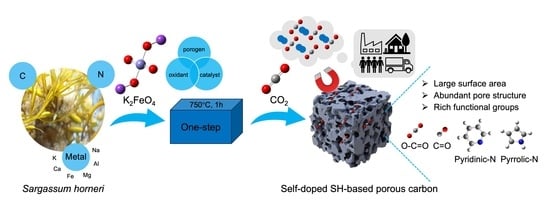
Turn Waste Golden Tide into Treasure: Bio-Adsorbent Synthesis for CO2 Capture with K2FeO4 as Catalytic Oxidative Activator
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Adsorbents
2.1.1. BET Analysis
2.1.2. SEM and TEM Analysis
2.1.3. FTIR Analysis
2.1.4. XPS Analysis
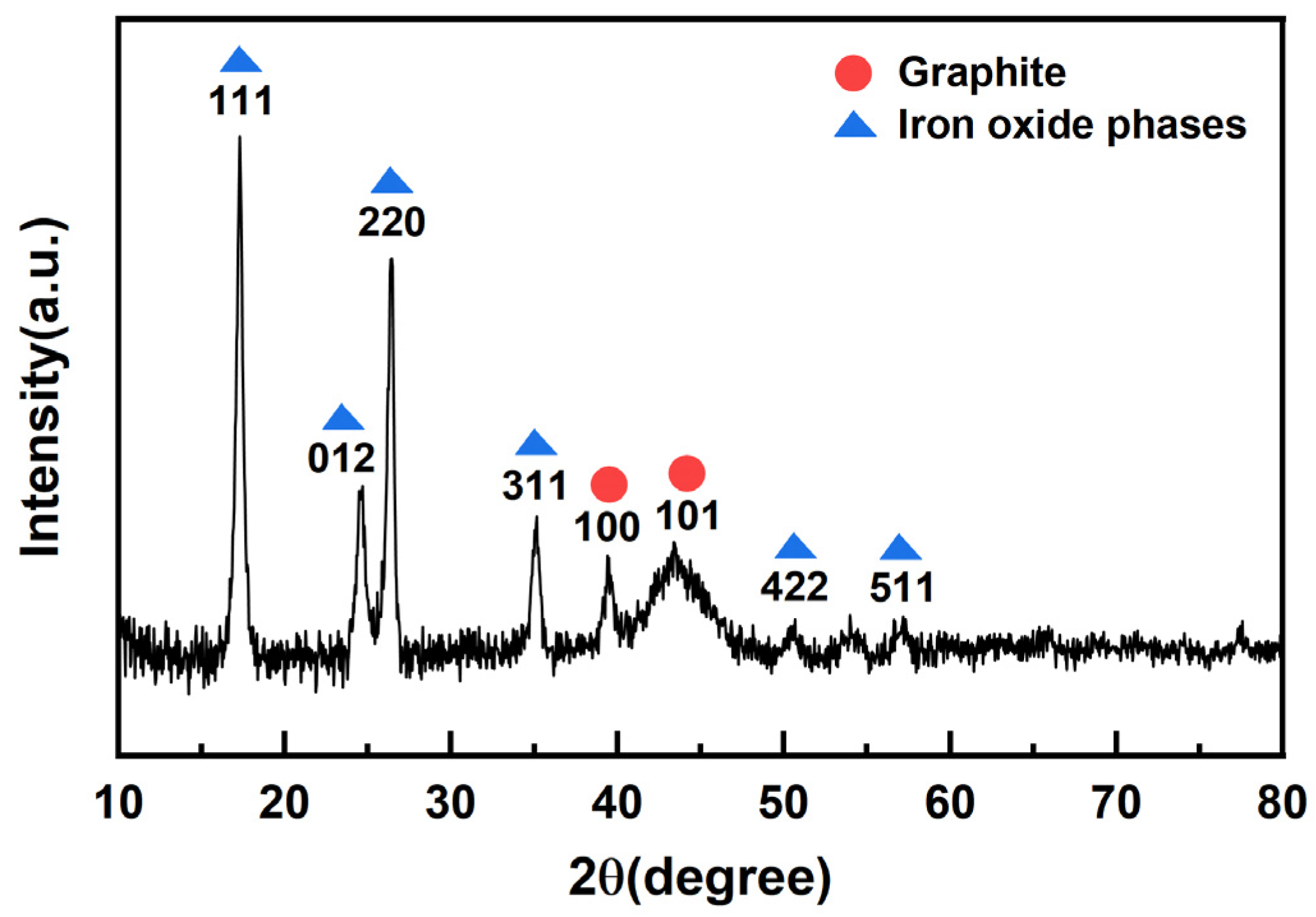
2.1.5. XRD Analysis
2.1.6. RAM Analysis
2.2. CO2 Adsorption Capacity
2.2.1. CO2 Uptake Capacity with Different Temperatures
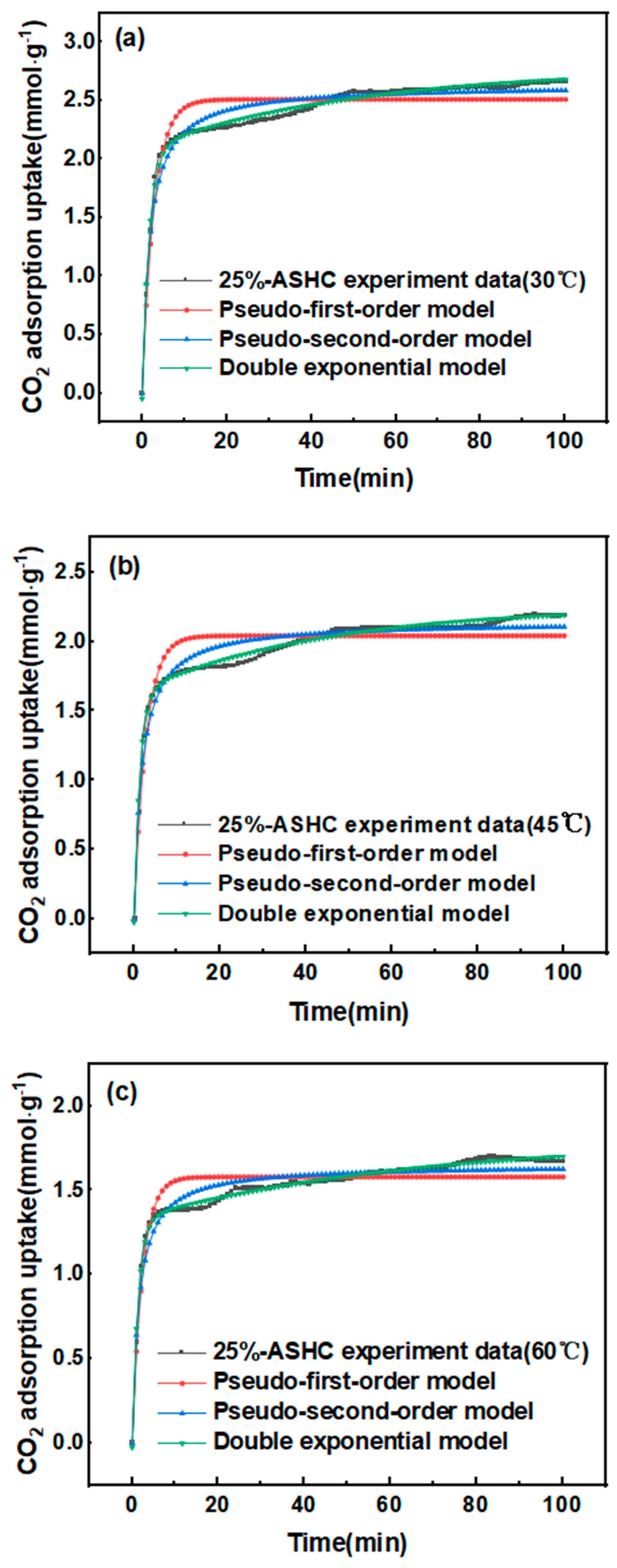
2.2.2. Adsorption Kinetics with Different Temperatures
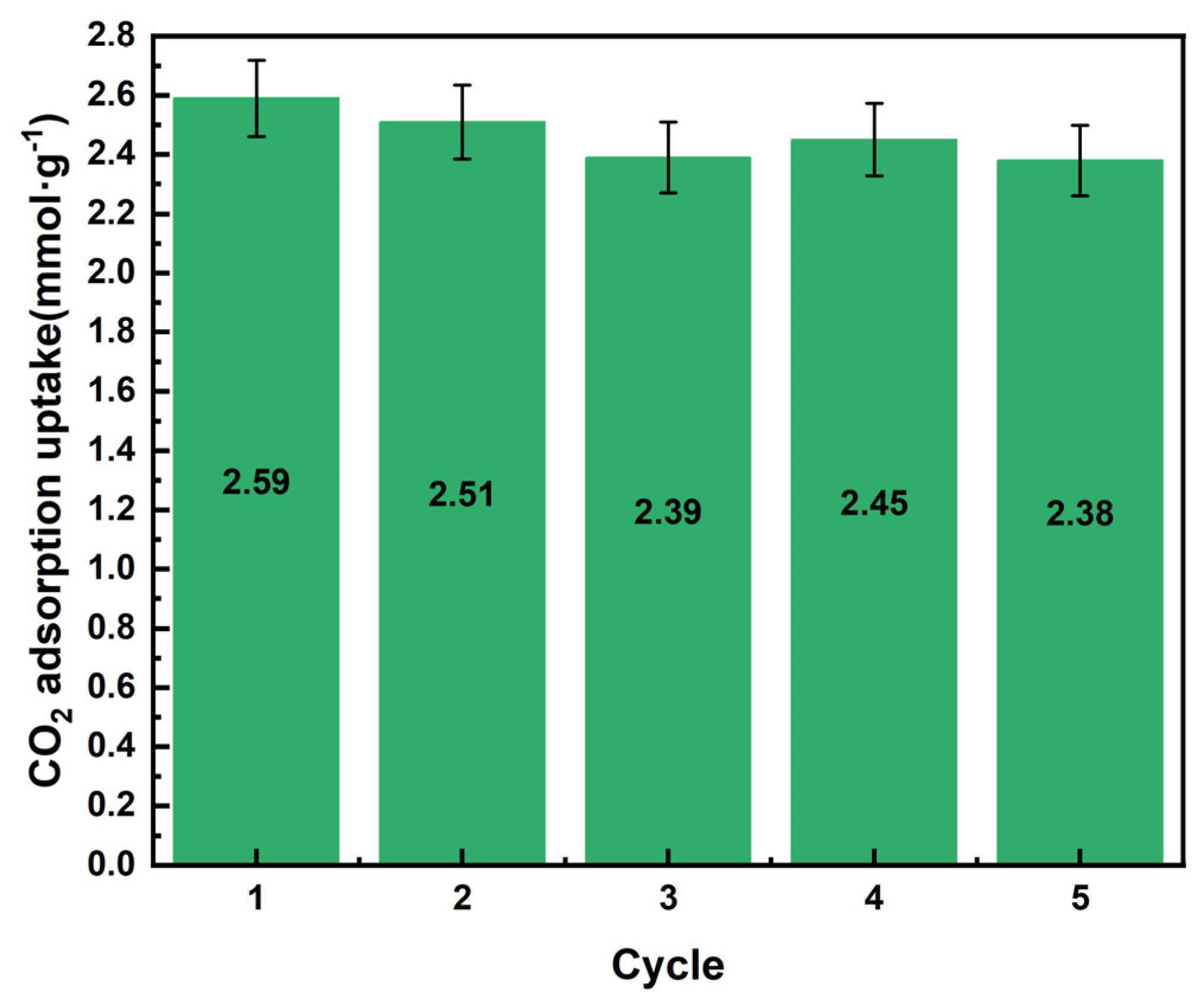
2.2.3. Adsorbent Regeneration
3. Materials and Methods
3.1. Materials
3.2. Preparation of Samples
3.3. Characterization of Samples
3.4. CO2 Adsorption Measurements
3.5. CO2 Adsorption Kinetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smetacek, V.; Zingone, A. Green and Golden Seaweed Tides on the Rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, R.E.; Medina-Valmaseda, A.E.; Blanchon, P.; Monroy-Velázquez, L.V.; Almazán-Becerril, A.; Delgado-Pech, B.; Vásquez-Yeomans, L.; Francisco, V.; García-Rivas, M.C. Faunal Mortality Associated with Massive Beaching and Decomposition of Pelagic Sargassum. Mar. Pollut. Bull. 2019, 146, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The Great Atlantic Sargassum Belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.K.; Lee, S.; Ha, D.; Park, C. Sargassum Golden Tides in the Shinan-Gun and Jeju Island, Korea. Korean J. Fish. Aquat. Sci. 2016, 49, 689–693. [Google Scholar] [CrossRef]
- Lapointe, B.; Burkholder, J.; Van Alstyne, K. Harmful Macroalgal Blooms in a Changing World: Causes, Impacts, and Management. In Harmful Algal Blooms: A Compendium Desk Reference; Wiley: Hoboken, NJ, USA, 2018; pp. 515–560. ISBN 978-1-118-99465-8. [Google Scholar]
- Resiere, D.; Kallel, H.; Florentin, J.; Banydeen, R.; Compton, K.; Gueye, P.; Mehdaoui, H.; Neviere, R. Sargassum Seaweed in the Caribbean: A Major Public Health Problem Still Unsolved. J. Glob. Health 2023, 13, 03017. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Guo, R.; Wu, L.; An, D.; Cong, M.; Qin, S.; Li, X. High-Resolution Satellite Observations of a New Hazard of Golden Tides Caused by Floating Sargassum in Winter in the Yellow Sea. IEEE Geosci. Remote Sens. Lett. 2017, 14, 1815–1819. [Google Scholar] [CrossRef]
- Su, L.; Shan, T.; Pang, S.; Jing, L. Analyses of the Genetic Structure of Sargassum Horneri in the Yellow Sea: Implications of the Temporal and Spatial Relations among Floating and Benthic Populations. J. Appl. Phycol. 2018, 30, 1417–1424. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Wang, M.; Shang, S.; Wilson, C. Floating Algae Blooms in the East China Sea. Geophys. Res. Lett. 2017, 44, 11,501–11,509. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Wang, Y.; Jin, Z.; Moejes, F.; Sun, S. Insights on the Sargassum Horneri Golden Tides in the Yellow Sea Inferred from Morphological and Molecular Data. Limnol. Oceanogr. 2018, 63, 1762–1773. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Barnes, B.; Lapointe, B.; Chen, Y.; Xie, Y.; Wang, M. Climate and Anthropogenic Controls of Seaweed Expansions in the East China Sea and Yellow Sea. Geophys. Res. Lett. 2022, 49, e2022GL098185. [Google Scholar] [CrossRef]
- Fidai, Y.A.; Machado, C.B.; Almela, V.D.; Oxenford, H.A.; Jayson-Quashigah, P.-N.; Tonon, T.; Dash, J. Innovative Spectral Characterisation of Beached Pelagic Sargassum towards Remote Estimation of Biochemical and Phenotypic Properties. Sci. Total Environ. 2024, 914, 169789. [Google Scholar] [CrossRef]
- Mincer, T.J.; Bos, R.P.; Zettler, E.R.; Zhao, S.; Asbun, A.A.; Orsi, W.D.; Guzzetta, V.S.; Amaral-Zettler, L.A. Sargasso Sea Vibrio Bacteria: Underexplored Potential Pathovars in a Perturbed Habitat. Water Res. 2023, 242, 120033. [Google Scholar] [CrossRef]
- Fang, R.-E.; Wei, Y.-J.; Fang, S.-Y.; Huang, C.-H. Effects of Sargassum-Derived Oligosaccharides, Polysaccharides and Residues on Ameliorating Enteritis and Dysbiosis in a Murine Model of Food Allergy. J. Funct. Foods 2023, 110, 105844. [Google Scholar] [CrossRef]
- Kar, T.; González-Escobar, C.; Ramos-Hernández, J.J.; Casales-Díaz, M.; Flores-Rodríguez, M.F.; Pérez, R.; Kesarla, M.K. A Comprehensive Analysis of Sargassum Natans-Derived Inorganic Carbon Composite for Electrochemical Charge Storage. J. Energy Storage 2024, 82, 110600. [Google Scholar] [CrossRef]
- Rekha, A.; Srinivasan, L.; Pavithra, S.; Gomathi, T.; Sudha, P.N.; Lavanya, G.; Arumugam, N.; Vidhya, A. Biosorption Efficacy Studies of Sargassum Wightii and Its Biochar on the Removal of Chromium from Aqueous Solution. J. Taiwan Inst. Chem. Eng. 2023, 105241. [Google Scholar] [CrossRef]
- Saldarriaga-Hernández, S.; Hernández-Vargas, G.; Iqbal, H.; Barceló, D.; Parra, R. Bioremediation Potential of Sargassum Sp. Biomass to Tackle Pollution in Coastal Ecosystems: Circular Economy Approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef]
- Santos, S.; Ungureanu, G.; Volf, I.; Boaventura, R.; Botelho, C. Macroalgae Biomass as Sorbent for Metal Ions. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018; pp. 69–112. ISBN 978-0-444-63774-1. [Google Scholar]
- González Fernández, L.A.; Navarro Frómeta, A.E.; Carranza Álvarez, C.; Flores Ramírez, R.; Díaz Flores, P.E.; Castillo Ramos, V.; Sánchez Polo, M.; Carrasco Marín, F.; Medellín Castillo, N.A. Valorization of Sargassum Biomass as Potential Material for the Remediation of Heavy-Metals-Contaminated Waters. Int. J. Environ. Res. Public Health 2023, 20, 2559. [Google Scholar] [CrossRef] [PubMed]
- Jafarian, S.; Bolouk, A.M.L.; Norouzian, R.; Taghavi, S.; Mousavi, F.; Kianpour, E.; Signoretto, M. Sargassum Macro-Algae-Derived Activated Bio-Char as a Sustainable and Cost-Effective Adsorbent for Cationic Dyes: A Joint Experimental and DFT Study. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132397. [Google Scholar] [CrossRef]
- Ding, S.; Liu, Y. Adsorption of CO2 from Flue Gas by Novel Seaweed-Based KOH-Activated Porous Biochars. Fuel 2020, 260, 116382. [Google Scholar] [CrossRef]
- Ying, H.; Zeng, G.; He, Y.; Hou, Y.; Ai, N. Enhanced Assembling of N-and-K-Riched Macroalgae as Carbon Adsorbent for CO2 Capture with Ni(NO3)2/KOH as Co-Catalysts. Molecules 2023, 28, 6242. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K. Potassium Ferrate(VI): An Environmentally Friendly Oxidant. Adv. Environ. Res. 2002, 6, 143–156. [Google Scholar] [CrossRef]
- Dong, D.; Xiao, Y.; Xing, J. Facile Wet Mechanochemistry Coupled K2FeO4 Activation to Prepare Functional Coal-Derived Hierarchical Porous Carbon for Supercapacitors. J. Clean. Prod. 2023, 428, 139474. [Google Scholar] [CrossRef]
- Cheng, A.; Wang, X.; Liu, X.; He, C. Wet-Process-Modified Blue-Green Algae Biochar by K2FeO4 for the Efficient Adsorption of Cr(VI) from Water. Processes 2023, 11, 1489. [Google Scholar] [CrossRef]
- Pei, T.; Shi, F.; Liu, C.; Lu, Y.; Lin, X.; Hou, D.; Yang, S.; Li, J.; Zheng, Z.; Zheng, Y. Bamboo-Derived Nitrogen-Doping Magnetic Porous Hydrochar Coactivated by K2FeO4 and CaCO3 for Phenol Removal: Governing Factors and Mechanisms. Environ. Pollut. 2023, 331, 121871. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ni, L.; Gao, Q.; Ren, H.; Su, M.; Hou, Y.; Liu, Z. Activated Carbon with Ultrahigh Specific Surface Derived from Bamboo Shoot Shell through K2FeO4 Oxidative Pyrolysis for Adsorption of Methylene Blue. Molecules 2023, 28, 3410. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly Porous Graphitic Biomass Carbon as Advanced Electrode Materials for Supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Ai, N.; Lou, S.; Lou, F.; Xu, C.; Wang, Q.; Zeng, G. Facile Synthesis of Macroalgae-Derived Graphene Adsorbents for Efficient CO2 Capture. Process Saf. Environ. Prot. 2021, 148, 1048–1059. [Google Scholar] [CrossRef]
- Zeng, G.; Lou, S.; Lou, F.; Du, M.; Luo, H.; Ai, N. Insights into Forming Behavior and CO2 Adsorption Properties of Graphene from Sargassum Horneri by Fe(NO3)3/KOH Activation. J. Chem. Technol. Biotechnol. 2022, 97, 2844–2851. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- de Souza, L.K.C.; Wickramaratne, N.P.; Ello, A.S.; Costa, M.J.F.; da Costa, C.E.F.; Jaroniec, M. Enhancement of CO2 Adsorption on Phenolic Resin-Based Mesoporous Carbons by KOH Activation. Carbon 2013, 65, 334–340. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, L.-Z.; Zhou, M.-Q.; Guan, H.; Qiao, S.; Antonietti, M.; Titirici, M.-M. Nitrogen-Containing Hydrothermal Carbons with Superior Performance in Supercapacitors. Adv. Mater. 2010, 22, 5202–5206. [Google Scholar] [CrossRef]
- Shi, S.; Liu, Y. Nitrogen-Doped Activated Carbons Derived from Microalgae Pyrolysis by-Products by Microwave/KOH Activation for CO2 Adsorption. Fuel 2021, 306, 121762. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH Activation of Carbon-Based Materials for Energy Storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Rash, T.; Firlej, L.; Kuchta, B.; Yu, P.; Suppes, G.; Wexler, C.; Pfeifer, P. Nanospace Engineering of KOH Activated Carbon. Nanotechnology 2011, 23, 015401. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Tian, K.; He, Y.-R.; Jiang, H.; Yu, H.-Q. High-Yield Harvest of Nanofibers/Mesoporous Carbon Composite by Pyrolysis of Waste Biomass and Its Application for High Durability Electrochemical Energy Storage. Environ. Sci. Technol. 2014, 48, 13951–13959. [Google Scholar] [CrossRef] [PubMed]
- Da, Y.; Xu, M.; Ma, J.; Gao, P.; Zhang, X.; Yang, G.; Wu, J.; Song, C.; Long, L.; Chen, C. Remediation of Cadmium Contaminated Soil Using K2FeO4 Modified Vinasse Biochar. Ecotoxicol. Environ. Saf. 2023, 262, 115171. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, J.; Li, D.; Chen, H.; Chen, Y. 5 Ultramicropore-Rich Renewable Porous Carbon from Biomass Tar with Excellent Adsorption Capacity and Selectivity for CO2 Capture. Chem. Eng. J. 2019, 373, 171–178. [Google Scholar] [CrossRef]
- Hong, S.-M.; Yoon, H.J.; Choi, Y.; Cho, Y.-Z.; Mun, S.; Pol, V.G.; Lee, K.B. Solving Two Environmental Problems Simultaneously: Scalable Production of Carbon Microsheets from Structured Packing Peanuts with Tailored Microporosity for Efficient CO2 Capture. Chem. Eng. J. 2020, 379, 122219. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Wang, C.; Wei, W.; Liu, Z.; Tian, Y.; Zong, P.; Qiao, Y.; Qin, S. Golden Seaweed Tides from Beach Inundations as a Valuable Sustainable Fuel Resource: Fast Pyrolysis Characteristics, Product Distribution and Pathway Study on Sargassum Horneri Based on Model Compounds. Algal Res. 2020, 48, 101888. [Google Scholar] [CrossRef]
- Tiwari, D.; Goel, C.; Bhunia, H.; Bajpai, P.K. Melamine-Formaldehyde Derived Porous Carbons for Adsorption of CO2 Capture. J. Environ. Manag. 2017, 197, 415–427. [Google Scholar] [CrossRef]
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Adsorption of CO2 on KOH Activated, N-Enriched Carbon Derived from Urea Formaldehyde Resin: Kinetics, Isotherm and Thermodynamic Studies. Appl. Surf. Sci. 2018, 439, 760–771. [Google Scholar] [CrossRef]
- Lahuri, A.H. Comparative Studies on Adsorption Isotherm and Kinetic for CO2 Capture Using Iron Oxide Impregnated Activated Carbon. Catal. Today 2023, 418, 114111. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhang, M.; Yao, B.; Li, Y.; Huang, L.; Li, C.; Shi, G. Water-Enhanced Oxidation of Graphite to Graphene Oxide with Controlled Species of Oxygenated Groups. Chem. Sci. 2016, 7, 1874–1881. [Google Scholar] [CrossRef]
- Kumar, S.; Baruah, B.; Kumar, A. Tunable Degree of Oxidation through Variation of H2O2 Concentration and Its Effect on Structural, Optical and Supercapacitive Properties of Graphene Oxide Powders Synthesized Using Improved Method. Mater. Today Commun. 2017, 13, 26–35. [Google Scholar] [CrossRef]
- Phadungbut, P.; Koo-amornpattana, W.; Bumroongsri, P.; Ratchahat, S.; Kunthakudee, N.; Jonglertjunya, W.; Chalermsinsuwan, B.; Hunsom, M. Adsorptive Purification of CO2/H2 Gas Mixtures of Spent Disposable Wooden Chopstick-Derived Activated Carbon: Optimal Synthesis Condition. Sep. Purif. Technol. 2022, 291, 120948. [Google Scholar] [CrossRef]
- Liu, X.; Sun, C.; Liu, H.; Tan, W.H.; Wang, W.; Snape, C. Developing Hierarchically Ultra-Micro/Mesoporous Biocarbons for Highly Selective Carbon Dioxide Adsorption. Chem. Eng. J. 2019, 361, 199–208. [Google Scholar] [CrossRef]
- Singh, M.G.; Lakhi, K.S.; Park, D.-H.; Srivastava, P.; Naidu, R.; Vinu, A. Facile One-Pot Synthesis of Activated Porous Biocarbons with a High Nitrogen Content for CO2 Capture. ChemNanoMat 2018, 4, 281–290. [Google Scholar] [CrossRef]
- Alazmi, A.; Nicolae, S.A.; Modugno, P.; Hasanov, B.E.; Titirici, M.M.; Costa, P.M.F.J. Activated Carbon from Palm Date Seeds for CO2 Capture. Int. J. Environ. Res. Public Health 2021, 18, 12142. [Google Scholar] [CrossRef] [PubMed]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Metal Incorporated Biochar as a Potential Adsorbent for High Capacity CO2 Capture at Ambient Condition. J. CO2 Util. 2018, 26, 281–293. [Google Scholar] [CrossRef]
- Vargas, D.P.; Giraldo, L.; Silvestre-Albero, J.; Moreno-Piraján, J.C. CO2 Adsorption on Binderless Activated Carbon Monoliths. Adsorption 2011, 17, 497–504. [Google Scholar] [CrossRef]
- Boonpoke, A.; Chiarakorn, S.; Laosiripojana, N.; Towprayoon, S.; Chidthaisong, A. Synthesis of Activated Carbon and MCM-41 from Bagasse and Rice Husk and Their Carbon Dioxide Adsorption Capacity. J. Sustain. Energy Environ. 2011, 2, 77–81. [Google Scholar]
- Isahak, W.N.R.W.; Hasan, S.Z.; Ramli, Z.A.C.; Ba-Abbad, M.M.; Yarmo, M.A. Enhanced Physical and Chemical Adsorption of Carbon Dioxide Using Bimetallic Copper–Magnesium Oxide/Carbon Nanocomposite. Res. Chem. Intermed. 2018, 44, 829–841. [Google Scholar] [CrossRef]
- Loganathan, S.; Tikmani, M.; Mishra, A.; Ghoshal, A. Amine Tethered Pore-Expanded MCM-41 for CO2 Capture: Experimental, Isotherm and Kinetic Modeling Studies. Chem. Eng. J. 2016, 303, 89–99. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, C.; Xu, Q.; Zou, X.; Cheng, H.; Lu, X. In Situ XRD, Raman Characterization, and Kinetic Study of CO2 Capture by Alkali Carbonate-Doped Na4SiO4. Separations 2022, 9, 428. [Google Scholar] [CrossRef]
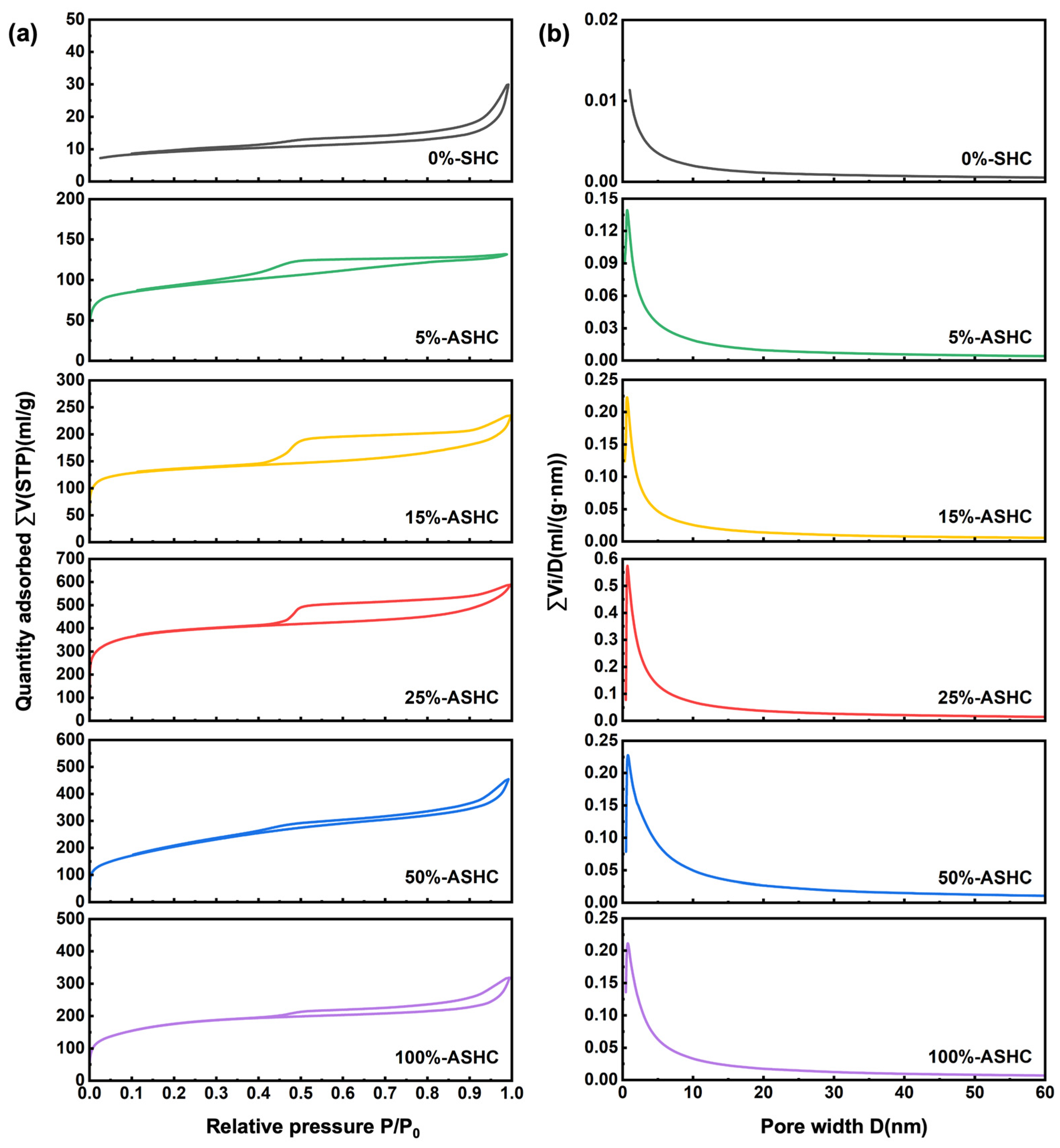

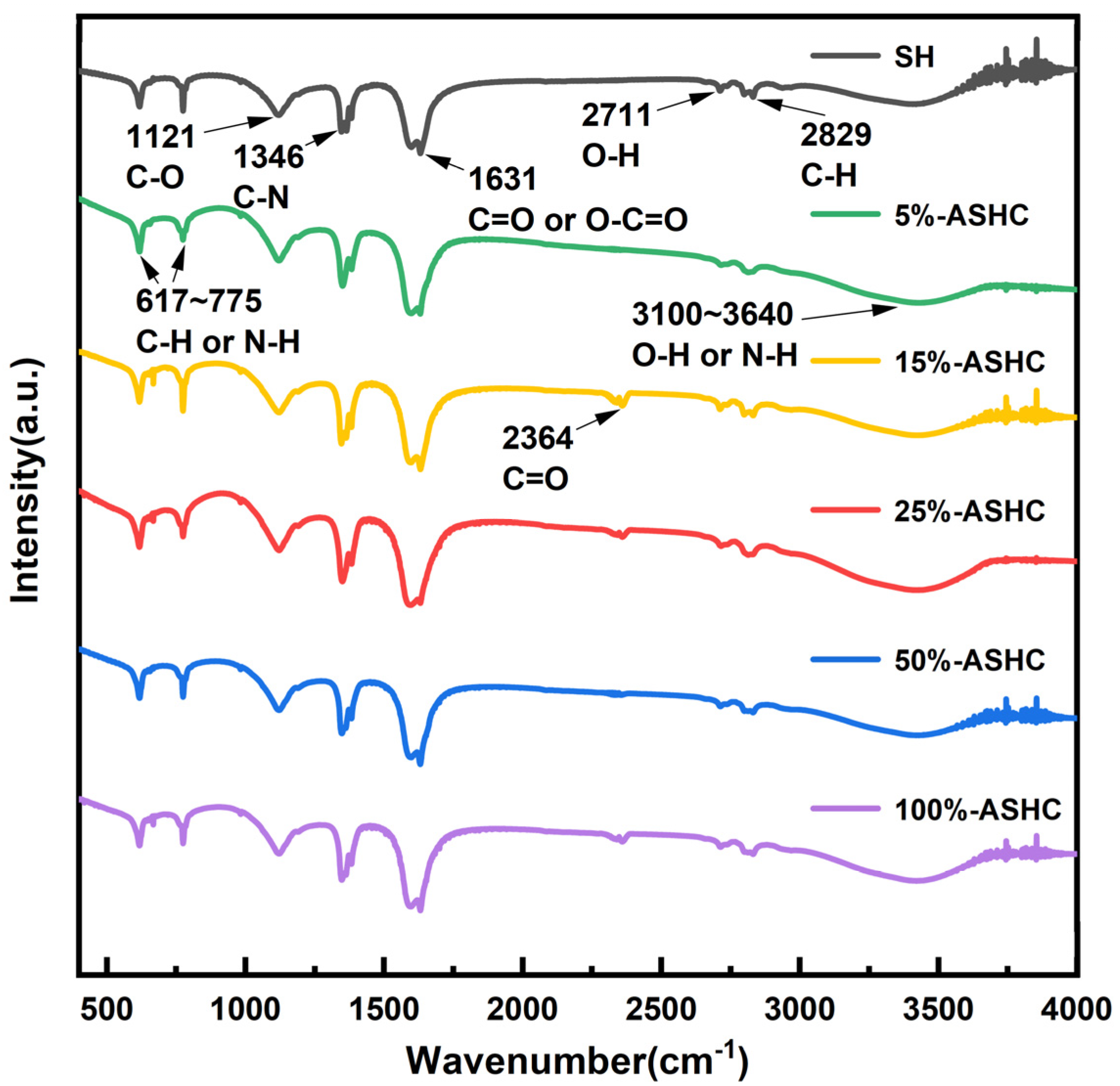
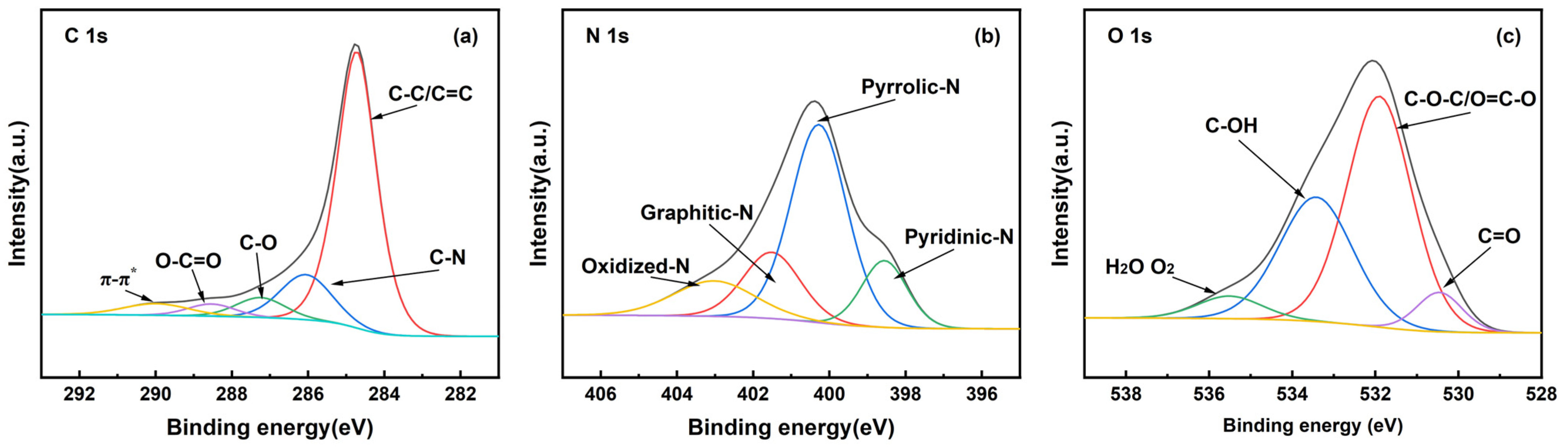

| Adsorbents | Surface Area | Pore Volume | Average Pore Diameter (nm) | Microporous Average Pore Diameter (nm) | ||
|---|---|---|---|---|---|---|
| SBET (m2·g−1) | Vtotal (cm3·g−1) | Vmicro (cm3·g−1) | Vmicro /Vtolal | |||
| 0%-SHC | 30.3827 | 0.0446 | 0.0133 | 0.2982 | 5.8718 | 1.2198 |
| 5%-ASHC | 297.1456 | 0.2046 | 0.1049 | 0.5127 | 2.7542 | 0.6864 |
| 15%-ASHC | 417.9733 | 0.3352 | 0.1539 | 0.4591 | 3.2079 | 0.6570 |
| 25%-ASHC | 1245.0812 | 0.8827 | 0.5682 | 0.6437 | 2.8358 | 0.7266 |
| 50%-ASHC | 730.5373 | 0.6926 | 0.3284 | 0.4742 | 3.7923 | 0.7994 |
| 100%-ASHC | 586.6224 | 0.4702 | 0.2379 | 0.5060 | 3.2062 | 0.7480 |
| Sample | C=O | C-O-C/O=C-O | C-OH | H2O-O2 |
|---|---|---|---|---|
| 25%-ASHC | 6.74 | 58.98 | 28.51 | 5.77 |
| Samples | CO2 Adsorption Capacity (mmol·g−1) | ||
|---|---|---|---|
| 30 °C | 45 °C | 60 °C | |
| 0%-SHC | 0.94 | 0.78 | 0.63 |
| 25%-ASHC | 2.67 | 2.17 | 1.70 |
| Feedstock | Activation | SBET (m2·g−1) | Vtotal (cm3·g−1) | Vmicro (cm3·g−1) | CO2 Uptakes (mmol·g−1) | Ref. | |
|---|---|---|---|---|---|---|---|
| 25 °C/1 bar | 30 °C/1 bar | ||||||
| Wooden chopstick | KOH | N/A | N/A | N/A | 2.63 | N/A | [47] |
| Dried rice husk | KOH/PEI | 1190 | 0.777 | 0.422 | 1.90 | N/A | [48] |
| Arundo donax | KOH/ZnCl2 | 982 | 0.62 | N/A | 2.20 | N/A | [49] |
| Palm date seeds | H3PO4 | 1439 | 0.60 | N/A | 4.40 | N/A | [50] |
| Walnut shell | Mg(NO3)2 | 292 | 0.157 | 0.118 | 1.86 | N/A | [51] |
| Coconut | H3PO4 | 1322 | 0.61 | 0.49 | N/A | 3.7 | [52] |
| Rice husk | ZnCl2/HCl | 927 | 0.56 | N/A | N/A | 1.3 | [53] |
| Nypha fruticans | Mg(NO3)2/Cu(NO3)2 | 727.7 | 0.50 | 0.26 | N/A | 1.91 | [54] |
| Sargassum horneri | K2FeO4 | 1245 | 0.8827 | 0.5682 | N/A | 2.67 | This work |
| Kinetic Models | Parameters | Adsorption Temperatures | ||
|---|---|---|---|---|
| 30 °C | 45 °C | 60 °C | ||
| Pseudo-first-order | qm,exp (mmol·g−1) | 2.67 | 2.17 | 1.70 |
| qe,cal (mmol·g−1) | 2.51 | 2.04 | 1.58 | |
| k1 (min−1) | 0.3549 | 0.3668 | 0.4229 | |
| R2 | 0.8499 | 0.8029 | 0.8190 | |
| E% | 2.87 | 2.66 | 1.90 | |
| Pseudo-second-order | qe,cal (mmol·g−1) | 2.63 | 2.14 | 1.65 |
| k2 (g/(mg·min)) | 0.2092 | 0.2577 | 0.3860 | |
| R2 | 0.9453 | 0.9283 | 0.9287 | |
| E% | 2.63 | 5.68 | 1.57 | |
| qe,cal (mmol·g−1) | 2.78 | 2.25 | 1.80 | |
| Double Exponential | A1 | 2.1343 | 1.6579 | 1.3535 |
| k3 | 0.6026 | 0.7312 | 0.7211 | |
| A2 | 0.6903 | 0.6208 | 0.4737 | |
| k4 | 0.0197 | 0.0227 | 0.0157 | |
| R2 | 0.9913 | 0.9905 | 0.9920 | |
| E% | 1.60 | 3.58 | 4.47 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, H.; Jia, C.; Zeng, G.; Ai, N. Turn Waste Golden Tide into Treasure: Bio-Adsorbent Synthesis for CO2 Capture with K2FeO4 as Catalytic Oxidative Activator. Molecules 2024, 29, 1345. https://doi.org/10.3390/molecules29061345
Ying H, Jia C, Zeng G, Ai N. Turn Waste Golden Tide into Treasure: Bio-Adsorbent Synthesis for CO2 Capture with K2FeO4 as Catalytic Oxidative Activator. Molecules. 2024; 29(6):1345. https://doi.org/10.3390/molecules29061345
Chicago/Turabian StyleYing, Huijuan, Chenglin Jia, Ganning Zeng, and Ning Ai. 2024. "Turn Waste Golden Tide into Treasure: Bio-Adsorbent Synthesis for CO2 Capture with K2FeO4 as Catalytic Oxidative Activator" Molecules 29, no. 6: 1345. https://doi.org/10.3390/molecules29061345
APA StyleYing, H., Jia, C., Zeng, G., & Ai, N. (2024). Turn Waste Golden Tide into Treasure: Bio-Adsorbent Synthesis for CO2 Capture with K2FeO4 as Catalytic Oxidative Activator. Molecules, 29(6), 1345. https://doi.org/10.3390/molecules29061345