell growth and differentiation have ester groups located at their C-terminal ends [41]. Lowe et al. explored various methods for synthesizing peptide methyl esters, a process needed in peptide chemistry [42]. Traditional approaches involved using different resins like Merrifield, 3-thiopropionic acid [43], oxime [44], or hydroxymethyl benzoic acid (HMBA) resin. These methods required specific conditions, such as the presence of tertiary bases, methanol, and sodium methoxide, to cleave the methyl ester from the resin [42]. However, a technique emerged, employing the aryl hydrazide linker [45], which allowed for direct ester generation from the solid support [46]. This innovative strategy utilizes oxidative cleavage in a one-step process, employing copper(II) acetate alongside a suitable nucleophile [47]. Furthermore, NBS can also be employed as an oxidizing agent, proving to be an efficient approach to synthesizing peptide methyl esters [48].
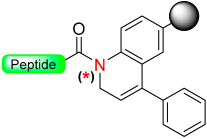
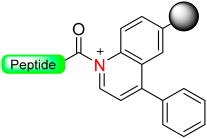
1.6. Dihydroquinoline (DHQ)
Mioskowski et al. developed a resin-bound compound called 1,2-dihydroquinoline (DHQ) that can generate amides or carboxylic acids peptides (Scheme 7) [49]. The DHQ resin in its N-acylated form was proven to be stable even under various conditions, such as basic, acidic, and mild reducing environments. It also proves to be compatible with Boc/Fmoc standard deprotection conditions, although no peptides were synthesized. To activate the linker, oxidative aromatization was performed using substances like 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ), cerium (IV) ammonium nitrate (CAN), or triphenylcarbenium tetrafluoroborate (Ph3CBF4). This process led to the formation of an activated acyl quinolinium intermediate. The final amides or carboxylic acid peptide was released by displacing the quinolinium moiety using the nucleophiles BnNH2 or H2O [49].
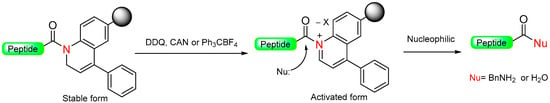
Scheme 7.
Oxidative aromatization safety-catch linker.
1.7. The Reductive-Acidolytic Safety-Catch Linkers
Although not green at all, TFA is an excellent reagent to remove the Boc group in a Boc/Bzl strategy and carry out the final global deprotection (removal of the side-chain protecting groups and cleavage of the peptide from the resin). In this regard, several linkers have been developed where the lability in front of the TFA is masked during the elongation of the peptide on the resin, and at the end of the synthetic process, the modification of the linker makes it labile to TFA. Most of them are based on the pair methylsulfinylbenzyl/methylthiobenzyl, depicted in Figure 1e,f. As discussed in the introduction, the sulfinyl/sulfoxide linker exhibits stability towards TFA, but it is converted to TFA labile after reduction to the thiobenzyl derivative. However, at the end of synthesis, a reductive treatment converts the sulfinyl/sulfoxide into a thio (Table 1), which is an electron-donating group and, therefore, makes the ester bond cleavable with TFA. Table 1 shows the different reduction methods for C-terminus acid peptides.

Table 1.
Reduction and cleavage conditions of sulfoxide to sulfide.
Various naturally existing peptides, particularly hormones like oxytocin, secretin, and calcitonin, possess a C-terminal amide function. As a result, Patek and Lebl [63,66] developed safety-catch amide-anchor groups that could be removed using TFA-based methods at the end. Several safety-catch systems have been developed by studying the electronic properties of ortho- and para-substituents in the context of heterolytic benzyl-oxygen or benzyl-nitrogen cleavage dependence [15]. Patek and Lebl introduced a novel safety-catch acid-labile (SCAL) mechanism based on 2-alkoxy-4,4′-bis(methylthio) benzhydrylamine (Scheme 8) [31,67,68]. Additionally, it enables the simultaneous utilization of both Fmoc and Boc groups in peptide synthesis. The oxidized form of the linker, in its sulfoxide state, displays exceptional stability against both acids (such as TFA and thioanisole/TFA) and bases (including aqueous NaOH and piperidine). Activation of the linker occurs through the reduction of both sulfoxide groups to their respective sulfides, achieved either with PPh3/Me3SiCl/CH2Cl2 or (EtO)2P(S)SH/DMPU [31]. Acidolytic cleavage is subsequently carried out by treating it with TFA in the presence of scavengers, resulting in the formation of C-terminal peptide amides [14,63].
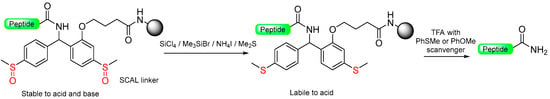
Scheme 8.
Patek safety-catch acid-labile (SCAL) linker.
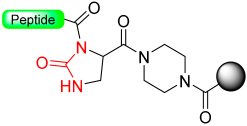
Portal et al. [69] developed a new safety-catch acid-labile linker (SCAL-2) (Figure 2) with a simplified molecular architecture, easier chemical accessibility, and improved stability compared to the original Patek and Lebl SCAL-1.
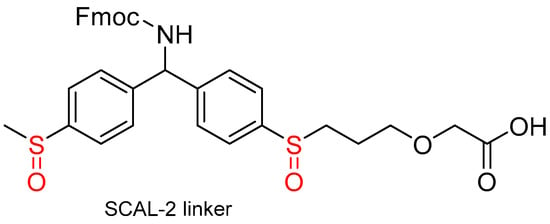
Figure 2.
Portal safety-catch acid-labile (SCAL-2) linker.
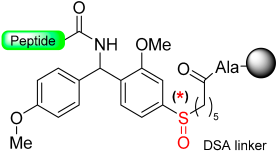
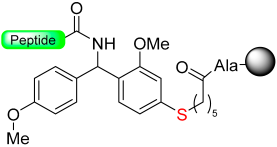
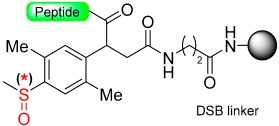
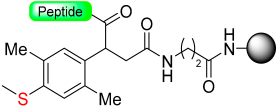
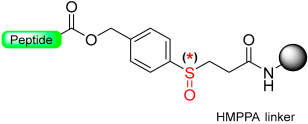
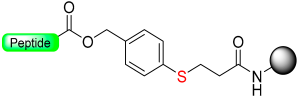
Kiso and colleagues employed similar strategies in SPPS while designing DSA [61] (4-(4-methoxyphenyl-aminomethyl)-3-methoxyphenylsulfinyl-6-hexanoic acid) and DSB [58] (4-(2,5-dimethyl-4-methylsulfinylphenyl)-4-hydroxybutanoic acid) (Scheme 9). In the DSA linker, amino acids are linked together through an amide bond. This results in the formation of peptide amides once activation occurs, followed by cleavage using TFA [61]. The stability of the DSB linker before reduction was tested using a γ-endorphin peptide comprising 17 amino acid residues. Thus, the peptide resin-DSB-resin was treated with TFA/anisole for 24 h, leading to the cleavage of only 3% of Leu. In contrast, under reductive acidolysis with SiCl4-thioanisole-anisole-TFA for 3 h, 94% of Leu was successfully cleaved from the resin [58]. Likewise, Undén and Erlandsson [57] utilized comparable methods in developing the HMPPA (3-(4-hydroxymethylphenylsulfanyl) propanoic acid) linker. The significant application of HMPPA SCLs lies in the synthesis of side-chain-to-side-chain cyclic peptides, as demonstrated by the synthesis of the model peptide Fmoc-K(Boc)FDAPE(OtBu)G-O-HMPPA(O) on aminomethyl resin.
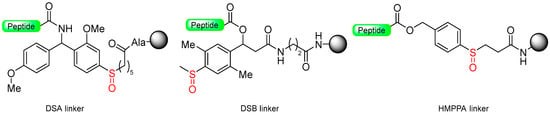
Scheme 9.
DSA (for amide peptides), and DSA and HMPPA (for acid peptides) as safety-catch linkers.
Our group introduced the Mmsb linker [13], which is compatible with both Boc and Fmoc chemistry SPPS. Peptide-O-Mmsb-resin was synthesized, and the linker exhibited stability to the treatments, with piperidine and TFA used for the removal of Fmoc and Boc protecting groups, respectively. The peptide can be detached using a two-step protocol. Firstly, the sulfoxide moiety is reduced to the sulfide using Me3SiCl and Ph3P. Subsequently, treatment with TFA completes the detachment process. The Mmsb linker was applied for the synthesis of somatostatin (Scheme 10).
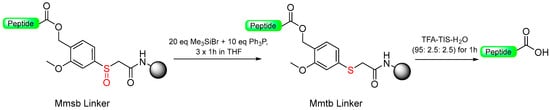
Scheme 10.
Mmsb safety-catch linker.
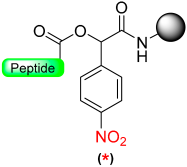
Our group described a p-nitrobenzyl alcohol (p-nitromandelic acid) linker that forms an ester with the first amino acid stable to TFA and, therefore, is compatible with a Boc strategy but not compatible with Fmoc chemistry [70]. At the end of the elongation of the peptide sequence, the nitro group is reduced very smoothly to amines using SnCl2 in HCl/dioxane. The reduced linker liberates the acid peptide via a 1,6-electron pair shift by microwave irradiation at 50 °C with 5% TFA in dioxane (Scheme 11). Cleavage was also attempted in basic media (DIEA), but the yield reported was very low, probably due to the high reactivity of the quinonimine methide generated with nucleophiles [70].
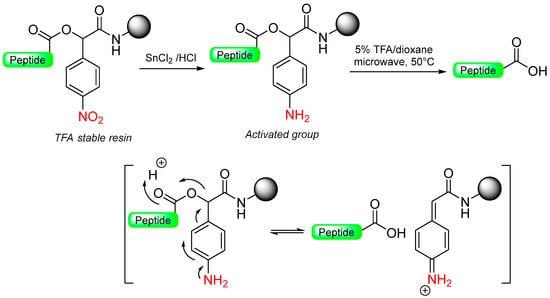
Scheme 11.
p-Nitromandelic-based safety-catch linker.
1.8. Heterocycle Formation—Via Intramolecular Cyclization—As Leaving Group
The high stability of the five-member ring heterocycles allows their easy preparation by intramolecular cyclization. The presence of an atom of N in the heterocycle makes these heterocycles acidic and, therefore, could be suitable leaving groups, which are the liberation of some acids (acid peptides). This chemical strategy was developed many years ago for the preparation of C-terminus modified peptides, in recent years, the need to prepare a thio ester peptide readily usable in native chemical ligation has emerged [40].
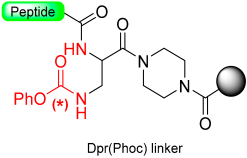
1.9. Dpr(Phoc) Linker–Imidazolidinone (Cyclic Urea)
Pascal et al. [71] introduced the Dpr(Phoc) safety-catch linker (Dpr = L-2,3-diaminopropionic acid, Phoc = Phenyloxycarbonyl), derived from β-aminophenyloxycarbonyl-2,3-diaminopropionic acid, which is stable in both basic and acidic conditions [72]. Once the peptide elongation has been concluded, the treatment of the peptide resin under mild alkaline conditions (2 equiv. of 0.04M NaOH for 1 h) renders the electrophilic isocyanate, which suffers an intramolecular cyclization toward the adjacent secondary amide group [73]. Once the N-acyl-2-imidazolidione has been formed, the linker undergoes cleavage through alkaline hydrolysis (0.03M NaOH) or ammonolysis (satd. NH3 in iPrOH) or potentially other nucleophiles (Scheme 12). This linker’s stability is notable under various conditions, including 1M in TFA at 0 °C, 50% TFA, and 6 M aq. HCl [74]. The Dpr(Phoc) linker is an aqueous media cleavable linker that can be useful for biological applications of synthetic peptides. It was successfully used to avoid diketopiperazine formation during the synthesis of Tyr-Asp-Pro-Ala-(Pro)6-OH. The Dpr(Phoc) linker can also be applied for the preparation of peptide conjugates and cyclic peptides.
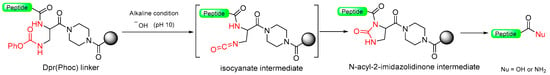
Scheme 12.
Dpr(Phoc) safety-catch linker.
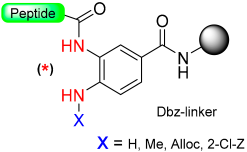
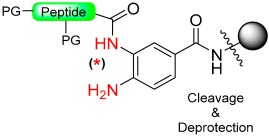
1.10. Diamino Benzoic Acid (Dbz)–Benzimidazolinone (Cyclic Urea)
The generation of C-terminal peptide thioesters constitutes a crucial step in the native chemical ligation method for creating fully or partially synthetic proteins [75]. However, efficiently producing these thioesters through Fmoc solid-phase peptide synthesis proves challenging due to the labile nature of thioester linkage under basic conditions. The functionality of C-terminal N-acylureas (imidazolinones) has been previously investigated in peptide synthesis, as described in the previous section, and similar reports have been made regarding the use of thioacylbenzimidazolinones [76,77]. Blanco-Canosa et al. have developed the 3,4-diamino benzoic acid (Dbz) linker (Scheme 13, X=H) based on that strategy [78]. At the end of peptide elongation, the peptide resin can be chemically modified by treatment with p-nitrophenyl chloroformate to yield a N-acyl-benzimidazolinone (Nbz) (N-acylurea) moiety on the resin. After TFA cleavage of the side-protecting groups, this can cause resin transthioesterification to render the thioester ready to be used in NCL. Alternatively, if the Dbz linker is attached to Rink amide resin, after the cyclization, the N-acylurea peptide can be cleaved from the resin with TFA, which can also be used directly for NCL in the presence of a thiol catalyst or transthioesterified.
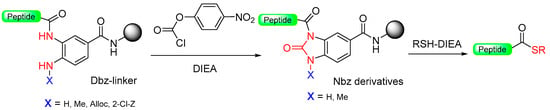
Scheme 13.
Dbz safety-catch linker.
The important aspect of this approach lies in regulating the chain extension process to ensure that acylation takes place exclusively at one of the two unprotected amines on the Dbz linker (Scheme 13, X = H) [78]. There have been reports indicating that within Gly-rich sequences, acylation of the unprotected o-aminoanilide can take place, particularly in the presence of an excess of bases [79]. The introduction of the protected Fmoc-Dbz (Alloc) effectively eliminates these side-products by employing orthogonal allyloxycarbonyl (Alloc) protection on a single Dbz amine (Scheme 13, X = Alloc) [79]. Additionally, a novel N-acylurea linker incorporating an o-amino(methyl)aniline (MeDbz) [40] moiety has been introduced to enhance the robustness of peptide chain assembly (Scheme 13, X = Me). The MeDbz linker has been demonstrated to be superior to the Dbz. It has been utilized in the synthesis of cysteine-rich proteins, such as the cyclotides Kalata B1 and MCoTI-II, through a single cyclization/folding step within the ligation/folding buffer [40]. Furthermore, the MeDbz linker has been used for the on-resin cyclization of side-chain (SH of Cys, NH2 of Dab, OH of Tyr)-to-tail cyclic peptides [80,81,82] and for the preparation of C-terminal modified peptides [83,84].
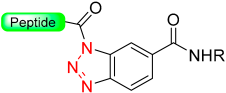
This concept of using unsymmetrical diamino benzoic resin has been further described by other groups who utilize different protecting groups for the second amine function [75,85,86]. Activation through a peptidyl-benzotriazole intermediate enables easy conversion to peptide thioesters for application in NCL [87]. Using the same Dbz linker, at the end of synthesis, the unprotected peptide can undergo treatment with sodium nitrite, resulting in an acyl benzotriazole intermediate. This intermediate can then be intercepted by a thiol, ultimately producing C-terminal thioester peptides [88] (Scheme 14). A similar linker and strategy were later proposed by Kao et al. [89] for the formation of the benzentriazole isoamylnitrile. Dawson et al. [90] utilized a comparable strategy (formation of the acybenzotriazole) for the in-solution activation of unprotected peptides for further NCL.
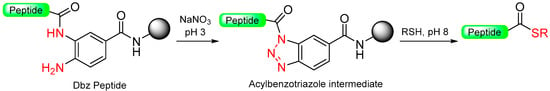
Scheme 14.
Dbz peptide to thioester peptide via ccylbenzotriazole intermediate.
1.11. Pyroglutamyl/Pyrrolidinone Amide
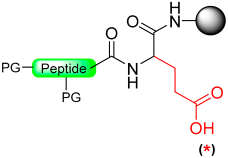
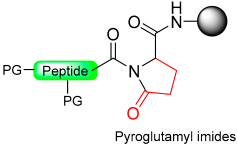
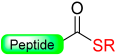
Jensen and co-workers introduced a new method for providing peptide thioesters based on the activation of a backbone amide in the peptide through the formation of a backbone pyroglutamyl imide (Scheme 15) [91].
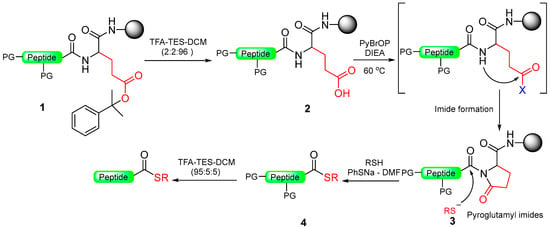
Scheme 15.
Peptide thioester synthesis through pyroglutamyl imides strategy.
The synthesis of C-terminal peptide thioesters using a pyroglutamyl imide as a linker strategy is carried out by first attaching a C-terminal glutamic acid residue with a selectively removable side-chain protecting group to a solid support 1. After the completion of the peptide chain elongation, the Glu side-chain was selectively deprotected 2. The crucial step involves the activation of the deprotected carboxylic acid with bromo-tris-pyrrolidino-phosphonium hexafluorophosphate (PyBrOP), leading to on-resin formation of the pyroglutamyl (pGlu) imide moiety 3. The next step was nucleophilic displacement by treatment with thiol (thiolysis) to release the protected peptide thioester 4 from the solid support, followed by the removal of the protecting group in solution (Scheme 15). This linker allows the preparation of thioester peptides with only moderate yields.
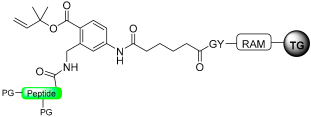
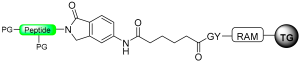
To address this issue of poor reactivity, Jensen’s group introduced a more reactive aromatic pyrrolidinone resin in a later publication [92]. This resin, based on the same five-member ring principle, exhibits enhanced reactivity in the presence of a nucleophile (Scheme 16).
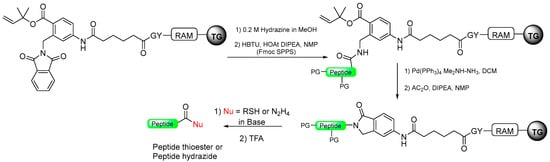
Scheme 16.
Peptide thioester and hydrazide synthesis through pyrrolidinone strategy (TG-RAM for Tenta Gel RinkAMide).
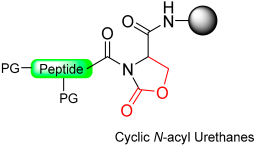
1.12. Cyclic Urethane Moiety
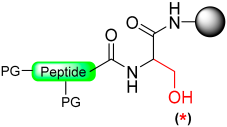
This five-member-ring journey ends with cyclic urethane after cyclic urea and a pyroglutanyl moiety. The method involves the selective activation of the backbone amide bond at the C-terminal Ser residue to generate a cyclic urethane moiety on a solid support [93]. The synthesis process includes anchoring a C-terminal serine residue with a removable side-chain protecting group to the solid support. After peptide chain elongation, Ser’s side-chain is deprotected and activated as an active carbonate with nitrophenyl chloroformate or disuccinidyl carbonate (DSC) or carbonyl diimiazole (CDI), leading to the formation of a cyclic urethane moiety on the resin. Subsequent nucleophilic displacement of the cyclic urethane moiety through treatment with a thiol releases the peptide thioester from the solid support, allowing for subsequent deprotection in solution (Scheme 17) [94]. The thioester produced is free of epimerization and is effectively applied to synthesize large peptides through NCL. (Table 2).
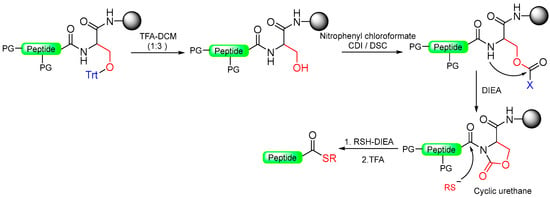
Scheme 17.
Peptide thioester synthesis through cyclic urethane strategy.

Table 2.
Summary of all the strategies discussed above.
2. Conclusions
Safety-catch linkers (SCLs) play an important role in solid-phase peptide synthesis by providing flexibility and stability during the elongation of the peptide chain and allowing safe release of the peptide from the solid support once the linker has been modified. Various types of SCLs have been developed, each with its own application and mechanism of action. The Kenner and Ellamn sulfonamide safety-catch linker, for example, utilizes acyl sulfonamides, which remain stable under various conditions due to their acidic SO2NH group. Activation of this linker involves N-methylation, leading to the formation of a labile N-methyl acyl sulfonamide that can be cleaved in the presence of nucleophiles. Similarly, the oxidative safety-catch linkers, introduced by Marshall, involve the oxidation of phenolsulfide resin to produce a sulfone linker, facilitating cleavage through alkali hydrolysis or ammonolysis. However, challenges such as lability in front of bases limit their practical use. Noki linkers overcome all these problems; the peptide linker bond is totally stable to the elongation of the peptide using a Fmoc/tBu strategy, and after oxidation, it liberates the peptide by treatment with secondary amines. This linker, when used in a multidetachable strategy, could be very useful for methodological studies. Other types of SCLs, such as aryl hydrazine and reductive-acidolytic linkers, offer alternative strategies for peptide synthesis, each with its own advantages and limitations. For instance, the aryl hydrazine linker developed by Wieland et al. undergoes oxidation with N-bromosuccinimide to generate a reactive diazene intermediate, allowing for the formation of functionalized acyl derivatives. Moreover, advancements in linker design, such as MeDbz linkers, provide improved stability and compatibility with peptide synthesis strategies like Boc and Fmoc chemistry.
Overall, safety-catch linkers continue to be a significant component in solid-phase peptide synthesis, offering flexibility and efficiency in the construction of peptide libraries and the synthesis of bioactive peptides with diverse functional groups. Due to the importance of the NCL for the chemical synthesis of large peptides and proteins, it is envisaged that other safety-catch linkers will be described, taking advantage of the stability of the corresponding heterocycles. The construction of these has been the driving force for the development of most parts of linkers suitable for the preparation of thioester peptides.
Author Contributions
S.N. contributed to the literature search and wrote the first draft of manuscript; B.G.d.l.T. and F.A. have reviewed and approved the final version for publication. All authors have read and agreed to the published version of the manuscript.
Funding
The work performed by the authors is funded by the National Research Foundation (NRF) of South africa and the University of KwaZulu-Natal.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Lloyd-Williams, P.; Albericio, F.; Giralt, E. Chemical Approaches to the Synthesis of Peptides and Proteins; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Nefkens, G.H.L.; Tesser, G.I. A novel activated ester in peptide syntheses. J. Am. Chem. Soc. 1961, 83, 1263. [Google Scholar] [CrossRef]
- Lam, K.S.; Salmon, S.E.; Hersh, E.M.; Hruby, V.J.; Kazmierski, W.M.; Knapp, R.J. A new type of synthetic peptide library for identifying ligand-binding activity. Nature 1991, 354, 82–84. [Google Scholar] [CrossRef]
- Dawson, P.E.; Muir, T.W.; Clark-Lewis, I.; Kent, S.B.H. Synthesis of Proteins by Native Chemical Ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.B.H. Total chemical synthesis of proteins. Chem. Soc. Rev. 2009, 38, 338–351. [Google Scholar] [CrossRef]
- Bruckdorfer, T.; Marder, O.; Albericio, F. From Production of Peptides in Milligram Amounts for Research to Multi-Tons Quantities for Drugs of the Future. Curr. Pharm. Biotechnol. 2004, 5, 29–43. [Google Scholar] [CrossRef]
- De la Torre, B.G.; Albericio, F. Peptide Therapeutics 2.0. Molecules 2020, 25, 2293. [Google Scholar] [CrossRef]
- Albericio, F. Orthogonal protecting groups for Nα-amino and C-terminal carboxyl functions in solid-phase peptide synthesis. Pept. Sci. 2000, 55, 123–139. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Synthesis (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1985, 24, 799–810. [Google Scholar] [CrossRef]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef]
- Barany, G.; Albericio, F. Three-dimensional orthogonal protection scheme for solid-phase peptide synthesis under mild conditions. J. Am. Chem. Soc. 1985, 107, 4936–4942. [Google Scholar] [CrossRef]
- Nandhini, K.P.; Albericio, F.; de la Torre, B.G. 2-Methoxy-4-methylsulfinylbenzyl Alcohol as a Safety-Catch Linker for the Fmoc/tBu Solid-Phase Peptide Synthesis Strategy. J. Org. Chem. 2022, 87, 9433–9442. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, S.; Patek, M. Safety-Catch Linker Units. In Linker Strategies in Solid-Phase Organic Synthesis; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 195–220. [Google Scholar]
- Sebastian Wiehn, M.; Jung, N.; Bräse, S. Safety-Catch and Traceless Linkers in Solid Phase Organic Synthesis. In The Power of Functional Resins in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 437–465. [Google Scholar]
- Agouridas, V.; El Mahdi, O.; Diemer, V.; Cargoët, M.; Monbaliu, J.-C.M.; Melnyk, O. Native Chemical Ligation and Extended Methods: Mechanisms, Catalysis, Scope, and Limitations. Chem. Rev. 2019, 119, 7328–7443. [Google Scholar] [CrossRef]
- Góngora-Benítez, M.; Tulla-Puche, J.; Albericio, F. Multifaceted Roles of Disulfide Bonds. Peptides as Therapeutics. Chem. Rev. 2014, 114, 901–926. [Google Scholar] [CrossRef]
- Kenner, G.W.; McDermott, J.R.; Sheppard, R.C. The safety catch principle in solid phase peptide synthesis. J. Chem. Soc. D 1971, 12, 636–637. [Google Scholar] [CrossRef]
- Backes, B.J.; Ellman, J.A. Carbon-Carbon Bond-Forming Methods on Solid Support. Utilization of Kenner’s Safety-Catch” Linker. J. Am. Chem. Soc. 1994, 116, 11171–11172. [Google Scholar] [CrossRef]
- Backes, B.J.; Virgilio, A.A.; Ellman, J.A. Activation Method to Prepare a Highly Reactive Acylsulfonamide “Safety-Catch” Linker for Solid-Phase Synthesis1. J. Am. Chem. Soc. 1996, 118, 3055–3056. [Google Scholar] [CrossRef]
- Suzuki, A. Organoboron compounds in new synthetic reactions. Pure Appl. Chem. 1985, 57, 1749–1758. [Google Scholar] [CrossRef]
- Backes, B.J.; Ellman, J.A. An Alkanesulfonamide “Safety-Catch” Linker for Solid-Phase Synthesis. J. Org. Chem. 1999, 64, 2322–2330. [Google Scholar] [CrossRef]
- He, Y.; Wilkins, J.P.; Kiessling, L.L. N-Acylsulfonamide Linker Activation by Pd-Catalyzed Allylation. Org. Lett. 2006, 8, 2483–2485. [Google Scholar] [CrossRef] [PubMed]
- Sewald, P.D.N. Peptide Synthesis. In Peptides: Chemistry and Biology; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 175–315. [Google Scholar]
- Ugi, I. The α-Addition of Immonium Ions and Anions to Isonitriles Accompanied by Secondary Reactions. Angew. Chem. Int. Ed. Engl. 1962, 1, 8–21. [Google Scholar] [CrossRef]
- Hulme, C.; Peng, J.; Morton, G.; Salvino, J.M.; Herpin, T.; Labaudiniere, R. Novel safety-catch linker and its application with a Ugi/De-BOC/Cyclization (UDC) strategy to access carboxylic acids, 1,4-benzodiazepines, diketopiperazines, ketopiperazines and dihydroquinoxalinones. Tetrahedron Lett. 1998, 39, 7227–7230. [Google Scholar] [CrossRef]
- Marshall, D.L.; Liener, I.E. Modified support for solid-phase peptide synthesis which permits the synthesis of protected peptide fragments. J. Org. Chem. 1970, 35, 867–868. [Google Scholar] [CrossRef]
- Flanigan, E.; Marshall, G.R. Synthesis of cyclic peptides on dual function supports. Tetrahedron Lett. 1970, 11, 2403–2406. [Google Scholar] [CrossRef]
- Fantauzzi, P.P.; Yager, K.M. Synthesis of diverse tetrahydro-β-carboline-3-carboxamides and -2,3-bis-lactams on a versatile 4-hydroxythiophenol-linked solid support. Tetrahedron Lett. 1998, 39, 1291–1294. [Google Scholar] [CrossRef]
- Guy Breitenbucher, J.; Johnson, C.R.; Haight, M.; Christopher Phelan, J. Generation of a piperazine-2-carboxamide library: A practical application of the phenol-sulfide react and release linker. Tetrahedron Lett. 1998, 39, 1295–1298. [Google Scholar] [CrossRef]
- Pátek, M.; Lebl, M. Safety-catch and multiply cleavable linkers in solid-phase synthesis. Pept. Sci. 1998, 47, 353–363. [Google Scholar] [CrossRef]
- Noki, S.; Saneii, H.; de la Torre, B.G.; Albericio, F. Safety-Catch Linkers for Solid Phase Peptide Synthesis. 144281-2, 12 December 2023. [Google Scholar]
- Schwyzer, R.; Felder, E.; Merli-Failli, P. The CAMET and CASET links for the synthesis of protected oligopeptides and oligodeoxynucleotides on solid and soluble supports. Helv. Chim. Acta 1984, 67, 1316–1327. [Google Scholar] [CrossRef]
- García-Echeverría, C. A base labile handle for solid phase organic chemistry. Tetrahedron Lett. 1997, 38, 8933–8934. [Google Scholar] [CrossRef]
- Wade, W.S.; Yang, F.; Sowin, T.J. Application of Base Cleavable Safety Catch Linkers to Solid Phase Library Production. J. Comb. Chem. 2000, 2, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Wieland, T.; Lewalter, J.; Birr, C. Subsequent activation of carboxylic derivatives by oxidation or elimination of water; application for cyclizing peptides. Justus Liebigs Ann. Chem. 1970, 740, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Wolman, Y.; Gallop, P.M.; Patchornik, A. Peptide synthesis via oxidation of hydrazides. J. Am. Chem. Soc. 1961, 83, 1263–1264. [Google Scholar] [CrossRef]
- Berst, F.; Holmes, A.B.; Ladlow, M.; Murray, P.J. A latent aryl hydrazine ‘safety-catch’ linker compatible with N-alkylation. Tetrahedron Lett. 2000, 41, 6649–6653. [Google Scholar] [CrossRef]
- Berst, F.; Holmes, A.B.; Ladlow, M. The development and preparation of the 2,4-dimethoxybenzyl arylhydrazine (DMBAH) “latent” safety-catch linker: Solid phase synthesis of ketopiperazines. Org. Biomol. Chem. 2003, 1, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canosa, J.B.; Nardone, B.; Albericio, F.; Dawson, P.E. Chemical Protein Synthesis Using a Second-Generation N-Acylurea Linker for the Preparation of Peptide-Thioester Precursors. J. Am. Chem. Soc. 2015, 137, 7197–7209. [Google Scholar] [CrossRef]
- Porter, J.A.; Young, K.E.; Beachy, P.A. Cholesterol Modification of Hedgehog Signaling Proteins in Animal Development. Science 1996, 274, 255–259. [Google Scholar] [CrossRef]
- Millington, C.R.; Quarrell, R.; Lowe, G. Aryl hydrazides as linkers for solid phase synthesis which are cleavable under mild oxidative conditions. Tetrahedron Lett. 1998, 39, 7201–7204. [Google Scholar] [CrossRef]
- Camarero, J.A.; Adeva, A.; Muir, T.W. 3-Thiopropionic acid as a highly versatile multidetachable thioester resin linker. Lett. Pept. Sci. 2000, 7, 17–21. [Google Scholar] [CrossRef]
- DeGrado, W.F.; Kaiser, E.T. Polymer-bound oxime esters as supports for solid-phase peptide synthesis. The preparation of protected peptide fragments. J. Org. Chem. 1980, 45, 1295–1300. [Google Scholar] [CrossRef]
- Peters, C.; Waldmann, H. Solid-Phase Synthesis of Peptide Esters Employing the Hydrazide Linker. J. Org. Chem. 2003, 68, 6053–6055. [Google Scholar] [CrossRef]
- Camarero, J.A.; Hackel, B.J.; de Yoreo, J.J.; Mitchell, A.R. Fmoc-Based Synthesis of Peptide α-Thioesters Using an Aryl Hydrazine Support. J. Org. Chem. 2004, 69, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Welsh, K.; Mitchell, A.R.; Camarero, J.A. Preparation of Peptide p-Nitroanilides Using an Aryl Hydrazine Resin. Org. Lett. 2004, 6, 3801–3804. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.-H.; Mitchell, A.R.; Camarero, J.A. The Use of Aryl Hydrazide Linkers for the Solid Phase Synthesis of Chemically Modified Peptides. Int. J. Pept. Res. Ther. 2007, 13, 181–190. [Google Scholar] [CrossRef]
- Arseniyadis, S.; Wagner, A.; Mioskowski, C. Resin-bound 4-phenyl-1,2-dihydroquinoline (DHQ): A new safety-catch linker for solid-phase organic synthesis (SPOS). Tetrahedron Lett. 2004, 45, 2251–2253. [Google Scholar] [CrossRef]
- Tanikaga, R.; Nakayama, K.; Tanaka, K.; Kaji, A.J.C.L. Rapid reaction between sulfonium ion and sulfide. Preparative reduction of sulfoxide to sulfide. Chem. Lett. 1977, 6, 395–396. [Google Scholar] [CrossRef]
- Drabowicz, J.; MikoŁAjczyk, M. A Rapid and Mild Reduction of Sulphoxides with Titanium(II) Chloride. Synthesis 1978, 1978, 138–139. [Google Scholar] [CrossRef]
- Soysa, H.S.D.; Weber, W.P. Facile reduction of sulfoxides by disilthianes. Tetrahedron Lett. 1978, 19, 235–238. [Google Scholar] [CrossRef]
- Numata, T.; Togo, H.; Oae, S. Facile reduction of sulfoxides and sulfimides with thiol/trimethylsilyl chloride system. J. Chem. Lett. 1979, 8, 329–332. [Google Scholar] [CrossRef]
- Samanen, J.M.; Brandeis, E. The p-(methylsulfinyl)benzyl group: A trifluoroacetic acid (TFA)-stable carboxyl-protecting group readily convertible to a TFA-labile group. J. Org. Chem. 1988, 53, 561–569. [Google Scholar] [CrossRef]
- Kiso, Y.; Tanaka, S.; Kimura, T.; Itoh, H.; Akaji, K. New hydroxyl protecting groups of safety-catch removal by reductive acidolysis. Chem. Pharm. Bull. 1991, 39, 3097–3099. [Google Scholar] [CrossRef][Green Version]
- Beck, W.; Jung, G. Convenient reduction of S-oxides in synthetic peptides, lipopeptides and peptide libraries. Lett. Pept. Sci. 1994, 1, 31–37. [Google Scholar] [CrossRef]
- Erlandsson, M.; Undén, A. 3-(4-Hydroxymethylphenylsulfanyl)propanoic acid (HMPPA) as a new safety catch linker in solid phase peptide synthesis. Tetrahedron Lett. 2006, 47, 5829–5832. [Google Scholar] [CrossRef]
- Kiso, Y.; Fukui, T.; Tanaka, S.; Kimura, T.; Akaji, K. A new reductive acidolysis final deprotection strategy in solid phase peptide synthesis. Use of a new safety-catch linker. Tetrahedron Lett. 1994, 35, 3571–3574. [Google Scholar] [CrossRef]
- Thennarasu, S.; Liu, C.-F. A new safety-catch protecting group and linker for solid-phase synthesis. Tetrahedron Lett. 2010, 51, 3218–3220. [Google Scholar] [CrossRef]
- Paradís-Bas, M.; Tulla-Puche, J.; Albericio, F. 2-Methoxy-4-methylsulfinylbenzyl: A Backbone Amide Safety-Catch Protecting Group for the Synthesis and Purification of Difficult Peptide Sequences. Chem. Eur. J. 2014, 20, 15031–15039. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Fukui, T.; Tanaka, S.; Akaji, K.; Kiso, Y. A Reductive Acidolysis Final Deprotection Strategy in Solid Phase Peptide Synthesis Based on Safety-Catch Protection. Chem. Pharm. Bull. 1997, 45, 18–26. [Google Scholar] [CrossRef]
- Fujii, N.; Otaka, A.; Sugiyama, N.; Hatano, M.; Yajima, H. Studies on Peptides. CLV. Evaluation of Trimethylsilyl Bromide as a Hard-Acid Deprotecting Reagent in Peptide Synthesis. Chem. Pharm. Bull. 1987, 35, 3880–3883. [Google Scholar] [CrossRef] [PubMed]
- Pátek, M.; Lebl, M. Safety-catch anchoring linkage for synthesis of peptide amides by Boc/Fmoc strategy. Tetrahedron Lett. 1991, 32, 3891–3894. [Google Scholar] [CrossRef]
- Brust, A.; Tickle, A.E. High-throughput synthesis of conopeptides: A safety-catch linker approach enabling disulfide formation in 96-well format. J. Pept. Sci. 2007, 13, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Noki, S.; Brasil, E.; Zhang, H.; Bruckdorfer, T.; de la Torre, B.G.; Albericio, F. Solid-Phase Peptide Synthesis Using a Four-Dimensional (Safety-Catch) Protecting Group Scheme. J. Org. Chem. 2022, 87, 9443–9453. [Google Scholar] [CrossRef] [PubMed]
- Lebl, M.; Patek, M.; Kocis, P.; Krchnak, V.; Hruby, V.J.; Salmon, S.E.; Lam, K. Multiple release of equimolar amounts of peptides from a polymeric carrier using orthogonal linkage-cleavage chemistry. Int. J. Pept. Protein Res. 1993, 41, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Patek, M. Multistep deprotection for peptide chemistry. Int. J. Pept. Protein Res. 1993, 42, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Patek, M.; Lebl, M. “Safety-Catch” Anchoring Linkages and Protecting Groups in Solid-Phase Peptide Synthesis; Peptides-American Symposium; Escom Science Publishers: Tucson, AZ, USA, 1994; p. 146. [Google Scholar]
- Portal, C.; Hintersteiner, M.; Barbeau, O.; Dodd, P.; Huggett, M.; Pérez-Pi, I.; Evans, D.; Auer, M. Facile Synthesis of a Next Generation Safety-Catch Acid-Labile Linker, SCAL-2, Suitable for Solid-Phase Synthesis, On-Support Display and for Post-Synthesis Tagging. ChemistrySelect 2017, 2, 6658–6662. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Alvarez, M.; Burger, K.; Spengler, J.; Albericio, F. p-Nitromandelic Acid as a Highly Acid-Stable Safety-Catch Linker for Solid-Phase Synthesis of Peptide and Depsipeptide Acids. Org. Lett. 2007, 9, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Pascal, R.; Chauvey, D.; Sola, R. Carboxyl-protecting groups convertible into activating groups. Carbamates of o-aminoanilides are precursors of reactive N-acylureas. Tetrahedron Lett. 1994, 35, 6291–6294. [Google Scholar] [CrossRef]
- Sola, R.; Saguer, P.; David, M.-L.; Pascal, R. A new type of safety-catch linker cleaved by intramolecular activation of an amide anchorage and allowing aqueous processing in solid-phase peptide synthesis. J. Chem. Soc. Chem. Commun. 1993, 23, 1786–1788. [Google Scholar] [CrossRef]
- Sola, R.; Méry, J.; Pascal, R. Fmoc-based solid-phase peptide synthesis using dpr(phoc) linker. Synthesis of a C-terminal proline peptide. Tetrahedron Lett. 1996, 37, 9195–9198. [Google Scholar] [CrossRef]
- Pascal, R.; Sola, R. Calcium-promoted hydrolysis of N-acylureas allows mild release of peptides anchored with the Dpr(Phoc) linker to hydrophilic resins. Tetrahedron Lett. 1997, 38, 4549–4552. [Google Scholar] [CrossRef]
- Mannuthodikayil, J.; Singh, S.; Biswas, A.; Kar, A.; Tabassum, W.; Vydyam, P.; Bhattacharyya, M.K.; Mandal, K. Benzimidazolinone-Free Peptide o-Aminoanilides for Chemical Protein Synthesis. Org. Lett. 2019, 21, 9040–9044. [Google Scholar] [CrossRef]
- Zacharie, B.; Sauvé, G.; Penney, C. Thioacylating agents. Use of thiobenzimidazolone derivatives for the preparation of thiotuftsin analogs. Tetrahedron 1993, 49, 10489–10500. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Grote, C.W.; Rapoport, H. Thiopeptide synthesis. {alpha}-amino thionoacid derivatives of nitrobenzotriazole as thioacylating agents. J. Org. Chem. 1996, 61, 9045–9048. [Google Scholar] [CrossRef]
- Blanco-Canosa, J.B.; Dawson, P.E. An Efficient Fmoc-SPPS Approach for the Generation of Thioester Peptide Precursors for Use in Native Chemical Ligation. Angew. Chem. Int. Ed. 2008, 47, 6851–6855. [Google Scholar] [CrossRef]
- Mahto, S.K.; Howard, C.J.; Shimko, J.C.; Ottesen, J.J. A Reversible Protection Strategy To Improve Fmoc-SPPS of Peptide Thioesters by the N-Acylurea Approach. ChemBioChem 2011, 12, 2386. [Google Scholar] [CrossRef] [PubMed]
- Acosta, G.A.; Royo, M.; de la Torre, B.G.; Albericio, F. Facile solid-phase synthesis of head-side chain cyclothiodepsipeptides through a cyclative cleavage from MeDbz-resin. Tetrahedron Lett. 2017, 58, 2788–2791. [Google Scholar] [CrossRef]
- Abdel Monaim, S.A.H.; Acosta, G.A.; Royo, M.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Solid-phase synthesis of homodetic cyclic peptides from Fmoc-MeDbz-resin. Tetrahedron Lett. 2018, 59, 1779–1782. [Google Scholar] [CrossRef]
- Acosta, G.A.; Murray, L.; Royo, M.; de la Torre, B.G.; Albericio, F. Solid-Phase Synthesis of Head to Side-Chain Tyr-Cyclodepsipeptides Through a Cyclative Cleavage From Fmoc-MeDbz/MeNbz-resins. Front. Chem. 2020, 8, 298. [Google Scholar] [CrossRef]
- Arbour, C.A.; Stamatin, R.E.; Stockdill, J.L. Sequence Diversification by Divergent C-Terminal Elongation of Peptides. J. Org. Chem. 2018, 83, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Arbour, C.A.; Belavek, K.J.; Tariq, R.; Mukherjee, S.; Tom, J.K.; Isidro-Llobet, A.; Kopach, M.E.; Stockdill, J.L. Bringing Macrolactamization Full Circle: Self-Cleaving Head-to-Tail Macrocyclization of Unprotected Peptides via Mild N-Acyl Urea Activation. J. Org. Chem. 2019, 84, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Uemura, T.; Mochizuki, M.; Nishio, H.; Yoshiya, T. Preparation of Peptide o-Aminoanilides Using a Modified Dawson’s Linker for Microwave-Assisted Peptide Synthesis. Synlett 2017, 28, 1956–1960. [Google Scholar] [CrossRef]
- Bondalapati, S.; Eid, E.; Mali, S.M.; Wolberger, C.; Brik, A. Total chemical synthesis of SUMO-2-Lys63-linked diubiquitin hybrid chains assisted by removable solubilizing tags. Chem. Sci 2017, 8, 4027–4034. [Google Scholar] [CrossRef]
- Weidmann, J.; Dimitrijević, E.; Hoheisel, J.D.; Dawson, P.E. Boc-SPPS: Compatible Linker for the Synthesis of Peptide o-Aminoanilides. Org. Lett. 2016, 18, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Fang, G.-M.; He, Y.; Qu, D.-L.; Yu, M.; Hong, Z.-Y.; Liu, L. Peptide o-Aminoanilides as Crypto-Thioesters for Protein Chemical Synthesis. Angew. Chem. Int. Ed. 2015, 54, 2194–2198. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, A.; Chen, H.-T.; Ya-Ting Huang, A.; Kao, C.-L. Expedient on-resin modification of a peptide C-terminus through a benzotriazole linker. Chem. Sci 2018, 9, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, J.; Schnölzer, M.; Dawson, P.E.; Hoheisel, J.D. Copying Life: Synthesis of an Enzymatically Active Mirror-Image DNA-Ligase Made of D-Amino Acids. Cell Chem. Biol. 2019, 26, 645–651.e3. [Google Scholar] [CrossRef]
- Tofteng, A.P.; Sørensen, K.K.; Conde-Frieboes, K.W.; Hoeg-Jensen, T.; Jensen, K.J. Fmoc solid-phase synthesis of C-terminal peptide thioesters by formation of a backbone pyroglutamyl imide moiety. Angew. Chem. 2009, 121, 7547–7550. [Google Scholar] [CrossRef]
- Paprocki, M.P.; Rasmussen, J.E.; Sørensen, K.K.; Jensen, K.J. Safety-catch linkers for Fmoc solid-phase synthesis of peptide thioesters and hydrazides by amide-to-imide activation. J. Pept. Sci. 2021, 27, e3364. [Google Scholar] [CrossRef]
- Elashal, H.E.; Raj, M. Site-selective chemical cleavage of peptide bonds. ChemComm 2016, 52, 6304–6307. [Google Scholar] [CrossRef]
- Elashal, H.E.; Sim, Y.E.; Raj, M. Serine promoted synthesis of peptide thioester-precursor on solid support for native chemical ligation. Chem. Sci 2017, 8, 117–123. [Google Scholar] [CrossRef]
- Shin, Y.; Winans, K.A.; Backes, B.J.; Kent, S.B.H.; Ellman, J.A.; Bertozzi, C.R. Fmoc-Based Synthesis of Peptide-αThioesters: Application to the Total Chemical Synthesis of a Glycoprotein by Native Chemical Ligation. J. Am. Chem. Soc. 1999, 121, 11684–11689. [Google Scholar] [CrossRef]
- Heidler, P.; Link, A. N-Acyl-N-alkyl-sulfonamide anchors derived from Kenner’s safety-catch linker: Powerful tools in bioorganic and medicinal chemistry. Bioorg. Med. Chem. 2005, 13, 585–599. [Google Scholar] [CrossRef]
- Zohrabi-Kalantari, V.; Heidler, P.; Larsen, T.; Link, A. O,N,N‘-Trialkylisoureas as Mild Activating Reagents for N-Acylsulfonamide Anchors. Org. Lett. 2005, 7, 5665–5667. [Google Scholar] [CrossRef]
- Ingenito, R.; Bianchi, E.; Fattori, D.; Pessi, A. Solid Phase Synthesis of Peptide C-Terminal Thioesters by Fmoc/t-Bu Chemistry. J. Am. Chem. Soc. 1999, 121, 11369–11374. [Google Scholar] [CrossRef]
- Quaderer, R.; Hilvert, D. Improved Synthesis of C-Terminal Peptide Thioesters on “Safety-Catch” Resins Using LiBr/THF. Org. Lett. 2001, 3, 3181–3184. [Google Scholar] [CrossRef] [PubMed]
- Burlina, F.; Morris, C.; Behrendt, R.; White, P.; Offer, J. Simplifying native chemical ligation with an N-acylsulfonamide linker. ChemComm 2012, 48, 2579–2581. [Google Scholar] [CrossRef] [PubMed]
- Merkx, R.; van Haren, M.J.; Rijkers, D.T.S.; Liskamp, R.M.J. Resin-Bound Sulfonyl Azides: Efficient Loading and Activation Strategy for the Preparation of the N-Acyl Sulfonamide Linker. J. Org. Chem. 2007, 72, 4574–4577. [Google Scholar] [CrossRef] [PubMed]
- Sheppeck Ii, J.E.; Kar, H.; Gosink, L.; Wheatley, J.B.; Gjerstad, E.; Loftus, S.M.; Zubiria, A.R.; Janc, J.W. Synthesis of a statistically exhaustive fluorescent peptide substrate library for profiling protease specificity. Bioorg. Med. Chem. Lett. 2000, 10, 2639–2642. [Google Scholar] [CrossRef] [PubMed]
- Overkleeft, H.S.; Bos, P.R.; Hekking, B.G.; Gordon, E.J.; Ploegh, H.L.; Kessler, B.M. Solid phase synthesis of peptide vinyl sulfone and peptide epoxyketone proteasome inhibitors. Tetrahedron Lett. 2000, 41, 6005–6009. [Google Scholar] [CrossRef]
- Mezzato, S.; Schaffrath, M.; Unverzagt, C. An Orthogonal Double-Linker Resin Facilitates the Efficient Solid-Phase Synthesis of Complex-Type N-Glycopeptide Thioesters Suitable for Native Chemical Ligation. Angew. Chem. Int. Ed. 2005, 44, 1650–1654. [Google Scholar] [CrossRef]
- Dressman, B.A.; Singh, U.; Kaldor, S.W. Solid phase synthesis of urea libraries using a diversifiable thiophenoxy carbonyl linker. Tetrahedron Lett. 1998, 39, 3631–3634. [Google Scholar] [CrossRef]
- Katti, S.B.; Misra, P.K.; Haq, W.; Mathur, K.B. A new base-labile linker for solid-phase peptide synthesis. J. Chem. Soc. Chem. Commun. 1992, 11, 843–844. [Google Scholar] [CrossRef]
- Kang, J.; Macmillan, D. Peptide and protein thioester synthesis via N→S acyl transfer. Org. Biomol. Chem. 2010, 8, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Arbour, C.A.; Saraha, H.Y.; McMillan, T.F.; Stockdill, J.L. Exploiting the MeDbz Linker To Generate Protected or Unprotected C-Terminally Modified Peptides. Chem. Eur. J. 2017, 23, 12484–12488. [Google Scholar] [CrossRef] [PubMed]
- Arbour, C.A.; Kondasinghe, T.D.; Saraha, H.Y.; Vorlicek, T.L.; Stockdill, J.L. Epimerization-free access to C-terminal cysteine peptide acids, carboxamides, secondary amides, and esters via complimentary strategies. Chem. Sci 2018, 9, 350–355. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).