Optimization and Application of the QuEChERS-UHPLC-QTOF-MS Method for the Determination of Broflanilide Residues in Agricultural Soils
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Separation Conditions of UHPLC-QTOF-MS
2.1.1. Chromatographic Column Optimization
2.1.2. Mobile Phase Optimization
2.2. QuEChERS Method Optimization
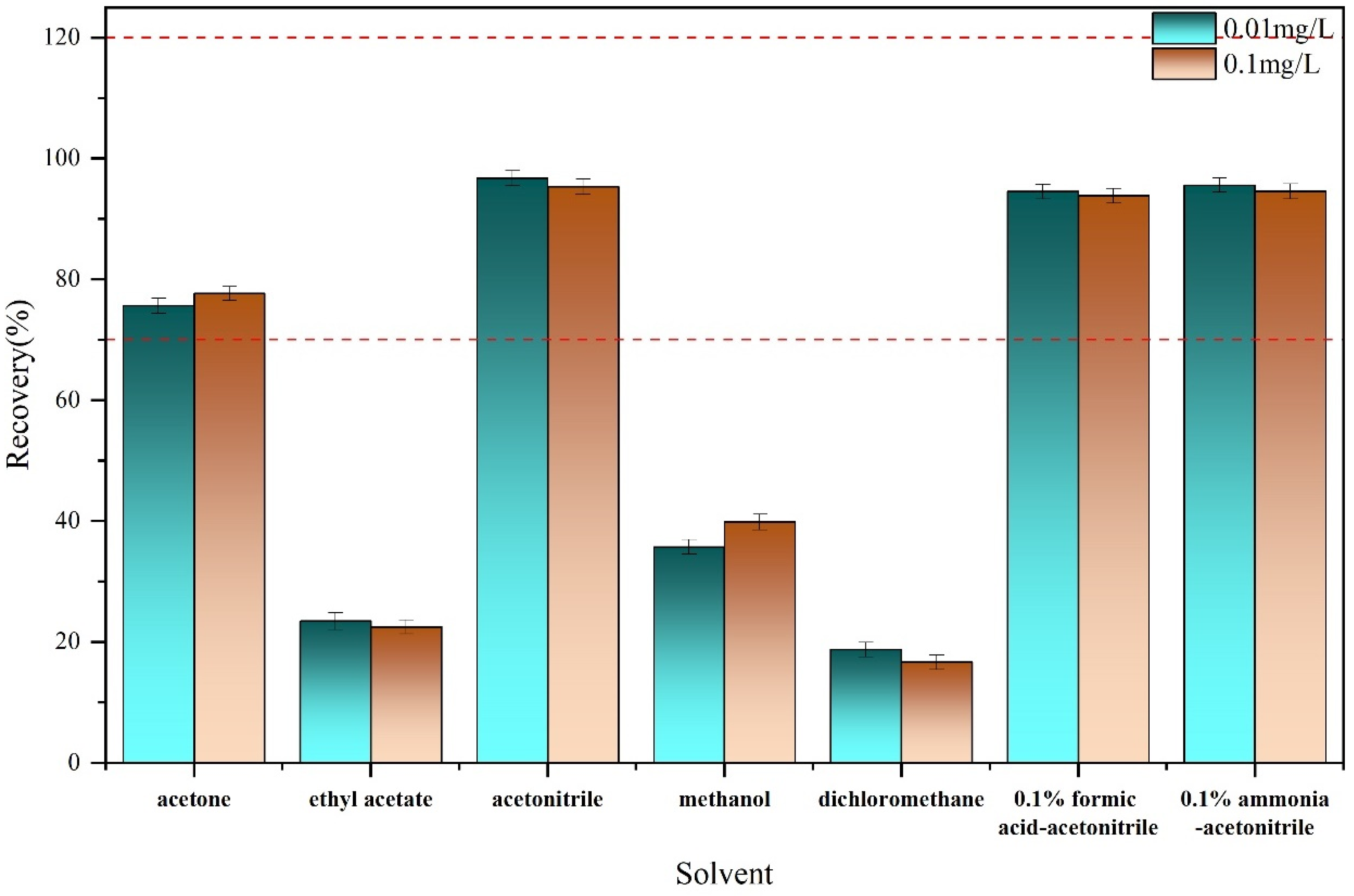
2.2.1. Solvent Optimization
2.2.2. Adsorbent Optimization
2.3. Verification of the Method
2.3.1. Linearity, Specificity, LOD, LOQ, and Matrix Effects
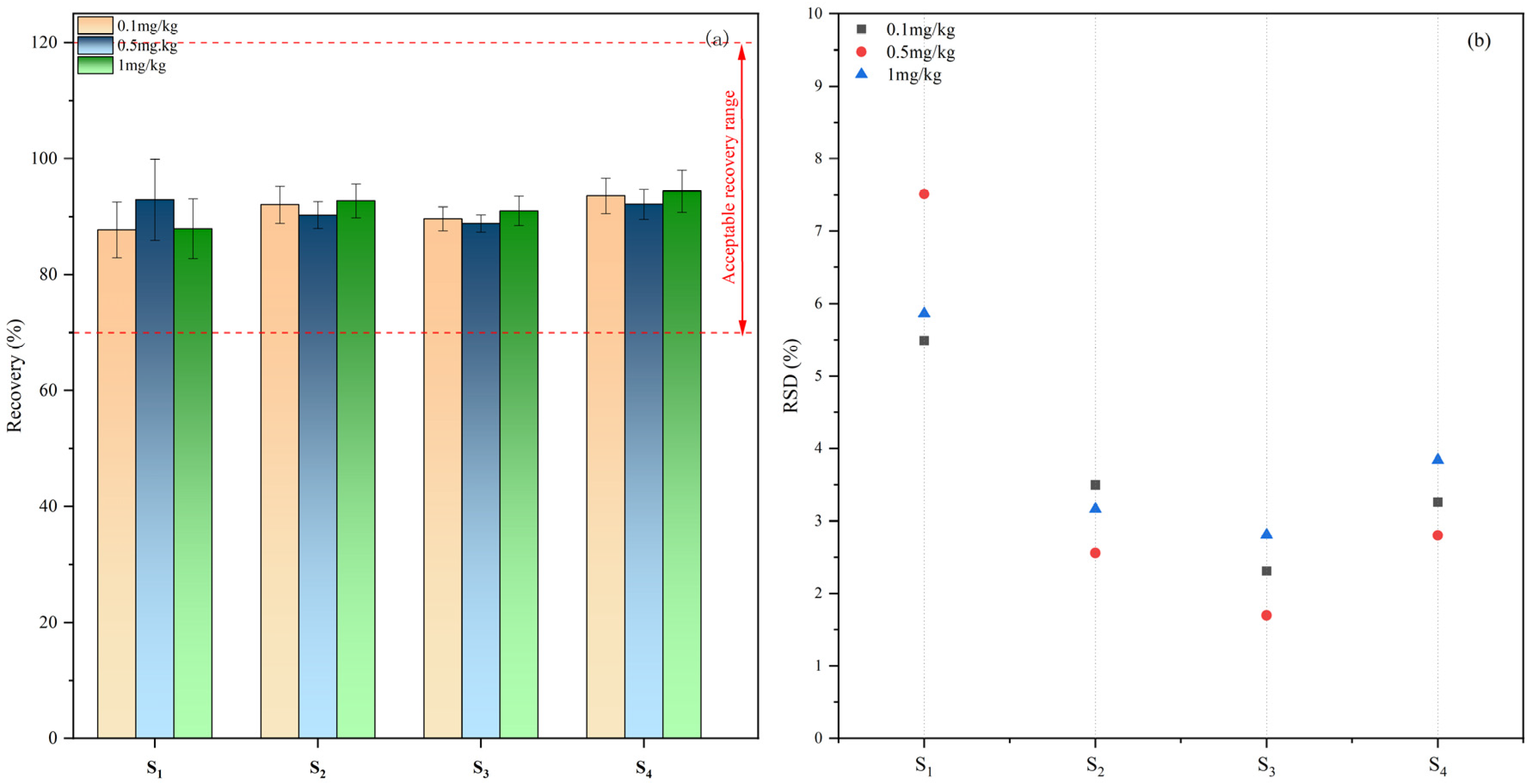
2.3.2. Accuracy and Precision
2.4. Application in Practical Research
2.4.1. Applicability Test
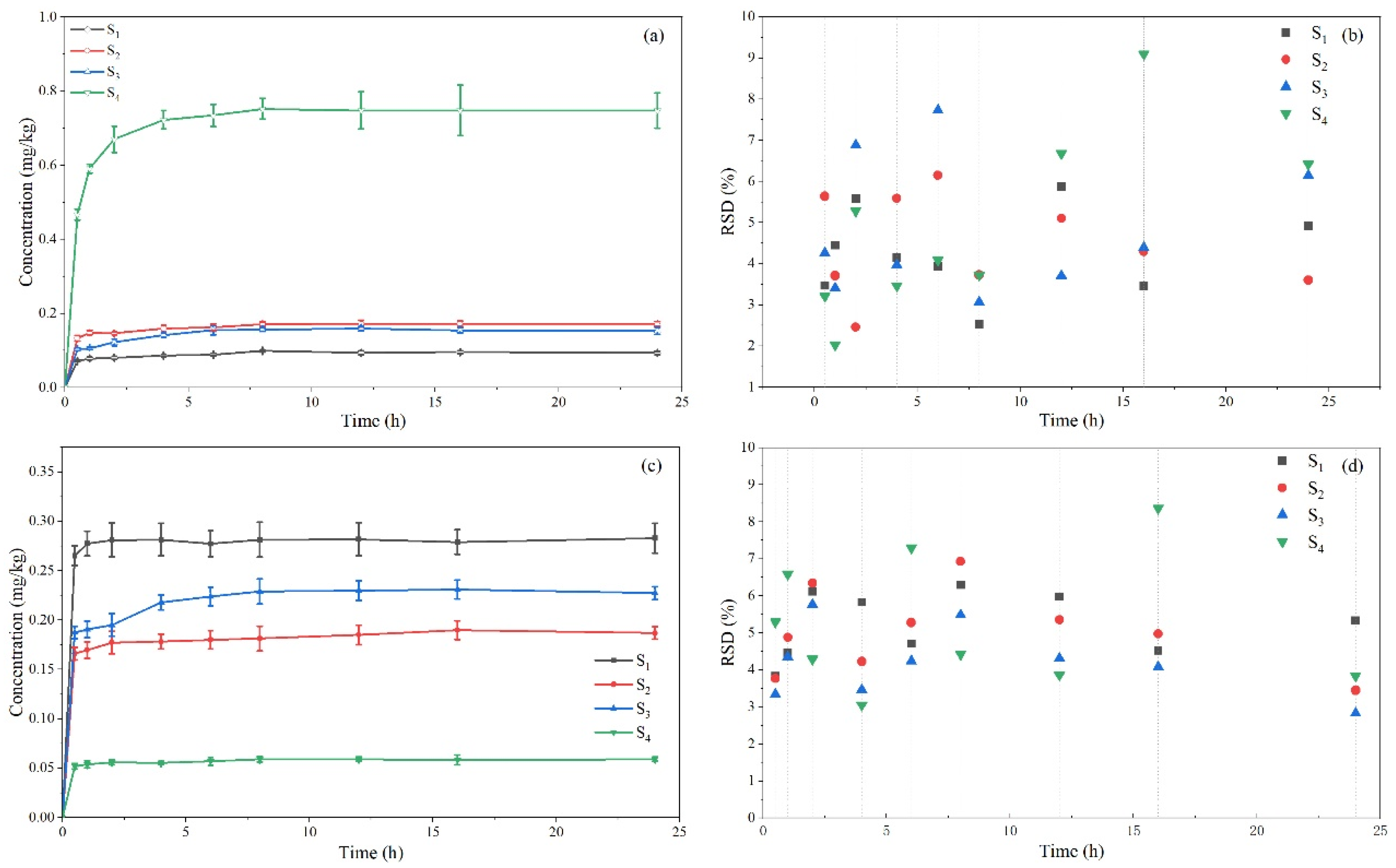
2.4.2. Application of Soil Adsorption-Desorption
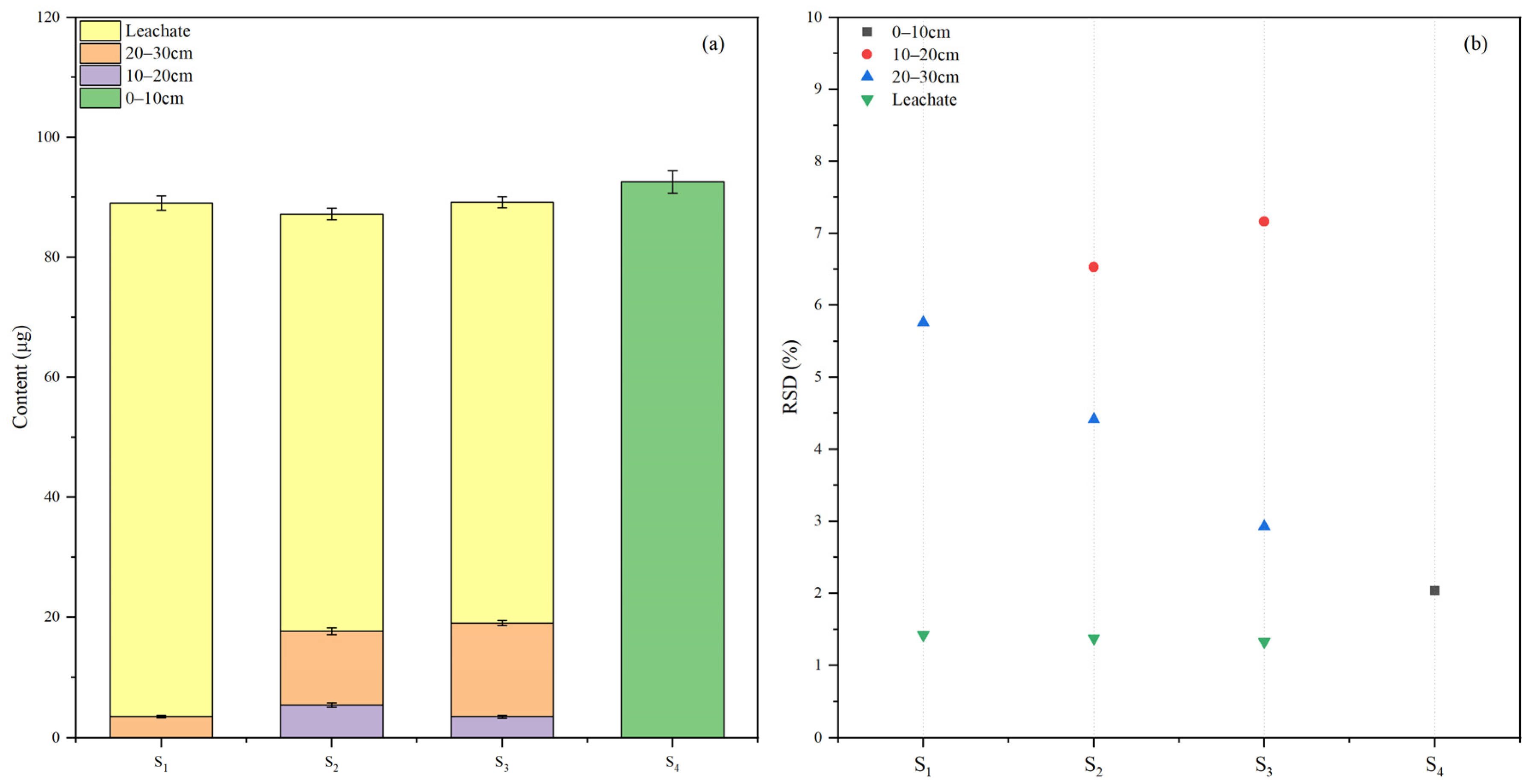
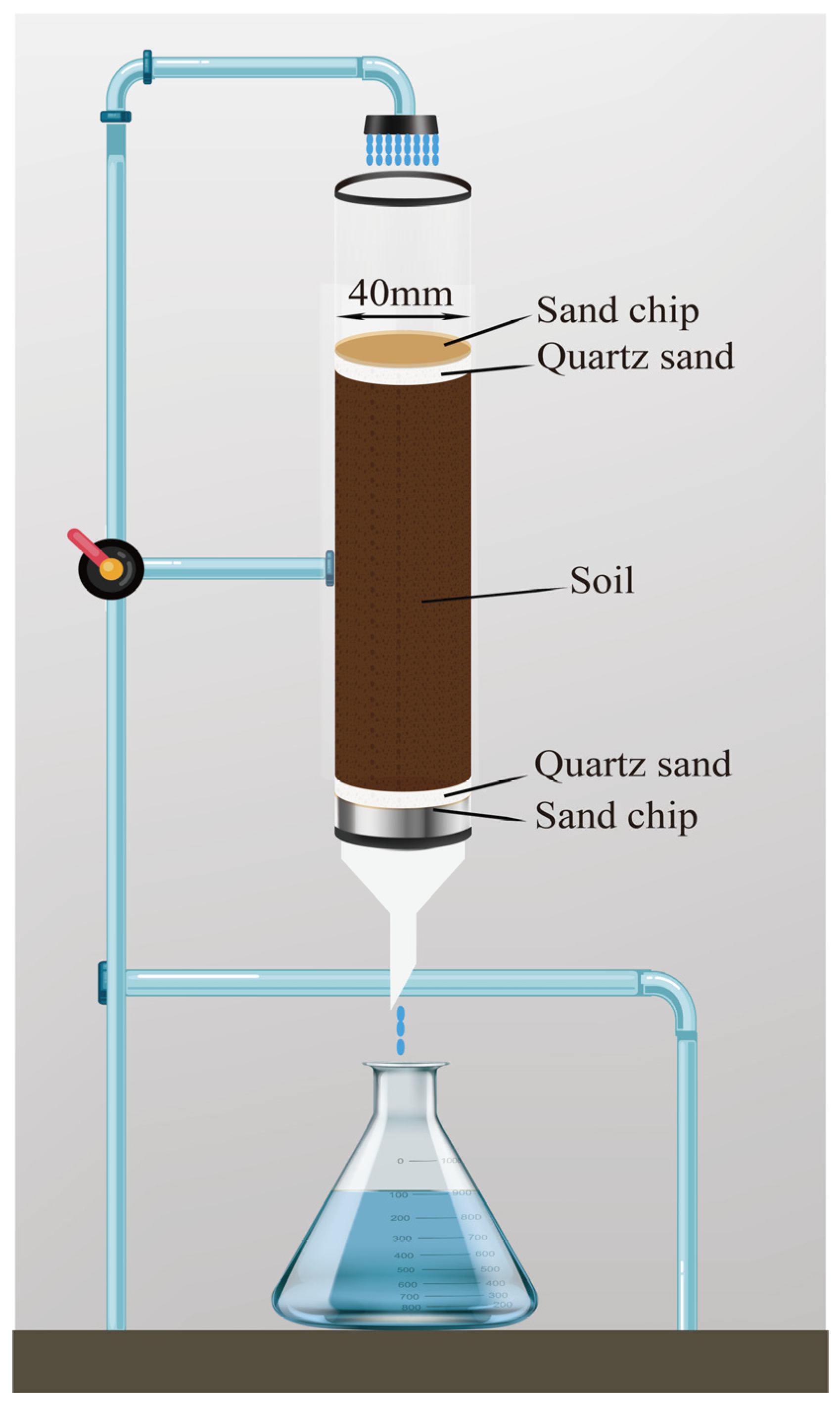
2.4.3. Application in Soil Leaching Experiment
3. Materials and Methods
3.1. Chemicals
3.2. Instrument and Conditions
3.3. Sample Collection and Pretreatment
3.4. Validation of Method and Data Analysis
3.5. Method Application
3.5.1. Applicability Experiment
3.5.2. Adsorption-Desorption Experiment
3.5.3. Leaching Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Sparks, T.C.; Hahn, D.R.; Garizi, N.V. Natural products, their derivatives, mimics and synthetic equivalents: Role in agrochemical discovery. Pest Manag. Sci. 2017, 73, 700–715. [Google Scholar] [CrossRef]
- Oerke, E.C.; Dehne, H.W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Protect. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- van der Werf, H.M. Assessing the impact of pesticides on the environment. Agric. Ecosyst. Environ. 1996, 60, 81–96. [Google Scholar] [CrossRef]
- Pimentel, D.; Burgess, M. Small Amounts of Pesticides Reaching Target Insects; Springer: Berlin/Heidelberg, Germany, 2012; Volume 14, pp. 1–2. [Google Scholar]
- Navarro, S.; Vela, N.; Navarro, G. An overview on the environmental behaviour of pesticide residues in soils. Span. J. Agric. Res. 2007, 5, 357–375. [Google Scholar] [CrossRef]
- Calderbank, A. The occurrence and significance of bound pesticide residues in soil. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 1989; pp. 71–103. [Google Scholar]
- Chiou, C.T.; Sheng, G.; Manes, M. A partition-limited model for the plant uptake of organic contaminants from soil and water. Environ. Sci. Technol. 2001, 35, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Cui, L.; Wang, Q.; Liu, F.; Rui, C. Toxicity of broflanilide to Plutella xylostella and its influence on the activities of related enzymes in P. xylostella. Plant Prot. 2017, 43, 112–116. [Google Scholar]
- Nakao, T.; Banba, S. Broflanilide: A meta-diamide insecticide with a novel mode of action. Biorg. Med. Chem. 2016, 24, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, S.; Mao, X.; Gao, X.; Ge, H.; Qu, S.; Qiao, X.; Jiang, X.; Wang, J.; Li, G. Hydrolytic Behavior of Novel Pesticide Broflanilide and Its Dissipative Properties in Different Types of Soils. Bull. Environ. Contam. Toxicol. 2023, 111, 8. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chow, W.; Leung, D. Applications of LC/ESI-MS/MS and UHPLC QqTOF MS for the determination of 148 pesticides in fruits and vegetables. Anal. Bioanal. Chem. 2010, 396, 1513–1538. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Begnini Konatu, F.; Sales Fontes Jardim, I.C. Development and validation of an analytical method for multiresidue determination of pesticides in lettuce using QuEChERS–UHPLC–MS/MS. J. Sep. Sci. 2018, 41, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, M.; Liu, X.; Xu, J.; Dong, F.; Wu, X.; Li, B.; Zheng, Y. Determination and dissipation of afidopyropen and its metabolite in wheat and soil using QuEChERS–UHPLC–MS/MS. J. Sep. Sci. 2018, 41, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhou, W.; Jin, M.; Yu, A.; Rao, L.; Jia, H.; Luo, J.; He, Y.; Li, B. Residues Analysis and Dissipation Dynamics of Broflanilide in Rice and Its Related Environmental Samples. Int. J. Anal. Chem. 2020, 2020, 8845387. [Google Scholar] [CrossRef] [PubMed]
- Žuvela, P.; Skoczylas, M.; Jay Liu, J.; Ba̧czek, T.; Kaliszan, R.; Wong, M.W.; Buszewski, B. Column Characterization and Selection Systems in Reversed-Phase High-Performance Liquid Chromatography. Chem. Rev. 2019, 119, 3674–3729. [Google Scholar] [CrossRef] [PubMed]
- Carr, P.W.; Doherty, R.M.; Kamlet, M.J.; Taft, R.W.; Melander, W.; Horvath, C. Study of temperature and mobile-phase effects in reversed-phase high-performance liquid chromatography by the use of the solvatochromic comparison method. Anal. Chem. 1986, 58, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Stalcup, A.M.; Martire, D.E.; Wise, S.A. Thermodynamic comparison of monomeric and polymeric C18 bonded phases using aqueous methanol and acetonitrile mobile phases. J. Chromatogr. A 1988, 442, 1–14. [Google Scholar] [CrossRef]
- Alvarez-Zepeda, A.; Barman, B.N.; Martire, D.E. Thermodynamic study of the marked differences between acetonitrile/water and methanol/water mobile-phase systems in reversed-phase liquid chromatography. Anal. Chem. 1992, 64, 1978–1984. [Google Scholar] [CrossRef]
- Rafferty, J.L.; Siepmann, J.I.; Schure, M.R. Mobile phase effects in reversed-phase liquid chromatography: A comparison of acetonitrile/water and methanol/water solvents as studied by molecular simulation. J. Chromatogr. A 2011, 1218, 2203–2213. [Google Scholar] [CrossRef]
- Sun, H.; Dong, X.; Lu, X.; Wang, H.; Han, J. Separation and determination of fluoroquinolones with a molecularly imprinted polymer. Chin. J. Chromatogr. 2003, 21, 233–238. [Google Scholar]
- Payá, P.; Anastassiades, M.; Mack, D.; Sigalova, I.; Tasdelen, B.; Oliva, J.; Barba, A. Analysis of pesticide residues using the Quick Easy Cheap Effective Rugged and Safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection. Anal. Bioanal. Chem. 2007, 389, 1697–1714. [Google Scholar] [CrossRef]
- Hajjo, R.; Afifi, F.; Battah, A. Multiresidue pesticide analysis of the medicinal plant Origanum syriacum. Food Addit. Contam. 2007, 24, 274–279. [Google Scholar] [CrossRef]
- Riaz, M.; Bilal Butt, S. Gamma radiolytic degradation of the endrin insecticide in methanol and monitoring of radiolytic degradation products by HPLC. J. Radioanal. Nucl. Chem. 2010, 285, 697–701. [Google Scholar] [CrossRef]
- Nordmeyer, K.; Thier, H.-P. Solid-phase extraction for replacing dichloromethane partitioning in pesticide multiresidue analysis. Z. Für Leb. Und-Forsch. A 1999, 208, 259–263. [Google Scholar] [CrossRef]
- Chen, G.; Cao, P.; Liu, R. A multi-residue method for fast determination of pesticides in tea by ultra performance liquid chromatography–electrospray tandem mass spectrometry combined with modified QuEChERS sample preparation procedure. Food Chem. 2011, 125, 1406–1411. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, C.; Wang, W.; Qian, M.; Xu, J.; Yang, H. Chromatography column comparison and rapid pretreatment for the simultaneous analysis of amantadine, rimantadine, acyclovir, ribavirin, and moroxydine in chicken muscle by ultra high performance liquid chromatography and tandem mass spectrometry. J. Sep. Sci. 2016, 39, 3998–4010. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, X.; Dong, F.; Xu, J.; Xu, H.; Hu, M.; Zheng, Y. Chemometric-assisted QuEChERS extraction method for the residual analysis of thiacloprid, spirotetramat and spirotetramat’s four metabolites in pepper: Application of their dissipation patterns. Food Chem. 2016, 192, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J. Quick, easy, cheap, effective, rugged, and safe approach for determining pesticide residues. Pestic. Protoc. 2006, 239–261. [Google Scholar]
- Brondi, S.H.; De Macedo, A.N.; de Souza, G.B.; Nogueira, A.R. Application of QuEChERS method and gas chromatography-mass spectrometry for the analysis of cypermethrin residue in milk. J. Environ. Sci. Health Part B 2011, 46, 671–677. [Google Scholar]
- Ministy of Agriculture and Rural Affairs of the People’s Republic of China. Guideline for the Testing of Pesticide Resudues in Crops; Ministy of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2018.
- Wang, Y.-Q.; Ye, D.-Q.; Zhu, B.-Q.; Wu, G.-F.; Duan, C.-Q. Rapid HPLC analysis of amino acids and biogenic amines in wines during fermentation and evaluation of matrix effect. Food Chem. 2014, 163, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, W.; Zhou, W.; Jia, H.; Li, B. Adsorption-desorption characteristics of pyraclonil in eight agricultural soils. J. Soils Sed. 2020, 20, 1404–1412. [Google Scholar] [CrossRef]
- Plakas, K.V.; Karabelas, A.J. Membrane retention of herbicides from single and multi-solute media: The effect of ionic environment. J. Membr. Sci. 2008, 320, 325–334. [Google Scholar] [CrossRef]
- Zhang, J.; Zhaojun, L.; Gaofei, G.; Wanchun, S.; Liang, Y.; Laosheng, W. Impacts of soil organic matter, pH and exogenous copper on sorption behavior of norfloxacin in three soils. J. Environ. Sci. 2009, 21, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, W.; Wang, M. Adsorption-desorption and leaching characteristics of fluazinam in soils. China Environ. Sci. 2013, 33, 669–673. [Google Scholar]
- Xie, G.; Li, B.; Tang, L.; Rao, L.; Dong, Z. Adsorption-desorption and leaching behaviors of broflanilide in four texturally different agricultural soils from China. J. Soils Sed. 2021, 21, 724–735. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Jia, H.; Zhou, W.; Li, B.; Huang, H. Residue analysis of tetraniliprole in rice and related environmental samples by HPLC/MS. Microchem. J. 2019, 150, 104168. [Google Scholar] [CrossRef]
- L’Huillier, L.; Dupont, S.; Dubus, I.; Becquer, T.; Bourdon, E.; Laubreaux, P.; Bonzon, B. Carence et fixation du phosphore dans les sols ferrallitiques ferritiques de Nouvelle-Calédonie. XVIe Congrès Mondial de Science du Sol 1998, 8, 20–26. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Scientific Publishers: Jodhpur, India, 2019. [Google Scholar]
- Deckers, J.; Driessen, P.; Nachtergaele, F.; Spaargaren, O. World reference base for soil resources. In Encyclopedia of Soil Science; Marcel Dekker: New York, NY, USA, 2002; pp. 1446–1451. [Google Scholar]
- Fanigliulo, A.; De Filippis, P.; Curcuruto, O.; Repeto, P.; Roveda, D.; Hartenstein, M.; Adams, E.; Cabooter, D. Development and validation of a stability indicating method for s-carboxymethyl-l-cysteine and related degradation products in oral syrup formulation. J. Pharm. Biomed. Anal. 2015, 115, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Seccia, S.; Albrizio, S.; Fidente, P.; Montesano, D. Development and validation of a solid-phase extraction method coupled to high-performance liquid chromatography with ultraviolet-diode array detection for the determination of sulfonylurea herbicide residues in bovine milk samples. J. Chromatogr. A 2011, 1218, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Qiu, F.; Kong, W.; Luo, J.; Cheng, H.; Yang, M. Simultaneous quantification of mycotoxins and pesticide residues in ginseng with one-step extraction using ultra-high performance liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. B 2013, 939, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Guideline, I.H.T. Validation of analytical procedures: Text and methodology. Q2 (R1) 2005, 1, 05. [Google Scholar]
- Zhu, Y.; Liu, X.; Xu, J.; Dong, F.; Liang, X.; Li, M.; Duan, L.; Zheng, Y. Simultaneous determination of spirotetramat and its four metabolites in fruits and vegetables using a modified quick, easy, cheap, effective, rugged, and safe method and liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2013, 1299, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Garrido Frenich, A.; González-Rodríguez, M.J.; Arrebola, F.J.; Martínez Vidal, J.L. Potentiality of gas chromatography−triple quadrupole mass spectrometry in vanguard and rearguard methods of pesticide residues in vegetables. Anal. Chem. 2005, 77, 4640–4648. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- Prevention, P. United States Environmental Protection Agency. Hospital 2020, 3862, 104. [Google Scholar]
- Guideline, P.-B.T. OECD guideline for the testing of chemicals. Hershberger 2001, 601, 858. [Google Scholar]
- Rao, L.; Luo, J.; Zhou, W.; Zou, Z.; Tang, L.; Li, B. Adsorption–desorption behavior of benzobicyclon hydrolysate in different agricultural soils in China. Ecotoxicol. Environ. Saf. 2020, 202, 110915. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I. Water treatment by reverse osmosis method. Environ. Water 2013, 117–134. [Google Scholar]


| Chemical Structure | Item | Value |
|---|---|---|
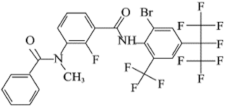 | IUPAC a name | Broflanilide (BFL) |
| Code name | MCI-8007 | |
| Molecular formula | C25H14F11BrN2O2 | |
| Molecular weight | 663.28 | |
| Formulation | WP 5% | |
| Density | 1.7 g/cm3 (296.15 K) | |
| Solubility | 0.71 mg/L (293.15 K, pure water) | |
| Vapor pressure | <9 × 10−9 Pa (298.15 K) | |
| Partition coefficient Log Pow | 5.2 (293.15 K, pH 4) |
| Sorbent | Recovery (%) | Average Value (%) | ||
|---|---|---|---|---|
| PSA | 93.62 | 95.25 | 92.55 | 93.81 |
| C18 | 92.73 | 93.56 | 91.55 | 92.61 |
| GCB | 89.69 | 88.32 | 91.54 | 89.85 |
| Matrix | Spiked Level (mg/kg) | Recovery (%) | Average Recovery (%) | Relative Standard Deviation (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| Paddy soil | 0.1 | 89.36 | 80.02 | 92.54 | 86.46 | 90.14 | 87.70 | 5.49 |
| 0.5 | 82.79 | 94.41 | 99.37 | 98.80 | 89.20 | 92.91 | 7.51 | |
| 1 | 89.73 | 80.54 | 94.64 | 86.15 | 88.45 | 87.90 | 5.86 | |
| Soil | Location (Latitude, Longitude) | Classification | Texture(%) | pH | CEC a (cmol/kg) | OC b (%) | OM c (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Slit | Clay | Type | |||||||
| S1 | Shijiazhuang, Hebei (N 39°45′, E 117°32′) | Luvisols | 40.62 | 35.91 | 23.47 | Silt loam | 6.58 | 26.15 | 0.14 | 0.48 |
| S2 | Ningbo, Zhejiang (N 29°14′, E 121°48′) | Anthrosols | 47.98 | 23.18 | 28.84 | Loam | 7.85 | 12.90 | 0.92 | 1.66 |
| S3 | Chengdu, Sichuan (N 30°56′, E 105°51′) | Gleysols | 45.83 | 40.83 | 13.34 | Silt loam | 8.48 | 25.40 | 0.07 | 0.17 |
| S4 | Haerbin, Heilongjiang (N 41°36′, E 127°53′) | Phaeozems | 13.08 | 32.50 | 54.42 | Sandy loam | 6.38 | 30.36 | 2.08 | 4.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Xie, G.; Huo, Z.; Zhang, B.; Lu, H.; Huang, Y.; Li, X.; Dai, L.; Huang, S.; Yu, A. Optimization and Application of the QuEChERS-UHPLC-QTOF-MS Method for the Determination of Broflanilide Residues in Agricultural Soils. Molecules 2024, 29, 1428. https://doi.org/10.3390/molecules29071428
Nie X, Xie G, Huo Z, Zhang B, Lu H, Huang Y, Li X, Dai L, Huang S, Yu A. Optimization and Application of the QuEChERS-UHPLC-QTOF-MS Method for the Determination of Broflanilide Residues in Agricultural Soils. Molecules. 2024; 29(7):1428. https://doi.org/10.3390/molecules29071428
Chicago/Turabian StyleNie, Xiaoli, Guai Xie, Zhitao Huo, Baoyu Zhang, Haifei Lu, Yi Huang, Xin Li, Liangliang Dai, Siyuan Huang, and Ailin Yu. 2024. "Optimization and Application of the QuEChERS-UHPLC-QTOF-MS Method for the Determination of Broflanilide Residues in Agricultural Soils" Molecules 29, no. 7: 1428. https://doi.org/10.3390/molecules29071428
APA StyleNie, X., Xie, G., Huo, Z., Zhang, B., Lu, H., Huang, Y., Li, X., Dai, L., Huang, S., & Yu, A. (2024). Optimization and Application of the QuEChERS-UHPLC-QTOF-MS Method for the Determination of Broflanilide Residues in Agricultural Soils. Molecules, 29(7), 1428. https://doi.org/10.3390/molecules29071428






