Gallium Trichloride Fluid: Dimer Dissociation Mechanism, Local Structure, and Atomic Dynamics
Abstract
1. Introduction
2. Results and Discussion
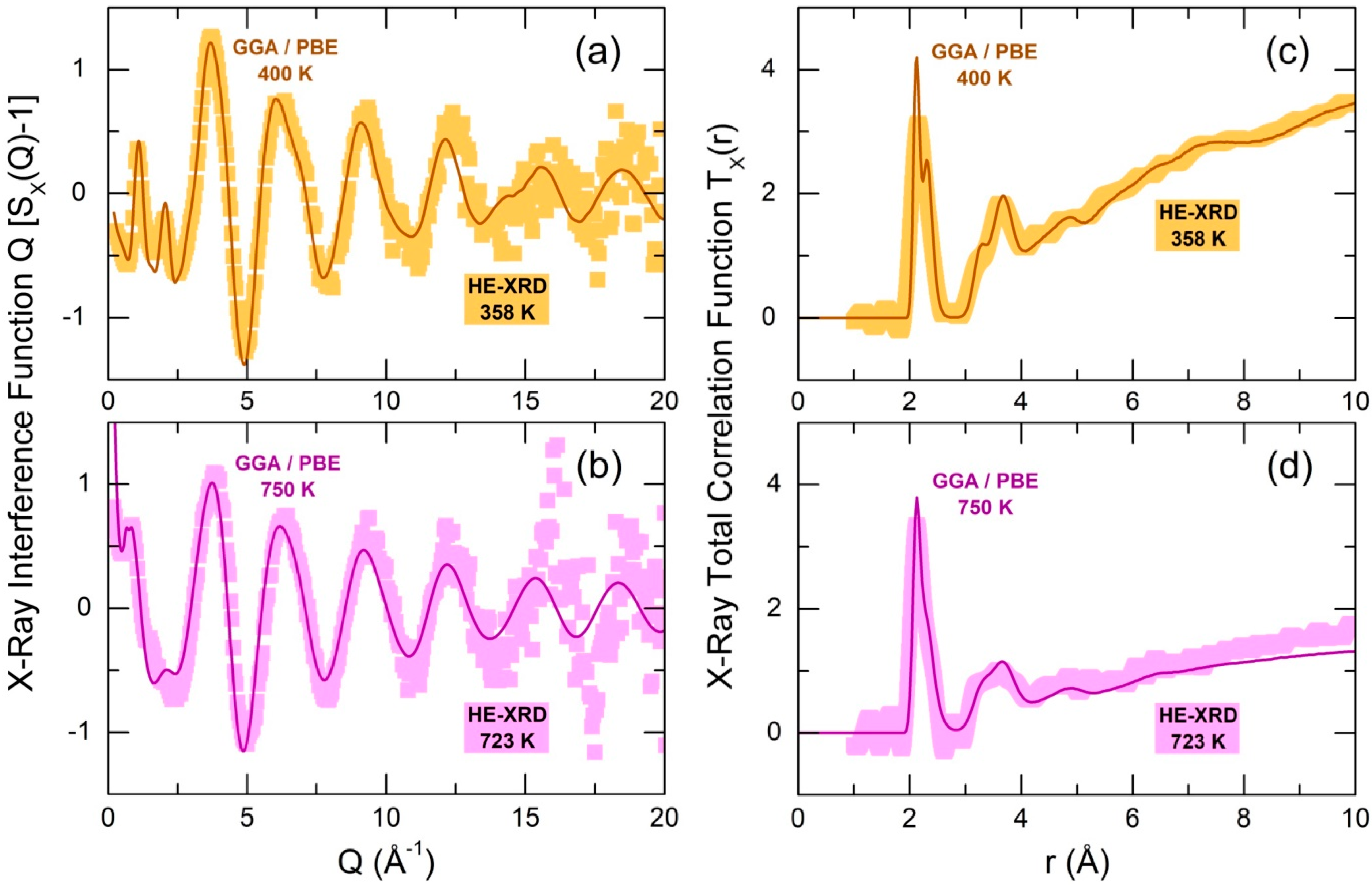
2.1. Validation of the FPMD Modeling by High-Energy X-ray Diffraction
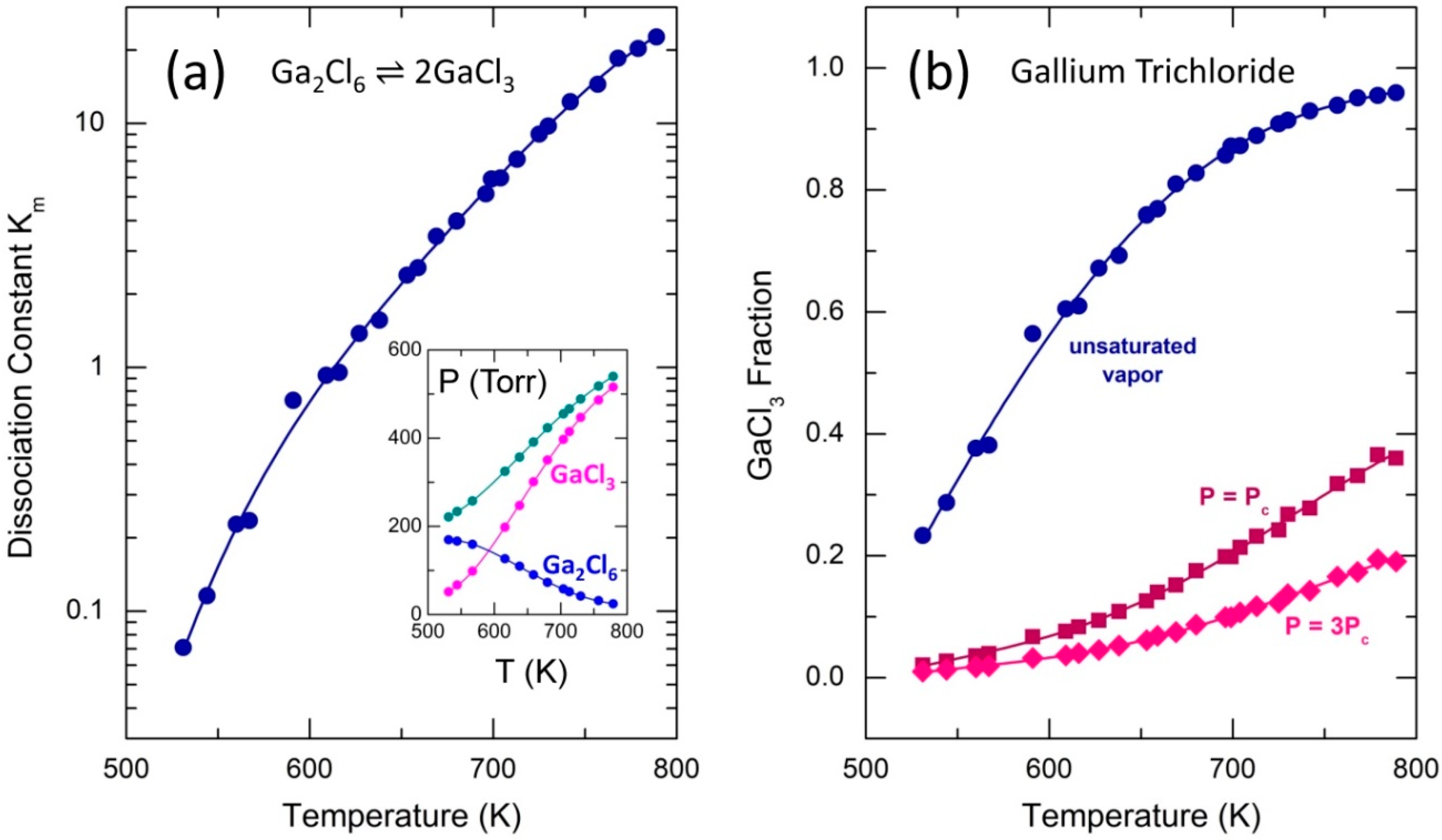
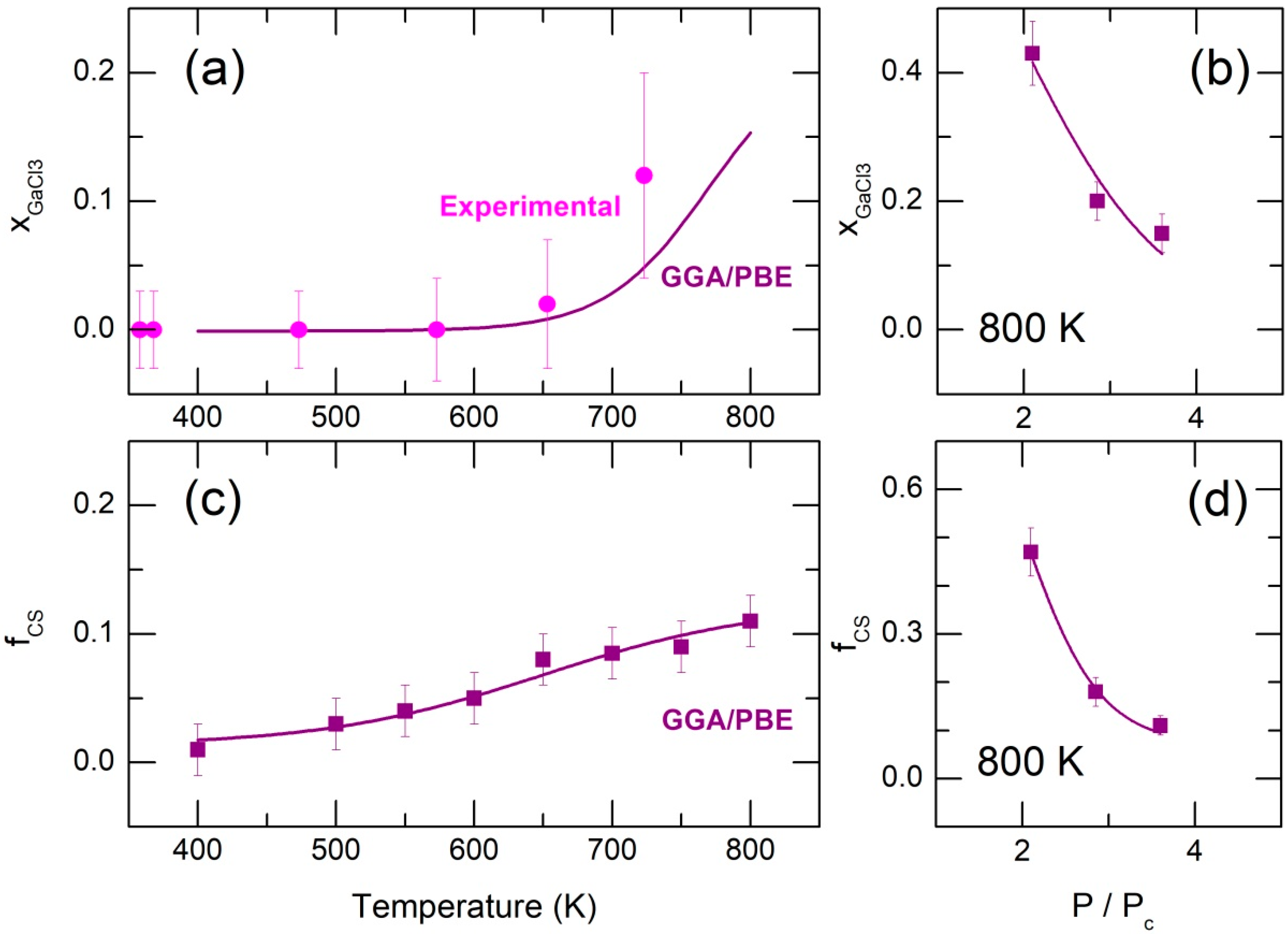
2.2. Dimer Dissociation in Supercritical Fluid
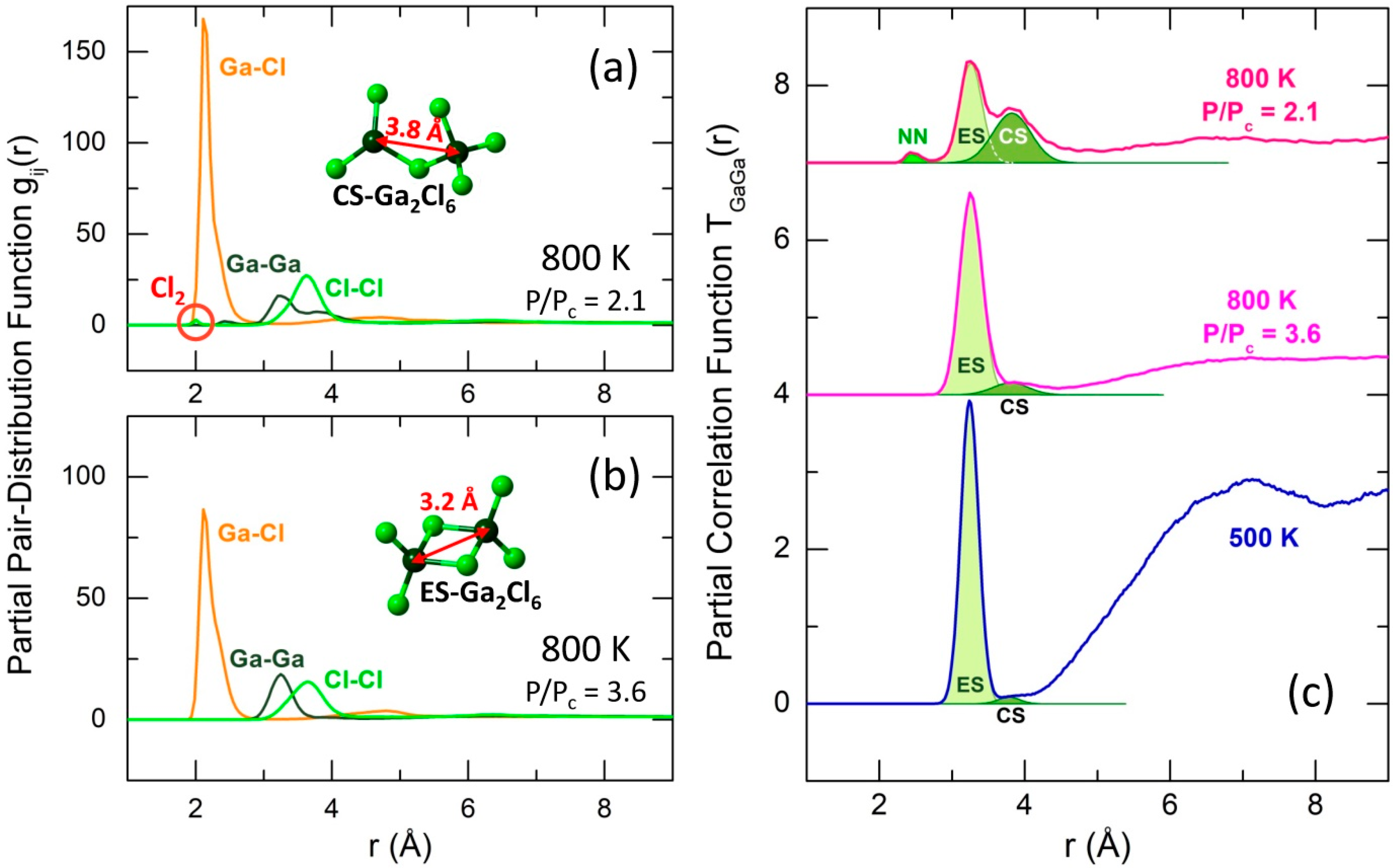
2.3. Local Geometry of Tetrahedral and Trigonal Units
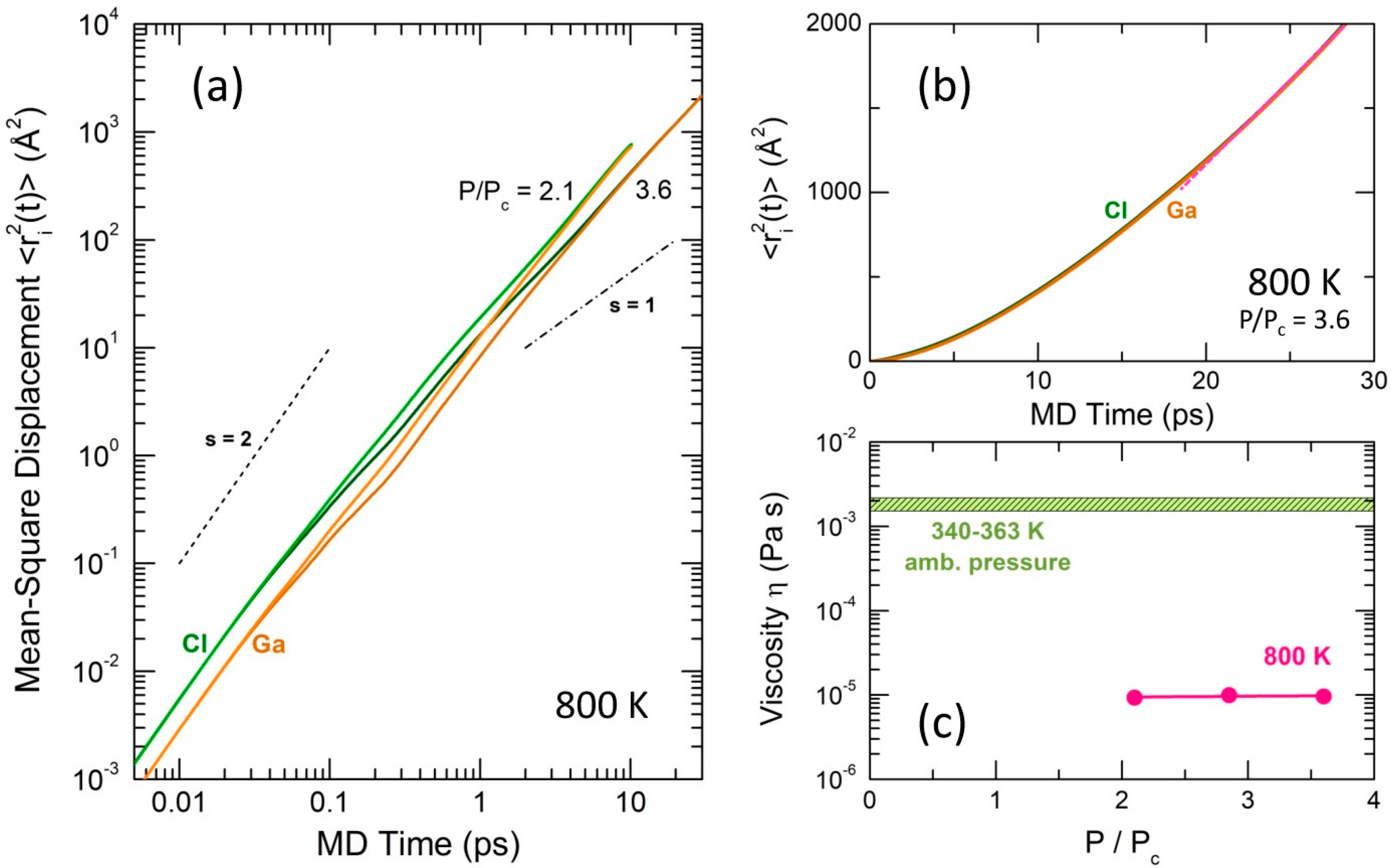
2.4. Dynamics in Supercritical Fluid
3. Simulation Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fetrow, T.V.; Cashman, B.K.; Carpenter, S.H.; Janicke, M.T.; Anderson, N.H.; Klamm, B.E.; Mason, H.E.; Tondreau, A.M. Oxidative Dissolution of Lanthanide Metals Ce and Ho in Molten GaCl3. Inorg. Chem. 2023. [Google Scholar] [CrossRef]
- Carpenter, S.H.; Klamm, B.E.; Fetrow, T.V.; Scott, B.L.; Gaunt, A.J.; Anderson, N.H.; Tondreau, A.M. Chlorination of Pu and U Metal Using GaCl3. Inorg. Chem. 2023, 62, 8462–8466. [Google Scholar] [CrossRef]
- Braunschweig, H.; Gruss, K.; Radacki, K. Reactivity of Pt0 Complexes toward Gallium(III) Halides: Synthesis of a Platinum Gallane Complex and Oxidative Addition of Gallium Halides to Pt0. Inorg. Chem. 2008, 47, 8595–8597. [Google Scholar] [CrossRef]
- Freudenmann, D.; Feldmann, C. [Bi3GaS5]2[Ga3Cl10]2[GaCl4]2·S8 Containing Heterocubane-Type [Bi3GaS5]2+, Star-Shaped [Ga3Cl10]−, Monomeric [GaCl4]− and Crown-Like S8. Dalton Trans. 2011, 40, 452–456. [Google Scholar] [CrossRef]
- Eich, A.; Schnakenburg, G.; Beck, J. Ga2SbCl7O − A Molecular Gallium Antimony Chloride Oxide Synthesized from a GaCl3 Melt. Z. Anorg. Allg. Chem. 2014, 640, 2431–2434. [Google Scholar] [CrossRef]
- Eich, A.; Hoffbauer, W.; Schnakenburg, G.; Bredow, T.; Daniels, J.; Beck, J. Double-Cube-Shaped Mixed Chalcogen/Pentele Clusters from GaCl3 Melts. Eur. J. Inorg. Chem. 2014, 2014, 3043–3052. [Google Scholar] [CrossRef]
- Li, X.; Binnemans, K. Oxidative Dissolution of Metals in Organic Solvents. Chem. Rev. 2021, 121, 4506–4530. [Google Scholar] [CrossRef]
- Chusova, T.P.; Zelenina, L.N.; Yu, G.; Stenin, Y.G.; Semenova, Z.I.; Titov, V.A. Thermodynamics of Vaporization of Gallium Trichloride. Rus. Chem. Bull. 2007, 56, 1313–1317. [Google Scholar] [CrossRef]
- Brunetti, B.; Piacente, V.; Scardala, P. Vapor Pressures of Gallium Trifluoride, Trichloride, and Triiodide and Their Standard Sublimation Enthalpies. J. Chem. Eng. Data 2010, 55, 98–102. [Google Scholar] [CrossRef]
- Morachevskii, A.G.; Sladkov, I.B. Physico-Chemical Properties of Molecular Inorganic Compounds: Experimental Data and Calculation Methods; Khimiya: St. Petersburg, FL, USA, 1996; p. 109. [Google Scholar]
- Haaland, A.; Hammel, A.; Martinsen, K.-G.; Tremmel, J.; Volden, H.V. Molecular Structures of Monomeric Gallium Trichloride, Indium Trichloride and Lead Tetrachloride by Gas Electron Diffraction. J. Chem. Soc. Dalton Trans. 1992, 2209–2214. [Google Scholar] [CrossRef]
- Petrov, V.M.; Giricheva, N.I.; Girichev, G.V.; Titov, V.A.; Chusova, T.P. Electron Diffraction Study of Saturated Vapor of Gallium Trichloride: Vapor Composition and Structure of Molecular Species. J. Struct. Chem. 1992, 32, 498–502. [Google Scholar] [CrossRef]
- Salyulev, A.B.; Zakiryanova, I.D. Raman Spectra of Solid, Molten, and Gaseous Gallium Trichloride. Russ. Metall. 2010, 2010, 108–111. [Google Scholar] [CrossRef]
- Usuki, T.; Bokova, M.; Kassem, M.; Ohara, K.; Hannon, A.C.; Bychkov, E. Dimeric Molecular Structure of Molten Gallium Trichloride and a Hidden Evolution toward a Possible Liquid–Liquid Transition. J. Phys. Chem. B 2019, 123, 10260–10266. [Google Scholar] [CrossRef]
- Tsirelnikov, V.I.; Lokshin, B.V.; Melnikov, P.; Nascimento, V.A. On the Existence of the Trimer of Gallium Trichloride in the Gaseous Phase. Z. Anorg. Allg. Chem. 2012, 638, 2335–2339. [Google Scholar] [CrossRef]
- Fischer, W.; Jübermann, O. Über thermische Eigenschaften von Halogeniden. 10. Dampfdrucke und Dampfdichten von Gallium III—Halogeniden. Z. Anorg. Allg. Chem. 1936, 227, 227–236. [Google Scholar] [CrossRef]
- Hillel, R.; Ait-Hou, A.; Berthet, M.P.; Bouix, J. Le Système Gazeux Gallium-Chlore: Etude par Spectrométrie Raman. J. Raman Spectrosc. 1987, 18, 259–264. [Google Scholar] [CrossRef]
- Bernard, C.; Chatillon, C. Thermodynamics of (Gallium + Chlorine) (g) I. Vapour-Pressure Measurements and Thermodynamic Stability of GaCl(g), GaCl2(g), GaCl3(g), Ga2Cl2(g), Ga2Cl4(g), and Ga2Cl6(g). J. Chem. Thermodynamics 1988, 20, 129–141. [Google Scholar] [CrossRef]
- Chau, P.-L.; Hardwick, A.J. A New Order Parameter for Tetrahedral Configurations. Mol. Phys. 1998, 93, 511–518. [Google Scholar] [CrossRef]
- Errington, J.R.; Debenedetti, P.G. Relationship between Structural Order and the Anomalies of Liquid Water. Nature 2001, 409, 318–321. [Google Scholar] [CrossRef]
- Caravati, S.; Bernasconi, M.; Kühne, T.D.; Krack, M.; Parrinello, M. Coexistence of Tetrahedral- and Octahedral-like Sites in Amorphous Phase Change Materials. Appl. Phys. Lett. 2007, 91, 171906. [Google Scholar] [CrossRef]
- Tverjanovich, A.; Khomenko, M.; Benmore, C.J.; Bokova, M.; Sokolov, A.; Fontanari, D.; Kassem, M.; Usuki, T.; Bychkov, E. Bulk Glassy GeTe2: A Missing Member of the tetrahedral GeX2 Family and a Precursor for the Next Generation of Phase-Change Materials. Chem. Mater. 2021, 33, 1031–1045. [Google Scholar] [CrossRef]
- Visintin, P.M.; Bessel, C.A.; White, P.S.; Schauer, C.K.; DeSimone, J.M. Oxidative Dissolution of Copper and Zinc Metal in Carbon Dioxide with Tert-Butyl Peracetate and a β-Diketone Chelating Agent. Inorg. Chem. 2005, 44, 316–324. [Google Scholar] [CrossRef]
- Huang, R.; Chavez, I.; Taute, K.M.; Lukić, B.; Jeney, S.; Raizen, M.G.; Florin, E.-L. Direct Observation of the Full Transition from Ballistic to Diffusive Brownian Motion in a Liquid. Nat. Phys. 2011, 7, 576–580. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Wade, K. Some Physical Properties of Molten and Supercooled Gallium Trichloride. J. Inorg. Nucl. Chem. 1957, 3, 349–356. [Google Scholar] [CrossRef]
- Watson, J.T.R.; Basu, R.S.; Sengers, J.V. An Improved Representative Equation for the Dynamic Viscosity of Water Substance. J. Phys. Chem. Ref. Data 1980, 9, 1255–1290. [Google Scholar] [CrossRef]
- Dalin, G.A.; West, J.R. The Viscosity of Sulfur Vapor. J. Phys. Chem. 1950, 54, 1215–1221. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Jacobsen, R.T. Viscosity and Thermal Conductivity Equations for Nitrogen, Oxygen, Argon, and Air. Intern. J. Thermophys. 2004, 25, 21–69. [Google Scholar] [CrossRef]
- Dhanuskodi, R.; Arunagiri, A.; Anantharaman, N. Analysis of Variation in Properties and its Impact on Heat Transfer in Sub and Supercritical Conditions of Water/Steam. Intern. J. Chem. Eng. Appl. 2011, 2, 320–325. [Google Scholar]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An Electronic Structure and Molecular Dynamics Software Package—Quickstep: Efficient and Accurate Electronic Structure Calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Soper, A.K. Partial Structure Factors from Disordered Materials Diffraction Data: An Approach using Empirical Potential Structure Refinement. Phys. Rev. B 2005, 72, 104204. [Google Scholar] [CrossRef]
- Soper, A.K. Computer Simulation as a Tool for the Interpretation of Total Scattering Data from Glasses and Liquids. Mol. Simul. 2012, 38, 1171–1185. [Google Scholar] [CrossRef]
- Goedecker, S.; Teter, M.; Hutter, J. Separable Dual-Space Gaussian Pseudopotentials. Phys. Rev. B 1996, 54, 1703–1710. [Google Scholar] [CrossRef]
- Nosé, S. A Molecular Dynamics Method for Simulations in the Canonical Ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical Dynamics: Equilibrium Phase-Space Distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, S.; Jund, P. Ring Statistics Analysis of Topological Networks: New Approach and Application to Amorphous GeS2 and SiO2 Systems. Comput. Mater. Sci. 2010, 49, 70–83. [Google Scholar] [CrossRef]
- Kohara, S.; Ohno, H.; Tabaka, M.; Usuki, T.; Morita, H.; Suzuya, K.; Akola, J.; Pusztai, L. Lead Silicate Glasses: Binary Network-Former Glasses with Large Amounts of Free Volume. Phys. Rev. B 2010, 82, 134209. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khomenko, M.; Sokolov, A.; Tverjanovich, A.; Bokova, M.; Kassem, M.; Usuki, T.; Bychkov, E. Gallium Trichloride Fluid: Dimer Dissociation Mechanism, Local Structure, and Atomic Dynamics. Molecules 2024, 29, 1358. https://doi.org/10.3390/molecules29061358
Khomenko M, Sokolov A, Tverjanovich A, Bokova M, Kassem M, Usuki T, Bychkov E. Gallium Trichloride Fluid: Dimer Dissociation Mechanism, Local Structure, and Atomic Dynamics. Molecules. 2024; 29(6):1358. https://doi.org/10.3390/molecules29061358
Chicago/Turabian StyleKhomenko, Maxim, Anton Sokolov, Andrey Tverjanovich, Maria Bokova, Mohammad Kassem, Takeshi Usuki, and Eugene Bychkov. 2024. "Gallium Trichloride Fluid: Dimer Dissociation Mechanism, Local Structure, and Atomic Dynamics" Molecules 29, no. 6: 1358. https://doi.org/10.3390/molecules29061358
APA StyleKhomenko, M., Sokolov, A., Tverjanovich, A., Bokova, M., Kassem, M., Usuki, T., & Bychkov, E. (2024). Gallium Trichloride Fluid: Dimer Dissociation Mechanism, Local Structure, and Atomic Dynamics. Molecules, 29(6), 1358. https://doi.org/10.3390/molecules29061358







