Hydrated Electron Dynamics and Stimulated Raman Scattering in Water Induced by Ultrashort Laser Pulses
Abstract
1. Introduction
2. Result
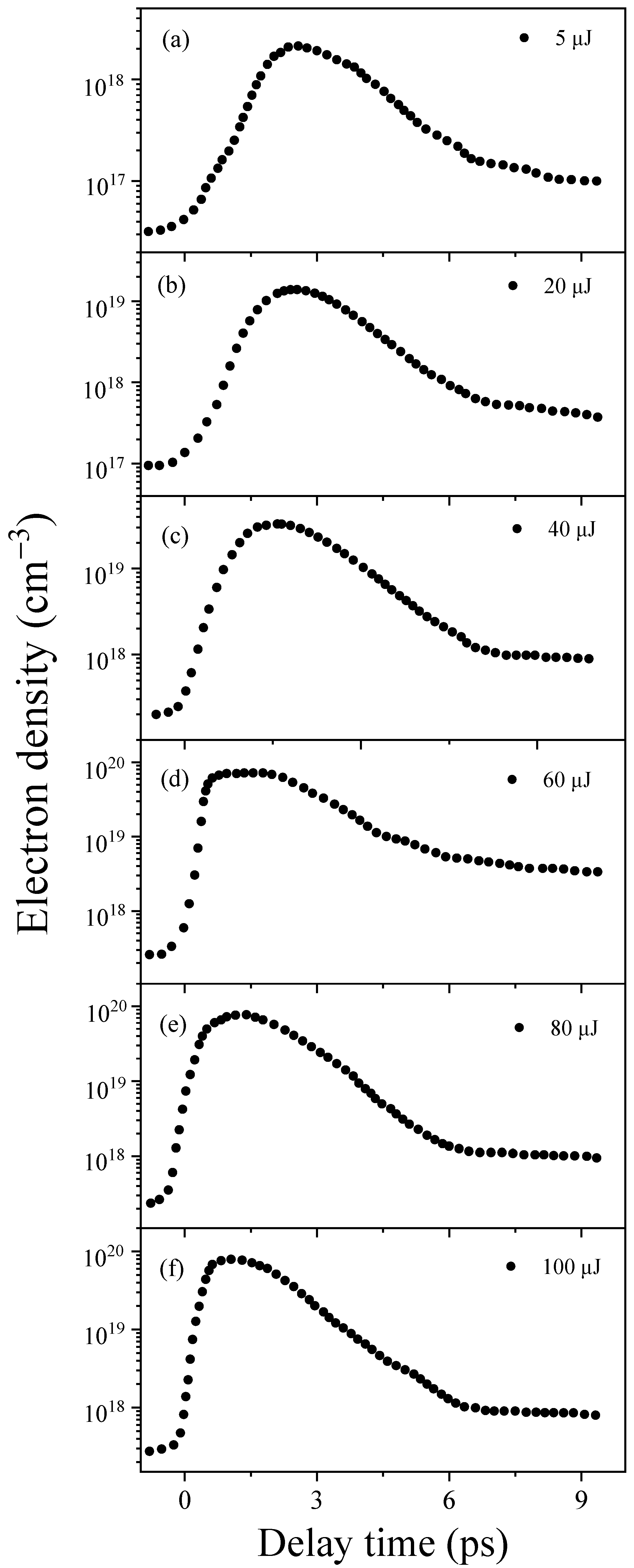
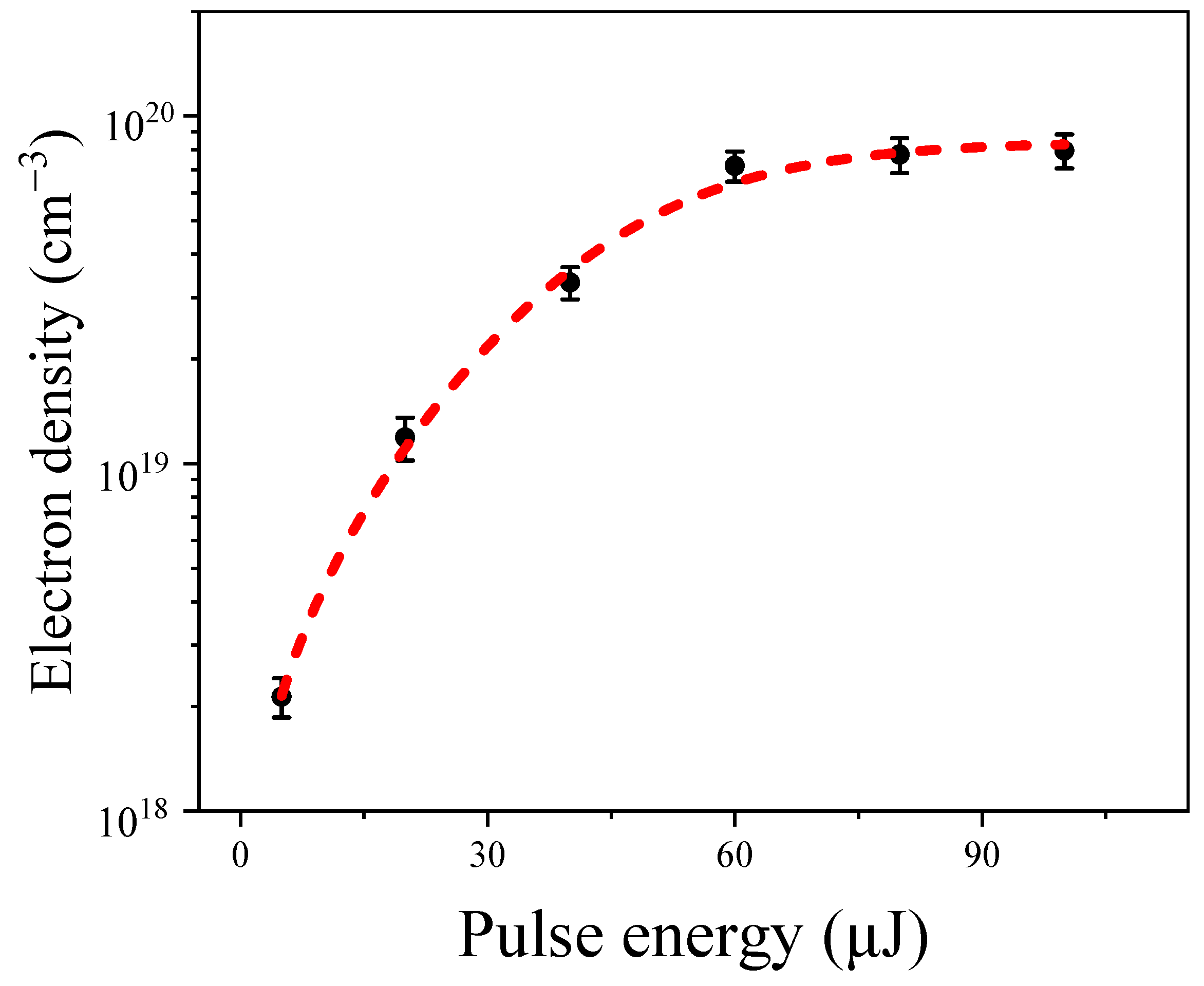
2.1. The Dynamics of Hydrated Electrons in Water
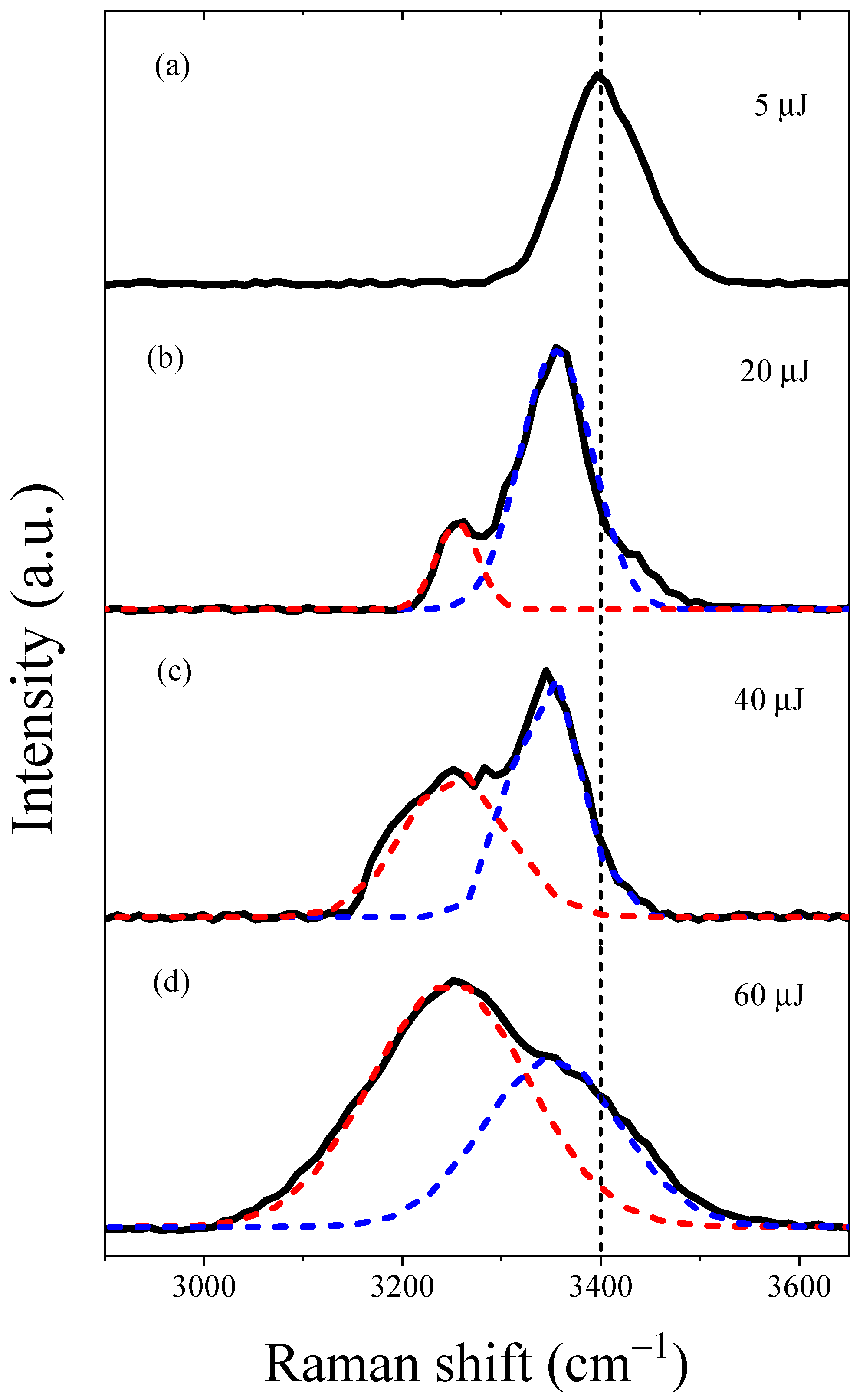
2.2. The Forward SRS Spectrum in Water
3. Discussion
- (i)
- The anomalous Raman shift at 3350 cm−1 is attributed to the enhanced Raman cross-section of the OH groups in water molecules, corresponding to an increasing region of electron generation in the hydrated electron dynamics process. The distinctive features of the SRS spectrum and the similarities in the vibrational spectrum to the vibrational spectra of water anion clusters indicate that the excess electrons effectively generated in the forward region strongly enhanced the Raman cross-section of the water molecules. The enhancement of Raman scattering occurred in the presence of strong excitation laser fields, prior to the saturated region and recombination of excess electrons. Therefore, the excess electrons responsible for Raman enhancement were not fully hydrated electrons.
- (ii)
- The anomalous Raman shift at 3260 cm−1 is attributed to the contribution of hydrated electrons in water. As shown in Figure 3b–d, with an increase in input pump energy, the intensity of the anomalous Raman shift at 3260 cm−1 gradually rises, corresponding to a non-linear increase in the hydrated electron density as incident energy rises, eventually reaching a saturation region. Furthermore, under the present experimental conditions, we noted that the saturated hydrated electrons reached about 7.17 × 1019 cm−3 under a 60 µJ input pump energy. The ratio of water molecules to electrons was about 465:1, indicating that the interaction with electrons drastically enhanced the Raman cross-section of the OH groups in the water molecules.
4. Experiments
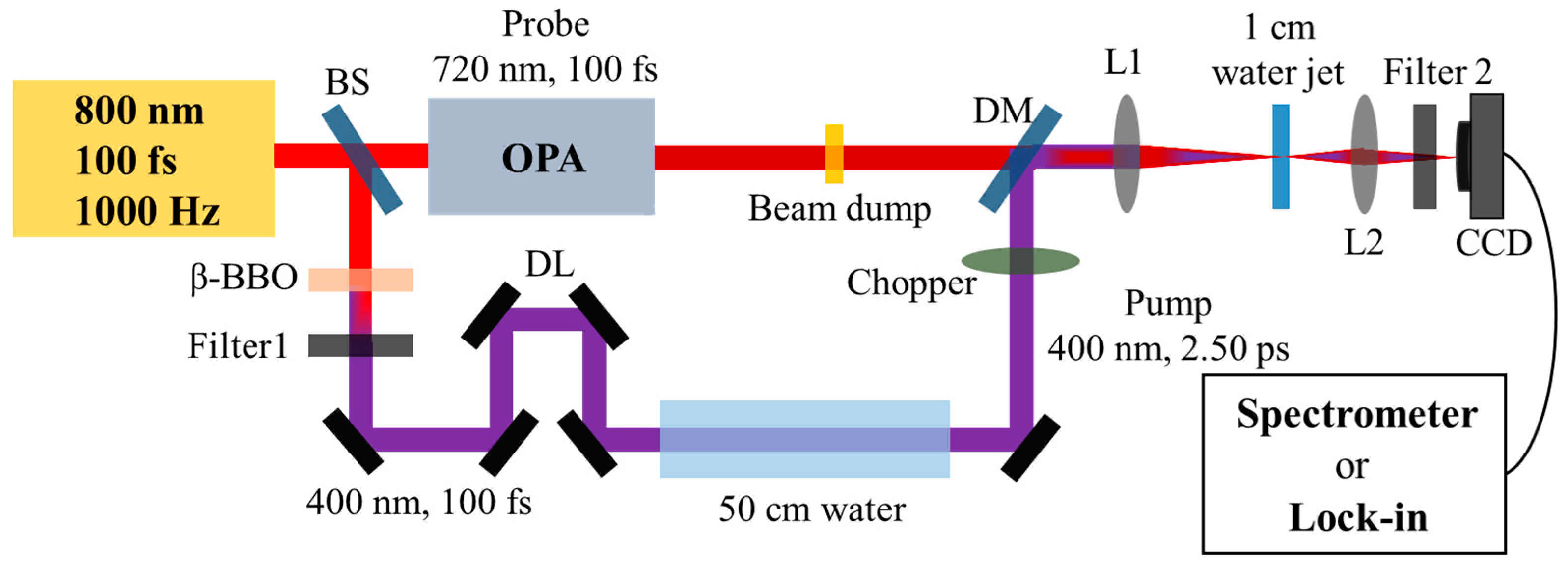
4.1. Experimental Measured the Dynamic of Hydrated Electrons in Water
4.2. Experimental Measured the Forward SRS in Water
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weyl, W. Ueber Metallammonium—Verbindungen. Ann. Phys. 1864, 197, 601–612. [Google Scholar] [CrossRef]
- Stein, G. Some aspects of the radiation chemistry of organic solutes. Discuss. Farad. Soc. 1952, 12, 227–234. [Google Scholar] [CrossRef]
- Hart, E.J. Biophysics. Physical and chemical aspects of basic mechanisms in radiobiology. Arch. Biochem. 1955, 54. [Google Scholar]
- Hart, E.J.; Boag, J.W. Absorption Spectrum of the Hydrated Electron in Water and in Aqueous Solutions. J. Am. Chem. Soc. 1962, 84, 4090–4095. [Google Scholar] [CrossRef]
- Boag, J.W.; Hart, E. Absorption spectra in irradiated water and some solutions. Part i. Absorption spectra of ‘hydrated’ electron. Nature 1963, 197, 45. [Google Scholar] [CrossRef]
- Keene, J.P. Absorption Spectra in Irradiated Water and Some Solutions: Optical Absorptions in Irradiated Water. Nature 1963, 197, 47–48. [Google Scholar] [CrossRef]
- Yui, H.; Sawada, T. Interaction of Excess Electrons with Water Molecules at the Early Stage of Laser-Induced Plasma Generation in Water. Phys. Rev. Lett. 2000, 85, 3512–3515. [Google Scholar] [CrossRef]
- Yui, H. Electron-enhanced Raman scattering: A history of its discovery and spectroscopic applications to solution and interfacial chemistry. Anal. Bioanal. Chem. 2010, 397, 1181–1190. [Google Scholar] [CrossRef]
- Yui, H.; Tomai, T.; Sawada, M.; Terashima, K. Generation of laser-induced plasma in supercritical water and vibrational spectroscopic study of accompanying stimulated Raman scattering. Appl. Phys. Lett. 2011, 99, 3942. [Google Scholar] [CrossRef]
- Schaffer, C.; Nishimura, N.; Glezer, E.; Kim, A.; Mazur, E. Dynamics of femtosecond laser-induced breakdown in water from femtoseconds to microseconds. Opt. Express 2002, 10, 196. [Google Scholar] [CrossRef]
- Ehrler, O.T.; Neumark, D.M. Dynamics of electron solvation in molecular clusters. Acc. Chem. Res. 2009, 42, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Park, I.; Lee, S.; Cho, K.; Lee, J.Y.; Kim, J.; Joannopoulos, J.D. The Nature of a Wet Electron. Phys. Rev. Lett. 1996, 76, 956. [Google Scholar] [CrossRef] [PubMed]
- Men, Z.; Fang, W.; Li, Z.; Sun, C.; Li, Z.; Wang, X. Hydrated-electron resonance enhancement O-H stretching vibration of water hexamer at air-water interface. Opt. Lett. 2015, 40, 7. [Google Scholar] [CrossRef] [PubMed]
- Bratos, S.; Leicknam, J.C.; Borgis, D.; Staib, A. Subpicosecond pump-probe absorption of the hydrated electron: Nonlinear response theory and computer simulation. Phys. Rev. E 1997, 55, 7217–7227. [Google Scholar] [CrossRef]
- Svoboda, V.; Michiels, R.; LaForge, A.C.; Stienkemeier, F.; Slavíček, P.; Wörner, H.J. Real-time observation of water radiolysis and hydrated electron formation induced by extreme-ultraviolet pulses. Sci. Adv. 2020, 6, eaaz0385. [Google Scholar] [CrossRef] [PubMed]
- Loh, Z.-H.; Doumy, G.; Arnold, C.; Kjellsson, L.; Southworth, S.; Al Haddad, A.; Kumagai, Y.; Tu, M.-F.; Ho, P.; March, A. Observation of the fastest chemical processes in the radiolysis of water. Science 2020, 367, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Neria, E.; Nitzan, A.; Barnett, R.N.; Landman, U. Quantum dynamical simulations of nonadiabatic processes: Solvation dynamics of the hydrated electron. Phys. Rev. Lett. 1991, 67, 1011. [Google Scholar] [CrossRef]
- Webster, F.J.; Schnitker, J.; Friedrichs, M.S.; Friesner, R.A.; Rossky, P.J. Solvation dynamics of the hydrated electron: A nonadiabatic quantum simulation. Phys. Rev. Lett. 1991, 66, 3172. [Google Scholar] [CrossRef]
- Yokoyama, K.; Silva, C.; Son, D.H.; Walhout, P.K.; Barbara, P.F. Detailed Investigation of the Femtosecond PumpProbe Spectroscopy of the Hydrated Electron. J. Phys. Chem. A 1998, 102, 6957–6966. [Google Scholar] [CrossRef]
- Baltuka, A.; Emde, M.F.; Pshenichnikov, M.S.; Wiersma, D.A. Early-Time Dynamics of the Photoexcited Hydrated Electron. J. Phys. Chem. A 1999, 103, 10065–10082. [Google Scholar] [CrossRef][Green Version]
- Kambhampati, P.; Son, D.H.; Kee, T.W.; Barbara, P.F. Solvation Dynamics of the Hydrated Electron Depends on Its Initial Degree of Electron Delocalization. J. Phys. Chem. A 2002, 106, 2374–2378. [Google Scholar] [CrossRef]
- Baldacchino, G.; Waele, V.D.; Monard, H.; Sorgues, S.; Mostafavi, M. Hydrated electron decay measurements with picosecond pulse radiolysis at elevated temperatures up to 350 °C. Chem. Phys. Lett. 2006, 424, 77–81. [Google Scholar] [CrossRef]
- Walrafen, G.E. Raman Spectral Studies of the Effects of Temperature on Water and Electrolyte Solutions. J. Chem. Phys. 1966, 44, 1546–1558. [Google Scholar] [CrossRef]
- Nikogosyan, D.N.; Oraevsky, A.A.; Rupasov, V.I. Two-photon ionization and dissociation of liquid water by powerful laser UV radiation. Chem. Phys. 1983, 77, 131–143. [Google Scholar] [CrossRef]
- Noack, J.; Vogel, A. Laser-induced plasma formation in water at nanosecond to femtosecond time scales: Calculation of thresholds, absorption coefficients, and energy density. IEEE J. Quantum Electron. 1999, 35, 1156–1167. [Google Scholar] [CrossRef]
- Bloembergen, N. Nonlinear Optics, 4th ed.; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Fan, C.H.; Sun, J.; Longtin, J.P. Breakdown threshold and localized electron density in water induced by ultrashort laser pulses. J. Appl. Phys. 2002, 91, 2530–2536. [Google Scholar] [CrossRef]
- Feng, Q.; Moloney, J.V.; Newell, A.C.; Wright, E.M.; Cook, K.; Kennedy, P.K.; Hammer, D.X.; Rockwell, B.A.; Thompson, C.R. Theory and simulation on the threshold of water breakdown induced by focused ultrashort laser pulses. IEEE J. Quantum Electron. 1997, 33, 127–137. [Google Scholar] [CrossRef]
- Wittmann, M.; Penzkofer, A. Spectral Superbroadening of Femtosecond Laser Pulses. Opt. Commun. 1996, 126, 308–317. [Google Scholar] [CrossRef]
- Liu, F.; Yuan, S.; He, B.; Nan, J.; Khan, A.Q.; Ding, L.E.; Zeng, H. Enhanced stimulated Raman scattering by femtosecond ultraviolet plasma grating in water. Appl. Phys. Lett. 2018, 112, 094101. [Google Scholar] [CrossRef]
- Tang, J.; Gao, Q.; Chen, X.; Xiao, K.; Sun, J.; Wang, A. Self-guided group velocity matched stimulated Raman conversion of X-waves in water. Photonics Res. 2024, submitted.
- Bailey, C.G.; Kim, J.; Johnson, M.A. Infrared Spectroscopy of the Hydrated Electron Clusters (H2O)n-, n = 6, 7: Evidence for Hydrogen Bonding to the Excess Electron. J. Phys. Chem. 1996, 100, 16782–16785. [Google Scholar] [CrossRef]
- Chen, X.; Tang, J.; Wang, Z. Dynamic nonlinear X-waves for femtosecond filamentation in Kerr media. Phys. Rev. A. 2024; in preparation. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Wang, Z. Hydrated Electron Dynamics and Stimulated Raman Scattering in Water Induced by Ultrashort Laser Pulses. Molecules 2024, 29, 1245. https://doi.org/10.3390/molecules29061245
Tang J, Wang Z. Hydrated Electron Dynamics and Stimulated Raman Scattering in Water Induced by Ultrashort Laser Pulses. Molecules. 2024; 29(6):1245. https://doi.org/10.3390/molecules29061245
Chicago/Turabian StyleTang, Jun, and Zhongyang Wang. 2024. "Hydrated Electron Dynamics and Stimulated Raman Scattering in Water Induced by Ultrashort Laser Pulses" Molecules 29, no. 6: 1245. https://doi.org/10.3390/molecules29061245
APA StyleTang, J., & Wang, Z. (2024). Hydrated Electron Dynamics and Stimulated Raman Scattering in Water Induced by Ultrashort Laser Pulses. Molecules, 29(6), 1245. https://doi.org/10.3390/molecules29061245




