CO2/CH4 Separation in Amino Acid Ionic Liquids, Polymerized Ionic Liquids, and Mixed Matrix Membranes
Abstract
1. Introduction
2. Results
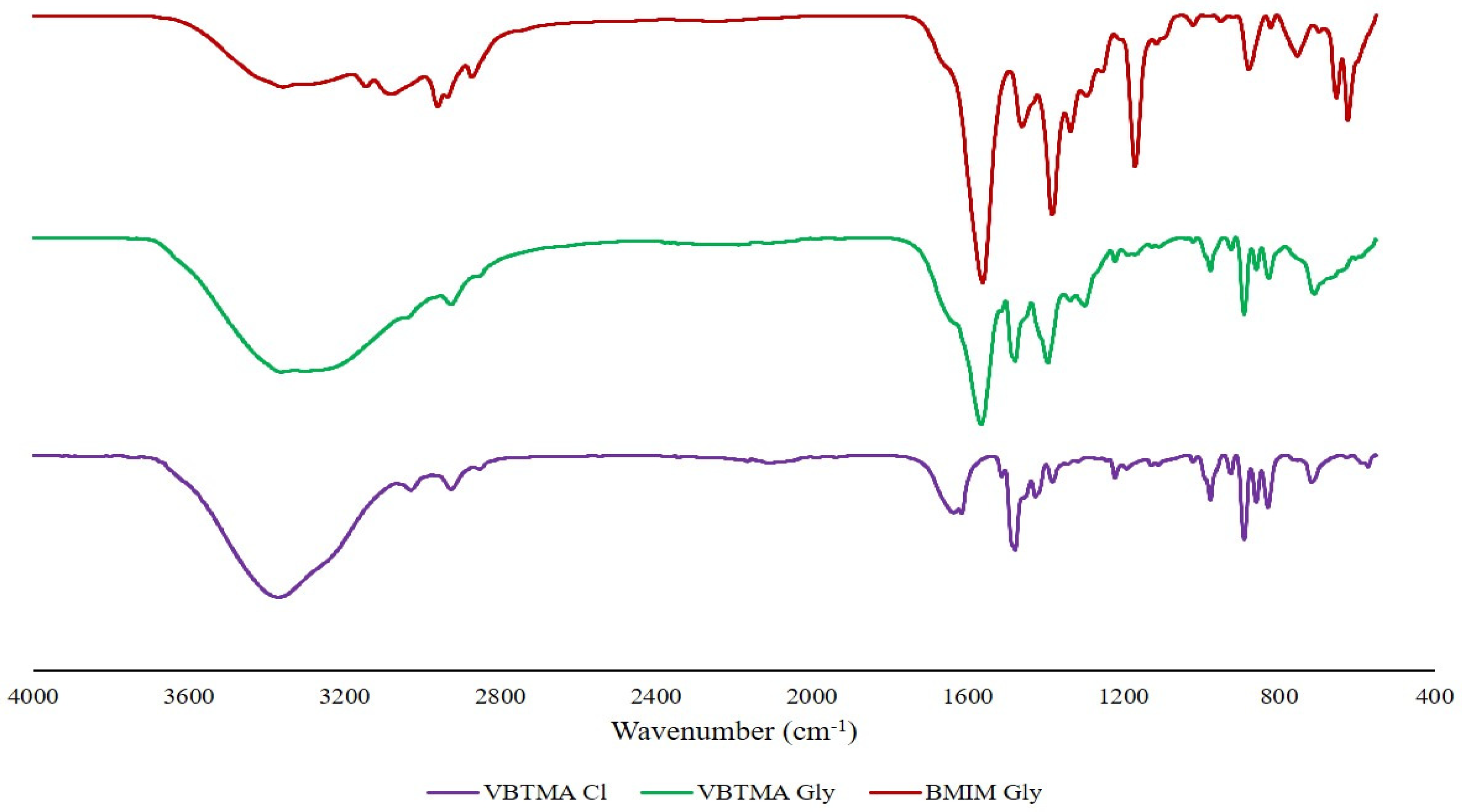
2.1. Characterization of [VBTMA][Gly] and [BMIM][Gly] Ionic Liquids
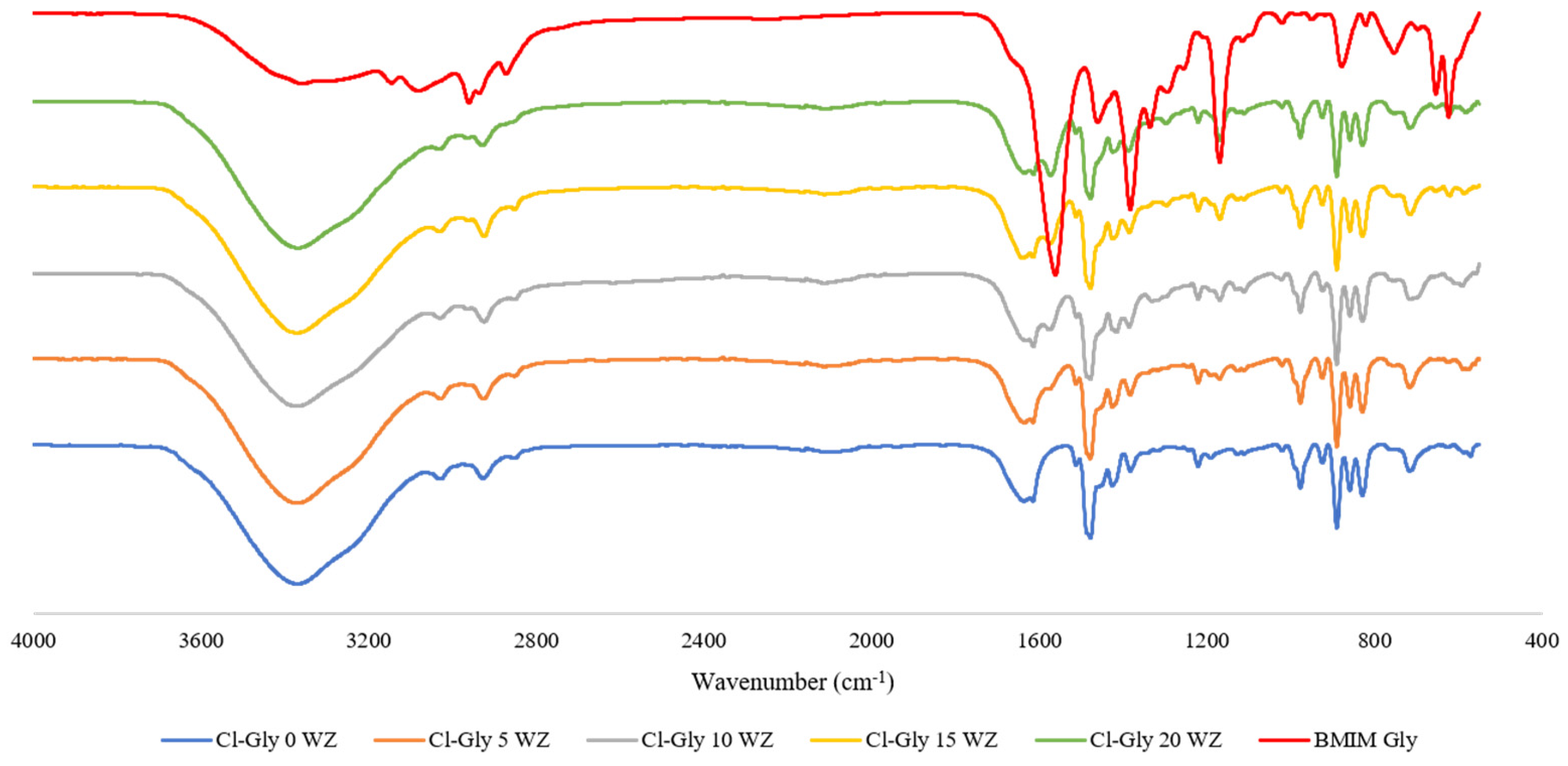
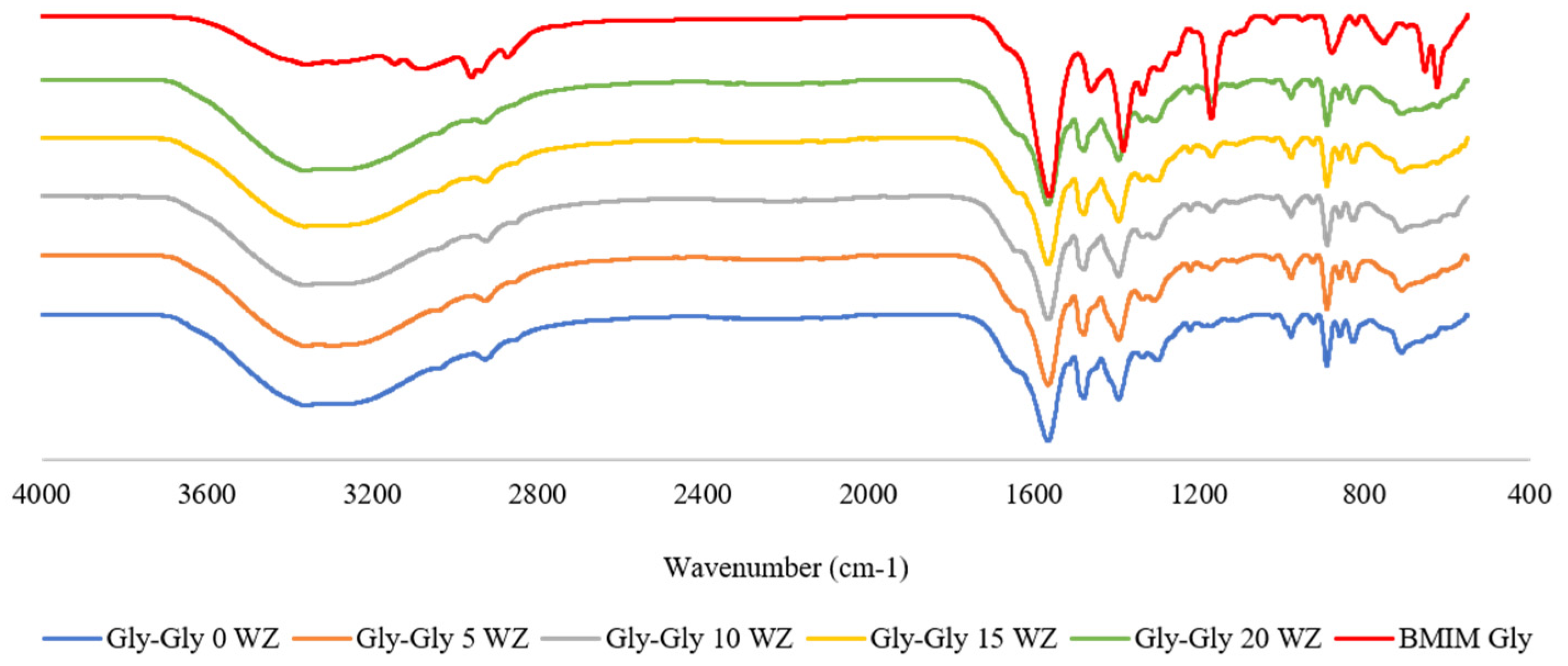
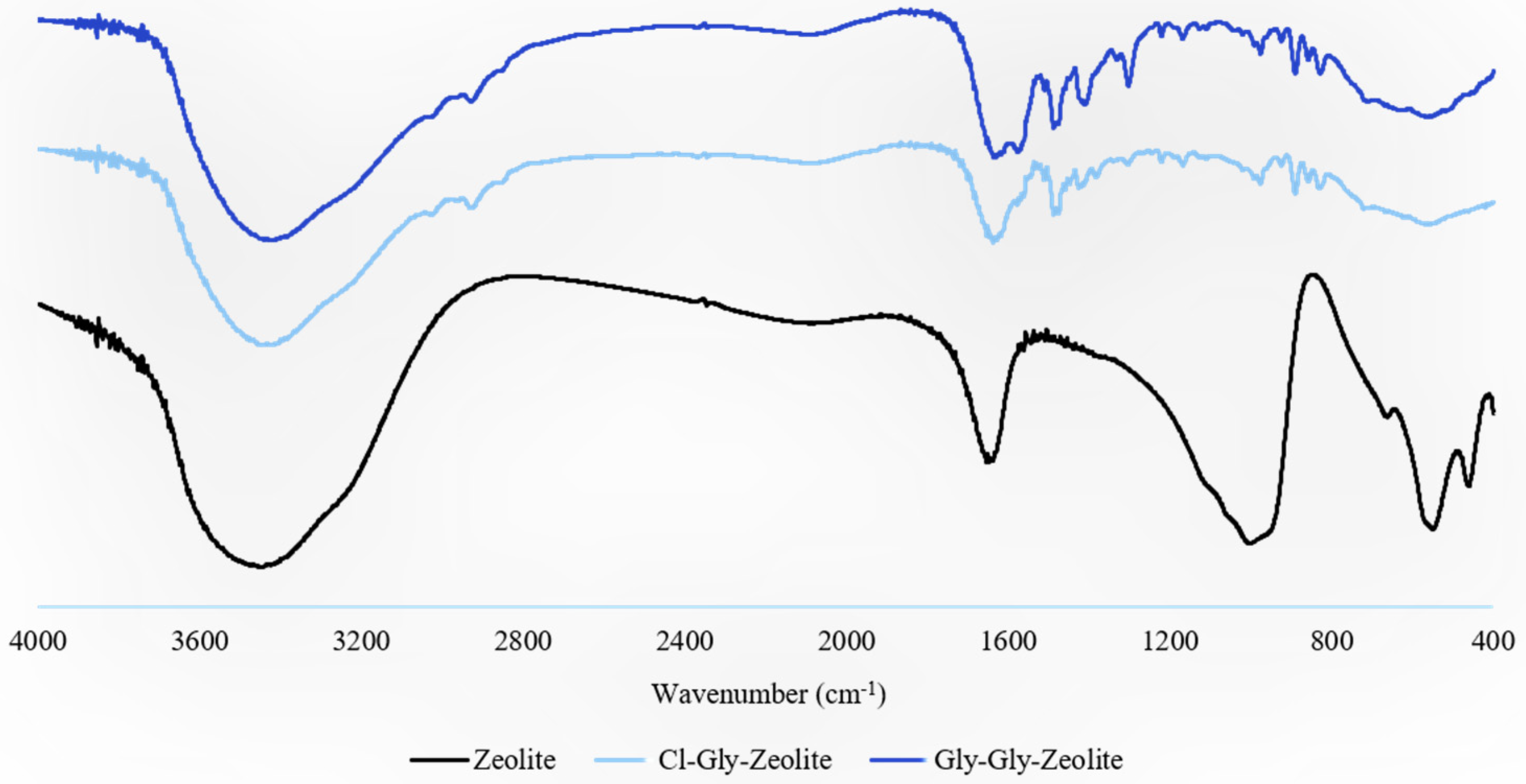
2.2. Characterization of Mixed Matrix Membranes
2.2.1. FTIR Analyses
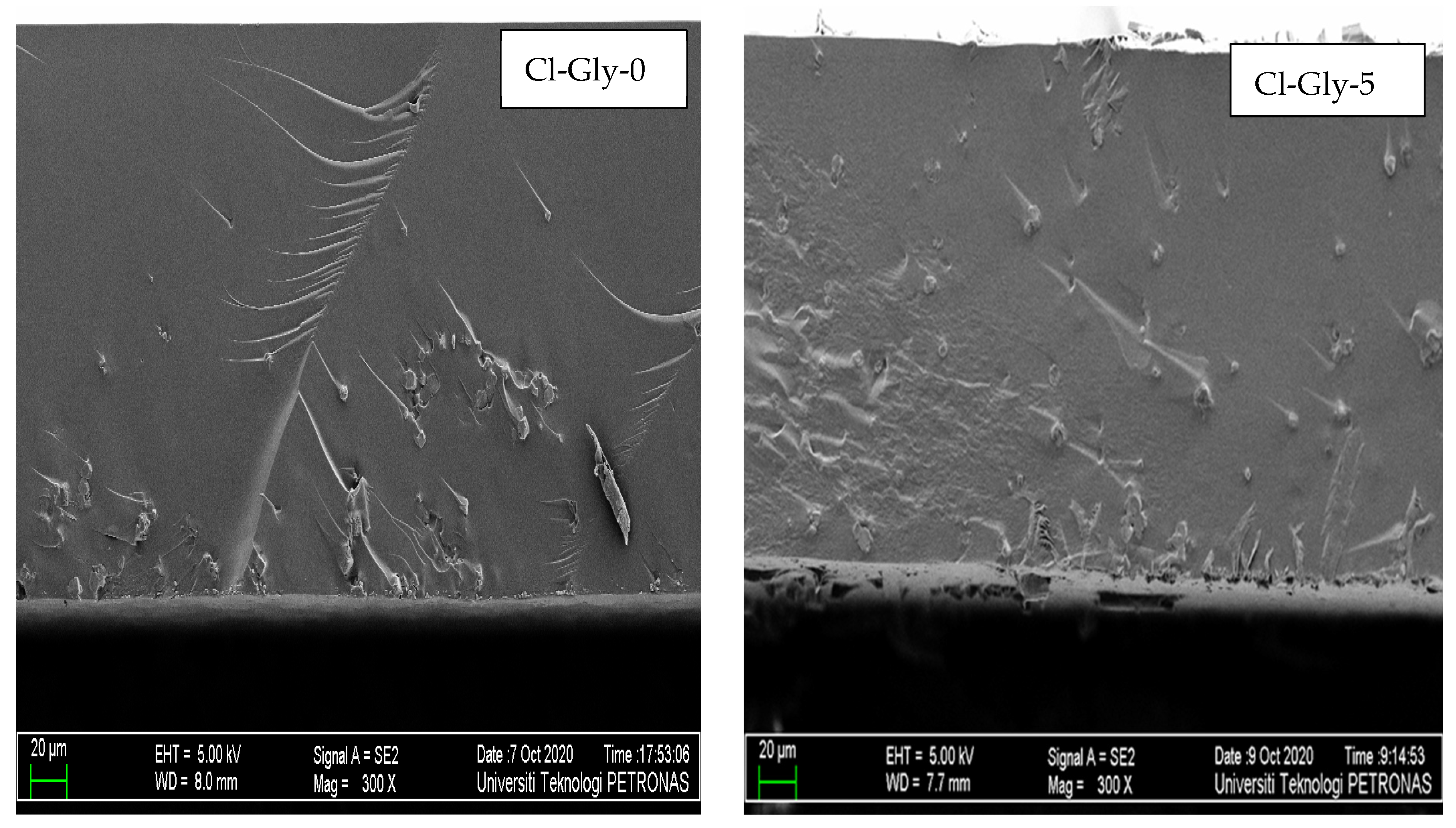
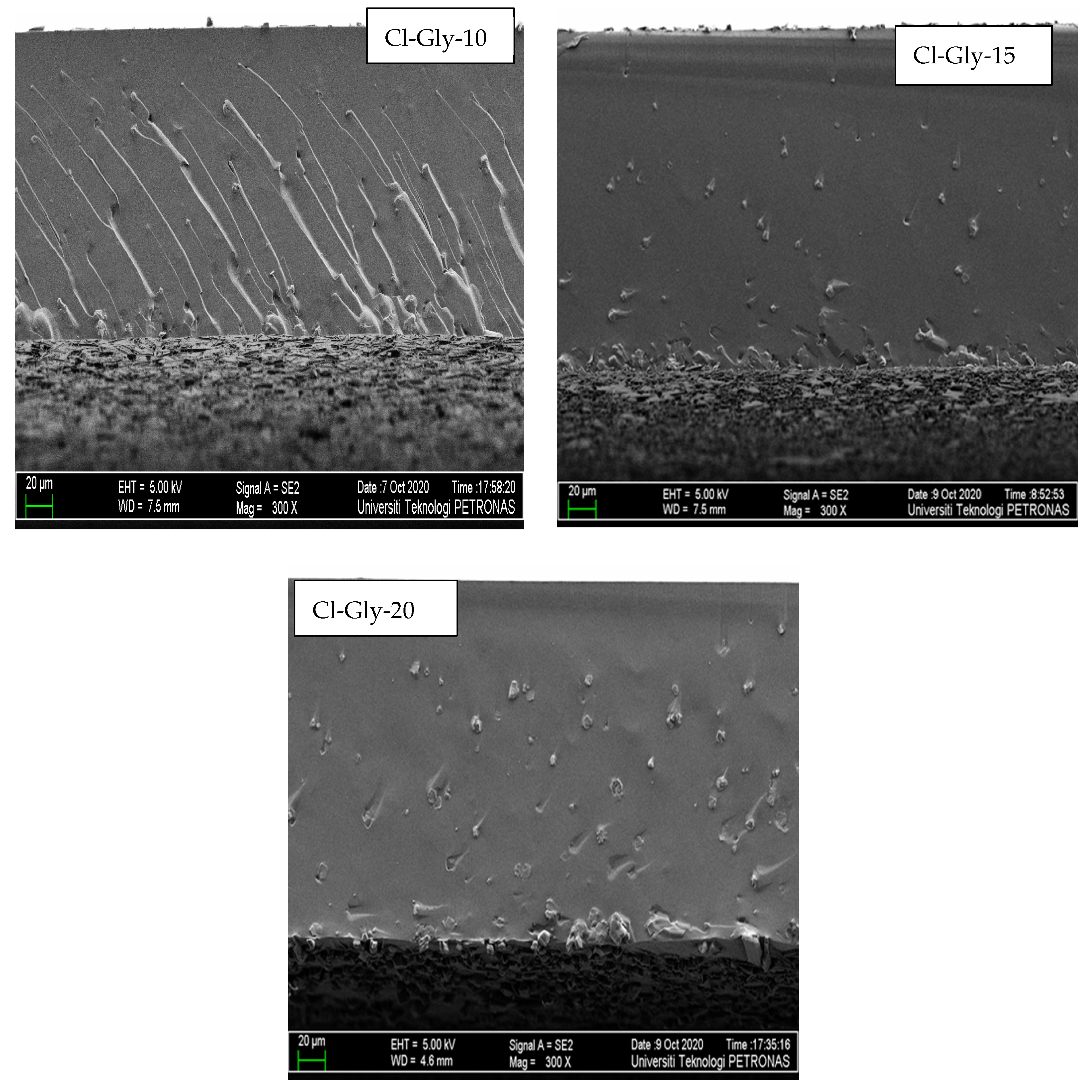
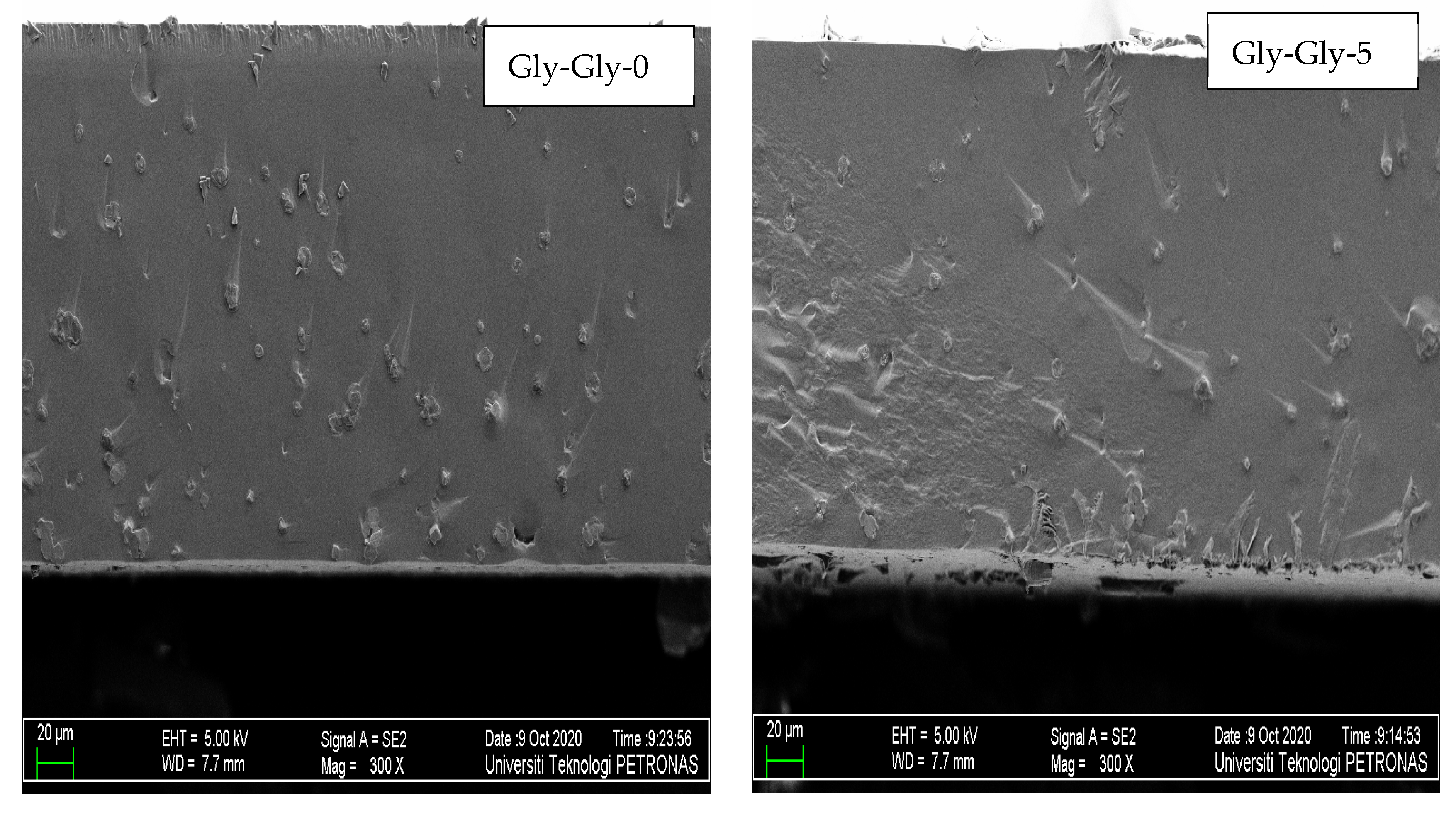
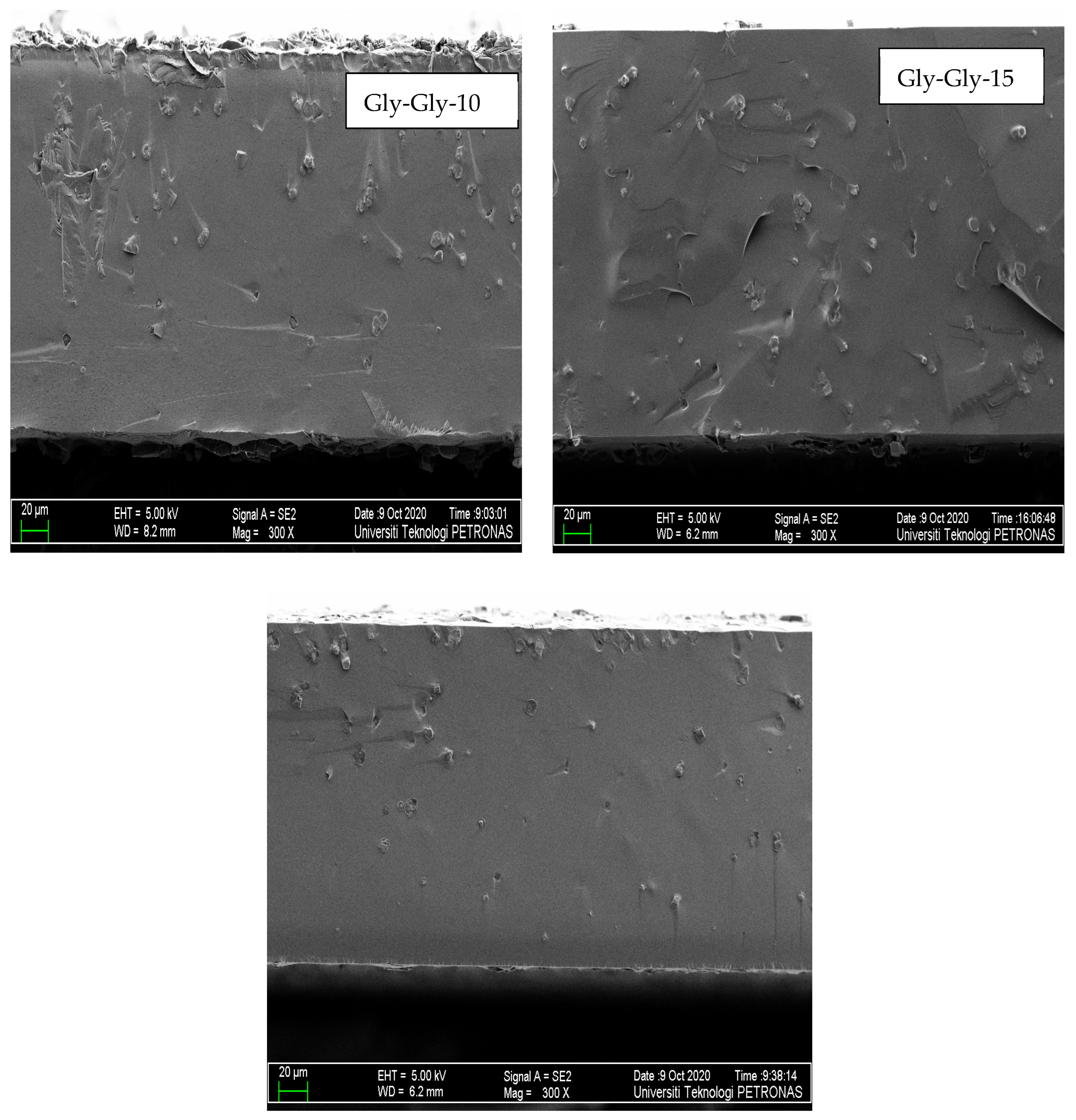
2.2.2. Morphology Studies of MMMs
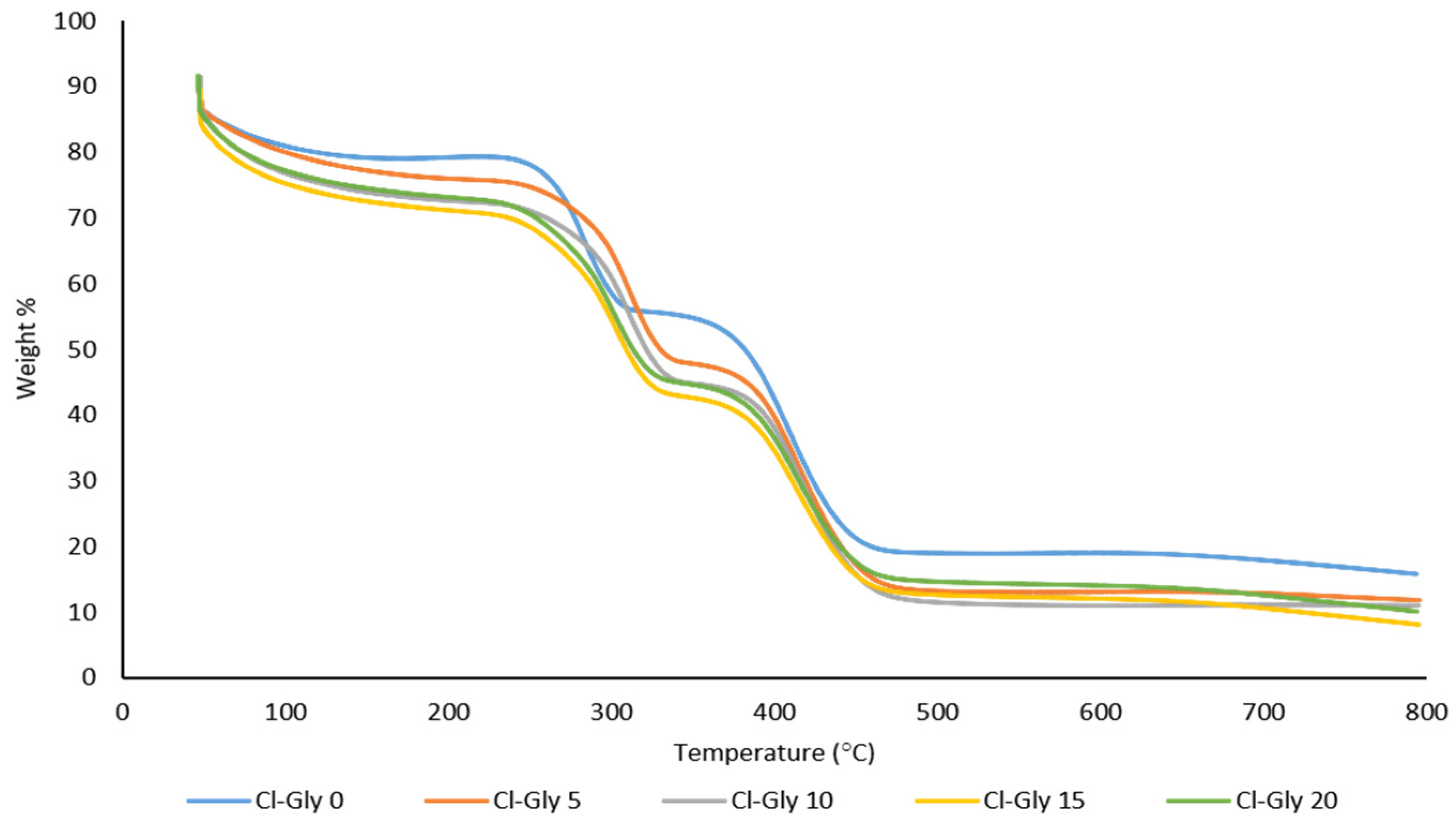
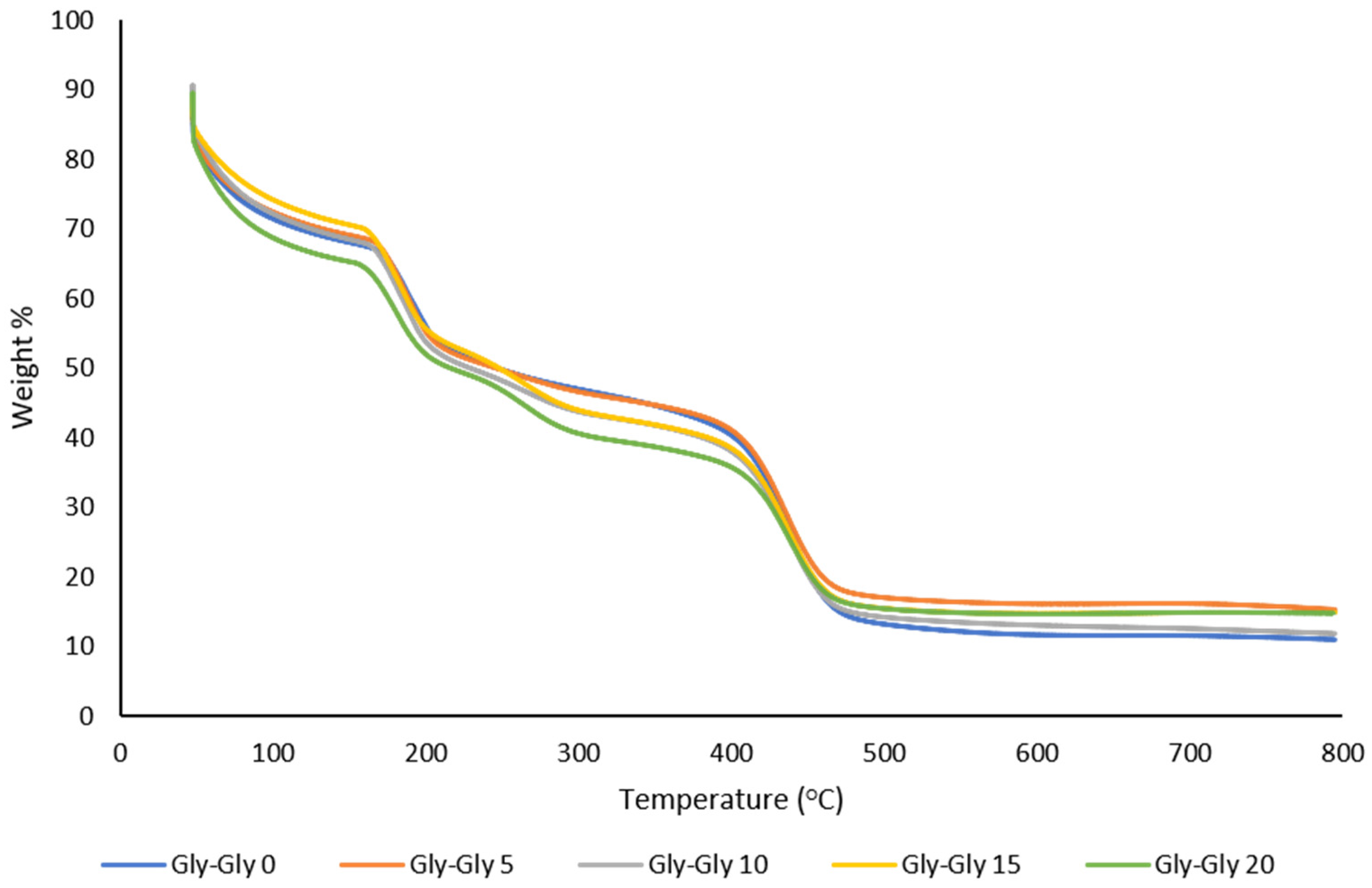
2.2.3. Thermal Analysis of MMMs
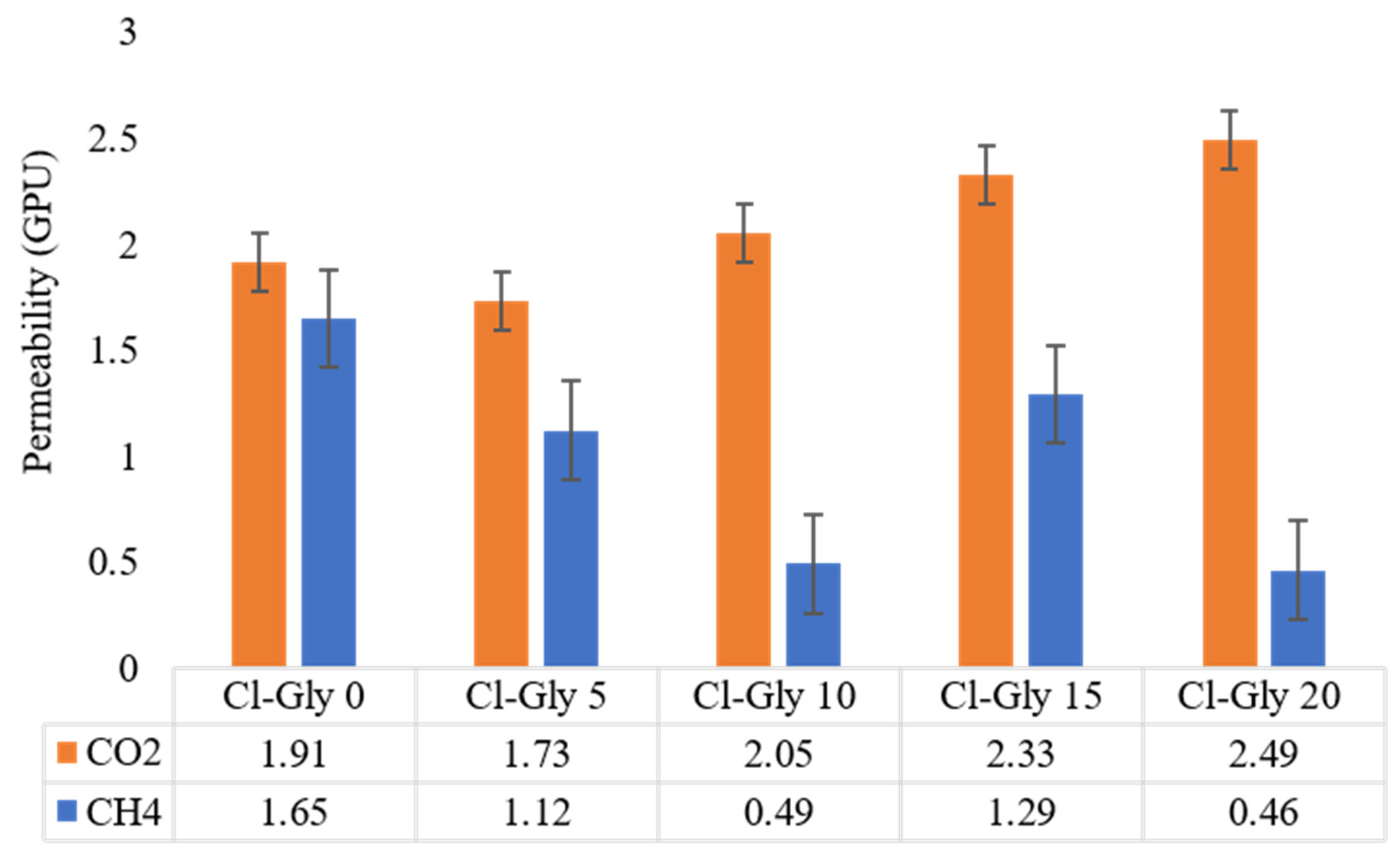
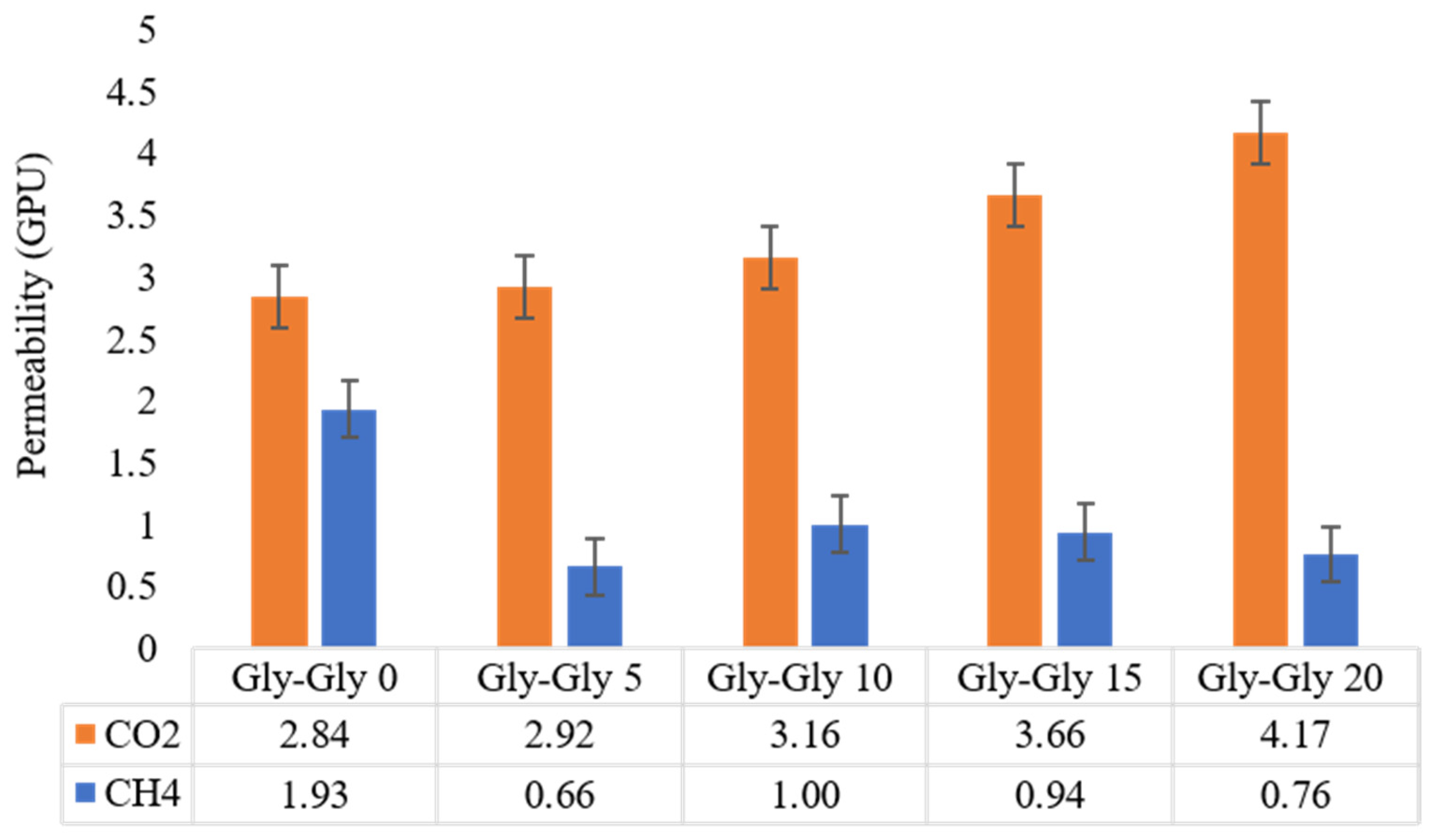
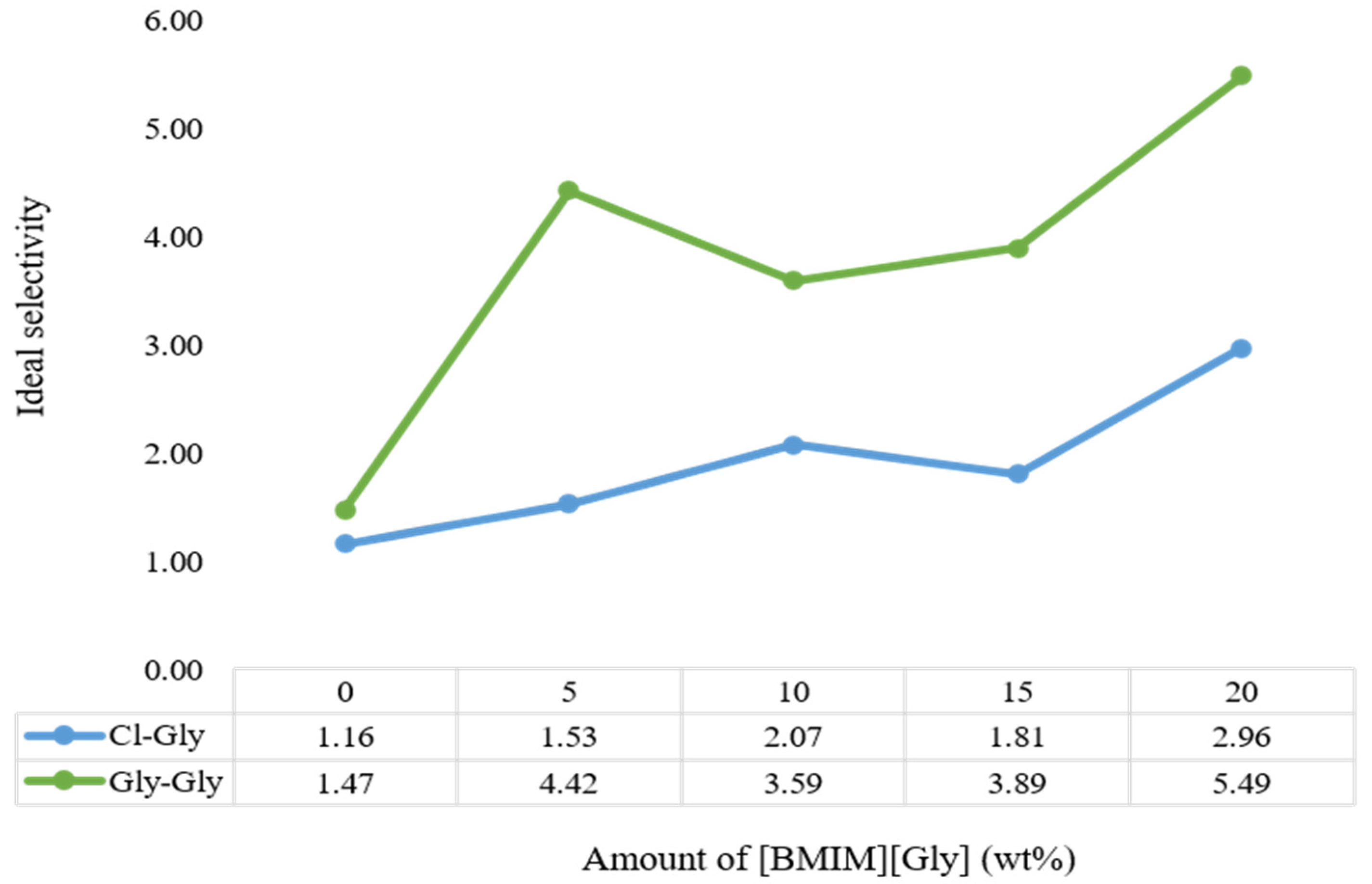
2.3. CO2 Permeability and CO2/CH4 Selectivity Studies
3. Materials and Methods
3.1. Materials
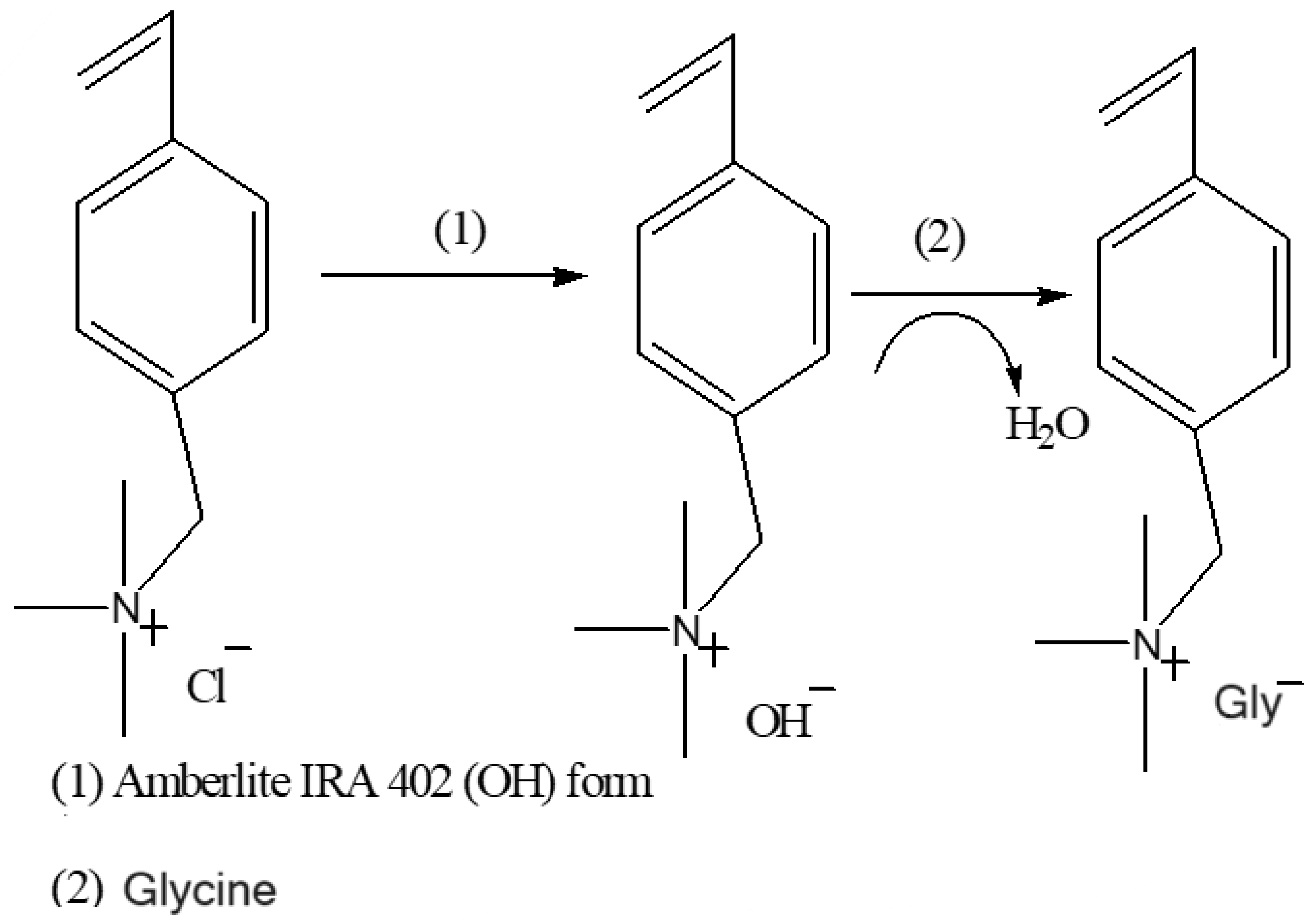
3.2. Synthesis of [VBTMA][Gly] and [BMIM][Gly] Ionic Liquids
3.2.1. Anion Exchange from Cl− to OH−
3.2.2. Neutralization Using Amino Acids
3.2.3. Characterization of [VBTMA][Gly] and [BMIM][Gly]
3.3. Fabrication of Mixed Matrix Membranes through Photopolymerization
3.4. Characterization of Mixed Matrix Membranes
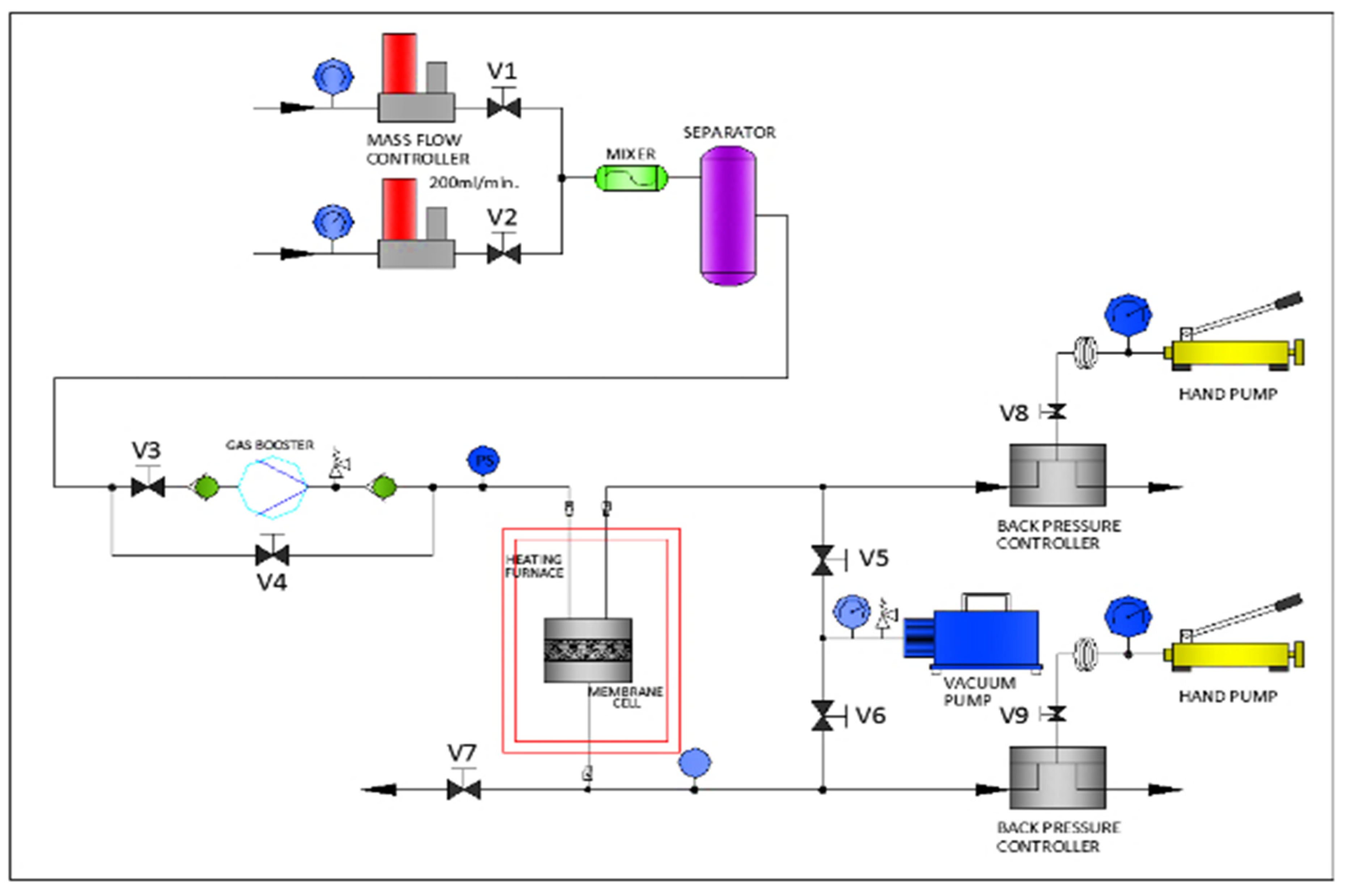
3.5. CO2/CH4 Permeability and Selectivity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, Y.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, S.; Yao, X.; Zhang, J.; Dong, K.; Dai, W.; Mori, R. Design of task-specific ionic liquids for capturing CO2: A molecular orbital study. Ind. Eng. Chem. Res. 2006, 45, 2875–2880. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennel, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Ali, A.; Pothu, R.; Siyal, S.H.; Phulpoto, S.; Sajjad, M.; Thebo, K.H. Graphene-based membranes for CO2 separation. Mater. Sci. Energy Technol. 2019, 2, 83–88. [Google Scholar] [CrossRef]
- Duan, Y.; Li, L.; Shen, Z.; Cheng, J.; He, K. Engineering Metal-Organic-Framework (MOF)-Based Membranes for Gas and Liquid Separation. Membranes 2023, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, H.; Jia, H.; Xu, J.; Ma, L.; Zang, Y.; Jiang, P.; Ma, W.; Zhang, Y.; Zhao, W.; et al. Preparation and High Performance of Cellulose Acetate Films by Grafting with Imidazole Ionic Liquid. ACS Omega 2021, 6, 12500–12506. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, M.; Fong, Y.Y.; Leng, C.T.; Keong, L.K. Enhanced Gases Separation of Cellulose Acetate Membrane Using N-Methyl-1-2 Pyrrolidone as Fabrication Solvent. Int. J. Automot. Mech. Eng. 2018, 15, 4978–4986. [Google Scholar] [CrossRef]
- George, G.; Bhoria, N.; AlHallaq, S.; Abdala, A.; Mittal, V. Polymer membranes for acid gas removal from natural gas. Sep. Purif. Technol. 2016, 158, 333–356. [Google Scholar] [CrossRef]
- Rehman, A.; Jahan, Z.; Sher, F.; Noor, T.; Niazi, M.B.K.; Akram, M.A.; Sher, E.K. Cellulose acetate based sustainable nanostructured membranes for environmental remediation. Chemosphere 2022, 307, 135736. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Ohno, H.; Fukumoto, K. Amino acid ionic liquids. Acc. Chem. Res. 2007, 40, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Dong, K.; Zhang, Y.; Shen, Y.; Lv, X. Supported absorption of CO2 by tetrabutylphosphonium amino acid ionic liquids. Chem. Eur. J. 2006, 12, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, B.E.; de la Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 absorption by anion functionalized ionic liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef]
- Bratton, A.F.; Kim, S.-S.; Ellison, C.J.; Miller, K.M. Thermomechanical and Conductive Properties of Thiol–Ene Poly(ionic liquid) Networks Containing Backbone and Pendant Imidazolium Groups. Ind. Eng. Chem. Res. 2018, 57, 16526–16536. [Google Scholar] [CrossRef]
- Blasig, A.; Tang, J.; Hu, X.; Shen, Y.; Radosz, M. Magnetic suspension balance study of carbon dioxide solubility in ammonium-based polymerized ionic liquids: Poly(p-vinylbenzyltrimethyl ammonium tetrafluoroborate) and poly([2-(methacryloyloxy)ethyl] trimethyl ammonium tetrafluoroborate). Fluid Phase Equilibria 2007, 256, 75–80. [Google Scholar] [CrossRef]
- Zajac, A.; Szpecht, A.; Zielinski, D.; Rola, K.; Hoppe, J.; Komorowska, K.; Smiglak, M. Synthesis and characterization of potentially polymerizable amine-derived ionic liquids bearing 4-vinylbenzyl group. J. Mol. Liq. 2019, 283, 427–439. [Google Scholar] [CrossRef]
- Shahrom, M.S.R. Studies of Novel Amino Acid Polymerized Ionic Liquids for Carbon Dioxide Capture. Ph.D. Thesis, Universiti Teknologi PETRONAS, Perak, Malaysia, 2017. [Google Scholar]
- Gao, T.; Itliong, J.; Kumar, S.P.; Hjorth, Z.; Nakamura, I. Polarization of ionic liquid and polymer and its implications for polymerized ionic liquids: An overview towards a new theory and simulation. J. Polym. Sci. 2021, 59, 2434–2457. [Google Scholar] [CrossRef]
- Bara, J.E.; Noble, R.D.; Gin, G.L. Effect of “Free” cation substituent on gas separation performance of polymer− room-temperature ionic liquid composite membranes. Ind. Eng. Chem. Res. 2009, 48, 4607–4610. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Bastani, D.N.; Esmaeili, M. Asadollahi, Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Li, S.; Falconer, J.L.; Noble, R.D. Improved SAPO-34 membranes for CO2/CH4 separations. Adv. Mater. 2006, 18, 2601–2603. [Google Scholar] [CrossRef]
- Tai-Shung, C.; Jiang, L.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2008, 32, 483–507. [Google Scholar] [CrossRef]
- Shahrom, M.S.R.; Wilfred, C.D. Synthesis and thermal properties of amino acids ionic liquids (AAILS). J. Appl. Sci. 2014, 14, 1067–1072. [Google Scholar] [CrossRef]
- Abidin, A.Z.; Bakar, N.A.; Ng, E.P.; Tan, W.L. Rapid degradation of methyl orange by Ag doped zeolite X in the presence of borohydride. J. Taibah Univ. Sci. 2017, 11, 1070–1079. [Google Scholar] [CrossRef]
- Colar, L.A.; Jakab, A.; Manea, F.; Pode, R.; Orha, C. Photocatalytic performance of Ag-modified natural zeolite catalyst for photocatalysis degradation of Methylene Blue (MB) under VIS irradiation. In Handbook of Water Pollution XI; Brebbia, C.A., Ed.; Wessex Institute of Technology: Southampton, UK, 2014; Volume 164, p. 335. [Google Scholar]
- Ng, E.P.; Delmotte, L.; Mintova, S. Selective capture of water using microporous adsorbents to increase the lifetime of lubricants. ChemSusChem 2009, 2, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Kusworo, T.D.; Mustafa, A. Enhanced gas permeation performance of polyethersulfone mixed matrix hollow fiber membranes using novel Dynasylan Ameo silane agent. J. Membr. Sci. 2008, 319, 306–312. [Google Scholar] [CrossRef]
- Yong, H.H.; Park, H.C.; Kang, Y.S.; Won, J.; Kim, W.N. Zeolite-filled polyimide membrane containing 2, 4, 6-triaminopyrimidine. J. Membr. Sci. 2001, 188, 151–163. [Google Scholar] [CrossRef]
- Mathew, M.E.; Ahmad, I.; Thomas, S.; Daik, R.; Kassim, M. Synthesis and characterization of poly(benzyl trimethylammonium chloride) ionic polymer. AIP Conf. Proc. 2018, 1940, 020108. [Google Scholar] [CrossRef]
- Guan, W.; Xue, W.F.; Li, N.; Tong, J. Enthalpy of solution of amino acid ionic liquid 1-butyl-3-methylimidazolium glycine. J. Chem. Eng. Data 2008, 53, 1401–1403. [Google Scholar] [CrossRef]
- Sattar, M.A.; Patnaik, A. Role of Interface Structure and Chain Dynamics on the Diverging Glass Transition Behavior of SSBR-SiO2-PIL Elastomers. ACS Omega 2020, 5, 21191–21202. [Google Scholar] [CrossRef] [PubMed]
- Rykl, D.; Pechar, F. Thermal decomposition of the natural zeolite stilbite. Zeolites 1985, 5, 389–392. [Google Scholar] [CrossRef]
- Shahrom, M.S.R.; Wilfred, C.D.; MacFarlane, D.R.; Vijayraghavan, R.; Chong, F.K. Amino acid based poly (ionic liquid) materials for CO2 capture: Effect of anion. J. Mol. Liq. 2019, 279, 644–652. [Google Scholar] [CrossRef]
- Mohshim, D.F.; Mukhtar, H.; Man, Z. Composite blending of ionic liquid–poly (ether sulfone) polymeric membranes: Green materials with potential for carbon dioxide/methane separation. J. Appl. Polym. Sci. 2016, 133, 43999. [Google Scholar] [CrossRef]
- Mohamed, A.A. Synthesis of Polymerized Ionic Liquids for Carbon Dioxide Capture. Ph.D. Thesis, Universiti Teknologi PETRONAS, Perak, Malaysia, 2011. [Google Scholar]
- Airong, X.; Zhang, Y.; Lu, W.; Yao, K.; Wang, J. Transport properties of some 1-butyl-3-methylimidazolium carboxylate ionic liquids. J. Chem. Eng. Data 2015, 60, 580–585. [Google Scholar] [CrossRef]
- Oral, E.E.; Yilmaz, L.; Kalipcilar, H. Effect of gas permeation temperature and annealing procedure on the performance of binary and ternary mixed matrix membranes of polyethersulfone, SAPO-34, and 2-hydroxy 5-methyl aniline. J. Appl. Polym. Sci. 2014, 131, 40679. [Google Scholar] [CrossRef]
- Şen, D.; Kalıpçılar, H.; Yilmaz, L. Development of polycarbonate based zeolite 4A filled mixed matrix gas separation membranes. J. Membr. Sci. 2007, 303, 194–203. [Google Scholar] [CrossRef]
- Klepić, M.; Fuoco, A.; Monteleone, M.; Esposito, E.; Friess, K.; Petrusová, Z.; Izák, P.; Jansen, J.C. Tailoring the Thermal and Mechanical Properties of PolyActiveTM Poly (Ether-Ester) Multiblock Copolymers Via Blending with CO2-Phylic Ionic Liquid. Polymers 2020, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Kusworo, T.D.; Ismail, A.F.; Mustafa, A.; Li, K. The effect of type zeolite on the gas transport properties of polyimide-based mixed matrix membranes. J. REAKTOR 2008, 12, 68–77. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J. Membr. Sscience 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Tong, Z.; Ho, W.S.W. Facilitated transport membranes for CO2 separation and capture. Sep. Sci. Technol. 2017, 52, 156–167. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Leo, C.P.; Ahmad, A.L.; Kamal, S.N.M.; Chew, T.L. Carbon dioxide separation using asymmetric polysulfone mixed matrix membranes incorporated with SAPO-34 zeolite. Fuel Process. Technol. 2018, 118, 125–132. [Google Scholar] [CrossRef]
- Nasir, R.; Ahmad, N.N.R.; Mukhtar, H.; Mohshim, D.F. Effect of ionic liquid inclusion and amino–functionalized SAPO-34 on the performance of mixed matrix membranes for CO2/CH4 separation. J. Environ. Chem. Eng. 2018, 6, 2363–2368. [Google Scholar] [CrossRef]
| Membranes | [VBTMA][Cl] (g) | [VBTMA][Gly] (g) | [BMIM][Gly] (g) | Zeolite (g) |
|---|---|---|---|---|
| Cl-Gly-0 | 2.00 | 0.00 | 0.00 | 0.10 |
| Cl-Gly-5 | 2.00 | 0.00 | 0.10 | 0.10 |
| Cl-Gly-10 | 2.00 | 0.00 | 0.20 | 0.10 |
| Cl-Gly-15 | 2.00 | 0.00 | 0.30 | 0.10 |
| Cl-Gly-20 | 2.00 | 0.00 | 0.40 | 0.10 |
| Gly-Gly-0 | 0.00 | 2.00 | 0.00 | 0.10 |
| Gly-Gly-5 | 0.00 | 2.00 | 0.10 | 0.10 |
| Gly-Gly-10 | 0.00 | 2.00 | 0.20 | 0.10 |
| Gly-Gly-15 | 0.00 | 2.00 | 0.30 | 0.10 |
| Gly-Gly-20 | 0.00 | 2.00 | 0.40 | 0.10 |
| Degradation Temperature, Tg (°C) | Degradation Temperature, Tg (°C) | |
|---|---|---|
| Poly[VBTMA]Cl | 230 | |
| [BMIM]Gly | 210.35 °C | |
| Membranes | ||
| Cl-Gly-0 | 284 | 410 |
| Cl-Gly-5 | 314 | 415 |
| Cl-Gly-10 | 316 | 419 |
| Cl-Gly-15 | 303 | 417 |
| Cl-Gly-20 | 289 | 418 |
| Degradation Temperature, Tg (°C) | Degradation Temperature, Tg (°C) | |
|---|---|---|
| Poly[VBTMA]Gly | 192 | |
| [BMIM]Gly | 210.35 | |
| Membranes | ||
| Gly-Gly-0 | 192 | 439 |
| Gly-Gly-5 | 186 | 436 |
| Gly-Gly-10 | 183 | 437 |
| Gly-Gly-15 | 182 | 440 |
| Gly-Gly-20 | 181C | 437 |
| Membrane | Tg (°C) |
|---|---|
| Cl-Gly-0 | 89.90 |
| Cl-Gly-5 | 89.82 |
| Cl-Gly-10 | 89.28 |
| Cl-Gly-15 | 89.19 |
| Cl-Gly-20 | 90.23 |
| Gly-Gly-0 | 89.44 |
| Gly-Gly-5 | 88.41 |
| Gly-Gly-10 | 89.15 |
| Gly-Gly-15 | 89.34 |
| Gly-Gly-20 | 87.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvaraj, G.; Wilfred, C.D. CO2/CH4 Separation in Amino Acid Ionic Liquids, Polymerized Ionic Liquids, and Mixed Matrix Membranes. Molecules 2024, 29, 1357. https://doi.org/10.3390/molecules29061357
Selvaraj G, Wilfred CD. CO2/CH4 Separation in Amino Acid Ionic Liquids, Polymerized Ionic Liquids, and Mixed Matrix Membranes. Molecules. 2024; 29(6):1357. https://doi.org/10.3390/molecules29061357
Chicago/Turabian StyleSelvaraj, Gowri, and Cecilia Devi Wilfred. 2024. "CO2/CH4 Separation in Amino Acid Ionic Liquids, Polymerized Ionic Liquids, and Mixed Matrix Membranes" Molecules 29, no. 6: 1357. https://doi.org/10.3390/molecules29061357
APA StyleSelvaraj, G., & Wilfred, C. D. (2024). CO2/CH4 Separation in Amino Acid Ionic Liquids, Polymerized Ionic Liquids, and Mixed Matrix Membranes. Molecules, 29(6), 1357. https://doi.org/10.3390/molecules29061357






