Comprehensive Review on Chiral Stationary Phases in Single-Column Simultaneous Chiral–Achiral HPLC Separation Methods
Abstract
1. Introduction
2. Enantio- and Chemoselective Methods
3. Discussion
3.1. Different CSP Types and Their Applications in Simultaneous Chiral–Achiral Separations
3.2. Chemoselective Separation Mechanisms Involved in Achiral Separations on CSPs
3.3. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | cyclodextrin |
| CE | capillary electrophoresis |
| CS | chiral selector |
| CSP | chiral stationary phase |
| EMA | European Medical Agency |
| ESI | electrospray ionization |
| Eur Ph | European Pharmacopoeia |
| FDA | Food and Drug Administration |
| HILIC | hydrophilic interaction chromatography |
| HPLC | high-performance liquid chromatography |
| I.S | internal standard |
| LLE | liquid–liquid extraction |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MRM | multiple reaction monitoring |
| MS | mass spectrometry |
| NP | normal phase |
| PI | polar ionic |
| PO | polar organic |
| PPI | proton pump inhibitor |
| RP | reverse phase |
| SPE | solid phase extraction |
| SSRI | selective serotonin reuptake inhibitor |
| USP | United States Pharmacopoeia |
References
- Agranat, I.; Caner, H.; Caldwell, J. Putting chirality to work: The strategy of chiral switches. Nat. Rev. Drug Discov. 2002, 1, 753–768. [Google Scholar] [CrossRef]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Hancu, G.; Modroiu, A. Chiral Switch: Between therapeutical benefit and marketing strategy. Pharmaceuticals 2022, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- FDA’S policy statement for the development of new stereoisomeric drugs. Chirality 1992, 4, 338–340. [CrossRef] [PubMed]
- Committee for Proprietary Medicinal Products. Working Parties on Quality, Safety, and Efficacy of Medicinal Products, Note for Guidance: Investigation of Chiral Active Substances; Committee for Proprietary Medicinal Products: London, UK, 1993. [Google Scholar]
- Moldoveanu, S.; David, V. Essentials in Modern HPLC Separations; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780123850133. [Google Scholar]
- Taylor, D.R.; Maher, K. Chiral separations by high-performance liquid chromatography. J. Chromat. Sci. 1992, 30, 67–85. [Google Scholar] [CrossRef]
- León-González, M.E.; Rosales-Conrado, N.; Pérez-Arribas, L.V.; Guillén-Casla, V. Two-dimensional liquid chromatography for direct chiral separations: A review. Biomed. Chromatogr. 2014, 28, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Iguiniz, M.; Heinisch, S. Two-dimensional liquid chromatography in pharmaceutical analysis. Instrumental aspects, trends and applications. J. Pharm. Biomed. Anal. 2017, 145, 482–503. [Google Scholar] [CrossRef]
- Chankvetadze, B. Polysaccharide-based chiral stationary phases for enantioseparations by high-performance liquid chromatography: An overview. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1985, pp. 93–126. [Google Scholar]
- Fieger, H.; Blaschke, G. Direct determination of the enantiomeric ratio of verapamil, its major metabolite norverapamil and gallopamil in plasma by chiral high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1992, 575, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Stagni, G.; Gillespie, W.R. Simultaneous analysis of verapamil and norverapamil enantiomers in human plasma by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1995, 667, 349–354. [Google Scholar] [CrossRef]
- Berthod, A.; Liu, Y.; Bagwill, C.; Armstrong, D.W. Facile liquid chromatographic enantioresolution of native amino acids and peptides using a teicoplanin chiral stationary phase. J. Chromatogr. A 1996, 731, 123–137. [Google Scholar] [CrossRef]
- Brandšteterová, E.; Wainer, I.W. Achiral and chiral high-performance liquid chromatography of verapamil and its metabolites in serum samples. J. Chromatogr. B Biomed. Sci. Appl. 1999, 732, 395–404. [Google Scholar] [CrossRef]
- Zhang, M.; Fawcett, J.P.; Shaw, J.P. Rapid chiral high-performance liquid chromatographic assay for salmeterol and α-hydroxysalmeterol. Application to in vitro metabolism studies. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 225–230. [Google Scholar] [CrossRef]
- Yanagihara, Y.; Ohtani, M.; Kariya, S.; Uchino, K.; Aoyama, T.; Yamamura, Y.; Iga, T. Stereoselective high-performance liquid chromatographic determination of ketamine and its active metabolite, norketamine, in human plasma. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 227–231. [Google Scholar] [CrossRef]
- Volosov, A.; Bialer, M.; Xiaodong, S.; Perucca, E.; Sintov, A.; Yagen, B. Simultaneous stereoselective high-performance liquid chromatographic determination of 10-hydroxycarbazepine and its metabolite carbamazepine- 10,11-trans-dihydrodiol in human urine. J. Chromatogr. B Biomed. Sci. Appl. 2000, 738, 419–425. [Google Scholar] [CrossRef]
- Kaddoumi, A.; Nakashima, M.N.; Nakashima, K. Fluorometric determination of DL-fenfluramine, DL-norfenfluramine and phentermine in plasma by achiral and chiral high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 2001, 763, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Petritis, K.; Valleix, A.; Elfakir, C.; Dreux, M. Simultaneous analysis of underivatized chiral amino acids by liquid chromatography-ion spray tandem mass spectrometry using a teicoplanin chiral stationary phase. J. Chromatogr. A 2001, 913, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Brunnenberg, M.; Kovar, K.A. Stereospecific analysis of ecstasy-like N-ethyl-3,4-methylenedioxyamphetamine and its metabolites in humans. J. Chromatogr. B Biomed. Sci. Appl. 2001, 751, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, H.; Hamada, A.; Nakamura, C.; Arimori, K.; Nakano, M. High-performance liquid chromatographic assay for the simultaneous determination of lansoprazole enantiomers and metabolites in human liver microsomes. J. Chromatogr. B Biomed. Sci. Appl. 2001, 757, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B.; Kartozia, I.; Blaschke, G. Simultaneous enantioseparation of cis-diltiazem hydrochloride and its metabolite cis-desacetyldiltiazem using high-performance liquid chromatography and capillary electrophoresis. J. Pharm. Biomed. Anal. 2002, 27, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Buechler, J.; Schwab, M.; Mikus, G.; Fischer, B.; Hermle, L.; Marx, C.; Grön, G.; Spitzer, M.; Kovar, K.A. Enantioselective quantitation of the ecstasy compound (R)- and (S)-N-ethyl-3,4-methylenedioxyamphetamine and its major metabolites in human plasma and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 793, 207–222. [Google Scholar] [CrossRef]
- Gatti, G.; Bonomi, I.; Marchiselli, R.; Fattore, C.; Spina, E.; Scordo, G.; Pacifici, R.; Perucca, E. Improved enantioselective assay for the determination of fluoxetine and norfluoxetine enantiomers in human plasma by liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 784, 375–383. [Google Scholar] [CrossRef]
- Miura, M.; Tada, H.; Suzuki, T. Simultaneous determination of lansoprazole enantiomers and their metabolites in plasma by liquid chromatography with solid-phase extraction. J. Chromatogr. B 2004, 804, 389–395. [Google Scholar] [CrossRef]
- Miura, M.; Tada, H.; Satoh, S.; Habuchi, T.; Suzuki, T. Determination of rabeprazole enantiomers and their metabolites by high-performance liquid chromatography with solid-phase extraction. J. Pharm. Biomed. Anal. 2006, 41, 565–570. [Google Scholar] [CrossRef]
- Srinivasu, M.K.; Rao, B.M.; Sridhar, G.; Kumar, P.R.; Chandrasekhar, K.B.; Islam, A. A validated chiral LC method for the determination of zolmitriptan and its potential impurities. J. Pharm. Biomed. Anal. 2005, 37, 453–460. [Google Scholar] [CrossRef]
- Sellers, J.A.; Olsen, B.A.; Owens, P.K.; Gavin, P.F. Determination of the enantiomer and positional isomer impurities in atomoxetine hydrochloride with liquid chromatography using polysaccharide chiral stationary phases. J. Pharm. Biomed. Anal. 2006, 41, 1088–1094. [Google Scholar] [CrossRef]
- Alves, G.; Figueiredo, I.; Castel-Branco, M.; Loureiro, A.; Falcão, A.; Caramona, M. Simultaneous and enantioselec-tive liquid chromatographic determination of eslicarbazepine acetate, S-licarbazepine, R-licarbazepine and ox-carbazepine in mouse tissue samples using ultraviolet detection. Anal. Chim. Acta 2007, 596, 132–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alves, G.; Figueiredo, I.; Castel-Branco, M.; Loureiro, A.; Fortuna, A.; Falcão, A.; Caramona, M. Enantioselective HPLC-UV method for determination of eslicarbazepine acetate (BIA 2-093) and its metabolites in human plasma. Biomed. Chromatogr. 2007, 21, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.N.; Talluri, M.V.N.K.; Maurya, P.K. Separation of stereoisomers of sertraline and its related enantiomeric impurities on a dimethylated β-cyclodextrin stationary phase by HPLC. J. Pharm. Biomed. Anal. 2009, 50, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Ferretti, R.; Gallinella, B.; Turchetto, L.; Zanitti, L.; La Torre, F. Development and validation of an enantioselective and chemoselective HPLC method using a Chiralpak IA column to simultaneously quantify (R)-(+)- and (S)-(-)-lansoprazole enantiomers and related impurities. J. Pharm. Biomed. Anal. 2009, 50, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Ferretti, R.; Gallinella, B.; De Santis, E.; Zanitti, L.; La Torre, F. High-performance liquid chromatography enantioseparation of proton pump inhibitors using the immobilized amylose-based Chiralpak IA chiral stationary phase in normal-phase, polar organic and reversed-phase conditions. J. Chromatogr. A 2008, 1177, 105–113. [Google Scholar] [CrossRef]
- Zanitti, L.; Ferretti, R.; Gallinella, B.; La Torre, F.; Sanna, M.L.; Mosca, A.; Cirilli, R. Direct HPLC enantioseparation of omeprazole and its chiral impurities: Application to the determination of enantiomeric purity of esomeprazole magnesium trihydrate. J. Pharm. Biomed. Anal. 2010, 52, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhu, L.; Deng, J.; Liu, A.; Guo, B.; Tan, W.; Dai, R. Simultaneous analysis of bambuterol and its active metabolite terbutaline enantiomers in rat plasma by chiral liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010, 52, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Shiohira, H.; Yasui-Furukori, N.; Tateishi, T.; Uno, T. Chiral assay of omeprazole and metabolites and its application to a pharmacokinetics related to CYP2C19 genotypes. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Materazzo, S.; Carradori, S.; Ferretti, R.; Gallinella, B.; Secci, D.; Cirilli, R. Effect of the water content on the retention and enantioselectivity of albendazole and fenbendazole sulfoxides using amylose-based chiral stationary phases in organic-aqueous conditions. J. Chromatogr. A 2014, 1327, 73–79. [Google Scholar] [CrossRef]
- Balamurugan, P.; Anver Basha, K.; Jayachandran, J.; Gangrade, M.; Parthiban, P. Simultaneous Chemo/Enantioseparation and Assay of R-(+)-Rabeprazole and Related Impurities in Pharmaceutical Formulations. Chromatographia 2015, 78, 1367–1375. [Google Scholar] [CrossRef]
- Servais, A.C.; Janicot, B.; Takam, A.; Crommen, J.; Fillet, M. Mass spectrometry detection of basic drugs in fast chiral analyses with vancomycin stationary phases. Application to their analysis after in vitro metabolism. J. Chromatogr. A 2016, 1467, 306–311. [Google Scholar] [CrossRef]
- Sadutto, D.; Ferretti, R.; Zanitti, L.; Casulli, A.; Cirilli, R. Analytical and semipreparative high performance liquid chromatography enantioseparation of bicalutamide and its chiral impurities on an immobilized polysaccharide-based chiral stationary phase. J. Chromatogr. A 2016, 1445, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, R.; Zanitti, L.; Casulli, A.; Cirilli, R. Green high-performance liquid chromatography enantioseparation of lansoprazole using a cellulose-based chiral stationary phase under ethanol/water mode. J. Sep. Sci. 2016, 39, 1418–1424. [Google Scholar] [CrossRef]
- Gallinella, B.; Ferretti, R.; Zanitti, L.; Sestili, I.; Mosca, A.; Cirilli, R. Comparison of reversed-phase enantioselective HPLC methods for determining the enantiomeric purity of (S)-omeprazole in the presence of its related substances. J. Pharm. Anal. 2016, 6, 132–136. [Google Scholar] [CrossRef]
- Ferretti, R.; Zanitti, L.; Cirilli, R. Development of a high-performance liquid chromatography method for the simultaneous determination of chiral impurities and assay of (S)-clopidogrel using a cellulose-based chiral stationary phase in methanol/water mode. J. Sep. Sci. 2018, 41, 1208–1215. [Google Scholar] [CrossRef]
- Guo, H.; Wahab, M.F.; Berthod, A.; Armstrong, D.W. Mass spectrometry detection of basic drugs in fast chiral analyses with vancomycin stationary phases. J. Pharm. Anal. 2018, 8, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Niedermeier, S.; Matarashvili, I.; Chankvetadze, B.; Scriba, G.K.E. Simultaneous determination of dextromepromazine and related substances 2-methoxyphenothiazine and levomepromazine sulfoxide in levomepromazine on a cellulose tris(4-methylbenzoate) chiral column. J. Pharm. Biomed. Anal. 2018, 158, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M.A.; Yehia, A.M.; Aboul-Enein, H.Y. Simultaneous determination of guaifenesin enantiomers and ambroxol HCl using 50-mm chiral column for a negligible environmental impact. Chirality 2019, 31, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Sanganyado, E.; Lu, Z.; Fu, Q.; Schlenk, D.; Gan, J. Chiral pharmaceuticals: A review on their environmental occurrence and fate processes. Water Res. 2017, 124, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Qu, H.; Wang, B.; Wang, F.; Yu, Y.; Yu, G. Simultaneous enantiomeric analysis of non-steroidal anti-inflammatory drugs in environment by chiral LC-MS/MS: A pilot study in Beijing, China. Ecotoxicol. Environ. Saf. 2019, 174, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Rosetti, A.; Ferretti, R.; Zanitti, L.; Casulli, A.; Villani, C.; Cirilli, R. Single-run reversed-phase HPLC method for determining sertraline content, enantiomeric purity, and related substances in drug substance and finished product. J. Pharm. Anal. 2020, 10, 610–616. [Google Scholar] [CrossRef]
- Rosetti, A.; Villani, C.; Pierini, M.; Cirilli, R. Comparison of coated and immobilized chiral stationary phases based on amylose tris-[(S)-α-methylbenzylcarbamate] for the HPLC enantiomer separation of α-lipoic acid and its reduced form. Molecules 2021, 26, 1747. [Google Scholar] [CrossRef]
- Tóth, G.; Fogarasi, E.; Bartalis-Fábián, Á.; Foroughbakhshfasaei, M.; Boldizsár, I.; Darcsi, A.; Lohner, S.; Scriba, G.K.E.; Szabó, Z.I. Liquid chromatographic method for the simultaneous determination of achiral and chiral impurities of dapoxetine in approved and counterfeit products. J. Chromatogr. A 2020, 1626, 461388. [Google Scholar] [CrossRef]
- Ferencz, E.; Kovács, B.; Boda, F.; Foroughbakhshfasaei, M.; Kelemen, É.K.; Tóth, G.; Szabó, Z.I. Simultaneous determination of chiral and achiral impurities of ivabradine on a cellulose tris(3-chloro-4-methylphenylcarbamate) chiral column using polar organic mode. J. Pharm. Biomed. Anal. 2020, 177, 112851. [Google Scholar] [CrossRef]
- Rosetti, A.; Ferretti, R.; Villani, C.; Pierini, M.; Cirilli, R. Simultaneous enantio- and diastereoselective high-performance liquid chromatography separation of paroxetine on an immobilized amylose-based chiral stationary phase under green reversed-phase conditions. J. Chromatogr. A 2021, 1653, 462406. [Google Scholar] [CrossRef]
- Camilo, K.; Foley, J.P. Simultaneous Achiral/Chiral HPLC Separation of Ketoprofen, Ibuprofen, Flurbiprofen, and Naproxen. Chromatographia 2021, 84, 371–379. [Google Scholar] [CrossRef]
- Szabó, Z.I.; Bartalis-Fábián, Á.; Tóth, G. Simultaneous determination of escitalopram impurities including the R-enantiomer on a cellulose tris(3,5-dimethylphenylcarbamate)-based chiral column in reversed-phase mode. Molecules 2022, 27, 9022. [Google Scholar] [CrossRef]
- Cantatore, C.; Bertocchi, P.; De Orsi, D.; Panusa, A.; Cirilli, R. Enantioselective HPLC analysis of escitalopram oxalate and its impurities using a cellulose-based chiral stationary phase under normal- and green reversed-phase conditions. J. Sep. Sci. 2022, 45, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Mammone, F.R.; Rotundo, P.; Ferretti, R.; Puxeddu, M.; Silvestri, R.; Cirilli, R. Chemo- and enantio-selective reversed-phase HPLC analysis of rosuvastatin using a cellulose-based chiral stationary phase in gradient elution mode. J. Pharm. Biomed. Anal. 2023, 225, 115239. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.A.; Hancu, G.; Szabó, Z.I. Simultaneous determination of enantiomeric and organic impurities of vildagliptin on a cellulose tris(3-chloro-4-methylphenylcarbamate) column under revered-phase conditions. J. Pharm. Biomed. Anal. 2023, 234, 115495. [Google Scholar] [CrossRef]
- Hesse, G.; Hagel, R. Eine vollständige Recemattennung durch eluitons-chromagographie an cellulose-tri-acetat. Chromatographia 1973, 6, 277–280. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kawashima, M.; Hatada, K. Useful chiral packing materials for high-performance liquid chromatographic resolution of enantiomers: Phenylcarbamates of polysaccharides coated on silica gel. J. Am. Chem. Soc. 1984, 106, 5357–5359. [Google Scholar] [CrossRef]
- Ghanem, A.; Aboul-Enein, H.Y. Comparison, applications, advantages, and limitations of immobilized and coated amylose tris-(3,5-dimethylphenylcarbamate) chiral stationary phases in HPLC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2863–2874. [Google Scholar] [CrossRef]
- Ghanem, A.; Hoenen, H.; Aboul-Enein, H.Y. Application and comparison of immobilized and coated amylose tris-(3,5-dimethylphenylcarbamate) chiral stationary phases for the enantioselective separation of β-blockers enantiomers by liquid chromatography. Talanta 2006, 68, 602–609. [Google Scholar] [CrossRef]
- Thunberg, L.; Hashemi, J.; Andersson, S. Comparative study of coated and immobilized polysaccharide-based chiral stationary phases and their applicability in the resolution of enantiomers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 875, 72–80. [Google Scholar] [CrossRef]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral stationary phases for liquid chromatography: Recent developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Ferretti, R.; Gallinella, B.; Zanitti, L. Retention behavior of proton pump inhibitors using immobilized polysaccharide-derived chiral stationary phases with organic-aqueous mobile phases. J. Chromatogr. A 2013, 1304, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, R.; Zanitti, L.; Casulli, A.; Cirilli, R. Unusual retention behavior of omeprazole and its chiral impurities B and E on the amylose tris (3-chloro-5-methylphenylcarbamate) chiral stationary phase in polar organic mode. J. Pharm. Anal. 2018, 8, 234–239. [Google Scholar] [CrossRef]
- Kalíková, K.; Riesová, M.; Tesařová, E. Recent chiral selectors for separation in HPLC and CE. Cent. Eur. J. Chem. 2012, 10, 450–471. [Google Scholar]
- Ward, K.D.; Bravenec, A.D.; Ward, T.J. Chiral Separations by high-performance liquid chromatography. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 1–28. [Google Scholar]
- Scriba, G.K.E. Chiral recognition in separation sciences. Part I: Polysaccharide and cyclodextrin selectors. TrAC Trends Anal. Chem. 2019, 120, 115639. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xie, S.M.; Yuan, L.M. Recent progress in the development of chiral stationary phases for high-performance liquid chromatography. J. Sep. Sci. 2022, 45, 51–77. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Chang, L.W.; Chang, S.C.; Wang, X.; Ibrahim, H.; Reid, G.R.; Beesley, T.E. Comparison of the enantioselectivity of β-cyclodextrin vs. heptakis-2, 3-O-dimethyl-β-cyclodextrin LC stationary phases. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 3279–3295. [Google Scholar] [CrossRef]
- Dobó, M.; Ádám, M.; Fiser, B.; Papp, L.A.; Dombi, G.; Sekkoum, K.; Szabó, Z.I.; Tóth, G. Enantioseparation and molecular docking study of selected chiral pharmaceuticals on a commercialized phenylcarbamate-β-cyclodextrin column using polar organic mode. Sci. Rep. 2023, 13, 14778. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.V.; Rosenau, T.; Hettegger, H. Polysaccharide-and β-cyclodextrin-based chiral selectors for enantiomer resolution: Recent developments and applications. Molecules 2021, 26, 4322. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Z.; Yuan, H.; Meng, Z. Preparation and chiral recognition of a mono[6A-N-1-(2-hydroxy)- phe-nylethylimino-6A-deoxy]-β-cyclodextrin HPLC stationary phase. J. Chromatogr. Sci. 2008, 46, 777–782. [Google Scholar] [CrossRef]
- Qin, Q.; Zhang, S.; Zhang, W.G.; Zhang, Z.B.; Xiong, Y.J.; Guo, Z.Y.; Fan, J.; Run-Zheng, S.; Finlow, D.; Yin, Y. The impact of silica gel pore and particle sizes on HPLC column efficiency and resolution for an immobilized, cyclones-train based, chiral stationary phase. J. Sep. Sci. 2010, 33, 2582–2589. [Google Scholar] [CrossRef]
- Lin, C.; Fan, J.; Liu, W.; Chen, X.; Ruan, L.; Zhang, W. A new single-urea-bound 3,5-dimethylphenylcarbamoylated β-cyclodextrin chiral stationary phase and its enhanced separation performance in normal-phase liquid chromatography. Electrophoresis 2018, 39, 348–355. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, S.; Lin, Y.; Zhou, J.; Pang, L.; Nie, X.; Zhou, B.; Tang, W. Engineering cyclodextrin clicked chiral stationary phase for high-efficiency enantiomer separation. Sci. Rep. 2015, 5, 11523. [Google Scholar] [CrossRef] [PubMed]
- Estevez, P.; Flor, S.; Boscolo, O.; Tripodi, V.; Lucangioli, S. Development and validation of a capillary electrophoresis method for determination of enantiomeric purity and related substances of esomeprazole in raw material and pellets. Electrophoresis 2014, 35, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Fradi, I.; Servais, A.C.; Lamalle, C.; Kallel, M.; Abidi, M.; Crommen, J.; Fillet, M. Chemo- and enantio-selective method for the analysis of amino acids by capillary electrophoresis with in-capillary derivatization. J. Chromatogr. A 2012, 1267, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.; Stegander, F.; Marlin, N.; Wan, H.; Blomberg, L.G. Enantiomeric separation of omeprazole and its metabolite 5-hydroxyomeprazole using non-aqueous capillary electrophoresis. J. Chromatogr. A 2006, 1129, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Modroiu, A.; Krait, S.; Hancu, G.; Scriba, G.K.E. Quality by design-guided development of a capillary electrophoresis method for the simultaneous chiral purity determination and impurity profiling of tamsulosin. J. Sep. Sci. 2023, 46, 2300604. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Yang, S.H.; Wigginton, J.G.; Simpkins, J.W.; Schug, K.A. Retention behavior of estrogen metabolites on hydrophilic interaction chromatography stationary phases. J. Sep. Sci. 2010, 33, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Breitbach, Z.S.; Armstrong, D.W. Separations of cycloinulooligosaccharides via hydrophilic interaction chromatography (HILIC) and ligand-exchange chromatography. Sep. Sci. Technol. 2010, 45, 447–452. [Google Scholar] [CrossRef]
- Nowik, W.; Bonose-Crosnier de Bellaistre, M.; Tchapla, A.; Héron, S. Separation of 9,10-anthraquinone derivatives: Evaluation of functionalised stationary phases in reversed phase mode. J. Chromatogr. A 2011, 1218, 3636–3647. [Google Scholar] [CrossRef] [PubMed]
- Berkecz, R.; Tanács, D.; Péter, A.; Ilisz, I. Enantioselective liquid chromatographic separations using macrocyclic glycopeptide-based chiral selectors. Molecules 2021, 26, 3380. [Google Scholar] [CrossRef] [PubMed]
- Aslani, S.; Berthod, A.; Armstrong, D.W. Macrocyclic Antibiotics and Cyclofructans. In Chiral Separations and Stereochemical Elucidation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 247–272. [Google Scholar]
- Ilisz, I.; Orosz, T.; Péter, A. High-performance liqud chromatography enantioseparations using macrocyclic gly-peptide-based chiral stationary phases: An overview. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1985, pp. 201–237. [Google Scholar]
- Scriba, G.K.E.; Konjaria, M.-L.; Krait, S. Cyclodextrins. In Chiral Separations and Stereochemical Elucidation: Fundamentals, Methods, and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 273–323. [Google Scholar] [CrossRef]
- Ferencz, E.; Kelemen, É.K.; Obreja, M.; Tóth, G.; Urkon, M.; Zöldhegyi, A.; Sipos, E.; Szabó, Z.I. The Applicability of Chromatographic Retention Modeling on Chiral Stationary Phases in Reverse-Phase Mode: A Case Study for Ezetimibe and Its Impurities. Int. J. Mol. Sci. 2023, 24, 16097. [Google Scholar] [CrossRef] [PubMed]
- Wagdy, H.A.; Hanafi, R.S.; El-Nashar, R.M.; Aboul-Enein, H.Y. Predictability of enantiomeric chromatographic behavior on various chiral stationary phases using typical reversed phase modeling software. Chirality 2013, 25, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Wagdy, H.A.; Hanafi, R.S.; El-Nashar, R.M.; Aboul-Enein, H.Y. Enantiomeric separation of underivatized amino acids: Predictability of chiral recognition on ristocetin a chiral stationary phase. Chirality 2014, 26, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Galella, E.; Tomasella, F.P.; Fish, W.P. Separation of atropisomers by chiral liquid chromatography and thermodynamic analysis of separation mechanism. J. Pharm. Anal. 2017, 7, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Horváth, C.; Melander, W.; Molnár, I. Solvophobic interactions in liquid chromatography with nonpolar stationary phases. J. Chromatogr. A 1976, 125, 129–156. [Google Scholar] [CrossRef]
- Molnár, I. Searching for robust HPLC methods—Csaba Horváth and the Solvophobic Theory. Chromatographia 2005, 62, s7–s17. [Google Scholar] [CrossRef]
- Dispas, A.; Avohou, H.T.; Lebrun, P.; Hubert, P.; Hubert, C. ‘Quality by Design’ approach for the analysis of impurities in pharmaceutical drug products and drug substances. TrAC Trends Anal. Chem. 2018, 101, 24–33. [Google Scholar] [CrossRef]
- Chiarentin, L.; Gonçalves, C.; Augusto, C.; Miranda, M.; Cardoso, C.; Vitorino, C. Drilling into “Quality by Design” approach for analytical methods. Crit. Rev. Anal. Chem. 2023, 4, 1–42. [Google Scholar] [CrossRef]
- Catani, M.; Ismail, O.H.; Gasparrini, F.; Antonelli, M.; Pasti, L.; Marchetti, N.; Felletti, S.; Cavazzini, A. Recent advancements and future directions of superficially porous chiral stationary phases for ultrafast high-performance enantioseparations. Analyst 2017, 142, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Bezhitashvili, L.; Bardavelidze, A.; Mskhiladze, A.; Gumustas, M.; Ozkan, S.A.; Volonterio, A.; Farkas, T.; Chankvetadze, B. Application of cellulose 3,5-dichlorophenylcarbamate covalently immobilized on superficially porous silica for the separation of enantiomers in high-performance liquid chromatography. J. Chromatogr. A 2018, 1571, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.H.; Felletti, S.; De Luca, C.; Pasti, L.; Marchetti, N.; Costa, V.; Gasparrini, F.; Cavazzini, A.; Catani, M. The way to ultrafast, high-throughput enantioseparations of bioactive compounds in liquid and supercritical fluid chromatography. Molecules 2018, 23, 2709. [Google Scholar] [CrossRef] [PubMed]
- Ciogli, A.; Ismail, O.H.; Mazzoccanti, G.; Villani, C.; Gasparrini, F. Enantioselective ultra high performance liquid and supercritical fluid chromatography: The race to the shortest chromatogram. J. Sep. Sci. 2018, 41, 1307–1318. [Google Scholar] [CrossRef]
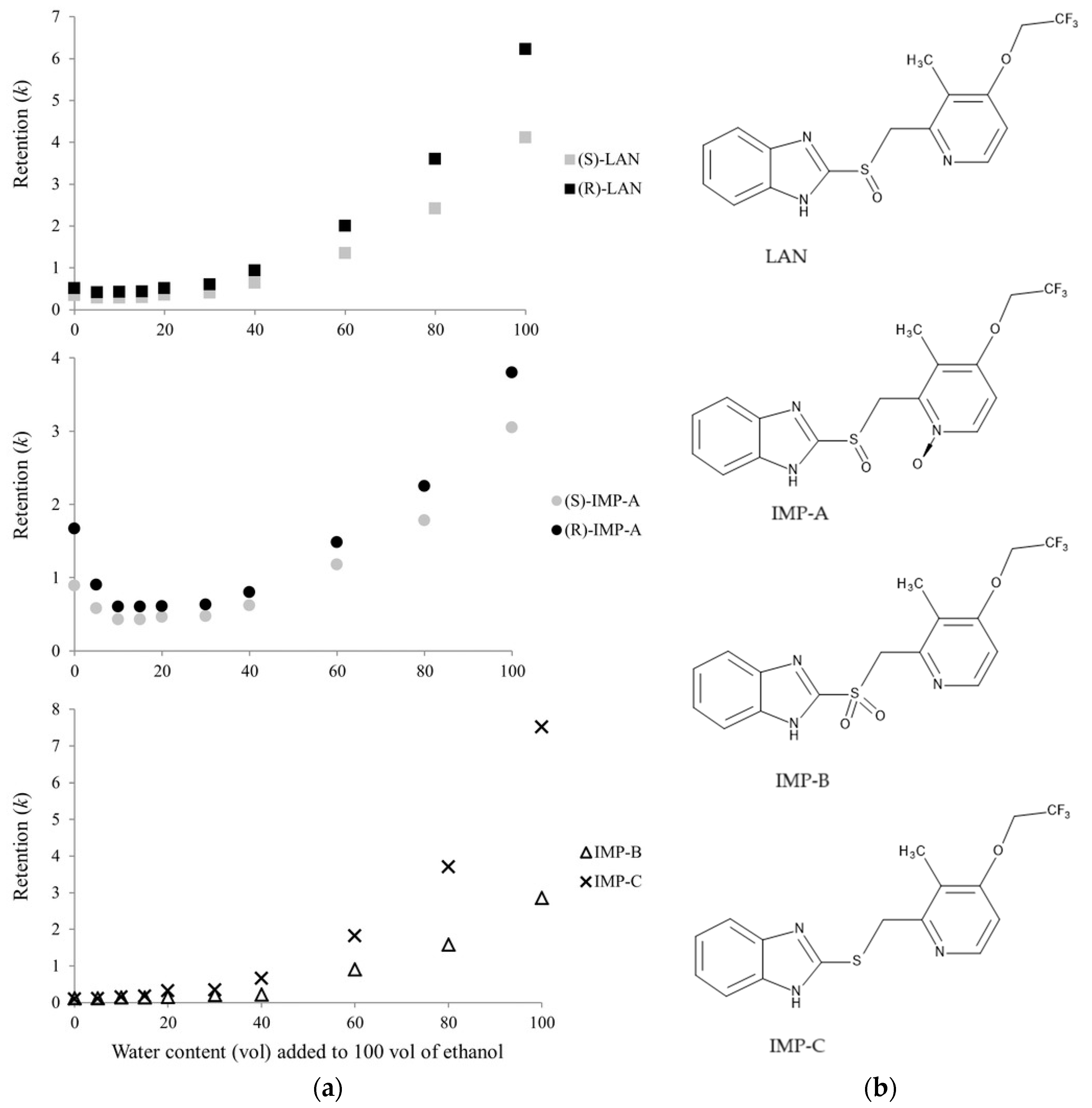
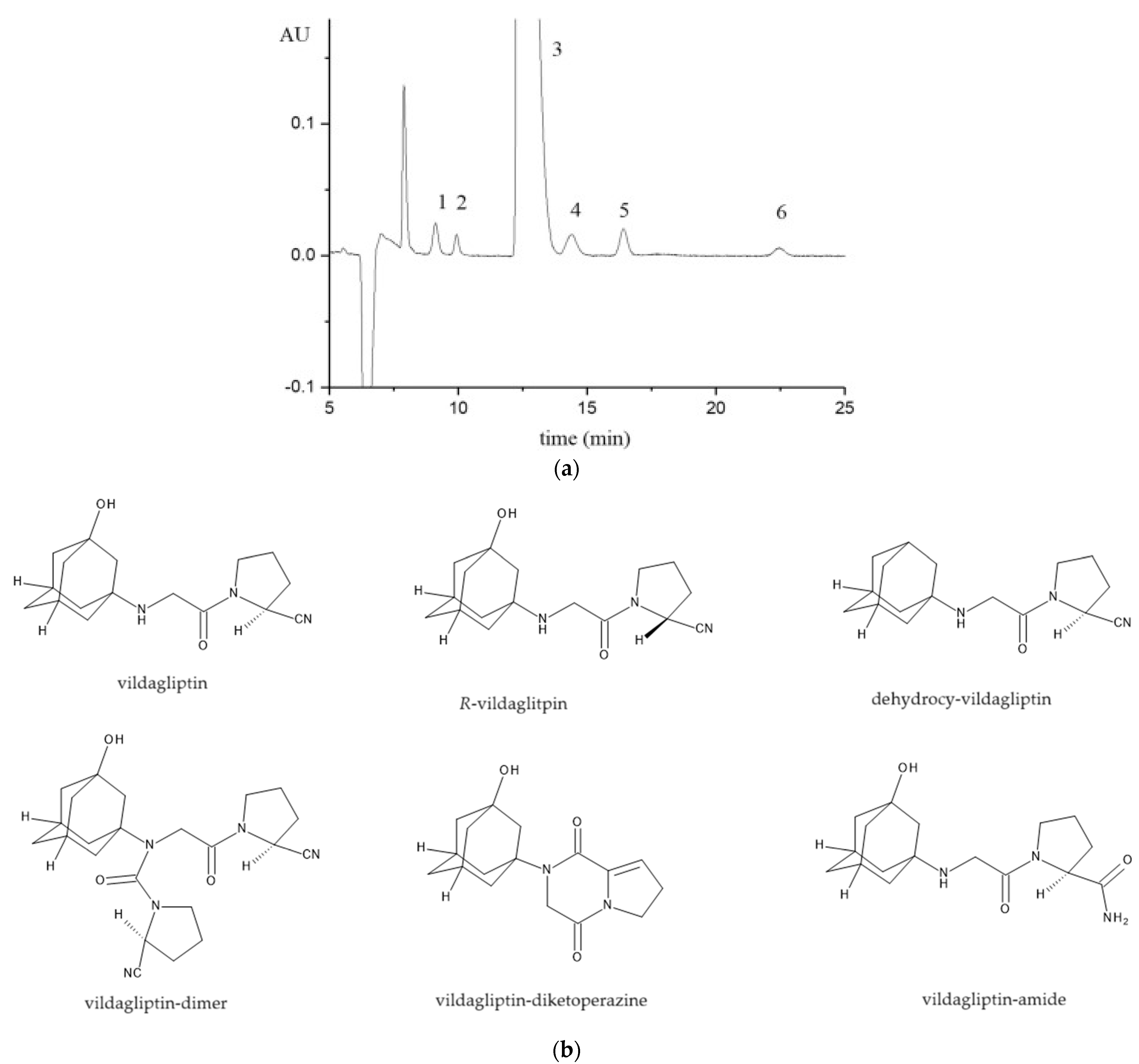
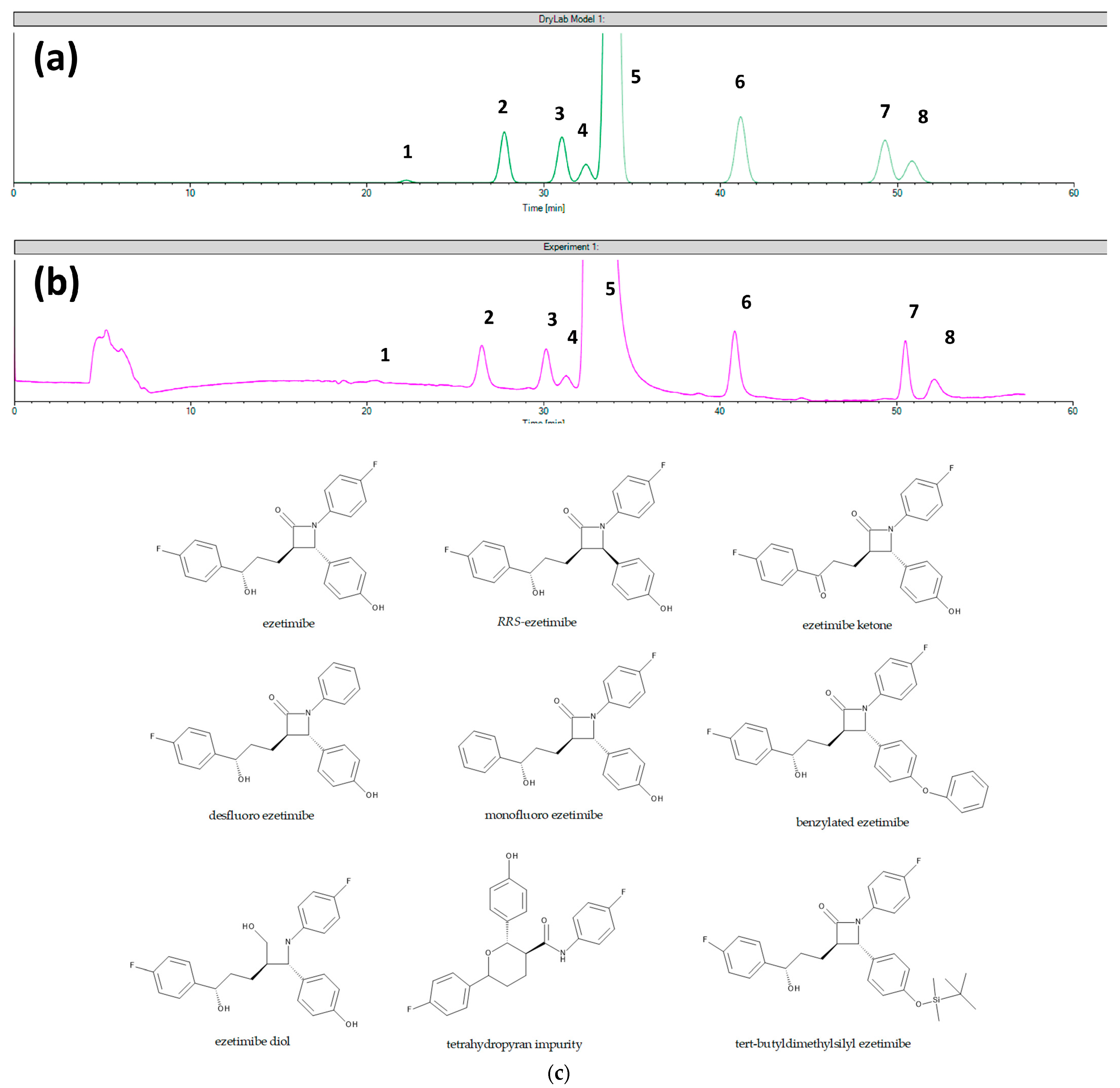
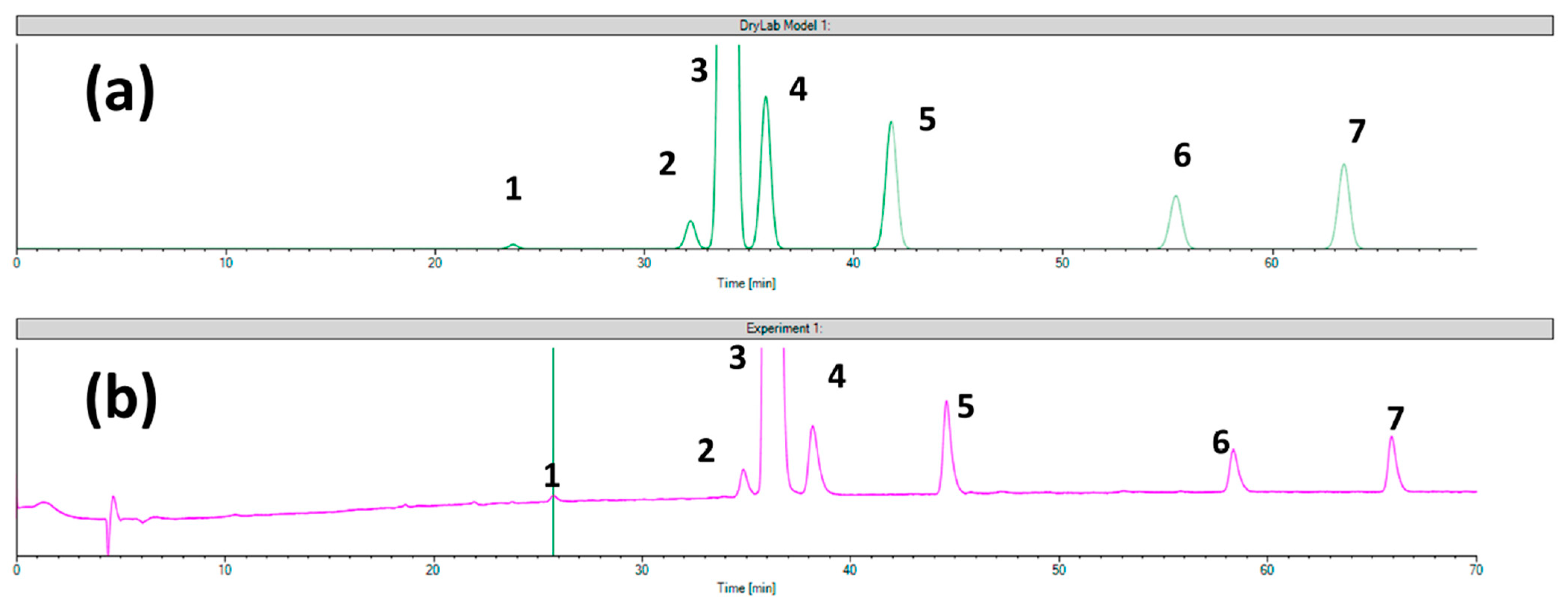
| Analytical Conditions | Column/CSP | Samples, Extraction Procedure | Analytes | Reference |
|---|---|---|---|---|
| mobile phase: n-hexane:isopropanol:diethylamine (90:10:0.1, v/v), flow rate: 1.0 mL/min, fluorescence detection: excitation 223 nm, without using an emission filter | Chiralpak AD (50 mm × 4.6 mm, 5 µm), CSP: amylose-tris(3,5-dimethylphenylcarbamate) | human plasma LLE | verapamil norverapamil | [11] |
| mobile phase: 0.01 M phosphate buffer (pH 6.65):acetonitrile (91:9, v/v), flow rate: 0.9 mL/min, fluorescence detection: excitation 227 nm, emission 308 nm | Chiral AGP (150 × 4.0 mm, 5 µm), CSP: α1-acid glycoprotein | human plasma LLE | verapamil N-acetyl-norverapamil | [12] |
| mobile phase: 0.01 M phosphate buffer (pH 7.0):acetonitrile (90:10, v/v), fluorescence detection: excitation 276 nm, emission 310 nm | Chiral–AGP (100 × 4 mm, 5 µm), CSP:α1-acid glycoprotein | human serum online SPE | verapamil, norverapamil, D617; D620; PR24; PR23; PR22; PR25 metabolites | [14] |
| mobile phase: 25 mm phosphate buffer) (pH 6):isopropanol (85:15, v/v), flow rate = 0.9 mL/min, coulometric detection | Chiral CBH (100 × 4.0 mm, 5 µm), CSP: cellobiohydrolase | microsomal incubation mixtures LLE | salmeterol α-hydroxysalmeterol | [15] |
| mobile phase: n-hexane:isopropanol (98:2, v/v), flow rate = 0.8 mL/min, 25 °C, UV detection 215 nm | Chiralcel OD column (250 × 4.6 mm, 5 µm) CSP: cellulose tris(3,5-dimethylcarbammate) | human plasma LLE | ketamine, norketamine bromoketamine (I.S.) | [16] |
| mobile phase: n-hexane:ethanol:isopropanol (18:2:1, v/v/v) + 0.1% of glacial acetic acid, flow rate = 1.0 mL/min, 25 °C, UV detection 215 nm | Chiralcel OD column (250 × 4.6 mm) CSP: cellulose tris(3,5-dimethylphenylcarbamate) | human urine LLE | 10-hydroxycarbazepine carbamazepine-10,11-transdihydrodiol oxcarbazepine oxime (achiral I.S) | [17] |
| mobile phase: gradient elution: solvent A (acetonitrile:0.01 M citrate buffer (pH 2.7):0.05 M perchlorate buffer (pH 2.0) (50:25:25 v/v/v), solvent B (acetonitrile), flow rate = 1 mL/min, 60 °C, fluorescence detection: excitation 325 nm, emission 430 nm | Chiralcel OD-R (250 × 4.6 mm, 5 μm) CSP: cellulose tris (3,5-dimethylphenylcarbamate) | plasma LLE | fenfluramine, norfenfluramine, phentermine (achiral) | [18] |
| mobile phase: acetonitrile:water, (75:25, v/v), flow rate = 0.8 mL/min, MS detection | Chirobiotic T (250 × 4.6 mm, 5 µm), CSP: teicoplanin | bulk | 15 different amino acids | [19] |
| mobile phase: 40 mM potassium dihydrogen phosphate buffer (pH 5.5):acetonitrile (91:9, v/v), flow rate = 0.8 mL/min, 20 °C, fluorescence detection: excitation 286 nm, emission 320 nm | ChiralDex (250 × 4 mm, 5 µm), CSP: β-cyclodextrin | human plasma SPE | N-ethyl-3,4-methylenedioxyamphetamine 3,4-methylenedioxyamphetamine | [20] |
| mobile phase: methanol:water (75:25, v/v), flow rate = 0.5 mL/min, UV detection 285 nm. | Chiralcel OD-R (250 × 4.6 mm, 5 μm) CSP: cellulose tris(3,5-dimethylphenylcarbamate) | human liver microsomes LLE | lansoprazole, 5-hydroxy-lansoprazole, lansoprazole-sulfone (achiral) | [21] |
| mobile phase: isopropanol, flow rate = 0.5 mL/min, UV detection 214 nm | homemade cellulose tris (3,5-dichlorophenylcarbamate) CSP (250 × 4.6 mm) | bulk | cis-diltiazem, desacetyl-cis-diltiazem | [22] |
| mobile phase: 20 mM phosphate buffer (pH 6.44) containing 50 mM EDTA:isopropanol (93:7, v/v), flow rate = 0.7 mL/min, 17.5 °C, fluorescence detection: excitation 286 nm, emission 322 nm | Chiral CBH (150 × 4 mm, 5 μm) CSP: cellobiohydrolase | human plasma, human urine SPE | N-ethyl-3,4-methylenedioxyamphetamine, N-ethyl-4-hydroxy-3-methoxyamphetamine, 3,4-methylenedioxyamphetamine | [23] |
| mobile phase: 100 mm potassium hexafluorophosphate (pH 3.0):acetonitrile (65:35, v/v), flow rate = 0.5 mL/min, 37 °C, UV detection 227 nm | Chiralcel OD-R column (250 × 4.6 mm, 10 μm) CSP: cellulose tris(3,5-dimethylphenylcarbamate) | plasma LLE | fluoxetine, norfluoxetine, fluvoxamine (achiral I.S.) | [24] |
| mobile phase: 0.5 M sodium perchlorate:acetonitrile:methanol (6:3:1, v/v/v), flow rate = 0.5 mL/min, ambient temperature, UV detection 285 nm | Chiral CD-Ph (250 × 4.6 mm, 5 μm), CSP: phenylcarbamated β-CD | human plasma SPE | lansoprazole, 5-hydroxy-lansoprazole, lansoprazole sulfone (achiral), S-omeprazole (I.S.) | [25] |
| mobile phase: 0.5 M sodium perchlo-rate:acetonitrile (6:4, v/v), flow rate = 0.5 mL/min, ambient temperature, UV detection 285 nm | Chiral CD-Ph (250 × 4.6 mm, 5 μm), CSP: phenylcarbamated β-CD-based | human plasma SPE | rabeprazole, rabeprazole-thioether (achiral), rabeprazole sulfone (achiral), S-omeprazole (I.S.) | [26] |
| mobile phase: hexane:isopronaol:methanol:diethylamine (75:10:15:0.1, v/v/v/v), flow rate =1.0 mL/min, 25 °C, UV detection 225 nm | Chiralpak AD-H (250 mm × 4.6 mm, 5 µm), CSP: amylose tris-(3,5-dimethylphenylcarbamate) | tablets | zolmitriptan, impurity-1 | [27] |
| mobile phase: hexane:isopropanol:diethylamine: 0,1% trifluoroacetic acid (85:15:0.15:0.2, v/v/v/v), flow rate = 1.0 mL/min, ambient temperature, UV detection 273 nm | Chiralpak OD-H column (250 × 4.6 mm, 5 μm) CSP: cellulose tris(3,5-dimethylphenylcarbamate) | bulk | atomoxetine, meta- and para-isomers of atomoxetine and desmethyl-atomoxetine | [28] |
| mobile phase: water:methanol (88:12, v/v), flow rate = 0.7 mL/min, 30 °C, UV detection 225 nm | Lichro-CART 250-4 ChiraDex (250 × 4 mm, 5 µm) CSP: β-cyclodextrin | mouse plasma and brain, liver, and kidney tissue homogenates, SPE | eslicarbazepine acetate, licarbazepine, oxcarbazepine (achiral) | [30] |
| mobile phase: 0.4% trifluoroacetic acid:acetonitrile (80:20, v/v), flow rate = 0.8 mL/min, 30 °C, UV detection 225 nm | Cyclobond I 2000 DM (250 × 4.6 mm, 5 µm) CSP: dimethyl β-cyclodextrin | bulk, capsules, tablets | sertraline and its 9 process-related impurities | [31] |
| mobile phase: methyl-tert-butyl ether:ethyl acetate:ethanol:diethylamine (60:40:5:0.1, v/v/v/v), flow rate: 1 mL/min (analytical) and 4 mL/min (semipreparative), 25 °C, UV detection 310 and 285 nm, circular dichroism detection 285 nm | Chiralpak IA (250 × 4.6 mm, analytical), (250 × 10 mm, semipreparative), CSP: amylose tris (3,5-dimethyl-phenylcarbamate) | bulk | lansoprazole, impurities A, B–E (achiral) | [32] |
| mobile phase: methyl tert-butylether:ethyl acetate:ethanol:diethylamine (60:40:5:0.1, v/v/v/v), flow rate = 1 mL/min (analytical), 4 mL/min (semipreparative), 25 °C, UV and circular dichroism detection 299 nm | Chiralpak IA (250 × 4.6 mm, analytical), (250 × 10 mm, semipreparative) CSP: amylose tris (3,5-dimethyl-phenylcarbamate) | bulk, tablets | esomeprazole, impurities B, F, H, and impurities A, C, D, F, G, and I (achiral) | [34] |
| mobile phase: phosphate buffer (pH–5.0):methanol (45:55, v/v), flow rate = 0.4 mL/min, UV detection 254 nm | Chiral CD-ph (150 mm × 4.6 mm, 5 μm) CSP: phenylcarbamated β-CD-based | human plasma LLE | omeprazole, 5-hydroxy-omeprazole, omeprazole sulfone (achiral) | [36] |
| mobile phase: ethanol:water (100:60, v/v), acetonitrile:water (100:100, v/v) (fenbendazole), flow rate = 1.0 mL/min, 25 °C, UV detection 254 nm | Chiralpak IA-3 (100 mm × 4.6 m, 3 μm) CSP: amylose-tris(3,5-dimethylphenylcarbamate) | bulk | albendazole (achiral), albendazole-sulfone (achiral), albendazole-sulfoxide, fenbendazole (achiral), fenbendazole-sulfone (achiral), and fenbendazole-sulfoxide | [37] |
| mobile phase: phosphate buffer (pH–8.0):acetonitrile-gradient elution, flow rate = 1.0 mL/min, 35 °C. UV detection 282 nm | Chiralpak IC column (250 mm × 4.6 mm, 5 μm) CSP: cellulose tris-(3,5-dichlorophenylcarbamate) | bulk | rabeprazole, impurities A, B, C (achiral), and impurities D and E | [38] |
| mobile phase: acetonitrile:methanol:acetic acid:diethylamine (95:5:0.2:0.07, v/v/v/v), flow rate = 1.0 mL/min, 40 °C, UV detection 240 nm. | Sepapak-2 (250 mm × 4.6 mm, 5 µm) CSP: cellulose tris(3-chloro-4-methylphenylcarbamate) | microsomal incubation mixtures SPE | eslicarbazepine acetate licarbazepine, oxcarbazepine (achiral) | [39] |
| mobile phase: analytical: ethanol:water (50:50, v/v), flow rate = 1 m/min, 25 °C, UV detection 280 nm mobile phase: semipreparative: mobile phase: n-hexane:ethanol:diethylamine (40:60:0.1, v/v/v) (Impurity A), n-hexane:ethanol:diethylamine (60:40:0.1 v/v/v) (lansoprazole), flow rate = 4.5 mL/min, 35 °C, UV detection 310 nm | Chiralpak IC-3 (100 × 4.6 mm, analytical) CSP: cellulose tris-(3,5-dichlorophenylcarbamate) | bulk | lansoprazole, impurities A, B–E (achiral) | [41] |
| mobile phase: acetonitrile:water (50:50, v/v), flow rate = 1 mL/min, 40 °C, UV detection 280 nm and 300 nm, circular dichroism detection 280 nm | Chiralpak ID-3 (100 × 4.6 mm, 3 μm) CSP: amylose tris-(3-chlorophenylcarbamate) | bulk | esomeprazole, impurities B, E, F (R-omeprazole), impurities A, C, D (achiral) | [42] |
| mobile phase: analytical: methanol:water (100:15, v/v), semipreparative: n-hexane:ethanol (70:30, v/v); flow rate = 4.5 mL/min, 25 °C; UV detection 280 nm | Chiralcel OJ-RH (150 mm × 4.6 mm, 5 μm, analytical), (250 mm × 10 mm, 5 μm, semipreparative), CSP: cellulose tris(4-methylbenzoate) | bulk, tablets | clopidogrel, impurities B, C | [43] |
| mobile phase: 20 mM ammonium acetate:methanol (10:90, v/v), flow rate = 0.4 mL/min, 25 °C, MS (MRM) detection | Chirobiotic T column (250 mm × 4.6 mm, 5 μm) CSP: teicoplanin | rat plasma LLE | bambuterol, terbutaline | [44] |
| mobile phase: 0.1% diethylamine in methanol, flow rate = 1.0 mL/min, ambient temperature, UV detection at 254 nm | Lux Cellulose-3 (250 mm × 4.6 mm, 3 µm) CSP: cellulose tris(4-methylbenzoate) | bulk, tablets, injection solution | levomepromazine, dextromepromazine, levomepromazine sulfoxide, 2-methoxyphenothiazine (achiral) | [45] |
| mobile phase: ethanol:water-gradient elution, flow rate = 1.0 mL/min, UV detection 270 nm | Lux Cellulose-1 (50 × 4.6 mm, 5 μm) CSP: cellulose tris(3,5-dimethylphenylcarbamate) | bulk, pharmaceutical syrup, | guaifenesin, ambroxol | [46] |
| mobile phase: 10 mM acetate buffer (pH–5.0):acetonitrile (65:35, v/v), flow rate = 0.4 mL/min, 25 °C, MS (MRM) detection | Chiralpak AD-RH (150 × 4.6 mm, 5 μm) CSP: amylose tris(3,5-dimethylphenylcarmabate) | surface water sample SPE | flurbiprofen, ibuprofen, naproxen | [48] |
| mobile phase: acetonitrile:water:diethylamine (75:25:0.1, v/v/v), flow rate = 1.0 mL/min, 30 °C | Chiralpak IG-3 (250 mm × 4.6 mm, 3 μm) CSP: amylose tris(3-chloro-5-methylphenylcarbamate) | bulk, tablets | sertraline, impurities A, B, C, E, F, and G | [49] |
| mobile phase: n-hexane:isopropanol: trifluoroacetic acid (80;20:0.1, v/v/v), flow rate = 1 mL/min, 5 °C, UV detection 330 nm | Chiralpak AS-H (250 mm × 4.6 mm, 5 μm) CSP: amylose tris[(S)-α-methylbenzylcarbamate] | bulk | α-lipoic acid, α-dihydrolipoic acid | [50] |
| mobile phase: ethanol:diethylamine:water-gradient elution and flow-programming, 40 °C, UV detection 210 nm and 224 nm | Lux Cellulose-3 (150 × 4.6 mm, 5 μm) CSP: cellulose tris(4-methylbenzoate) | bulk, tablets | dapoxetine, dapoxetine impurities, including R-dapoxetine, (3 S)-3-(dimethylamino-3-phenyl-1-propanol), S-3-amino-3-phenyl-1-propanol, 1-naphtol (achiral), 4-phenyl-2H,3H,4H-naphtho[1,2- b ]pyran (achiral), 1-(2 E)-Cinnamyloxynaphthalene (achiral) | [51] |
| mobile phase: acetonitrile:methanol (98:2, v/v) containing 0.06% DEA, flow rate = 0.45 mL/min, 12 °C, UV detection 286 nm | Lux Cellulose-2 (4.6 × 150 mm, 5 µm) CSP: cellulose tris(3-chloro-4-methylphenylcarbamate) | bulk, tablets | ivabradine, dehydro-S-ivabradine, N-demethyl-S-ivabradine, ((S)-3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-yl-methyl)-methyl-amine), 1-(7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepine-2-on-3-yl)-3-chloro-propane) (achiral) | [52] |
| mobile phase: analytical: ethanol:water:diethylamine (80:20:0.1, v/v/v), mobile phase semipreparative: ethanol, flow rate = 0.5 mL/min, 35 °C, UV detection 295 nm, 263 nm (impurity I), and 243 nm (impurity G) | Chiralpak IA-3 (250 mm × 4.6 mm, 3 μm—analytical) (250 mm × 10 mm—semipreparative) CSP: amylose-tris(3,5-dimethylphenylcarbamate) | bulk | paroxetine, impurities A, C, D, E, I, impurity G, sesamol (achiral) | [53] |
| mobile phase: water:acetonitrile:diethylamine (55:45:0.01, v/v/v), flow rate = 0.8 mL/min, 25 °C, UV detection 230 nm | Lux Cellulose-1 (150 × 4.6 mm, 5 µm) CSP: cellulose tris(3,5-dimethylphenylcarbamate) | bulk, tablets | escitalopram, R-ciralopram, impurities C, D, E | [55] |
| NP method: mobile phase: n-hexane:ethanol:diethylamine 90:10:0.1 (v/v/v), flow rate = 1.0 mL/min, 30 °C; UV detection 240 nm RP method: mobile phase: ethanol:water:diethylamine (70:30:0.1, v/v/v), flow rate = 0.4 mL/min, 30 °C, UV detection 240 nm | Chiralcel OJ-H (250 mm × 4.6 mm, 5 μm) CSP: cellulose tris(4-methylbenzoate) | bulk | escitalopram, impurities A, B, C, H, and K | [56] |
| mobile phase: acetonitrile:trifluoracetic acid (0.05%)-gradient elution, flow rate = 1.0 mL/min, 40 °C; UV detection 240 nm | Lux Cellulose-2 (250 mm × 4.6 mm, 3 μm) CSP: cellulose tris(3-chloro-4-methylphenylcarbamate) | bulk, tablets | rosuvastatin, impurities A, B, C, D, G, FP-A, FP-B | [57] |
| mobile phase: methanol:water:diethylamine (80:20:0.2, v/v/v), flow rate = 0.45 mL/min, 45 °C, UV detection 215 nm | Lux Cellulose-2 (250 ×4.6 mm, 5 μm) CSP: cellulose tris(3-chloro-4-methylphenylcarbamate) | bulk, tablets | vildagliptin, R-vildagliptin, dehydroxy-vildagliptin, vildagliptin-dimer, vildalgliptin-amide, vildalgliptin-diketopiperazine (achiral) | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papp, L.A.; Szabó, Z.I.; Hancu, G.; Farczádi, L.; Mircia, E. Comprehensive Review on Chiral Stationary Phases in Single-Column Simultaneous Chiral–Achiral HPLC Separation Methods. Molecules 2024, 29, 1346. https://doi.org/10.3390/molecules29061346
Papp LA, Szabó ZI, Hancu G, Farczádi L, Mircia E. Comprehensive Review on Chiral Stationary Phases in Single-Column Simultaneous Chiral–Achiral HPLC Separation Methods. Molecules. 2024; 29(6):1346. https://doi.org/10.3390/molecules29061346
Chicago/Turabian StylePapp, Lajos Attila, Zoltán István Szabó, Gabriel Hancu, Lénárd Farczádi, and Eleonora Mircia. 2024. "Comprehensive Review on Chiral Stationary Phases in Single-Column Simultaneous Chiral–Achiral HPLC Separation Methods" Molecules 29, no. 6: 1346. https://doi.org/10.3390/molecules29061346
APA StylePapp, L. A., Szabó, Z. I., Hancu, G., Farczádi, L., & Mircia, E. (2024). Comprehensive Review on Chiral Stationary Phases in Single-Column Simultaneous Chiral–Achiral HPLC Separation Methods. Molecules, 29(6), 1346. https://doi.org/10.3390/molecules29061346









