Magnetite/MXene (Fe3O4/Ti3C2) Nanocomposite as a Novel Adsorbent for Environmental Remediation of Malachite Green Dye
Abstract
1. Introduction
2. Results and Discussion
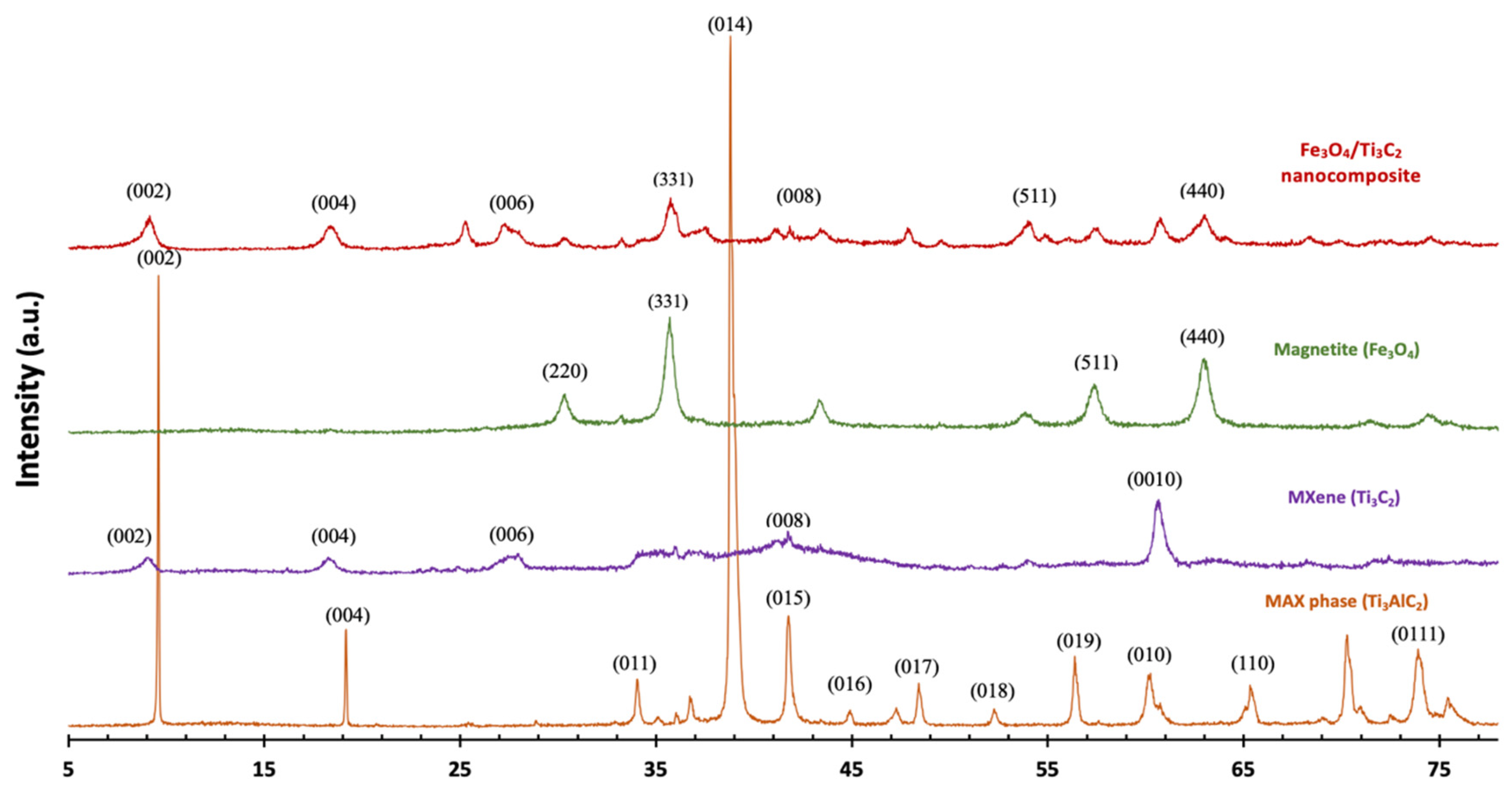
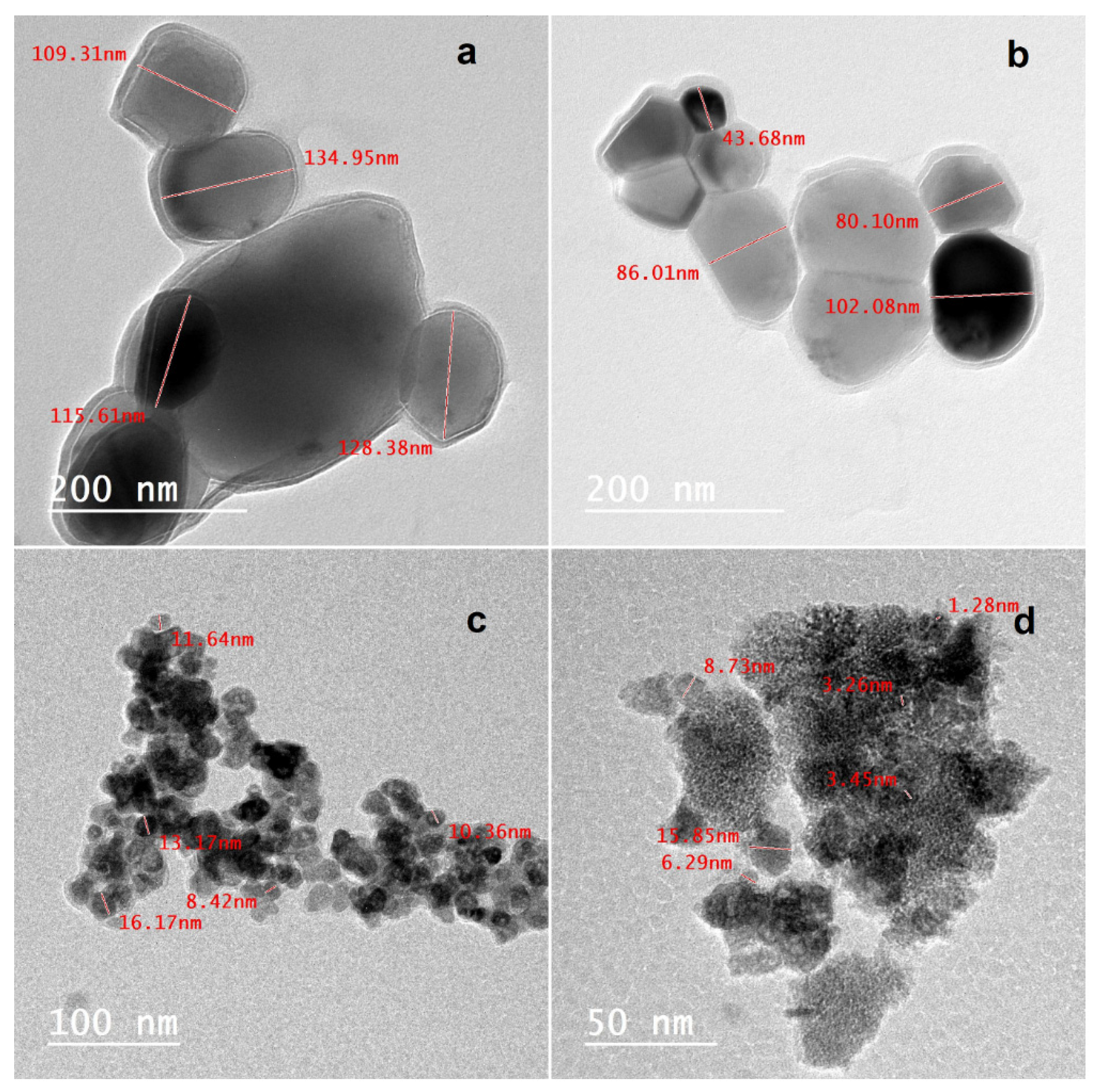
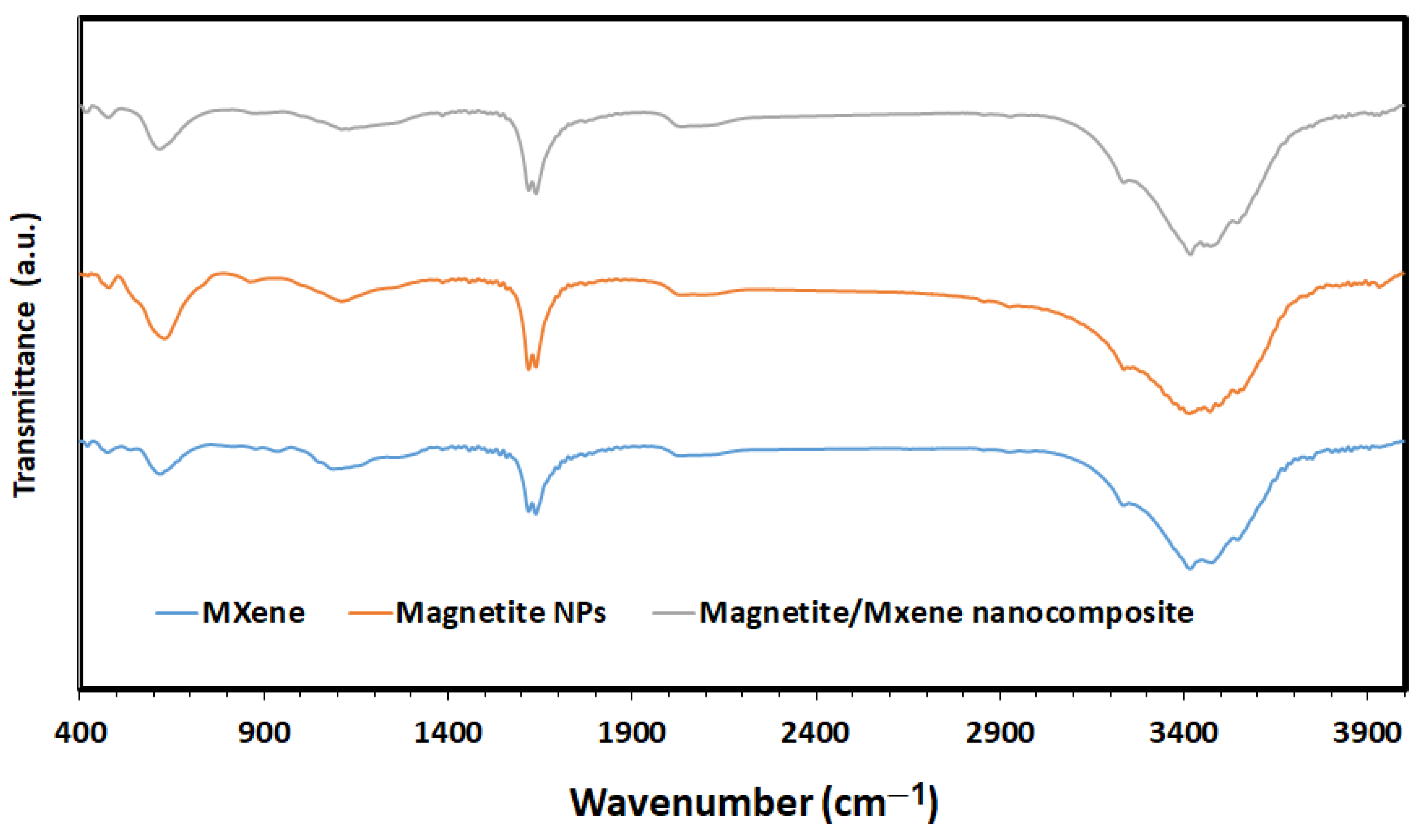
2.1. Characterization
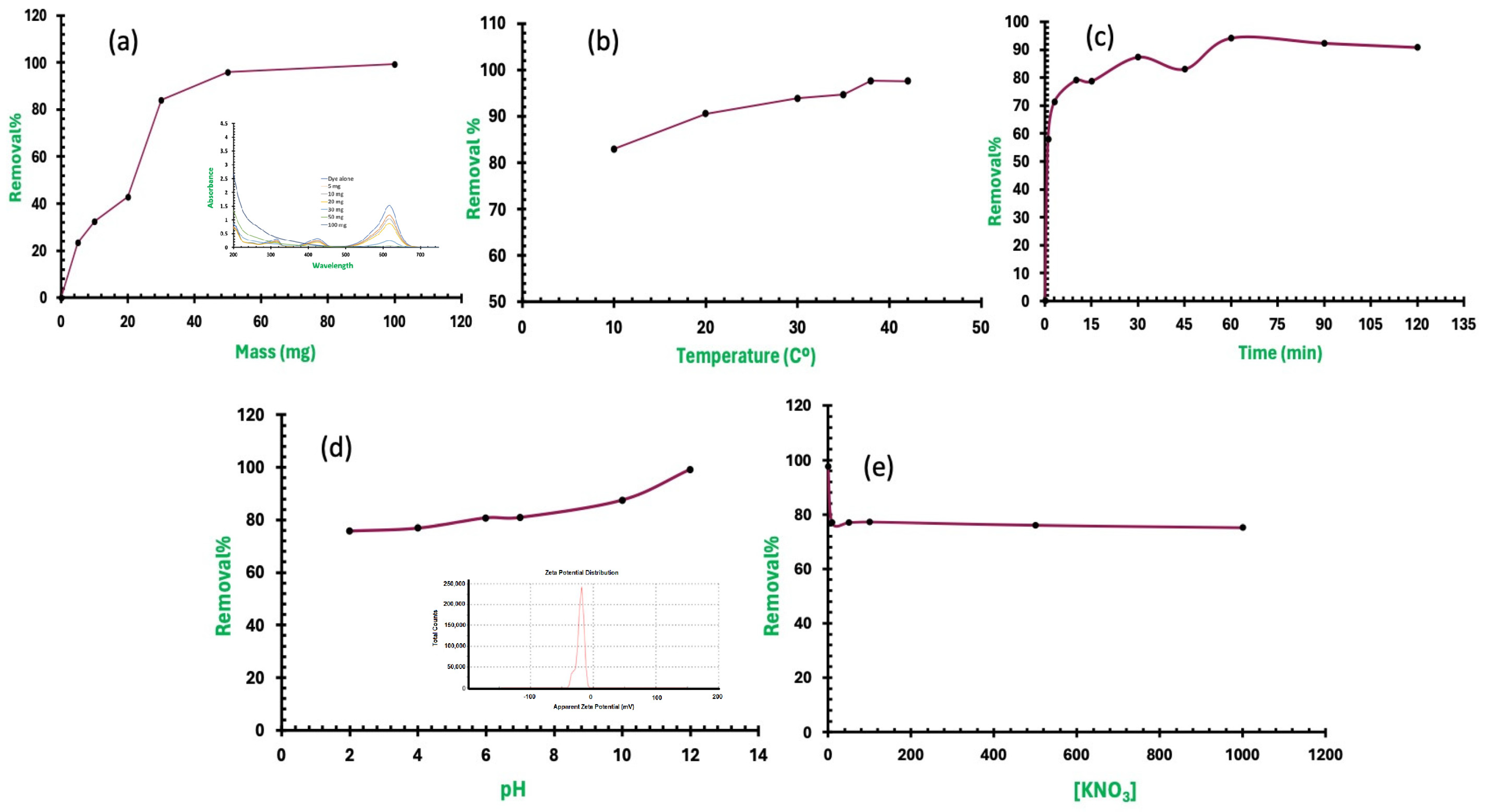
2.2. Adsorption Studies
2.3. Kinetics Study

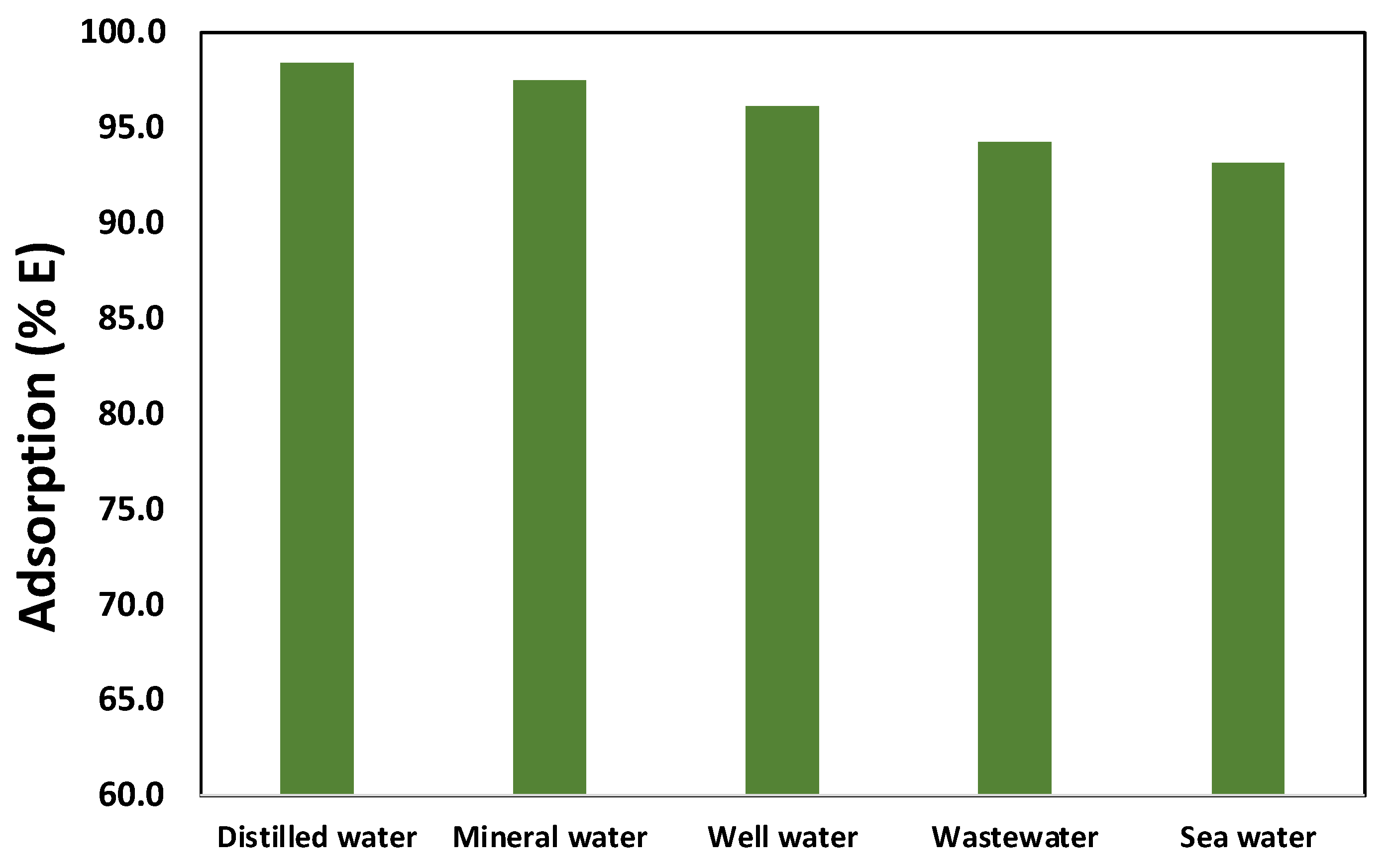
2.4. Environmental Applications
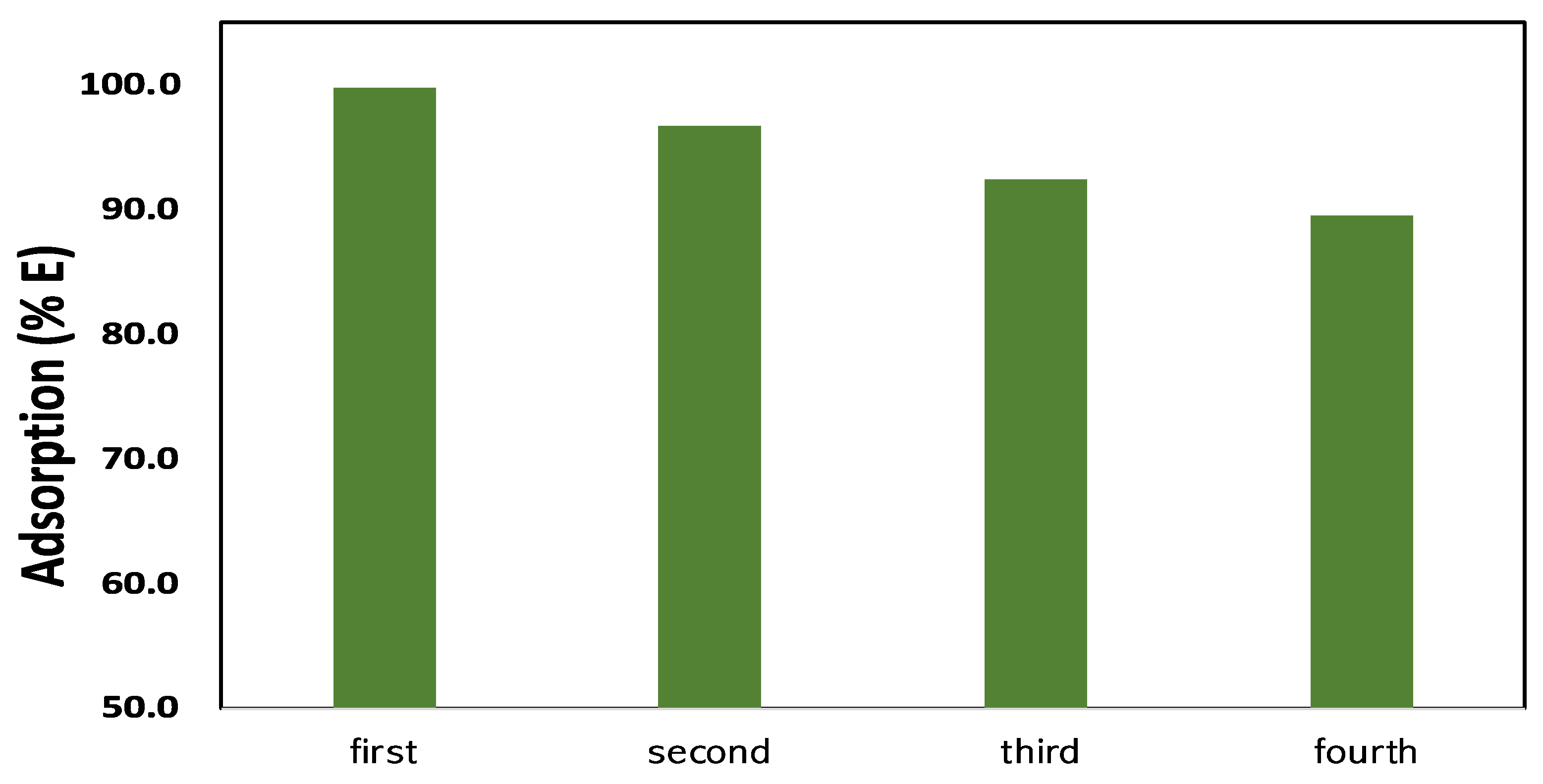
2.5. Reusability Studies
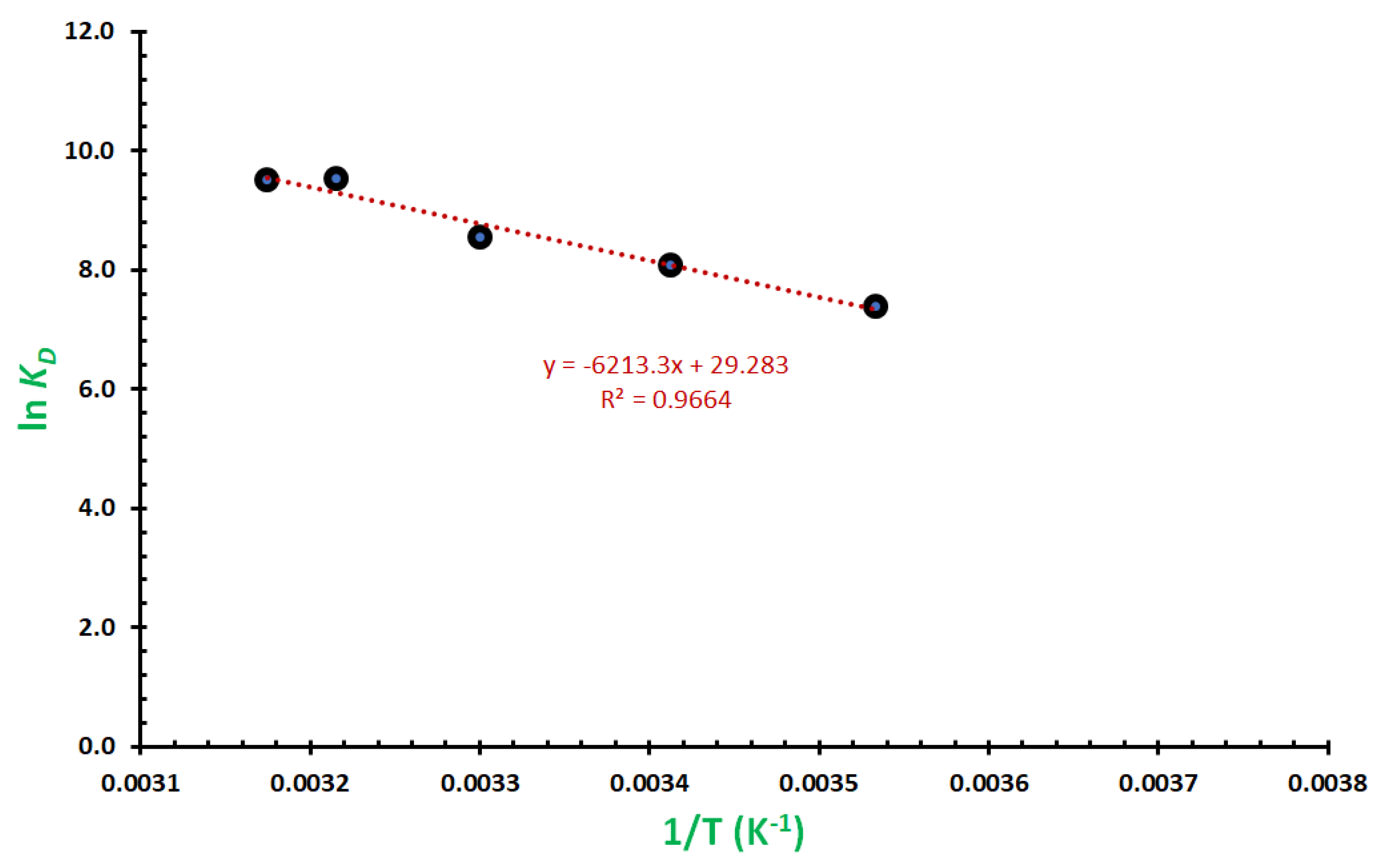
2.6. Studies on Thermodynamics of the Removal
3. Experimental
3.1. Substances
3.2. Fe3O4 Nanoparticles and MXene Nanosheet Synthesis
3.3. Synthesis of Fe3O4/Ti3C2 Nanocomposite
3.4. Characterizations
3.5. Adsorption Experiment
3.6. Collecting Real Water Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Dinh, D.M.; Hsieh, Y.-L. Adsorption, and desorption of cationic malachite green dye on cellulose nanofibril aerogels. Carbohydr. Polym. 2017, 173, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Chouli, F.; Ezzat, A.O.; Sabantina, L.; Benyoucef, A.; Zehhaf, A. Optimization Conditions of Malachite Green Adsorption onto Almond Shell Carbon Waste Using Process Design. Molecules 2024, 29, 54. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; Abd-Elhamid, A.I.; Arafa, W.A.A.; Ahmed, I.M.; El-Shanshory, A.A.; Abu-Saied, M.A.; Soliman, H.M.A.; Abdelgawad, M.A.; Ali, H.M.; Bräse, S. Chitosan-Functionalized-Graphene Oxide (GO@CS) Beads as an Effective Adsorbent to Remove Cationic Dye from Wastewater. Polymers 2022, 14, 4236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, T.; Ding, S.; Wang, X. Development of loose thin film nanofibrous composite nanofiltration membrane with modified g-C3N4 nanosheets barrier layer for efficient separation of salt/dye mixtures. Sep. Purif. Technol. 2023, 306 Pt A, 122661. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Advances in application of cotton-based adsorbents for heavy metals trapping, surface modifications and future perspectives. Ecotoxicol. Environ. Saf. 2020, 201, 110825. [Google Scholar] [CrossRef]
- He, H.; Wang, X.; Yu, Q.; Wu, W.; Feng, X.; Kong, D.; Ren, X.; Gao, J. In Situ Growth of Ti3C2/UiO-66-NH2 Composites for Photoreduction of Cr (VI). Catalysts 2023, 13, 876. [Google Scholar] [CrossRef]
- Yang, G.; Li, S.; Wang, X.; Ding, B.; Li, Y.; Lin, H.; Tang, D.; Ren, X.; Wang, Q.; Luo, S.; et al. A universal strategy boosting photoelectrochemical water oxidation by utilizing MXene nanosheets as hole transfer mediators. Appl. Catal. B Environ. 2021, 297, 120268. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Li, C.; Zhang, C.; Wu, C.; Li, Z.; An, X.; He, L. Niche Applications of MXene Materials in Photothermal Catalysis. Chemistry 2023, 5, 492–510. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Li, C.; Zhang, C.; Feng, K.; Wang, Z.; Wang, X.; Meira, D.M.; Cai, M.; Zhang, D.; et al. Mo2TiC2 MXene-Supported Ru Clusters for Efficient Photothermal Reverse Water–Gas Shift. ACS Nano 2023, 17, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-y.; Deng, Y.-f.; Huang, T.; Zhang, N.; Wang, Y. Polydopamine-assisted MXene decoration on electrospun polylactide fibers toward oil/water separation and organic dye adsorption. Sep. Purif. Technol. 2024, 328, 125040. [Google Scholar] [CrossRef]
- Yang, F.; Li, J.; Dong, J.; Chen, S.; Hu, W.; Zhang, Y.; Wang, H.; Li, Z.; Wang, Z. MX@MIL-125(Ti)-mediated sonocatalytic degradation for the dyes and microplastics. Sep. Purif. Technol. 2024, 337, 126488. [Google Scholar] [CrossRef]
- Tran, N.M.; Ta, Q.T.H.; Sreedhar, A.; Noh, J.-S. Ti3C2Tx MXene playing as a strong methylene blue adsorbent in wastewater. Appl. Surf. Sci. 2021, 537, 148006. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Zhang, S.; Liao, S.; Qi, F.; Liu, R.; Xiao, T.; Hu, J.; Li, K.; Wang, R.; Min, Y. Direct deposition of two-dimensional MXene nanosheets on commercially available filter for fast and efficient dye removal. J. Hazard. Mater. 2020, 384, 121367. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, C.; Li, Z.; Feng, K.; Cai, M.; Zhang, D.; Wang, S.; Chu, M.; Zhang, C.; Shen, J.; et al. Niobium and Titanium Carbides (MXenes) as Superior Photothermal Supports for CO2 Photocatalysis. ACS Nano 2021, 15, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Tawalbeh, M.; Mohammed, S.; Al-Othman, A.; Yusuf, M.; Mofijur, M.; Kamyab, H. MXenes and MXene-based materials for removal of pharmaceutical compounds from wastewater: Critical review. Environ. Res. 2023, 228, 115919. [Google Scholar] [CrossRef]
- Gopalram, K.; Kapoor, A.; Kumar, P.S.; Sunil, A.; Rangasamy, G. MXenes and MXene-Based Materials for Removal and Detection of Water Contaminants: A Review. Ind. Eng. Chem. Res. 2023, 62, 6559–6583. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Zhang, L.; Pei, L.; Xue, H.; Li, Z. Adsorption of methylene blue and Congo red from aqueous solution on 3D MXene/carbon foam hybrid aerogels: A study by experimental and statistical physics modeling. J. Environ. Chem. Eng. 2023, 11, 109206. [Google Scholar] [CrossRef]
- Elgarhy, G.S.; El-Subruiti, G.M.; Omer, A.M.; Eltaweil, A.S. 2D/3D MXene/NiFeMn-layered double hydroxide decorated gelatin for removal of Cr (VI) and Congo red: Performance and mechanism. J. Mol. Liq. 2024, 396, 123889. [Google Scholar] [CrossRef]
- Xue, H.; Gao, X.; Seliem, M.K.; Mobarak, M.; Dong, R.; Wang, X.; Fu, K.; Li, Q.; Li, Z. Efficient adsorption of anionic azo dyes on porous heterostructured MXene/biomass activated carbon composites: Experiments, characterization, and theoretical analysis via advanced statistical physics models. Chem. Eng. J. 2023, 451, 138735. [Google Scholar] [CrossRef]
- Liu, D.; Li, T.; Sun, W.; Zhou, W.; Zhang, G. Magnetic Ti3C2 MXene Nanomaterials for Doxorubicin Adsorption from Aqueous Solutions: Kinetic, Isotherms, and Thermodynamic Studies. ACS Omega 2022, 7 Pt 3, 31945–31953. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.J.; Nouralishahi, A.; Hallajisani, A. Fe3O4-chitosan nanocomposite as a magnetic biosorbent for removal of nickel and cobalt heavy metals from polluted water. Int. J. Biol. Macromol. 2023, 248, 125984. [Google Scholar] [CrossRef] [PubMed]
- Elgamal, A.M.; El-Ghany, N.A.A.; Saad, G.R. Synthesis and characterization of hydrogel-based magnetite nanocomposite adsorbents for the potential removal of Acid Orange 10 dye and Cr (VI) ions from aqueous solution. Int. J. Biol. Macromol. 2023, 227, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Zawrah, M.F.; El-Gammal, M.I.; Salem, M.; El-Sonbati, M.A.; Ahmed, M. Recycling of Rice Husk for Preparation of Activated Carbon/Magnetite Nanocomposites for Removal of Methylene Blue from Wastewater. Int. J. Environ. Res. 2023, 17, 51. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2 MXene hybrids with ultrahigh volumetric capacity as an anode material for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Jing, Y.; Ma, J.; Du, C.-F.; Yan, Q. Surface Modified MXene-Based Nanocomposites for Electrochemical Energy Conversion and Storage. Small 2019, 15, 1901503. [Google Scholar] [CrossRef]
- Rakhi, B.; Ahmed, B.; Hedhili, M.N.; Anjum, D.H.; Alshareef, H.N. Effect of Postetch Annealing Gas Composition on the Structural and Electrochemical Properties of Ti2CTx MXene Electrodes for Supercapacitor Applications. Chem. Mater. 2015, 27, 5314–5323. [Google Scholar] [CrossRef]
- Scheibe, B.; Kupka, V.; Peplińska, B.; Jarek, M.; Tadyszak, K. The Influence of Oxygen Concentration during MAX Phases (Ti3AlC2) Preparation on the α-Al2O3 Microparticles Content and Specific Surface Area of Multilayered MXenes (Ti3C2Tx). Materials 2019, 12, 353. [Google Scholar] [CrossRef]
- Mahmood, M.; Rasheed, A.; Ayman, I.; Rasheed, T.; Munir, S.; Ajmal, S.; Agboola, P.O.; Warsi, M.F.; Shahid, M. Synthesis of ultrathin MnO2 nanowire-intercalated 2D-MXenes for high-performance hybrid supercapacitors. Energy Fuels 2021, 35, 3469–3478. [Google Scholar] [CrossRef]
- Kiran, N.U.; Deore, A.B.; More, M.A.; Late, D.J.; Rout, C.S.; Mane, P.; Chakraborty, B.; Besra, L.; Chatterjee, S. Comparative Study of Cold Electron Emission from 2D Ti3C2TX MXene Nanosheets with Respect to Its Precursor Ti3SiC2 MAX Phase. ACS Appl. Electron. Mater. 2022, 4, 2656–2666. [Google Scholar] [CrossRef]
- Woo, S.-Y.; Lee, H.-S.; Kim, J.-S.; Kim, K.-H.; Ji, H.; Kim, Y.-D. Applicability assessment of functional adsorption zeolite materials in adsorption desalination cum cooling systems driven by low-grade heat source. Chem. Eng. J. 2022, 430, 131375. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nanocomposites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, R. Fractal Reaction Kinetics. Science 1988, 241, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.C.; Dehghani, M.H.; Guleria, A.; Sher, F.; Karri, R.R.; Dotto, G.L.; Tran, H.N. Adsorption: Fundamental aspects and applications of adsorption for effluent treatment. In Green Technologies for the Defluoridation of Water; Dehghani, M.H., Karri, R., Lima, E.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–88. [Google Scholar] [CrossRef]
- Haerifar, M.; Azizian, S. Fractal-like kinetics for adsorption on heterogeneous solid surfaces. J. Phys. Chem. C 2014, 118, 1129–1134. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Yao, C.; Chen, T. A film-diffusion-based adsorption kinetic equation and its application. Chem. Eng. Res. Des. 2017, 119, 87–92. [Google Scholar] [CrossRef]
- An, B. Cu (II) and As (V) Adsorption Kinetic Characteristic of the Multifunctional Amino Groups in Chitosan. Processes 2020, 8, 1194. [Google Scholar] [CrossRef]
- Owija, N.Y.; Salam, S.K.M.A. Removal of cadmium ions from aqueous solution by Zero valent iron nanoparticles: Equilibrium and thermodynamic studies. J. Mol. Liq. 2021, 342, 117462. [Google Scholar] [CrossRef]
- Li, C.; Kong, D.; Yao, X.; Ma, X.; Wei, C.; Wang, H. Adsorption Characteristics and Molecular Simulation of Malachite Green onto Modified Distillers’ Grains. Water 2022, 14, 171. [Google Scholar] [CrossRef]
- Gupta, V.; Mittal, A.; Krishnan, L.; Gajbe, V. Adsorption kinetics and column operations for the removal and recovery of malachite green from wastewater using bottom ash. Sep. Purif. Technol. 2004, 40, 87–96. [Google Scholar] [CrossRef]
- Chen, J.; Mao, J.; Mo, X.; Hang, J.; Yang, M. Study of adsorption behavior of malachite green on polyethylene glycol micelles in cloud point extraction procedure. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 345, 231–236. [Google Scholar] [CrossRef]
- Khan, A.A.; Ahmad, R.; Khan, A.; Mondal, P.K. Preparation of unsaturated polyester Ce (IV) phosphate by plastic waste bottles and its application for removal of malachite green dye from water samples. Arab. J. Chem. 2013, 6, 361–368. [Google Scholar] [CrossRef]
- Kaith, B.S.; Jindal, R.; Sharma, R. Synthesis of a Gum rosin alcohol-poly(acrylamide) based adsorbent and its application in removal of malachite green dye from waste water. RSC Adv. 2015, 5, 43092–43104. [Google Scholar] [CrossRef]
- Kaith, B.S.; Sukriti Sharma, J.; Kaur, T.; Sethi, S.; Shanker, U.; Jassal, V. Microwave-assisted green synthesis of hybrid nanocomposite: Removal of malachite green from waste water. Iran. Polym. J. 2016, 25, 787–797. [Google Scholar] [CrossRef]
- Khattri, S.; Singh, M. Removal of malachite green from dye wastewater using neem sawdust by adsorption. J. Hazard. Mater. 2009, 167, 1089–1094. [Google Scholar] [CrossRef]
- Abou-Gamra, Z.M.; Ahmed, M.A. TiO2 nanoparticles for removal of malachite green dye from waste water. Adv. Chem. Eng. Sci. 2015, 5, 373–388. [Google Scholar] [CrossRef]
- Lv, G.-Y.; Cheng, J.-H.; Chen, X.-Y.; Zhang, Z.-F.; Fan, L.-F. Biological decolorization of malachite green by Deinococcus radiodurans R1. Bioresour. Technol. 2013, 144, 275–280. [Google Scholar] [CrossRef]

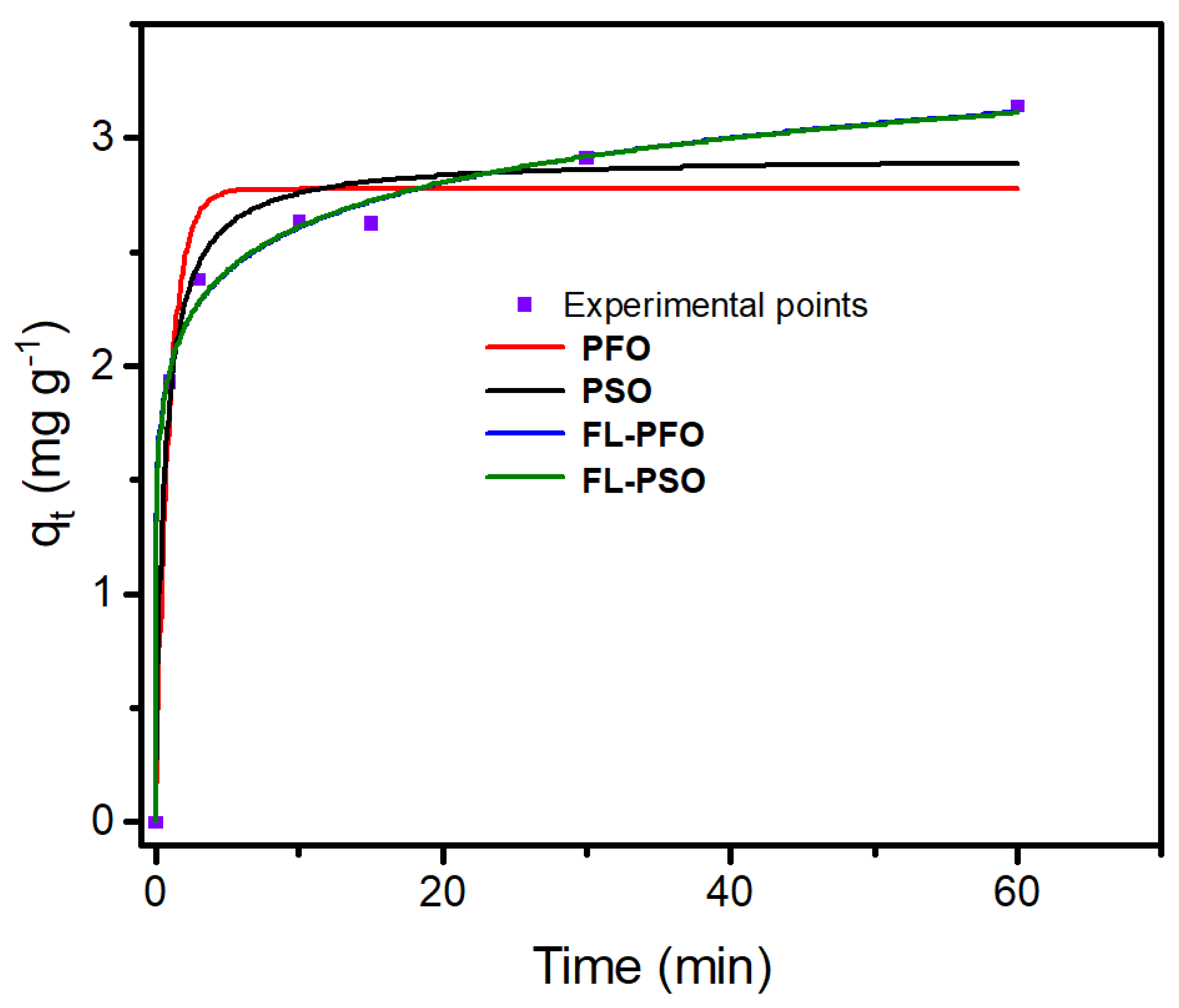
| Pseudo-First-Order Kinetics Model (PFO) | |||||||
| qe,exp (mg g−1) | qe,cal (mg g−1) | k1 (min−1) | R2 | SD | RSS | BIC | |
| 3.140 | 2.776 | 1.080 | 0.9573 | 0.9488 | 0.2392 | 0.2861 | −16.544 |
| Pseudo-second-order kinetics model (PSO) | |||||||
| qe,exp (mg g−1) | qe,cal (mg g−1) | k2 (g mg−1 min−1) | R2 | SD | RSS | BIC | |
| 3.140 | 2.917 | 0.5965 | 0.9813 | 0.9775 | 0.1584 | 0.1254 | −22.315 |
| Fractal-like Pseudo-first-order Kinetics model (FL-PFO) | |||||||
| qe,exp (mg g−1) | qe,cal (mg g−1) | k1,0 (min−1) | R2 | SD | RSS | BIC | |
| 3.140 | 4.446 | 0.0488 | 0.9963 | 0.9944 | 0.0790 | 0.0249 | −31.668 |
| Fractal-like Pseudo-second-order Kinetics model (FL-PSO) | |||||||
| qe,exp (mg g−1) | qe,cal (mg g−1) | k2,0 (g mg−1 min−1) | R2 | SD | RSS | BIC | |
| 3.140 | 5.358 | 0.1098 | 0.9963 | 0.9945 | 0.0784 | 0.0246 | −31.783 |
| Material | Removal Capacity (mg/g) | Removal Time | Reference |
|---|---|---|---|
| Bottom ash | 0.71 | 83 min | [44] |
| Polyethylene glycol micelles | 1.00 | 10 min | [45] |
| Unsaturated Polyester Ce(IV) phosphate | 1.01 | 35 min | [46] |
| Fum rosin alcoholpoly(acrylamide) | 1.40 | 300 min | [47] |
| Hybrid nanocomposite | 3.21 | 28 h | [48] |
| Neem sawdust | 4.35 | 14 min | [49] |
| Fe3O4/Ti3C2 nanocomposite | 4.68 | 60 min | [Current study] |
| TiO2 nanoparticles | 6.25 | 40 min | [50] |
| Deinococcus radiodurans | 7.63 | 30 min | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhudaydi, A.M.; Danish, E.Y.; Abdel Salam, M. Magnetite/MXene (Fe3O4/Ti3C2) Nanocomposite as a Novel Adsorbent for Environmental Remediation of Malachite Green Dye. Molecules 2024, 29, 1372. https://doi.org/10.3390/molecules29061372
Alkhudaydi AM, Danish EY, Abdel Salam M. Magnetite/MXene (Fe3O4/Ti3C2) Nanocomposite as a Novel Adsorbent for Environmental Remediation of Malachite Green Dye. Molecules. 2024; 29(6):1372. https://doi.org/10.3390/molecules29061372
Chicago/Turabian StyleAlkhudaydi, Amal M., Ekram Y. Danish, and Mohamed Abdel Salam. 2024. "Magnetite/MXene (Fe3O4/Ti3C2) Nanocomposite as a Novel Adsorbent for Environmental Remediation of Malachite Green Dye" Molecules 29, no. 6: 1372. https://doi.org/10.3390/molecules29061372
APA StyleAlkhudaydi, A. M., Danish, E. Y., & Abdel Salam, M. (2024). Magnetite/MXene (Fe3O4/Ti3C2) Nanocomposite as a Novel Adsorbent for Environmental Remediation of Malachite Green Dye. Molecules, 29(6), 1372. https://doi.org/10.3390/molecules29061372






