Targeting Cancer Hallmarks with Epigallocatechin Gallate (EGCG): Mechanistic Basis and Therapeutic Targets
Abstract
1. Introduction
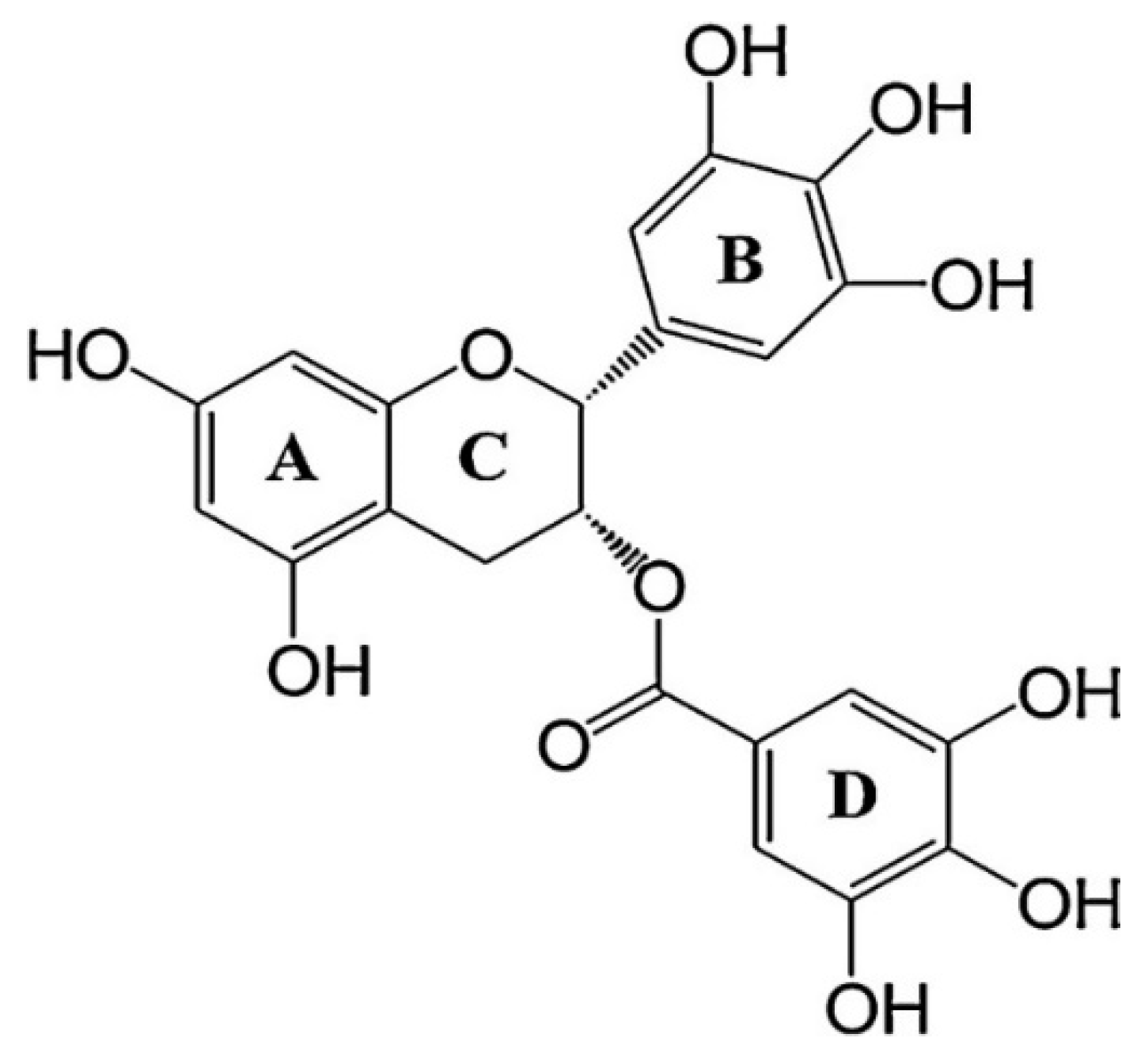
1.1. Pharmacokinetic Properties of EGCG
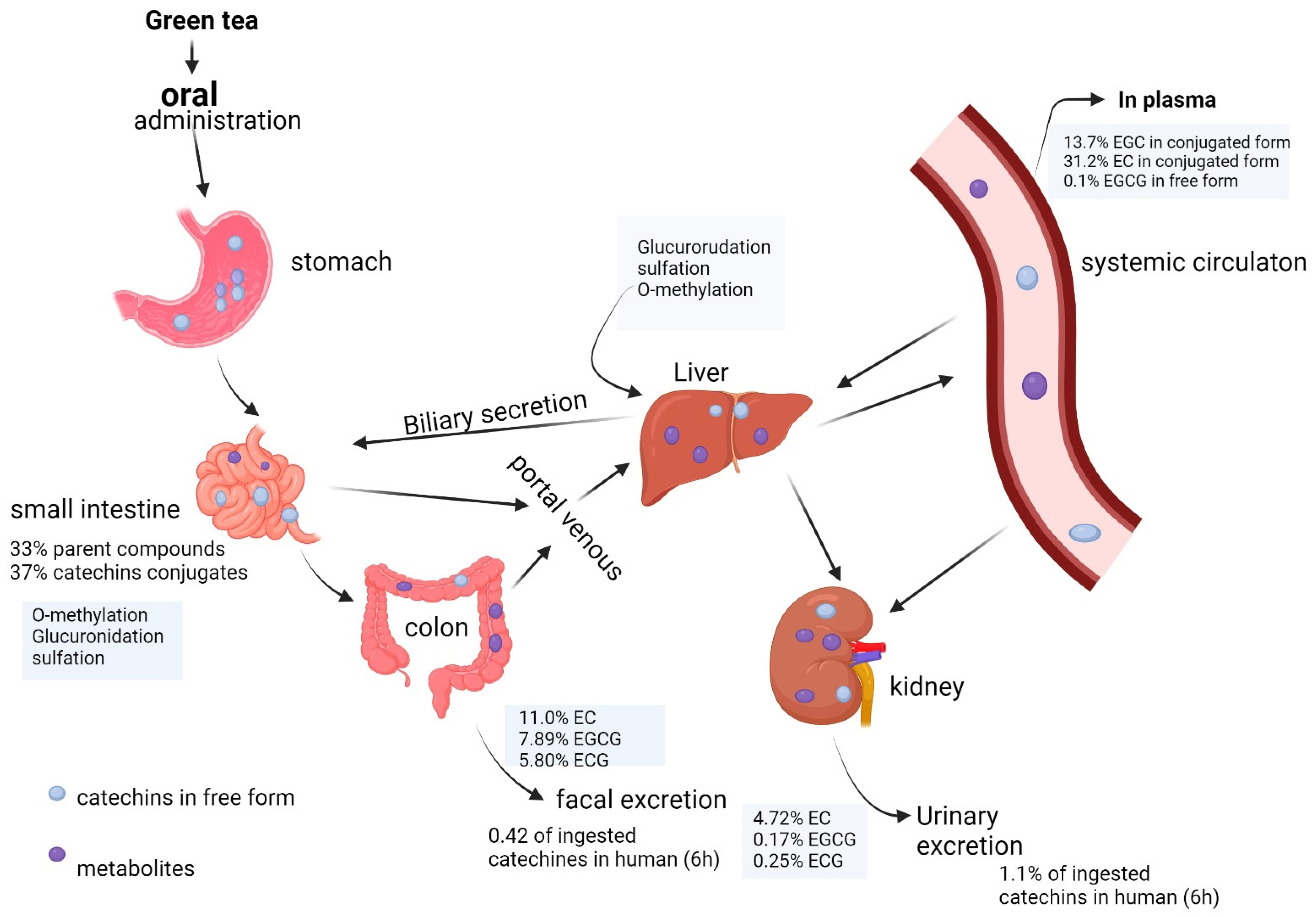
1.2. EGCG Bioavailability Obstacles
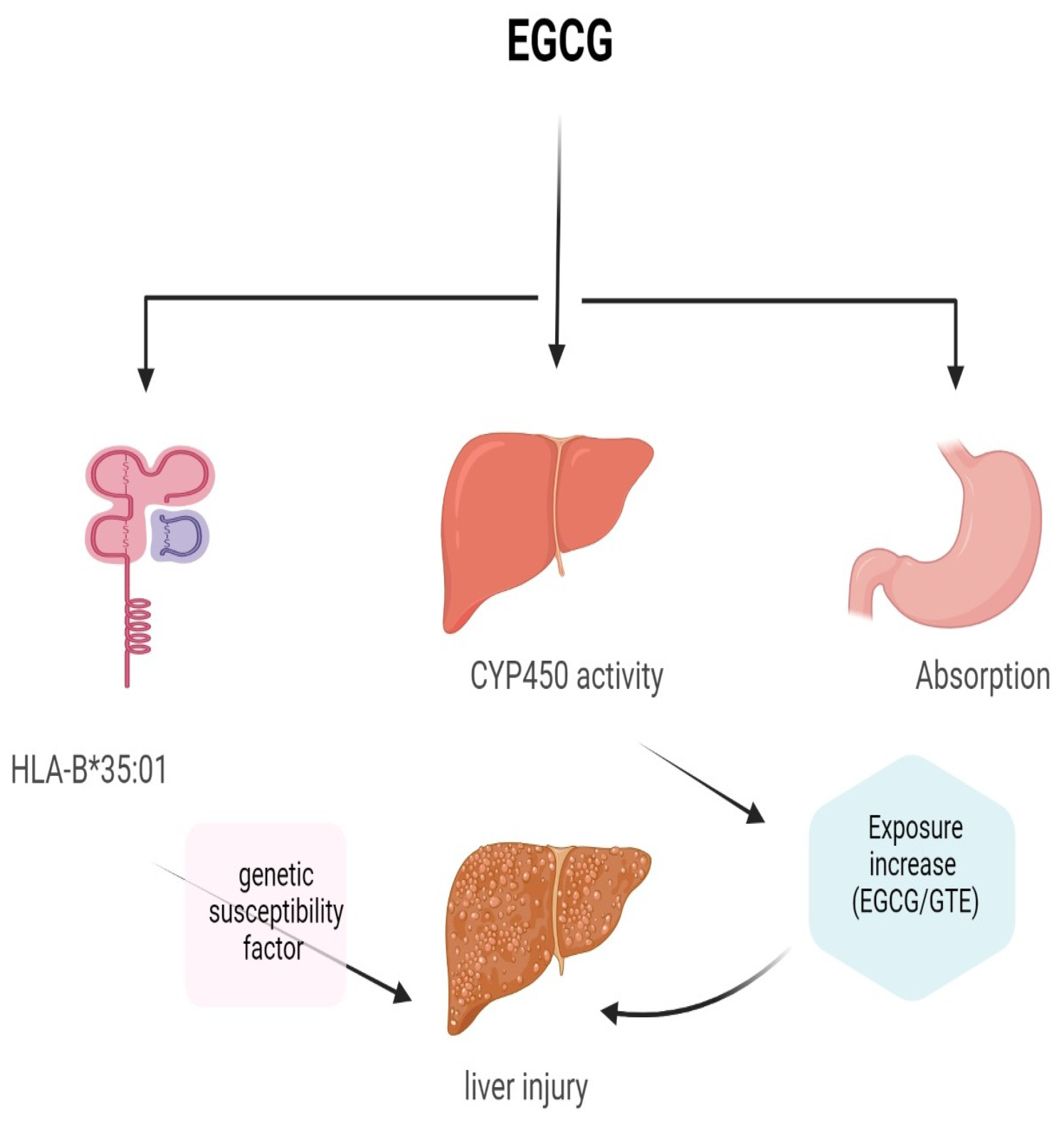
1.3. EGCG Toxicity
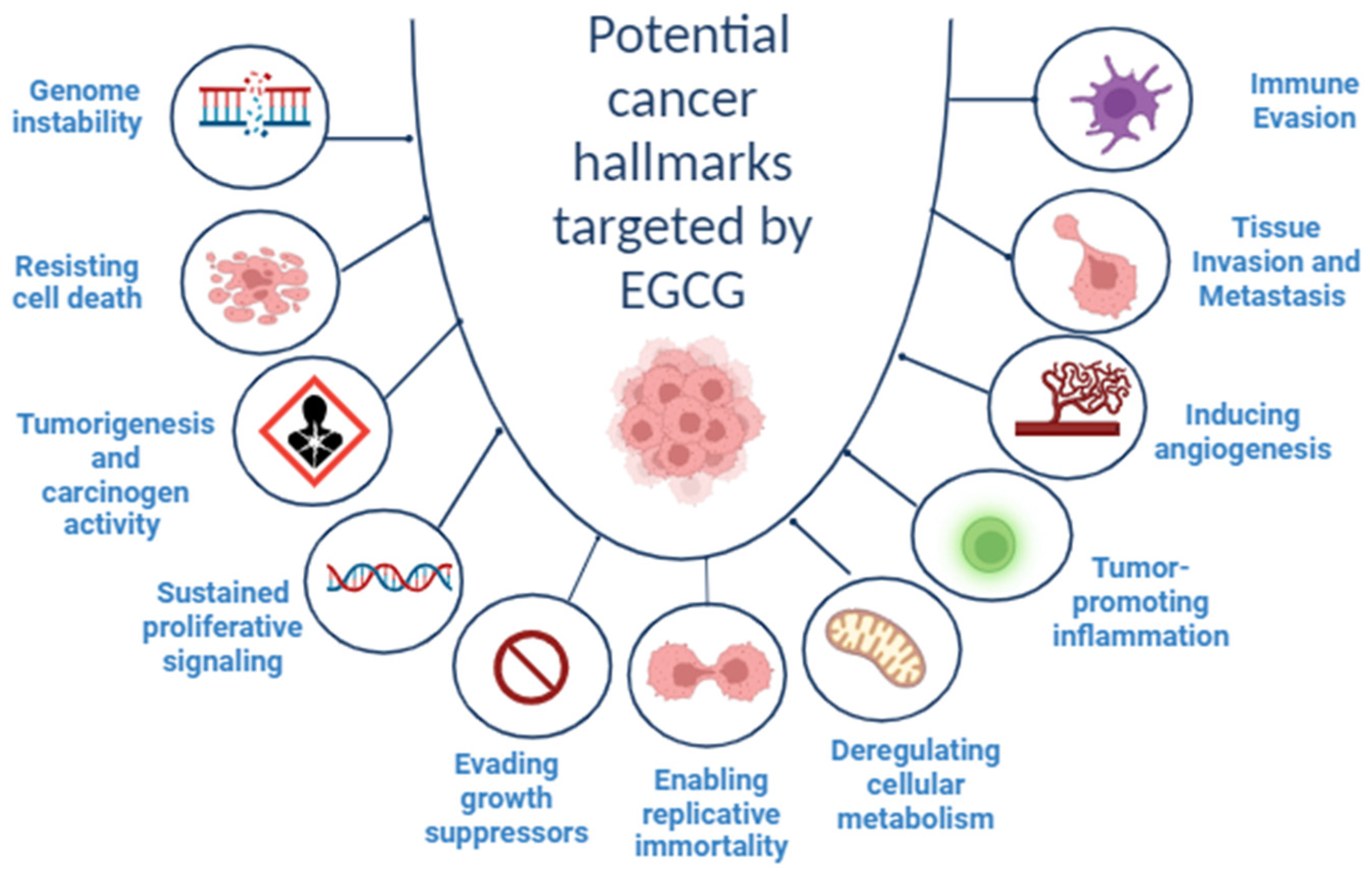
2. Cancer Hallmarks as Therapeutic Targets
3. EGCG and Cancer Hallmarks
3.1. Role of EGCG in Genomic Instability
3.2. Induction of Apoptosis
3.2.1. Caspase-Dependent Apoptosis
3.2.2. Caspase-Independent Apoptosis
3.3. Tumorigenesis and Carcinogen Activity Suppression
3.4. Role of EGCG in Sustained Proliferative Signaling
3.4.1. ERK and PI3K-Akt Pathways
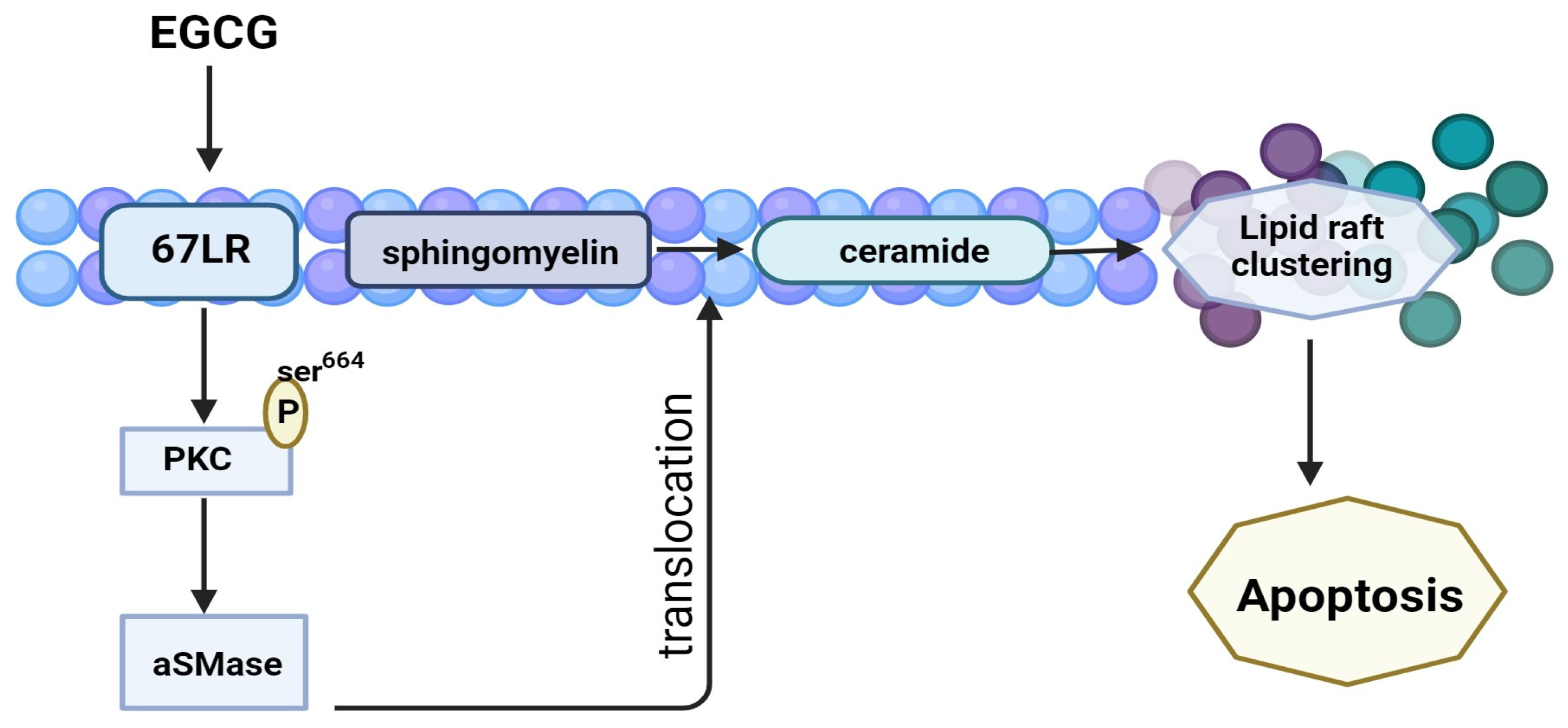
3.4.2. 67-LR Pathway
3.4.3. NF-κB Pathway
3.5. Role of EGCG in Evasion of Anti-Growth Signaling
3.6. Role of EGCG in Replicative Immortality
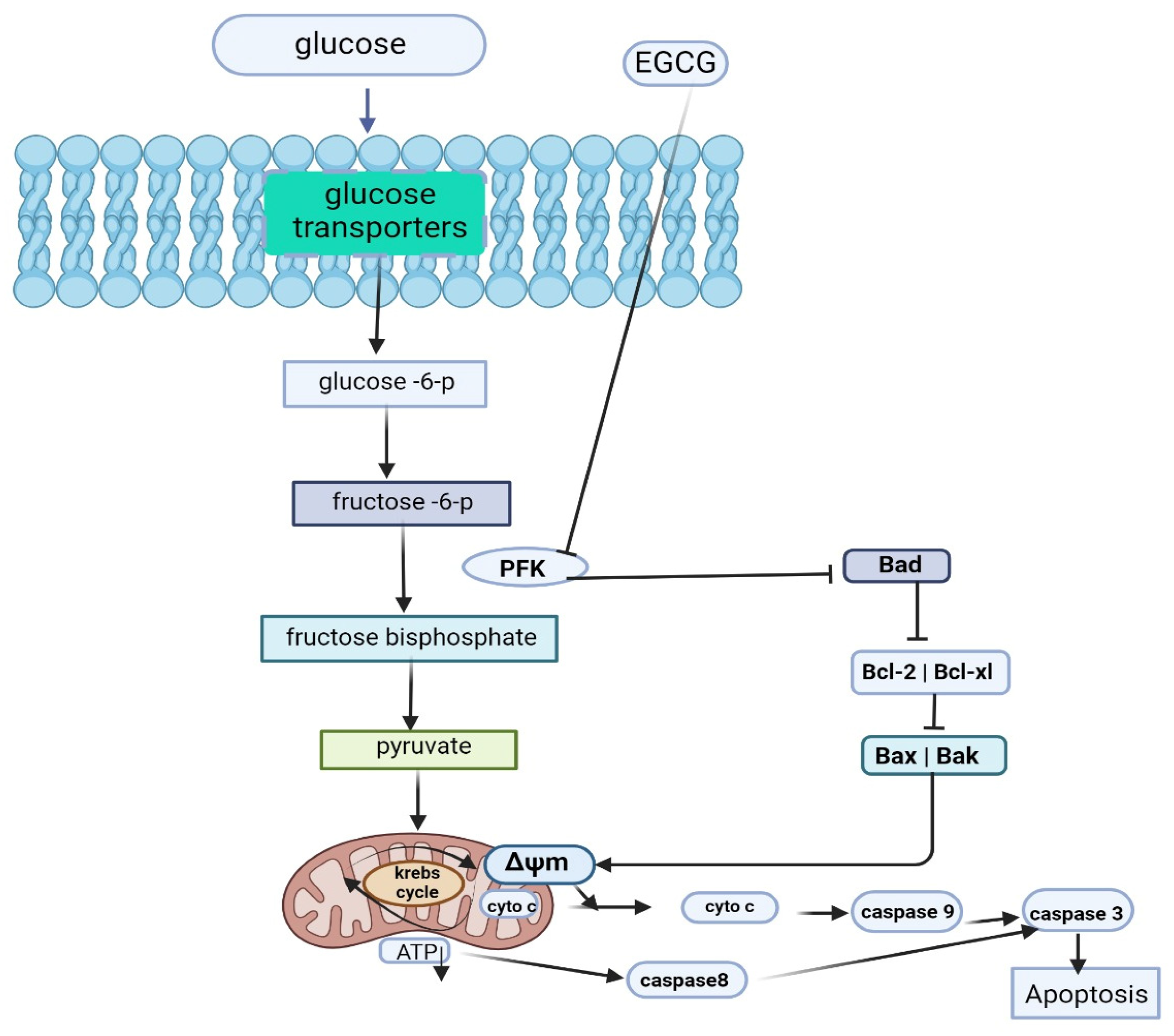
3.7. Role of EGCG in Tumor Dysregulated Metabolism
3.8. Role of EGCG in Tumor-Promoting Inflammation
3.9. Role of EGCG in Angiogenesis Inhibition
3.10. Role of EGCG in Tissue Invasion and Metastasis
3.11. Role of EGCG in Tumor-Associated Immune Evasion
3.11.1. Myeloid-Derived Suppressor Cells (MDSCs)
3.11.2. Programmed Cell Death Protein (PD) and Programmed Cell Death Ligand (PD-L)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fidler, M.M.; Bray, F.; Soerjomataram, I. The global cancer burden and human development: A review. Scand. J. Public Health 2017, 46, 27–36. [Google Scholar] [CrossRef]
- Talib, W.H.; Daoud, S.; Mahmod, A.I.; Hamed, R.A.; Awajan, D.; Abuarab, S.F.; Odeh, L.H.; Khater, S.; Al Kury, L.T. Plants as a source of anticancer agents: From bench to bedside. Molecules 2022, 27, 4818. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Kao, C.-L.; Liu, C.-M. The Cancer Prevention, Anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef]
- Lin, S.-R.; Fu, Y.-S.; Tsai, M.-J.; Cheng, H.; Weng, C.-F. Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy. Int. J. Mol. Sci. 2017, 18, 1412. [Google Scholar] [CrossRef]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; Da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The hallmarks of flavonoids in cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lee, M.-J.; Li, H.; Yang, C.S. Absorption, distribution, and elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Moore, R.J.; Jackson, K.G.; Minihane, A.M. Green tea (Camellia sinensis) catechins and vascular function. Br. J. Nutr. 2009, 102, 1790–1802. [Google Scholar] [CrossRef]
- Miyazawa, T. Absorption, metabolism and antioxidative effects of tea catechin in humans. BioFactors 2000, 13, 55–59. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Meng, X.; Sang, S.; Zhu, N.; Lu, H.; Sheng, S.; Lee, M.-J.; Ho, C.-T.; Yang, C.S. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. [Google Scholar] [CrossRef]
- Williamson, G.; Dionisi, F.; Renouf, M.J. Flavanols from green tea and phenolic acids from coffee: Critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol. Nutr. Food Res. 2011, 55, 864–873. [Google Scholar] [CrossRef]
- Schantz, M.; Erk, T.; Richling, E. Metabolism of green tea catechins by the human small intestine. Biotechnol. J. 2010, 5, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L. Bioavailability of tea catechins and its improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Klinski, E.; Semov, A.; Yan, X.; Alakhov, V.; Muyzhnek, E.; Kiselev, V.J. Block copolymer based composition of epigallocatechin-3-gallate with improved oral bioavailability as a way to increase its therapeutic activity. J. Nanomed. Biother. Discov. 2013, 3, 117. [Google Scholar] [CrossRef]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e14189. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Metabolism of (-)-epigallocatechin gallate by rat intestinal flora. J. Agric. Food Chem. 2010, 58, 1313–1321. [Google Scholar] [CrossRef]
- Slot, G.V.; Humpf, H.-U. Degradation and metabolism of catechin, epigallocatechin-3-gallate (EGCG), and related compounds by the intestinal microbiota in the pig cecum model. J. Agric. Food Chem. 2009, 57, 8041–8048. [Google Scholar] [CrossRef]
- Alexander, A.; Qureshi, A.; Kumari, L.; Vaishnav, P.; Sharma, M.; Saraf, S.; Saraf, S. Role of herbal bioactives as a potential bioavailability enhancer for active pharmaceutical ingredients. Fitoterapia 2014, 97, 1–14. [Google Scholar]
- Lambert, J.D.; Hong, J.; Kim, D.H.; Mishin, V.M.; Yang, C.S. Piperine enhances the bioavailability of the tea polyphenol (−)-epigallocatechin-3-gallate in mice. J. Nutr. 2004, 134, 1948–1952. [Google Scholar] [CrossRef]
- Peng, Y.; Meng, Q.; Zhou, J.; Chen, B.; Xi, J.; Long, P.; Zhang, L.; Hou, R. Nanoemulsion delivery system of tea polyphenols enhanced the bioavailability of catechins in rats. Food Chem. 2019, 242, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Guan, R.; Chen, X.; Tao, M.; Ma, J.; Zhao, J. Optimization on condition of epigallocatechin-3-gallate (EGCG) nanoliposomes by response surface methodology and cellular uptake studies in Caco-2 cells. Nanoscale Res. Lett. 2014, 9, 291. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Jing, H.; Ma, C.; Wang, H. Bilosomes as effective delivery systems to improve the gastrointestinal stability and bioavailability of epigallocatechin gallate (EGCG). Food Res. Int. 2021, 149, 110631. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Ochi, M.; Yamada, S. Development of (−)-epigallocatechin-3-gallate (EGCG)-loaded enteric microparticles with intestinal mucoadhesive property. Int. J. Pharm. 2011, 410, 111–113. [Google Scholar] [CrossRef]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother. Res. 2018, 32, 1163–1180. [Google Scholar] [CrossRef]
- Rasheed, N.O.A.; Ahmed, L.A.; Abdallah, D.M.; El-Sayeh, B.M. Nephro-toxic effects of intraperitoneally injected EGCG in diabetic mice: Involvement of oxidative stress, inflammation and apoptosis. Sci. Rep. 2017, 7, 40617. [Google Scholar] [CrossRef]
- Chiou, Y.-S.; John, J.A.; Ho, C.-T.; Pan, M.-H.; Shahidi, F. Evaluation of chemopreventive effects in colitis-associated colon tumourigenesis and oral toxicity of the lipophilic epigallocatechin gallate-docosahexaenoic acid. J. Funct. Foods 2016, 24, 48–56. [Google Scholar] [CrossRef]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2010, 48, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free. Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef]
- Kucera, O.; Mezera, V.; Moravcova, A.; Endlicher, R.; Lotkova, H.; Drahota, Z.; Cervinkova, Z. In vitro toxicity of epigallocatechin gallate in rat liver mitochondria and hepatocytes. Oxidative Med. Cell. Longev. 2015, 2015, 476180. [Google Scholar] [CrossRef] [PubMed]
- Isbrucker, R.; Edwards, J.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.J.E.J. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [PubMed]
- Rani, J.; Dhull, S.B.; Rose, P.K.; Kidwai, M.K. Drug-induced liver injury and anti-hepatotoxic effect of herbal compounds: A metabolic mechanism perspective. Phytomedicine 2024, 122, 155142. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.J. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Senga, S.S.; Grose, R.P. Hallmarks of cancer—The new testament. Open Biol. 2021, 11, 200358. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Zhou, S.-D.; Huang, L.; Meng, L.; Lin, Y.-F.; Xu, X.; Dong, M.-S. Soy protein isolate-(-)-epigallocatechin gallate conjugate: Covalent binding sites identification and IgE binding ability evaluation. Food Chem. 2020, 333, 127400. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- LaLandis-Piwowar, K.R.; Kuhn, D.J.; Wan, S.B.; Chen, D.; Chan, T.H.; Dou, Q.P. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (-)-EGCG analogs and their prodrugs. Int. J. Mol. Med. 2005, 15, 735–742. [Google Scholar]
- Li, H.; Zimmerman, S.E.; Weyemi, U. Genomic instability and metabolism in cancer. Int. Rev. Cell Mol. Biol. 2021, 364, 241–265. [Google Scholar] [PubMed]
- Wenzel, E.S.; Singh, A.T.K. Cell-cycle checkpoints and aneuploidy on the path to cancer. Vivo 2018, 32, 1–5. [Google Scholar]
- Ui, A.; Chiba, N.; Yasui, A. Relationship among DNA double-strand break (DSB), DSB repair, and transcription prevents genome instability and cancer. Cancer Sci. 2020, 111, 1443–1451. [Google Scholar] [CrossRef]
- Vodicka, P.; Musak, L.; Vodickova, L.; Vodenkova, S.; Catalano, C.; Kroupa, M.; Naccarati, A.; Polivkova, Z.; Vymetalkova, V.; Försti, A.; et al. Genetic variation of acquired structural chromosomal aberrations. Mutat. Res. Toxicol. Environ. Mutagen. 2018, 836, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015, 35, S5–S24. [Google Scholar] [CrossRef]
- Ni, J.; Guo, X.; Wang, H.; Zhou, T.; Wang, X. Differences in the effects of EGCG on chromosomal stability and cell growth between normal and colon cancer cells. Molecules 2018, 23, 788. [Google Scholar] [CrossRef]
- Pointner, A.; Mölzer, C.; Magnet, U.; Zappe, K.; Hippe, B.; Tosevska, A.; Tomeva, E.; Dum, E.; Gessner, D.; Lilja, S.; et al. The green tea polyphenol EGCG is differentially associated with telomeric regulation in normal human fibroblasts versus cancer cells. Funct. Foods Health Dis. 2021, 11, 73–91. [Google Scholar] [CrossRef]
- Morley, N.; Clifford, T.; Salter, L.; Campbell, S.; Gould, D.; Curnow, A. The green tea polyphenol (−)-epigallocatechin gallate and green tea can protect human cellular DNA from ultraviolet and visible radiation-induced damage. Photodermatol. Photoimmunol. Photomed. 2004, 21, 15–22. [Google Scholar] [CrossRef]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The potential of epigallocatechin gallate (EGCG) in targeting autophagy for cancer treatment: A narrative review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Mobbili, G.; Galeazzi, R.; Armeni, T. Effect of epigallocatechin-3-gallate on EGFR signaling and migration in non-small cell lung cancer. Int. J. Mol. Sci. 2021, 22, 11833. [Google Scholar] [CrossRef]
- Lei, B.; Zhang, M.; Chen, X.; Liang, B.; Xie, W.; Wang, H.; Li, B. The effect and mechanism of epigallocatechol gallate combined with trastuzumab on the proliferation of HER2 overexpressing breast cancer cells. J. Pharm. Pract. Serv. 2022, 40, 136–142. [Google Scholar]
- Suresh, S.V.; Byers, D.M.J. Combined curcumin and EGCG target key markers in hepatocellular and colorectal cancers. J. Restor. Med. 2021, 11, 1–18. [Google Scholar]
- Gan, L.; Zhong, L.; Shan, Z.; Xiao, C.; Xu, T.; Song, H.; Li, L.; Yang, R.; Liu, B. Epigallocatechin-3-gallate induces apoptosis in acute promyelocytic leukemia cells via a SHP-1-p38α MAPK-Bax cascade. Oncol. Lett. 2017, 14, 6314–6320. [Google Scholar] [CrossRef][Green Version]
- Huang, C.-Y.; Han, Z.; Li, X.; Xie, H.-H.; Zhu, S.-S. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol. Lett. 2017, 14, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yao, J.; Wang, H.; Cui, W.; Leng, J.; Ding, L.; Fan, K. Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1α and VEGF under a hypoxic state. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 155–161. [Google Scholar] [PubMed]
- Jiang, S.; Huang, C.; Zheng, G.; Yi, W.; Wu, B.; Tang, J.; Liu, X.; Huang, B.; Wu, D.; Yan, T.; et al. EGCG inhibits proliferation and induces apoptosis through downregulation of SIRT1 in nasopharyngeal carcinoma cells. Front. Nutr. 2022, 9, 851972. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Z.L.; Sun, L.; Liu, Y.; Li, C.C.; Li, H.M.; Zhang, W.; Li, C.J.; Qin, W. (-)-Epigallocatechin-3-gallate induces apoptosis in human pancreatic cancer cells via PTEN. Mol. Med. Rep. 2016, 14, 599–605. [Google Scholar] [CrossRef]
- Li, S.; Wu, L.; Feng, J.; Li, J.; Liu, T.; Zhang, R.; Xu, S.; Cheng, K.; Zhou, Y.; Zhou, S. In vitro and in vivo study of epigallocatechin-3-gallate-induced apoptosis in aerobic glycolytic hepatocellular carcinoma cells involving inhibition of phosphofructokinase activity. Sci. Rep. 2016, 6, 28479. [Google Scholar] [CrossRef]
- Khiewkamrop, P.; Phunsomboon, P.; Richert, L.; Pekthong, D.; Srisawang, P. Epistructured catechins, EGCG and EC facilitate apoptosis induction through targeting de novo lipogenesis pathway in HepG2 cells. Cancer Cell Int. 2018, 18, 46. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jeong, Y.-J.; Lee, S.-W.; Kim, D.; Oh, S.-J.; Lim, H.-S.; Oh, H.-K.; Kim, S.-H.; Kim, W.-J.; Jung, J.-Y. EGCG induces apoptosis in human laryngeal epidermoid carcinoma Hep2 cells via mitochondria with the release of apoptosis-inducing factor and endonuclease G. Cancer Lett. 2010, 290, 68–75. [Google Scholar] [CrossRef]
- Min, K.-j.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Mimoto, J.; Kiura, K.; Matsuo, K.; Yoshino, T.; Takata, I.; Ueoka, H.; Kataoka, M.; Harada, M. (–)-Epigallocatechin gallate can prevent cisplatin-induced lung tumorigenesis in A/J mice. Carcinogenesis 2000, 21, 915–919. [Google Scholar] [CrossRef]
- Shimizu, M.; Sakai, H.; Shirakami, Y.; Yasuda, Y.; Kubota, M.; Terakura, D.; Baba, A.; Ohno, T.; Hara, Y.; Tanaka, T. Preventive effects of (−)-epigallocatechin gallate on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Integr. Med. Res. 2011, 4, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Tachibana, H. Anti-cancer effect of EGCG and its mechanisms. Funct. Foods Health Dis. 2016, 6, 70–78. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Peng, S.; Guo, R.; Yao, L.; Mo, H.; Li, H.; Song, H.; Hu, L. EGCG alleviates oxidative stress and inhibits aflatoxin B1 biosynthesis via MAPK signaling pathway. Toxins 2021, 13, 693. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.-H.; Zhu, H.-Q.; Chen, Y.-Y.; Chen, R.-L.; Fu, L.-X.; Li, L.; Zhou, H.; Zhou, J.-L.; Liang, G.J. The epigallocatechin gallate derivative Y6 inhibits human hepatocellular carcinoma by inhibiting angiogenesis in MAPK/ERK1/2 and PI3K/AKT/HIF-1α/VEGF dependent pathways. J. Ethnopharmacol. 2020, 259, 112852. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2017, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.T.; Jasmer, K.J.; Forti, K.M.; Shanbhag, V.C.; Camden, J.M.; Erb, L.; Petris, M.J.; Weisman, G.A. P2Y2 receptors mediate nucleotide-induced EGFR phosphorylation and stimulate proliferation and tumorigenesis of head and neck squamous cell carcinoma cell lines. Oral Oncol. 2020, 109, 104808. [Google Scholar] [CrossRef]
- Liu, X.; Adorno-Cruz, V.; Chang, Y.-F.; Jia, Y.; Kawaguchi, M.; Dashzeveg, N.K.; Taftaf, R.; Ramos, E.K.; Schuster, E.J.; El-Shennawy, L. EGFR inhibition blocks cancer stem cell clustering and lung metastasis of triple negative breast cancer. Theranostics 2021, 11, 6632–6643. [Google Scholar] [CrossRef]
- Singh, S.; Sahadevan, R.; Roy, R.; Biswas, M.; Ghosh, P.; Kar, P.; Sonawane, A.; Sadhukhan, S. Structure-based design and synthesis of a novel long-chain 4′′-alkyl ether derivative of EGCG as potent EGFR inhibitor: In vitro and in silico studies. RSC Adv. 2022, 12, 17821–17836. [Google Scholar] [CrossRef]
- Qin, J.; Fu, M.; Wang, J.; Huang, F.; Liu, H.; Huangfu, M.; Yu, D.; Liu, H.; Li, X.; Guan, X.; et al. PTEN/AKT/mTOR signaling mediates anticancer effects of epigallocatechin-3-gallate in ovarian cancer. Oncol. Rep. 2020, 43, 1885–1896. [Google Scholar] [CrossRef]
- Pesapane, A.; Ragno, P.; Selleri, C.; Montuori, N. recent advances in the function of the 67 kda laminin receptor and its targeting for personalized therapy in cancer. Curr. Pharm. Des. 2017, 23, 4745–4757. [Google Scholar] [CrossRef]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Hirotsu, K.; Kumazoe, M.; Goto, Y.; Sugihara, K.; Suda, T.; Tsurudome, Y.; Suzuki, T.; Yamashita, S.; Kim, Y.; et al. Green tea polyphenol EGCG induces lipid-raft clustering and apoptotic cell death by activating protein kinase Cδ and acid sphingomyelinase through a 67 kDa laminin receptor in multiple myeloma cells. Biochem. J. 2012, 443, 525–534. [Google Scholar] [CrossRef]
- Porrini, C.; Ramarao, N.; Tran, S.-L. Dr. NO and Mr. Toxic—The versatile role of nitric oxide. Biol. Chem. 2020, 401, 547–572. [Google Scholar] [CrossRef]
- D’Acquisto, F.; May, M.J.; Ghosh, S. Inhibition of nuclear factor kappa B (NF-B). Mol. Interv. 2002, 2, 22. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Fuloria, N.K.; Fuloria, S.; Rahman, S.B.; Al-Malki, W.H.; Shaikh, M.A.J.; Thangavelu, L.; Singh, S.K.; Allam, V.S.R.R.; Jha, N.K.; et al. Nuclear factor-kappa B and its role in inflammatory lung disease. Chem. Interact. 2021, 345, 109568. [Google Scholar] [CrossRef] [PubMed]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, J.; Gan, R.; Wu, Z.; Luo, H.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Synergistic inhibition of lung cancer cells by EGCG and NF-κB inhibitor BAY11-7082. J. Cancer 2019, 10, 6543. [Google Scholar] [CrossRef]
- Luo, K.-W.; Chen, W.; Lung, W.-Y.; Wei, X.-Y.; Cheng, B.-H.; Cai, Z.-M.; Huang, W.-R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef]
- La, X.; Zhang, L.; Li, Z.; Li, H.; Yang, Y. (−)-Epigallocatechin Gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-κB/miR-155-5p/MDR1 pathway. J. Agric. Food Chem. 2019, 67, 2510–2518. [Google Scholar] [CrossRef]
- Tian, M.; Tian, D.; Qiao, X.; Li, J.; Zhang, L. Modulation of Myb-induced NF-kB-STAT3 signaling and resulting cisplatin resistance in ovarian cancer by dietary factors. J. Cell. Physiol. 2019, 234, 21126–21134. [Google Scholar] [CrossRef]
- Saedmocheshi, S.; Saghebjoo, M.; Vahabzadeh, Z.; Sheikholeslami-Vatani, D. Aerobic Training and Green Tea Extract Protect against N-methyl-N-nitrosourea–induced Prostate Cancer. Med. Sci. Sports Exerc. 2019, 51, 2210–2216. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef]
- Pan, H.; Chen, J.; Shen, K.; Wang, X.; Wang, P.; Fu, G.; Meng, H.; Wang, Y.; Jin, B. Mitochondrial modulation by Epigallocatechin 3-Gallate ameliorates cisplatin induced renal injury through decreasing oxidative/nitrative stress, inflammation and NF-KB in mice. PLoS ONE 2015, 10, e0124775. [Google Scholar] [CrossRef]
- Peters, J.M.; Gonzalez, F.J. The evolution of carcinogenesis. Toxicol. Sci. 2018, 165, 272–276. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical activities of epigallocatechin gallate in signaling pathways in cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef]
- Boumahdi, S.; de Sauvage, F.J. The great escape: Tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 2020, 19, 39–56. [Google Scholar] [CrossRef]
- Nirmaladevi, R.; Ilango, S.; Paital, B.; Jayachandran, P.; Padma, P.R. Epigenetic alterations in cancer. Front. Biosci. 2020, 25, 1058–1109. [Google Scholar] [CrossRef]
- Lin, L.L.; Choucair, K.; Patel, R. NF1 in Solid Tumors: The Unknown Soldier of Tumor Suppressor Genes? Genet. Mol. Med. 2019, 1, 1–13. [Google Scholar] [CrossRef]
- Amin, A.R.; Karpowicz, P.A.; Carey, T.E.; Arbiser, J.; Nahta, R.; Chen, Z.G.; Dong, J.-T.; Kucuk, O.; Khan, G.N.; Huang, G.S. Evasion of anti-growth signaling: A key step in tumorigenesis and potential target for treatment and prophylaxis by natural compounds. Semin. Cancer Biol. 2015, 35, S55–S77. [Google Scholar] [CrossRef]
- Singh, L.; Kashyap, S. Update on pathology of retinoblastoma. Int. J. Ophthalmol. 2018, 11, 2011. [Google Scholar]
- Ettl, T.; Schulz, D.; Bauer, R.J. The renaissance of cyclin dependent kinase inhibitors. Cancers 2022, 14, 293. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Kojima, A.; Moffatt, J.; Yamagiwa, H.; Yano, Y.; Hasuma, T.; Otani, S.; Matsui-Yuasa, I. Cellular thiol status-dependent inhibition of tumor cell growth via modulation of retinoblastoma protein phosphorylation by (−)-epigallocatechin. Cancer Lett. 2002, 179, 25–32. [Google Scholar] [CrossRef]
- Hastak, K.; Agarwal, M.K.; Mukhtar, H.; Agarwal, M.L. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005, 19, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Du, W.; Yang, D. Inhibition of green tea polyphenol EGCG((−)-epigallocatechin-3-gallate) on the proliferation of gastric cancer cells by suppressing canonical wnt/β-catenin signalling pathway. Int. J. Food Sci. Nutr. 2016, 67, 818–827. [Google Scholar] [CrossRef]
- Hegde, M.R.; Crowley, M.R. Genome and gene structure. In Emery and Rimoin’s Principles and Practice of Medical Genetics and Genomics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 53–77. [Google Scholar]
- Slusher, A.L.; Kim, J.J.; Ludlow, A.T. The role of alternative rna splicing in the regulation of htert, telomerase, and telomeres: Implications for cancer therapeutics. Cancers 2020, 12, 1514. [Google Scholar] [CrossRef]
- Eitsuka, T.; Nakagawa, K.; Kato, S.; Ito, J.; Otoki, Y.; Takasu, S.; Shimizu, N.; Takahashi, T.; Miyazawa, T. Modulation of telomerase activity in cancer cells by dietary compounds: A review. Int. J. Mol. Sci. 2018, 19, 478. [Google Scholar] [CrossRef]
- Udroiu, I.; Marinaccio, J.; Sgura, A. Epigallocatechin-3-gallate induces telomere shortening and clastogenic damage in glioblastoma cells. Environ. Mol. Mutagen. 2019, 60, 683–692. [Google Scholar] [CrossRef]
- Watanabe, L.M.; Noronha, N.Y.; Pinhel, M.A.; Nonino, C.B. Green Tea and Telomere Length Regulation in Health Conditions. In Tea as a Food Ingredient; CRC Press: Boca Raton, FL, USA, 2022; p. 207. [Google Scholar]
- Chen, X.; Tang, W.; Shi, J.B.; Liu, M.M.; Liu, X. Therapeutic strategies for targeting telomerase in cancer. Med. Res. Rev. 2019, 40, 532–585. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Y.; Wang, G.; Gu, W.; Zhao, S.; Hu, X.; Liu, W.; Cai, Y.; Ma, Z.; Gautam, R.K.; et al. Research progress of small-molecule drugs in targeting telomerase in human cancer and aging. Chem. Interact. 2023, 382, 110631. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Tauber, A.L.; Schweiker, S.S.; Levonis, S.M. From tea to treatment; epigallocatechin gallate and its potential involvement in minimizing the metabolic changes in cancer. Nutr. Res. 2019, 74, 23–36. [Google Scholar] [CrossRef]
- Chen, S.; Nishi, M.; Morine, Y.; Shimada, M.; Tokunaga, T.; Kashihara, H.; Takasu, C.; Yamada, S.; Wada, Y. Epigallocatechin-3-gallate hinders metabolic coupling to suppress colorectal cancer malignancy through targeting aerobic glycolysis in cancer-associated fibroblasts. Int. J. Oncol. 2022, 60, 1–13. [Google Scholar] [CrossRef]
- Wei, R.; Hackman, R.M.; Wang, Y.; Mackenzie, G.G. Targeting glycolysis with epigallocatechin-3-gallate enhances the efficacy of chemotherapeutics in pancreatic cancer cells and xenografts. Cancers 2019, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lai, X.; Xiang, L.; Li, Q.; Sun, L.; Lai, Z.; Li, Z.; Zhang, W.; Wen, S.; Cao, J.; et al. Aged green tea reduces high-fat diet-induced fat accumulation and inflammation via activating the AMP-activated protein kinase signaling pathway. Food Nutr. Res. 2022, 66, 7923. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, F.; Ren, X.; Wang, Y.; Huang, W.; Zhang, J.; Cui, Y. Targeting fatty acid synthase sensitizes human nasopharyngeal carcinoma cells to radiation via downregulating frizzled class receptor 10. Cancer Biol. Med. 2020, 17, 740–752. [Google Scholar] [CrossRef]
- Crous-Masó, J.; Palomeras, S.; Relat, J.; Camó, C.; Martínez-Garza, Ú.; Planas, M.; Feliu, L.; Puig, T. (−)-Epigallocatechin 3-gallate synthetic analogues inhibit fatty acid synthase and show anticancer activity in triple negative breast cancer. Molecules 2018, 23, 1160. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-K.; Park, K.-G. Targeting glutamine metabolism for cancer treatment. Biomol. Ther. 2018, 26, 19–28. [Google Scholar] [CrossRef]
- Li, C.; Feng, Y.; Wang, W.; Xu, L.; Zhang, M.; Yao, Y.; Wu, X.; Zhang, Q.; Huang, W.; Wang, X. Targeting Glutaminolysis to Treat Multiple Myeloma: An In Vitro Evaluation of Glutaminase Inhibitors Telaglenastat and Epigallocatechin-3-gallate. Anti-Cancer Agents Med. Chem. 2023, 23, 779–785. [Google Scholar]
- Zhou, Y.; Yu, H.; Cheng, S.; Chen, Y.; He, L.; Ren, J.; He, X.; Chen, J.; Zheng, L.; Li, F. Glutamate dehydrogenase 1 mediated glutaminolysis sustains HCC cells survival under glucose deprivation. J. Cancer 2022, 13, 1061–1072. [Google Scholar] [CrossRef]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Chen, A.; Jiang, P.; Zeb, F.; Wu, X.; Xu, C.; Chen, L.; Feng, Q. EGCG regulates CTR1 expression through its pro-oxidative property in non-small-cell lung cancer cells. J. Cell. Physiol. 2020, 235, 7970–7981. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, D.J. Low dose epigallocatechin-3-gallate revives doxorubicin responsiveness by a redox-sensitive pathway in A549 lung adenocarcinoma cells. J. Biochem. Mol. Toxicol. 2022, 36, e22999. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Waters, J.P.; Pober, J.S.; Bradley, J.R. Tumour necrosis factor and cancer. J. Pathol. 2013, 230, 241–248. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. The Role of EGCG in Green Tea Down Regulating Pro-inflammatory Cytokines Systemically. Int. J. Mol. Sci. 2020, 43, 340. [Google Scholar]
- Novilla, A.; Djamhuri, D.S.; Nurhayati, B.; Rihibiha, D.D.; Afifah, E.; Widowati, W. Anti-inflammatory properties of oolong tea (Camellia sinensis) ethanol extract and epigallocatechin gallate in LPS-induced RAW 264.7 cells. Asian Pac. J. Trop. Biomed. 2017, 7, 1005–1009. [Google Scholar] [CrossRef]
- Hussain, T.; Gupta, S.; Adhami, V.M.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int. J. Cancer 2005, 113, 660–669. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y. The impact of VEGF on cancer metastasis and systemic disease. Semin. Cancer Biol. 2022, 86, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Salajegheh, A. Angiogenesis in Health, Disease and Malignancy; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Rashidi, B.; Malekzadeh, M.; Goodarzi, M.; Masoudifar, A.; Mirzaei, H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017, 89, 949–956. [Google Scholar] [CrossRef]
- Luo, H.; Xu, M.; Zhong, W.; Cui, Z.; Liu, F.; Zhou, K.; Li, X. EGCG decreases the expression of HIF-1α and VEGF and cell growth in MCF-7 breast cancer cells. J. BUON 2014, 19, 435–439. [Google Scholar]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Yasuda, Y.; Kubota, M.; Adachi, S.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (−)-Epigallocatechin gallate inhibits growth and activation of the VEGF/VEGFR axis in human colorectal cancer cells. Chem. Biol. Interact. 2010, 185, 247–252. [Google Scholar] [CrossRef]
- Masuda, M.; Suzui, M.; Lim, J.T.; Deguchi, A.; Soh, J.W.; Weinstein, I.B. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J. Exp. Ther. Oncol. 2002, 2, 350–359. [Google Scholar] [CrossRef]
- Wang, J.; Man, G.C.W.; Chan, T.H.; Kwong, J.; Wang, C.C. A prodrug of green tea polyphenol (–)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer. Cancer Lett. 2018, 412, 10–20. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, J.; Du, Y.; Ding, J.; Liu, J.-Y. The green tea polyphenol EGCG potentiates the antiproliferative activity of sunitinib in human cancer cells. Tumor Biol. 2016, 37, 8555–8566. [Google Scholar] [CrossRef]
- Tang, H.; Zeng, L.; Wang, J.; Zhang, X.; Ruan, Q.; Wang, J.; Cui, S.; Yang, D. Reversal of 5-fluorouracil resistance by EGCG is mediate by inactivation of TFAP2A/VEGF signaling pathway and down-regulation of MDR-1 and P-gp expression in gastric cancer. Oncotarget 2017, 8, 82842–82853. [Google Scholar] [CrossRef]
- Xiang, L.-P.; Wang, A.; Ye, J.-H.; Zheng, X.-Q.; Polito, C.A.; Lu, J.-L.; Li, Q.-S.; Liang, Y.-R. Suppressive effects of tea catechins on breast cancer. Nutrients 2016, 8, 458. [Google Scholar] [CrossRef]
- Krakhmal, N.V.; Zavyalova, M.; Denisov, E.; Vtorushin, S.; Perelmuter, V. Cancer invasion: Patterns and mechanisms. Acta Naturae 2015, 7, 17–28. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Zhu, W.; Oteiza, P.I. NADPH oxidase 1: A target in the capacity of dimeric ECG and EGCG procyanidins to inhibit colorectal cancer cell invasion. Redox Biol. 2023, 65, 102827. [Google Scholar] [CrossRef]
- Lim, Y.C.; Park, H.Y.; Hwang, H.S.; Kang, S.U.; Pyun, J.H.; Lee, M.H.; Choi, E.C.; Kim, C.-H. (−)-Epigallocatechin-3-gallate (EGCG) inhibits HGF-induced invasion and metastasis in hypopharyngeal carcinoma cells. Cancer Lett. 2008, 271, 140–152. [Google Scholar] [CrossRef]
- Shankar, S.; Ganapathy, S.; Hingorani, S.R.; Srivastava, R.K. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front. Biosci. 2008, 13, 440–452. [Google Scholar] [CrossRef]
- Deng, Y.-T.; Lin, J.-K. EGCG inhibits the invasion of highly invasive CL1-5 lung cancer cells through suppressing MMP-2 expression via JNK signaling and induces G2/M arrest. J. Agric. Food Chem. 2011, 59, 13318–13327. [Google Scholar] [CrossRef]
- Farabegoli, F.; Papi, A.; Orlandi, M. (–)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9 and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant cells. Biosci. Rep. 2010, 31, 99–108. [Google Scholar] [CrossRef]
- Shore, N.D. Advances in the understanding of cancer immunotherapy. BJU Int. 2015, 116, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.S. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, M.; Xu, J.; Li, S.; Chen, Y.; Wang, W.; Yang, J.; Li, S.; Gu, M. The role of the programmed cell death protein-1/programmed death-ligand 1 pathway, regulatory T cells and T helper 17 cells in tumor immunity: A narrative review. Ann. Transl. Med. 2020, 8, 1526. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Ramachandran, I.; Youn, J.-I.; Gabrilovich, D.I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 2015, 66, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yan, F.; Zhao, Y.; Chen, X.; Sun, S.; Wang, Y.; Ying, L. Green tea polyphenol EGCG attenuates MDSCs-mediated immunosuppression through canonical and non-canonical pathways in a 4T1 murine breast cancer model. Nutrients 2020, 12, 1042. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Q.; Liu, Q.; Li, H.; Zhang, W.; Sun, C. Focus on immune checkpoint PD-1/PD-L1 pathway: New advances of polyphenol phytochemicals in tumor immunotherapy. Biomed. Pharmacother. 2022, 154, 113618. [Google Scholar] [CrossRef]
- Kantekure, K.; Yang, Y.; Raghunath, P.; Schaffer, A.; Woetmann, A.; Zhang, Q.; Odum, N.; Wasik, M. Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma (CTCL)/mycosis fungoides (MF). Am. J. Dermatopathol. 2012, 34, 126. [Google Scholar] [CrossRef]
- Blank, C.; Mackensen, A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: An update on implications for chronic infections and tumor evasion. Cancer Immunol. Immunother. 2007, 56, 739–745. [Google Scholar] [CrossRef]
- Liang, S.C.; Latchman, Y.E.; Buhlmann, J.E.; Tomczak, M.F.; Horwitz, B.H.; Freeman, G.J.; Sharpe, A.H. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 2003, 33, 2706–2716. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.; Faulhaber, E.; Caldwell, A.; Coy, J.; Kurihara, J.; Guth, A.; Regan, D.; Dow, S. Immune regulation of canine tumour and macrophage PD-L1 expression. Vet. Comp. Oncol. 2017, 15, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Hu, X.; Ding, Y.; Liu, M. Tumor-derived exosomes in the PD-1/PD-L1 axis: Significant regulators as well as promising clinical targets. J. Cell. Physiol. 2021, 236, 4138–4151. [Google Scholar] [CrossRef]
- Dong, C. Cytokine regulation and function in T cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef]
- Peña-Asensio, J.; Calvo, H.; Torralba, M.; Miquel, J.; Sanz-de-Villalobos, E.; Larrubia, J.-R. Anti-Pd-1/Pd-L1 based combination immunotherapy to boost antigen-specific CD8+ T cell response in hepatocellular carcinoma. Cancers 2021, 13, 1922. [Google Scholar] [CrossRef]
- Yu, X.; Fang, C.; Zhang, K.; Su, C. Recent advances in nanoparticles-based platforms targeting the PD-1/PD-L1 pathway for cancer treatment. Pharmaceutics 2022, 14, 1581. [Google Scholar] [CrossRef]
- Johnson, D.; Ma, B.B. Targeting the PD-1/PD-L1 interaction in nasopharyngeal carcinoma. Oral Oncol. 2021, 113, 105127. [Google Scholar] [CrossRef]
- Menon, D.R.; Li, Y.; Yamauchi, T.; Osborne, D.G.; Vaddi, P.K.; Wempe, M.F.; Zhai, Z.; Fujita, M. EGCG inhibits tumor growth in melanoma by targeting JAK-STAT signaling and its downstream PD-L1/PD-L2-PD1 axis in tumors and enhancing cytotoxic T-cell responses. Pharmaceuticals 2021, 14, 1081. [Google Scholar] [CrossRef]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green tea catechin is an alternative immune checkpoint inhibitor that inhibits PD-l1 expression and lung tumor growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef] [PubMed]
- Rawangkan, A.; Iida, K.; Sakai, R.; Fujiki, H.; Suganuma, M. Abstract 2665: Green tea catechin, EGCG, enhances antitumor immunity by down-regulation of PD-L1 expression in non-small human lung cancer cell lines. Cancer Res. 2017, 77, 2665. [Google Scholar] [CrossRef]
- Amin, A.R.; Wang, D.; Zhang, H.; Peng, S.; Shin, H.J.C.; Brandes, J.C.; Tighiouart, M.; Khuri, F.R.; Chen, Z.G.; Shin, D.M. Enhanced anti-tumor activity by the combination of the natural compounds (−)-epigallocatechin-3-gallate and luteolin: Potential role of p53. J. Biol. Chem. 2010, 285, 34557–34565. [Google Scholar] [CrossRef]
| Cancer Hallmark | Concentration Used | Type of Cells | Experimental Model | Outcomes of the Combination | Reference |
|---|---|---|---|---|---|
| Genomic Instability | 5–40 μg/mL | Colon adenocarcinoma | in vitro | EGCG exhibits different genetic and cytological effects on colon cancer cells. | [49] |
| 20 and 200 µM | Colorectal adenocarcinoma | in vitro | Telomeric modulation in cancer vs. primary cells and specific antioxidant activity of EGCG against oxidative damage to lipids in abnormal cells. | [50] | |
| 250 μM | Lung fibroblasts, skin fibroblasts, and epidermal keratinocytes | in vitro | Protection against UV-induced DNA damage in human cell cultures and human peripheral blood samples. | [51] | |
| Inducing Apoptosis | 30 µmol/L | Breast cancer | in vitro | EGCG suppressed the proliferation of human MCF-7 breast cancer cells and promoted apoptosis through the P53/Bcl-2 signaling pathway. | [58] |
| 80 μg/mL | Gastric cancer | in vitro | EGCG induces apoptosis of gastric cancer SGC7901 cells via down-regulating HIF-1α and VEGF under a hypoxic state. | [59] | |
| 40 μM | Nasopharyngeal cancer | in vitro | EGCG could inhibit the growth of nasopharyngeal cancer cells through the inhibition of the SIRT1-p53 signaling pathway. | [60] | |
| 40 µg/mL | Pancreatic cancer | in vitro | EGCG was able to inhibit proliferation and induce apoptosis in pancreatic cancer cells via PTEN, with the loss of PTEN reducing the ability of EGCG to inhibit proliferation and promote apoptosis in pancreatic cancer cells. | [61] | |
| 50 and 100 Μm 10 mg/kg/BW/day) | Hepatocellular carcinoma | in vitro in vivo | EGCG directly suppresses Phosphofructokinase activity and induces apoptosis by promoting a metabolic shift away from glycolysis in aerobic glycolytic hepatocellular carcinoma (HCC) cells. | [62] | |
| 0.5 mM | Hepatocellular carcinoma | in vitro | EGCG and epicatechin induced an inhibitory effect on the enzyme expression and activity of the de novo lipogenesis (DNL) pathway, which leads to the prominent activity of carnitine palmitoyl transferase-1 (CPT-1) mediating apoptosis in HepG2 cells. | [63] | |
| Caspase-independent apoptosis | 200 µM | Laryngeal epidermoid carcinoma | in vitro | P53-mediated mitochondrial pathway and the nuclear translocation of AIF and EndoG play a crucial role in EGCG-induced apoptosis of human laryngeal epidermoid carcinoma Hep2 cells, which proceeds through a caspase-independent pathway. | [64] |
| Tumorigenesis and carcinogen activity suppression | 1 mg/mL | Lung cancer | in vivo | EGCG inhibits cisplatin-induced weight loss and lung tumorigenesis in A/J mice | [66] |
| 0.1% | Liver cancer | in vivo | EGCG prevents obesity-related liver tumorigenesis by inhibiting the IGF/IGF-1R axis. | [67] | |
| Sustained Proliferative Signaling | (0, 5, 10, 20, 40 and 80 µg/mL) 10, 30 or 50 mg/kg | Ovarian cancer | in vitro in vivo | The involvement of PTEN/AKT/mTOR signaling pathway in the anti-ovarian cancer effects of EGCG in vitro and in vivo. | [76] |
| 40 µg/mL | Pancreatic cancer | in vitro | Inhibit proliferation and induce apoptosis in PC cells via PTEN. EGCG can downregulate the expression levels of p-Akt and p-mTOR to regulate the PI3K/Akt/mTOR pathway via PTEN. | [61] | |
| 5–20 μM 20 mg/kg | Multiple myeloma | in vitro in vivo | EGCG induces lipid-raft clustering and apoptotic cell death by activating protein kinase Cδ and acid sphingomyelinase through a 67 kDa laminin receptor. | [79] | |
| 160 μM 20 mg/kg | Lung cancer | in vitro in vivo | EGCG inhibits cell proliferation and migration and induces apoptosis in A549 and H1299 cells. EGCG inhibits lung cancer cell proliferation by suppressing NF-κB signaling. | [85] | |
| 1, 5, and 20 μM | Ovarian cancer | in vitro | Dietary factors EGCG and sulforaphane altered c-Myb-mediated ovarian cancer progression and chemoresistance. | [88] | |
| 1.34 mL of green tea extract | Prostate cancer | in vivo | Longstanding exercise training linked with the consumption of green tea extract may decrease levels of NF-κB and p53 in rats with prostate cancer. | [89] | |
| Evasion of Anti-Growth Signaling | 100 mM of EGC | Adenocarcinoma | in vitro | EGC caused a dose-dependent accumulation of cells in the G1 phase and a decrease in the phosphorylation of the retinoblastoma (Rb) protein, which was also in a cellular thiol-dependent manner. | [100] |
| 40 and 80 μM | Prostate cancer | in vitro | EGCG activates growth arrest and apoptosis primarily via a p53-dependent pathway that involves the function of both p21 and Bax. | [101] | |
| 30 μM 125 mg/kg | Head and neck and lung cancer | in vitro in vivo | A combination of EGCG and luteolin Significantly inhibits Ki-67 expression and boosts TUNEL-positive cells in xenografted tissues. A combination of EGCG and luteolin induced mitochondria-dependent apoptosis in some cell lines and mitochondria-independent apoptosis in others. More efficient stabilization and ATM-dependent Ser15 phosphorylation of p53 due to DNA damage by the combination. Ablation of p53 using shRNA strongly inhibited apoptosis as evidenced by decreased poly (ADP-ribose) polymerase and caspase-3 cleavage. Mitochondrial translocation of p53. | [168] | |
| 30μM 15, 30, and 45 μM | Gastric cancer | in vitro in vivo | EGCG suppressed gastric cancer cell proliferation and demonstrated that this inhibitory effect is related to canonical Wnt/β-catenin signaling. | [102] | |
| Replicative Immortality | 20 and 200 µM | Colorectal adenocarcinoma | in vitro | EGCG induced telomere shortening and decreased telomerase activity in Caco-2 cells. | [50] |
| 1, 5, and 10 µg/mL | Glioblastoma | in vitro | EGCG has the potential to stop the growth of U251 cells due to telomerase inhibition. The effect mainly was on cancerous cells and not normal ones. | [106] | |
| 80 µM | Breast cancer | in vitro | EGCG significantly diminished 0.8, 0.4, and 0.3 gene expression of hTERT. | [110] | |
| Tumor Dysregulated Metabolism | 20–100 μM 10 mg/kg | Pancreatic cancer | in vitro in vivo | EGCG and gemcitabine combination reduced pancreatic cancer cell growth by suppressing glycolysis. | [113] |
| 25, 50, or 100 µ | Colorectal cancer | in vitro | EGCG treatment of cancer-associated fibroblasts overcomes their tumor-promoting abilities by stopping their glycolytic activity. | [112] | |
| 25, 50, 100, and 150 μg/mL 30 μg/kg | Nasopharyngeal carcinoma | in vitro in vivo | EGCG may alert radio resistance by reducing fatty acid synthase and work as a radiosensitizer for better treatment of nasopharyngeal carcinoma. | [115] | |
| 10–140 µM | Breast cancer | in vitro | Significantly blocked fatty acid synthase activity in triple-negative breast cancer. | [116] | |
| 0, 25, 50 and 100 μM | Hepatocellular carcinoma | in vitro | EGCG suppressed cell growth under low glucose conditions. EGCG inhibited glutamate dehydrogenase 1. | [119] | |
| 30 μM and 40 μM 20 mg/kg | Non-small-cell lung cancer | in vitro in vivo | EGCG mediated ROS to regulate copper transporter 1 expression through the ERK1/2/ nuclear paraspeckle assembly transcript 1 (NEAT1) signaling pathway. | [122] | |
| 0.5 μM | Lung adenocarcinoma | in vitro | Low-dose EGCG enhanced Doxorubicin toxicity and revealed oxidative damage-mediated antineoplastic efficacy by reorienting the redox signaling in A549 cells. | [123] | |
| Tumor—Inflammation | 10 μM and 50 μM | Macrophage-like, Abelson leukemia virus-transformed cell line | in vitro | Oolong tea ethanol extract and EGCG decreased the assembly of NO, COX-2, IL-6, IL-1β, and TNF-α in active macrophages. | [128] |
| 10, 25, 50, or 100 μM | Prostate cancer | in vitro | EGCG selectively inhibits COX-2. | [129] | |
| Angiogenesis inhibition | 20, 40, and 60 µM | Endometrial cancer | in vivo | EGCG reduced the secretion of VEGFA from cancerous cells and also reduced tumor-associated macrophage-secreted VEGFA in endometrial cancer. | [137] |
| 50 μmol/L 50 mg/kg | Lung cancer Breast cancer | in vitro in vivo | EGCG along with sunitinib simultaneously inhibits the VEGFR2/ mTOR/VEGF signaling cascade. | [138] | |
| 20μM 25 mg/kg | Gastric cancer | in vitro in vivo | EGCG inhibited VEGF secretion and expression, and its up-stream signal regulator; it also down-regulated the transcription of factor activator protein 2A (TFAP2A). | [139] | |
| 30 μg/mL | Hepatocellular carcinoma | in vitro | EGCG inhibited tumor growth and angiogenesis by the intervention of MAPK/ERK1/2 and PI3K/AKT/HIF-1α/VEGF pathways. | [71] | |
| 20 μg/mL, 60 μg/mL, and 100 μg/mL | Gastric cancer | in vitro | With the increasing EGCG concentration, the expressions of HIF-1α and VEGF proteins were suppressed in hypoxic conditions. | [59] | |
| 25, 50, 100 mg/L | Breast cancer | in vitro | Protein expression of HIF-1α and VEGF declined in a dose-dependent manner in MCF-7 cells pretreated with increasing concentrations of EGCG. | [134] | |
| 0.01 and 0.1% | Colorectal cancer | in vivo | Restraining the activation of the VEGF/VEGFR axis by suppressing the expression of HIF-1 in the xenograft model. | [135] | |
| 30 μg/mL | Neck and breast | in vitro | Lowering VEGF by inhibiting EGFR-related pathways of signal transduction. | [136] | |
| Tissue Invasion and Metastasis | 15, 30, 60 μM | Colorectal cancer | in vitro | ECG and EGCG dimers restrained colorectal cancer cell invasion/metastasis, by downregulating MMP-2 and MMP-9 expression via a NOX1/EGFR-dependent mechanism, and through a direct inhibitory effect on MMPs enzyme activity. | [143] |
| 1 μM | Hypopharyngeal carcinoma | in vitro | EGCG inhibited HGF, MMP-9, and urokinase-type plasminogen activator activities, and also inhibited Akt and Erk pathway. | [144] | |
| 20, 40, and 60 μM 60 mg/kg | Pancreatic cancer | in vitro in vivo | EGCG slowed circulating endothelial growth factor receptor 2 (VEGF-R2). EGCG reduced ERK activity and enhanced p38 and JNK activities. EGCG prevented pancreatic cancer growth, invasion, metastasis, and angiogenesis. | [145] | |
| 2.5, 5, 10, 20, and 40 μM | Lung cancer | in vitro | EGCG suppresses the invasion ability of CL1-5 cells. EGCG induced G2/M arrest at higher doses (30, 40, and 50 μM). | [146] | |
| 50 μg/mL | Tamoxifen-resistant breast cancer | in vitro | EGCG prevented MCF-7 tamoxifen-resistant cells growth and in vitro invasion via down-regulation of EGFR and other molecules associated with aggressive biological behavior. | [147] | |
| Evading immune system | 50–350 μg/mL 50 μg/mL, 500 μg/mL, 1000 μg/mL, and 2000 μg/mL | Breast cancer | in vitro in vivo | EGCG reduced the production of related cytokines in MDSCs. | [153] |
| 10 µM 50 mg/kg | Melanoma | in vitro in vivo | EGCG improves anti-tumor immune responses by reducing JAK-STAT signaling in melanoma. EGCG targeted the PD-L1/PD-L2-PD-1 axis. | [165] | |
| 10 and 50 µM 0.85 g/L of catechins (14% EGCG) | Lung cancer | in vitro | EGCG partially restores T cell activity by inhibiting PD-L1/PD-1 signaling, resulting in the inhibition of lung cancer growth. | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talib, W.H.; Awajan, D.; Alqudah, A.; Alsawwaf, R.; Althunibat, R.; Abu AlRoos, M.; Al Safadi, A.; Abu Asab, S.; Hadi, R.W.; Al Kury, L.T. Targeting Cancer Hallmarks with Epigallocatechin Gallate (EGCG): Mechanistic Basis and Therapeutic Targets. Molecules 2024, 29, 1373. https://doi.org/10.3390/molecules29061373
Talib WH, Awajan D, Alqudah A, Alsawwaf R, Althunibat R, Abu AlRoos M, Al Safadi A, Abu Asab S, Hadi RW, Al Kury LT. Targeting Cancer Hallmarks with Epigallocatechin Gallate (EGCG): Mechanistic Basis and Therapeutic Targets. Molecules. 2024; 29(6):1373. https://doi.org/10.3390/molecules29061373
Chicago/Turabian StyleTalib, Wamidh H., Dima Awajan, Abdelrahim Alqudah, Razan Alsawwaf, Raha Althunibat, Mahmoud Abu AlRoos, Ala’a Al Safadi, Sharif Abu Asab, Rawan W. Hadi, and Lina T. Al Kury. 2024. "Targeting Cancer Hallmarks with Epigallocatechin Gallate (EGCG): Mechanistic Basis and Therapeutic Targets" Molecules 29, no. 6: 1373. https://doi.org/10.3390/molecules29061373
APA StyleTalib, W. H., Awajan, D., Alqudah, A., Alsawwaf, R., Althunibat, R., Abu AlRoos, M., Al Safadi, A., Abu Asab, S., Hadi, R. W., & Al Kury, L. T. (2024). Targeting Cancer Hallmarks with Epigallocatechin Gallate (EGCG): Mechanistic Basis and Therapeutic Targets. Molecules, 29(6), 1373. https://doi.org/10.3390/molecules29061373








