Biphasic Fermentation of Trapa bispinosa Shells by Ganoderma sinense and Characterization of Its Polysaccharides and Alcoholic Extract and Analysis of Their Bioactivity
Abstract
1. Introduction
2. Results
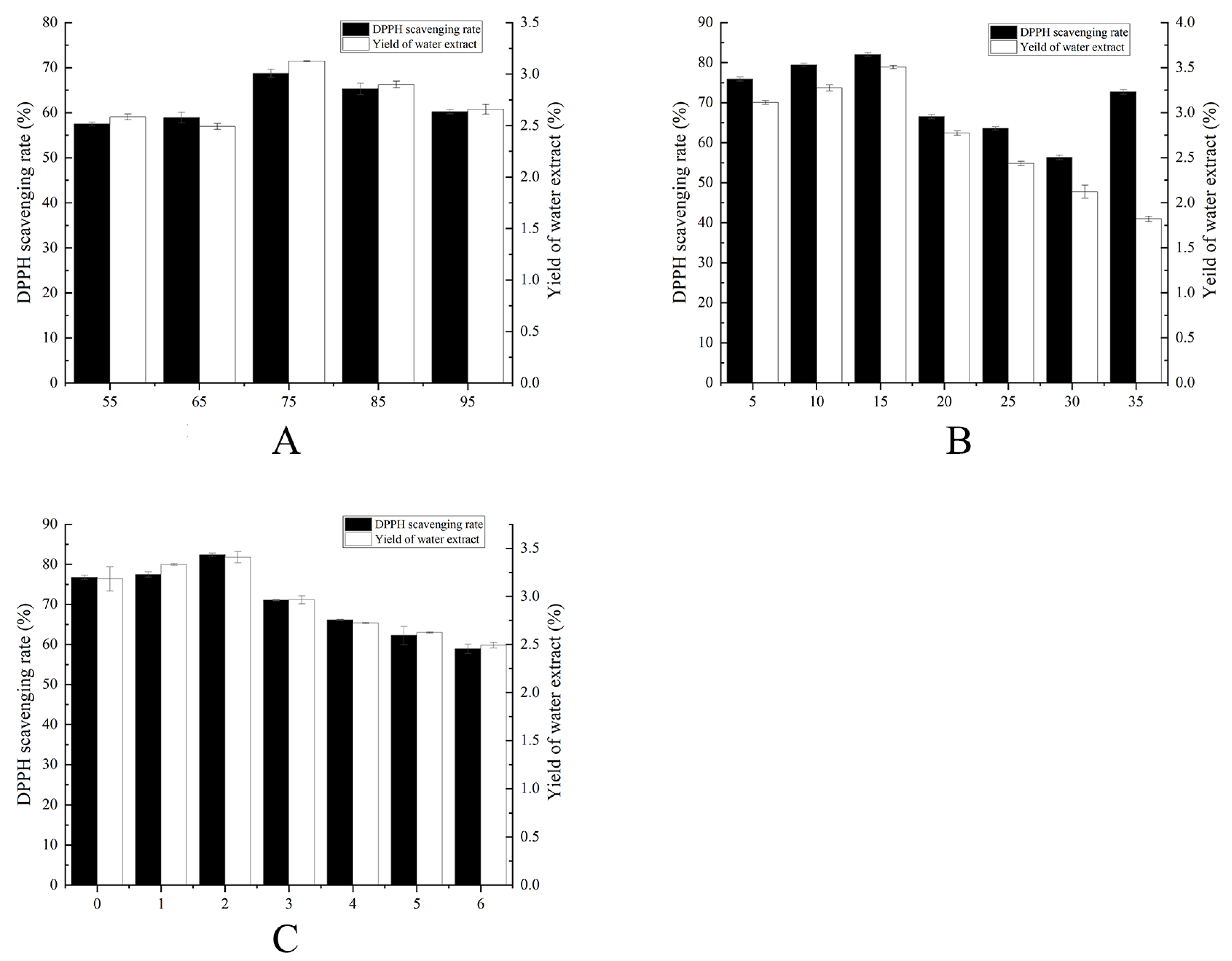
2.1. Single-Factor Fermentation Optimization Results
2.2. Response Surface Fermentation Optimization Results
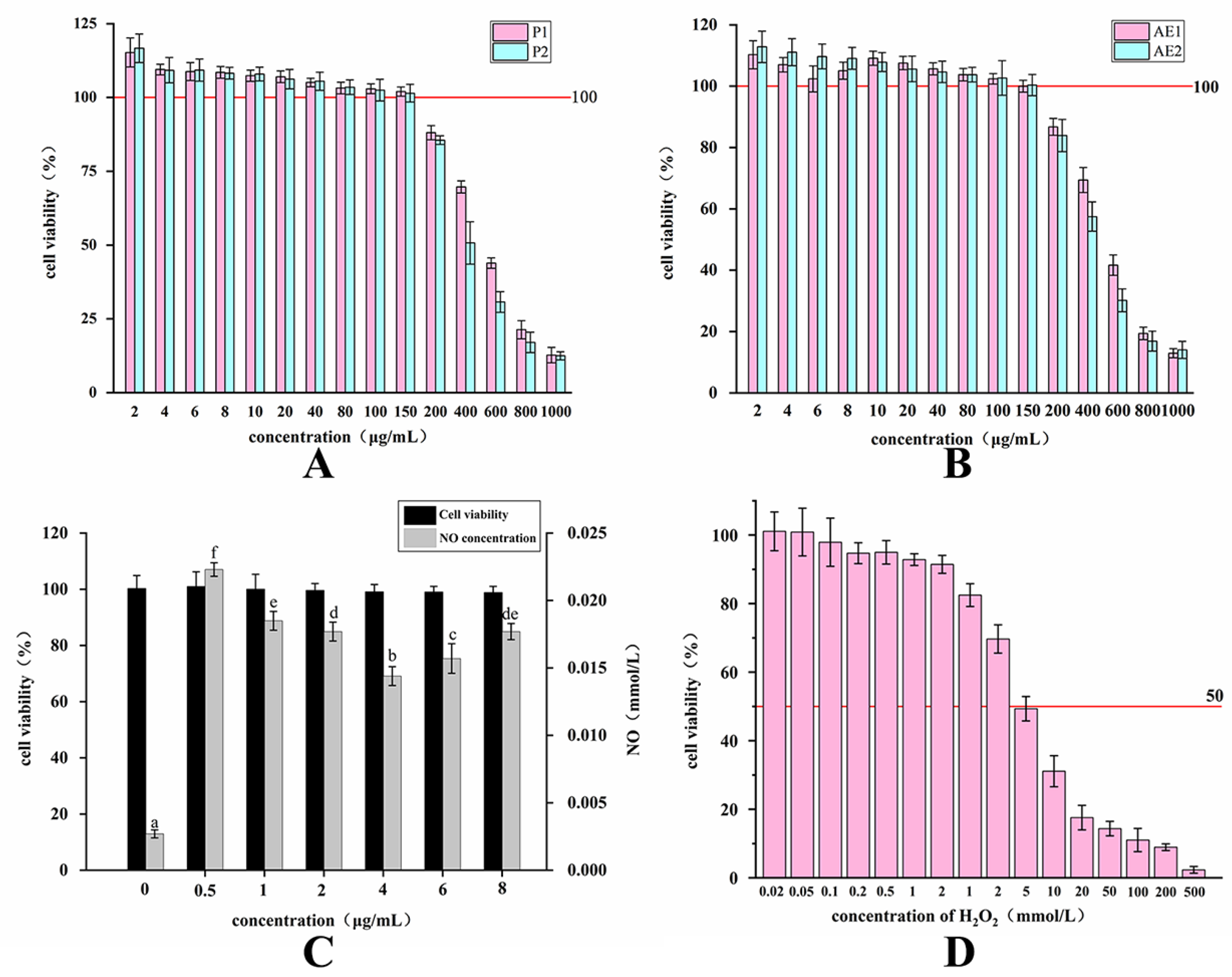
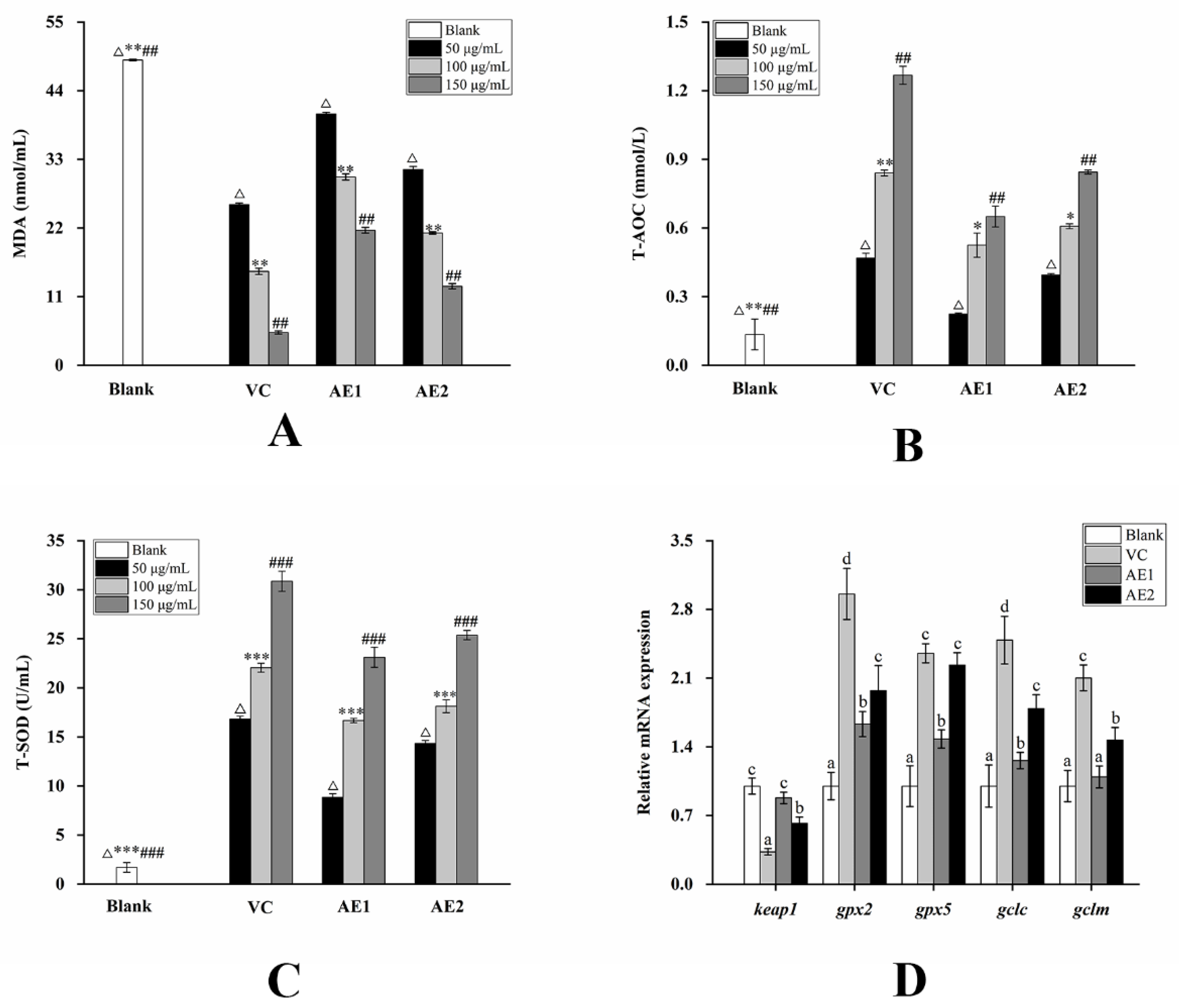
2.3. Changes in the Activity of Purified Polysaccharides and Alcoholic Extracts before and after Fermentation
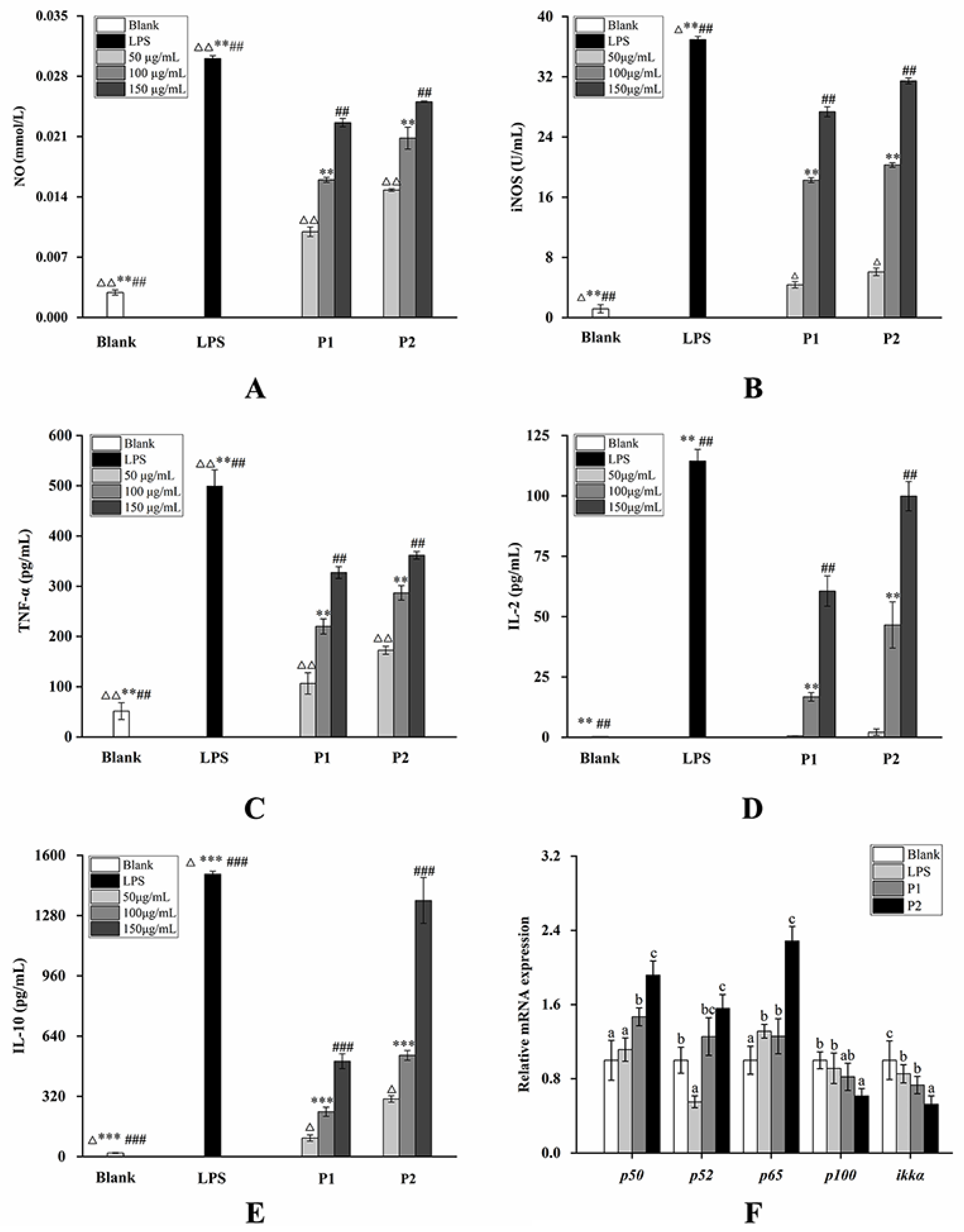
2.4. Changes in the mRNA Expression Level on the NF-κB Pathway and Nrf2/Keap1-ARE Pathway
2.5. Analysis of the Structural Characteristics of Polysaccharides after Fermentation
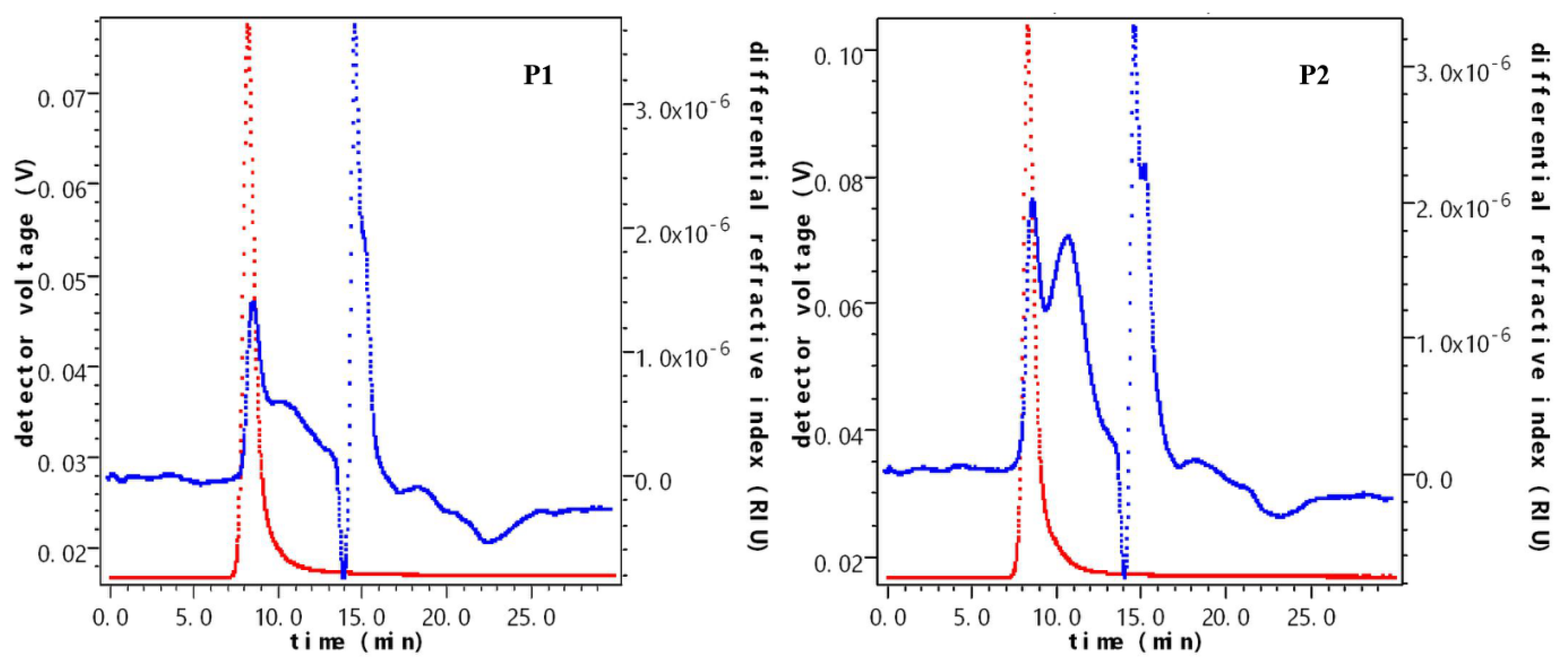
2.5.1. The Molecular Weight of Polysaccharides after Fermentation
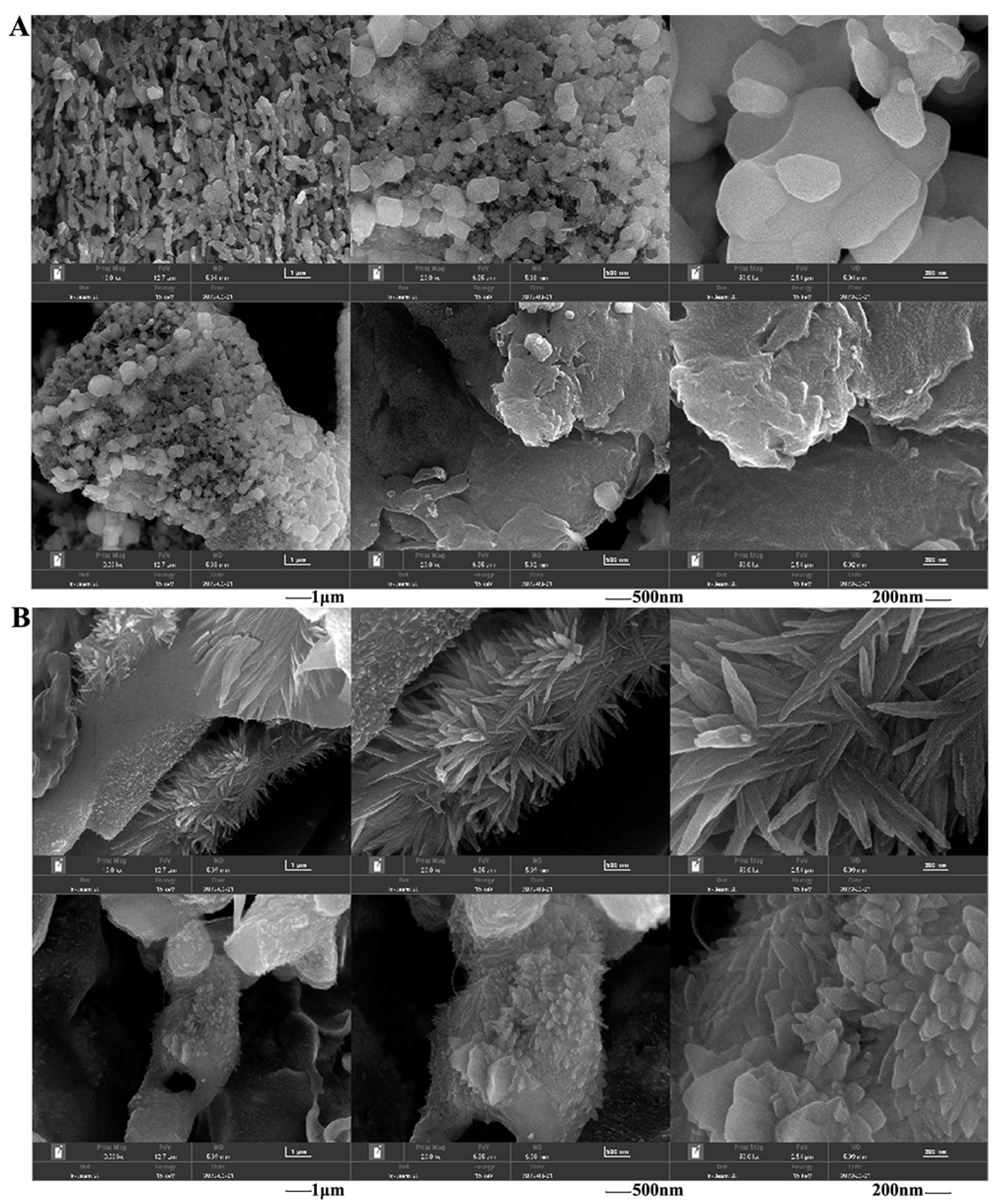
2.5.2. The Morphology of Polysaccharides after Fermentation
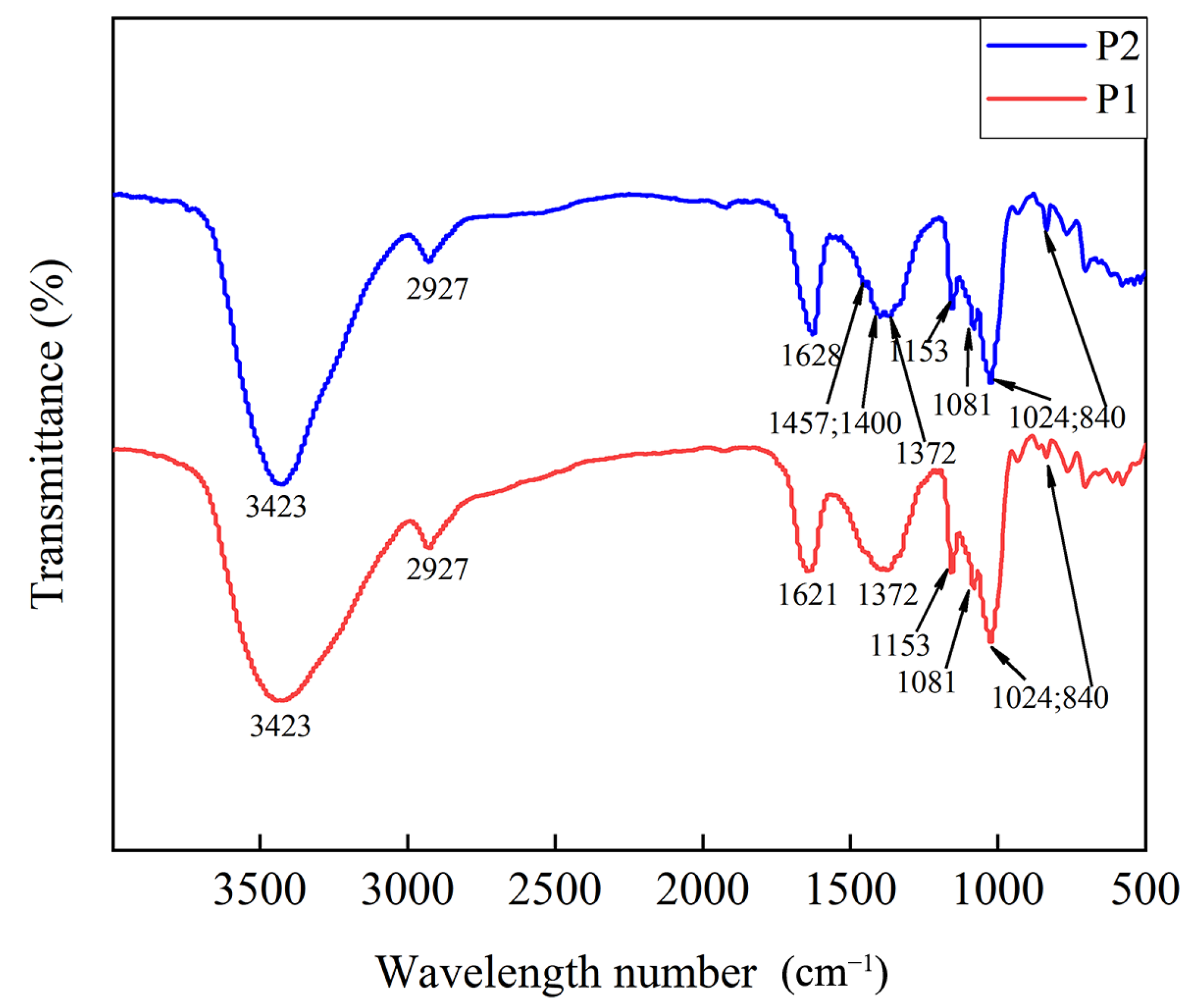
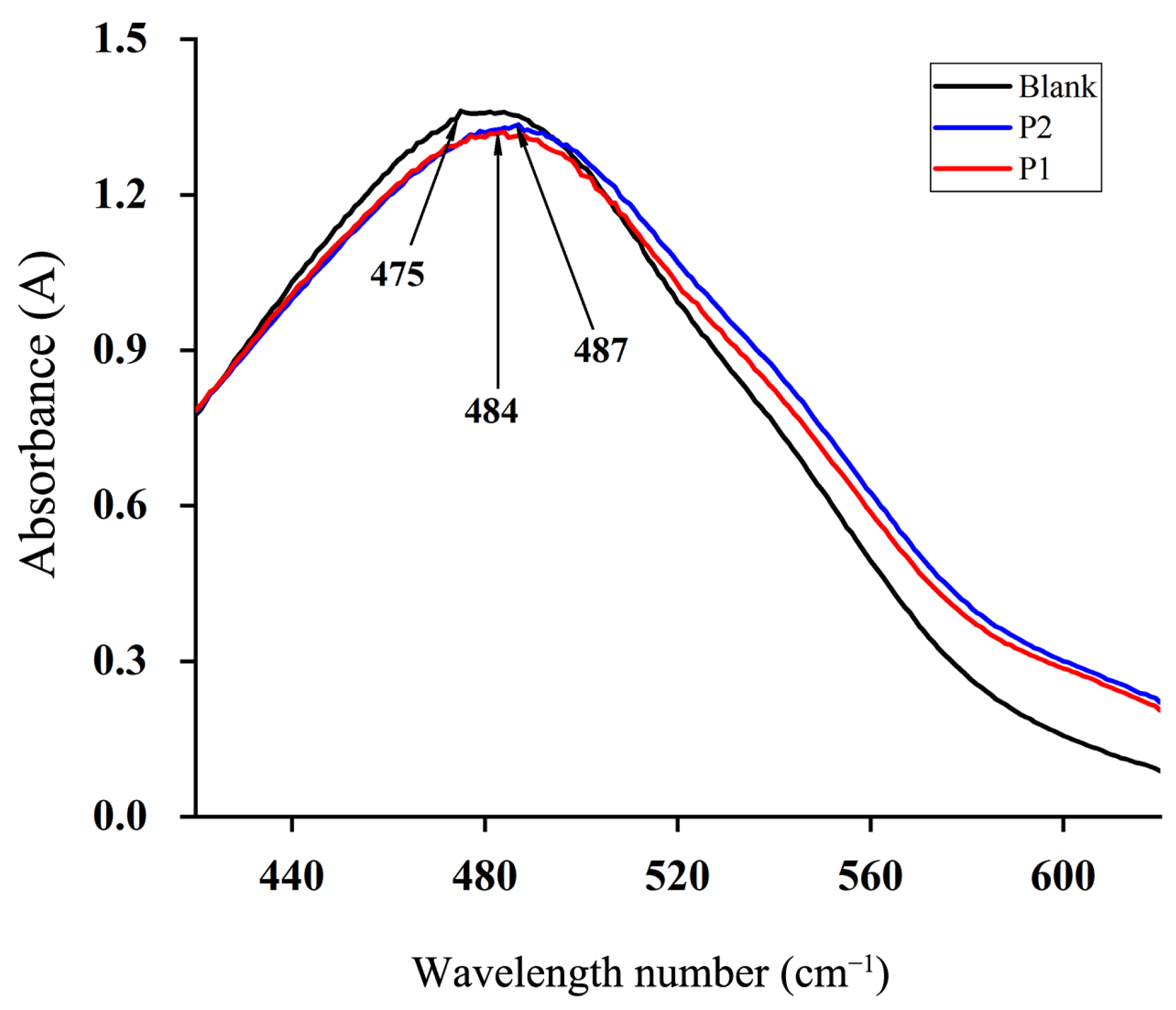
2.5.3. The Characteristic Functional Group Structure of Polysaccharides
2.5.4. The Triple Helix Structure of Polysaccharides
2.6. Analysis of the Components of Alcoholic Extracts after Fermentation
3. Materials and Methods
3.1. Experiment Materials
3.2. Optimization of the Fermentation Process
3.3. Preparation of Purified Polysaccharides and Alcoholic Extracts from TBs
3.4. Analysis of Changes in Immunomodulatory and Antioxidant Activities during TBs’ Fermentation
3.5. Polysaccharides Structure Characterization of TBs Fermented by GS
3.6. Component Analysis of Alcoholic Extract of TBs Fermented by GS
3.7. Analysis of the mRNA Expression Levels on the NF-κB Pathway and Nrf2/Keap1-ARE Pathway
3.8. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adkar, P.; Dongare, A.; Ambavade, S.; Bhaskar, V. Trapa bispinosa Roxb.: A review on nutritional and pharmacological aspects. Adv. Pharmacol. Pharm. Sci. 2014, 2014, 959830. [Google Scholar]
- Iwaoka, Y.; Suzuki, S.; Kato, N.; Hayakawa, C.; Kawabe, S.; Ganeko, N.; Uemura, T.; Ito, H. Characterization and identification of bioactive polyphenols in the Trapa bispinosa Roxb. pericarp extract. Molecules 2021, 26, 5802. [Google Scholar] [CrossRef]
- Wang, L.; Yin, D.; Fan, Y.; Min, T.; Yi, Y.; Wang, H. Molecular mechanism of the anti-gastric cancer activity of 1, 2, 3, 6-tetra-O-galloyl-β-D-glucose isolated from Trapa bispinosa Roxb. shell in vitro. PLoS ONE 2022, 17, e0269013. [Google Scholar] [CrossRef]
- Takeshita, S.; Ishioka, Y.; Yagi, M.; Uemura, T.; Yamada, M.; Yonei, Y. The effects of water chestnut (Trapa bispinosa Roxb.) on the inhibition of glycometabolism and the improvement in postprandial blood glucose levels in humans. Glycative Stress Res. 2016, 3, 24–132. [Google Scholar]
- Tania Wahed, T.W.; Islam, M.F.; Ratna Sarkar, R.S.; Sonia Wahed, W.S. Phytochemical, antioxidant and cytotoxicity study of the methanolic extracts of leaves of Trapa bispinosa Roxb. Int. J. Pharm. Sci. Res. 2014, 10, 4400–4405. [Google Scholar]
- Singh, S.; Badoni, H.; Kumar, V.; Madan, A.; Sharma, P.; Waheed, S.M. A comparitive study of antioxidant properties and phytochemical composition of Trapa bispinosa, Trigonella foenum-graecum, Syzygium cumini and Betula utilis. Int. J. Pharm. Sci. Rev. Res. 2016, 38, 83–88. [Google Scholar]
- Majee, C.; Mazumder, A.; Mazumder, R.; Choudhary, A.N. Determination of Total Phenolic Content and Total Antioxidant Activity in Various Parts of Trapa bispinosa. J. Pharm. Sci. Res. 2019, 11, 3625–3627. [Google Scholar]
- Chang, Y.-W.; Huang, W.-C.; Lin, C.-Y.; Wang, W.-H.; Hung, L.-C.; Chen, Y.-H. Tellimagrandin II, a type of plant polyphenol extracted from Trapa bispinosa inhibits antibiotic resistance of drug-resistant Staphylococcus aureus. Int. J. Mol. Sci. 2019, 20, 5790. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Xia, Z.; Wang, Q.; Liu, X.; Gong, Z. Discriminating geographical origins and determining active substances of water caltrop shells through near-infrared spectroscopy and chemometrics. Spectrochim. Acta Part A. 2023, 303, 123198. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Chao, C.-L.; Liaw, C.-C.; Hwang, S.-Y.; Kuo, Y.-H.; Chang, T.-C.; Chao, C.-H.; Chen, C.-J.; Kuo, Y.-H. Hypoglycemic constituents isolated from Trapa natans L. pericarps. J. Agric. Food Chem. 2016, 64, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shen, S. Phenolic composition, antioxidant, antimicrobial and antiproliferative activities of water caltrop pericarps extract. LWT—Food Sci. Technol. 2015, 61, 238–243. [Google Scholar] [CrossRef]
- Chiang, P.-Y.; Ciou, J.-Y. Effect of pulverization on the antioxidant activity of water caltrop (Trapa taiwanensis Nakai) pericarps. LWT—Food Sci. Technol. 2010, 43, 361–365. [Google Scholar] [CrossRef]
- Xia, J.; Yang, C.; Wang, Y.; Yang, Y.; Yu, J. Antioxidant and antiproliferative activities of the leaf extracts from Trapa bispinosa and active components. S. Afr. J. Bot. 2017, 113, 377–381. [Google Scholar] [CrossRef]
- Sarkar, R.; Nandan, C.K.; Sen, I.K.; Bhunia, S.K.; Behera, B.; Maiti, T.K.; Islam, S.S. Structural studies of an antioxidant, immunoenhancing polysaccharide isolated from the kernel of Trapa bispinosa fruit. J. Carbohydr. Chem. 2012, 31, 686–701. [Google Scholar] [CrossRef]
- Peng, X.; Qiu, M. Meroterpenoids from Ganoderma species: A review of last five years. Nat. Prod. Bioprospect. 2018, 8, 137–149. [Google Scholar] [CrossRef]
- Chen, T.-Q.; Zhao, X.-Y.; Wu, J.-Z.; Yu, D.-Y.; Wu, Y.-B. Supercritical fluid CO2 extraction, simultaneous determination of components in ultra-fine powder of Ganoderma sinense by HPLC–ESI-MS method. J. Taiwan Inst. Chem. Eng. 2011, 42, 428–434. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, F.; Chen, R. A novel alkaloid from the fruiting bodies of Ganoderma sinense Zhao, Xu et Zhang. Chin. Chem. Lett. 2010, 21, 197–199. [Google Scholar] [CrossRef]
- Wu, D.-T.; Xie, J.; Hu, D.-J.; Zhao, J.; Li, S.-P. Characterization of polysaccharides from Ganoderma spp. using saccharide mapping. Carbohydr. Polym. 2013, 97, 398–405. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, H. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol. Sin. 2004, 25, 1387–1395. [Google Scholar] [PubMed]
- Hasnat, M.A.; Pervin, M.; Cha, K.M.; Kim, S.K.; Lim, B.O. Anti-inflammatory activity on mice of extract of Ganoderma lucidum grown on rice via modulation of MAPK and NF-κB pathways. Phytochemistry 2015, 114, 125–136. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The application of fermentation technology in traditional Chinese medicine: A review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Waleed, A.; Mahdi, A.A. Microbial enzymes produced by fermentation and their applications in the food industry—A review. Int. J. Agric. Innov. Res. 2019, 8, 62–82. [Google Scholar]
- Li, R.; Zhang, Z.; Su, X.; Yu, J.; Lu, L.; Liu, T. Nontargeted metabolomics study and pharmacodynamic evaluation of bidirectional fermentation for Ganoderma lucidum with Marsdenia tenacissima. Front. Pharmacol. 2022, 13, 1012063. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Garcia, I.L.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.L.; Yin, J.Y.; Dong, W.Y.; Wang, X.D. Separation, purification and structural identification of anti-tumour compound in Trapa manshurica Fler. Chem. J. Chin. Univ.-Chin. 2005, 26, 852–855. [Google Scholar]
- Bao, X.F.; Dong, Q.; Fang, J.N. Structure and conformation behavior of a glucan from spores of Ganoderma lucidum (Fr.) Karst. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao Acta Biochim. Biophys. Sin. 2000, 32, 557–561. [Google Scholar]
- Kim, H.W.; Shin, M.-S.; Lee, S.J.; Park, H.-R.; Jee, H.S.; Yoon, T.J.; Shin, K.-S. Signaling pathways associated with macrophage-activating polysaccharides purified from fermented barley. Int. J. Biol. Macromol. 2019, 131, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, M.; Sakaeda, Y.; Yamaguchi, H.; Ohmori, Y. Anti-inflammatory cytokine interleukin-4 inhibits inducible nitric oxide synthase gene expression in the mouse macrophage cell line RAW264.7 through the repression of octamer-dependent transcription. Mediat. Inflamm. 2013, 2013, 369693. [Google Scholar] [CrossRef]
- Hong, S.H.; Ku, J.M.; Kim, H.I.; Ahn, C.-W.; Park, S.-H.; Seo, H.S.; Shin, Y.C.; Ko, S.-G. The immune-enhancing activity of Cervus nippon mantchuricus extract (NGE) in RAW264.7 macrophage cells and immunosuppressed mice. Food Res. Int. 2017, 99, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Trotta, E.; Simeonov, D.R.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef]
- Cruz, J.S.D.; Morrison, S.L.; Penichet, M.L. Insights into the mechanism of anti-tumor immunity in mice vaccinated with the human HER2/neu extracellular domain plus anti-HER2/neu IgG3-(IL-2) or anti-HER2/neu IgG3-(GM-CSF) fusion protein. Vaccine 2005, 23, 4793–4803. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Liu, Z.-J.; Kawahara, A.; Minami, Y.; Yamada, K.; Tsujimoto, Y.; Barsoumian, E.L.; Perlmutter, R.M.; Taniguchi, T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell 1995, 81, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, H.; Cicero, A.F.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kumar Rajendran, N.; George, B.P.; Chandran, R.; Tynga, I.M.; Houreld, N.; Abrahamse, H. The influence of light on reactive oxygen species and NF-кB in disease progression. Antioxidants 2019, 8, 640. [Google Scholar] [CrossRef]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-κB, IκB, and IKK: Integral components of immune system signaling. Struct. Immunol. 2019, 1172, 207–226. [Google Scholar]
- Wei, X.; Liu, H.; Sun, X.; Fu, F.; Zhang, X.; Wang, J.; An, J.; Ding, H. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci. Lett. 2005, 386, 58–62. [Google Scholar] [CrossRef]
- Pinmanee, P.; Sompinit, K.; Arnthong, J.; Suwannarangsee, S.; Jantimaporn, A.; Khongkow, M.; Nimchua, T.; Sukyai, P. Enhancing the productivity and stability of superoxide dismutase from Saccharomyces cerevisiae TBRC657 and its application as a free radical scavenger. Fermentation 2022, 8, 169. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Hawkes, H.-J.K.; Karlenius, T.C.; Tonissen, K.F. Regulation of the human thioredoxin gene promoter and its key substrates: A study of functional and putative regulatory elements. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 303–314. [Google Scholar] [CrossRef]
- Sun, H.; Ni, X.; Zeng, D.; Zou, F.; Yang, M.; Peng, Z.; Zhou, Y.; Zeng, Y.; Zhu, H.; Wang, H.; et al. Bidirectional immunomodulating activity of fermented polysaccharides from Yupingfeng. Res. Vet. Sci. 2017, 110, 22–28. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, C.; Yan, S.; Wang, P.; Wu, B.; Jiang, C.; Li, X.; Liu, Y. A novel polysaccharide from plant fermentation extracts and its immunomodulatory activity in macrophage RAW264. 7 cells. Food Agric. Immunol. 2021, 32, 54–77. [Google Scholar] [CrossRef]
- Xie, L.; Huang, Z.; Meng, H.; Shi, X.; Xie, J. Immunomodulation effect of polysaccharides from liquid fermentation of Monascus purpureus 40269 via membrane TLR-4 to activate the MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2022, 201, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zha, Z.; Tan, Y.; Li, Y.; Jiao, Y.; Yang, B.; Xiong, Q.; Yin, H.; Wang, H. Extraction and characterization of polysaccharide from fermented mycelia of Coriolus versicolor and its efficacy for treating nonalcoholic fatty liver disease. Int. J. Biol. Macromol. 2023, 248, 125951. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Song, M.; Wang, C.; Guo, Z.; Li, Y.; Wang, D. Structural characterization of polysaccharide purified from Hericium erinaceus fermented mycelium and its pharmacological basis for application in Alzheimer’s disease: Oxidative stress related calcium homeostasis. Int. J. Biol. Macromol. 2021, 193, 358–369. [Google Scholar] [CrossRef]
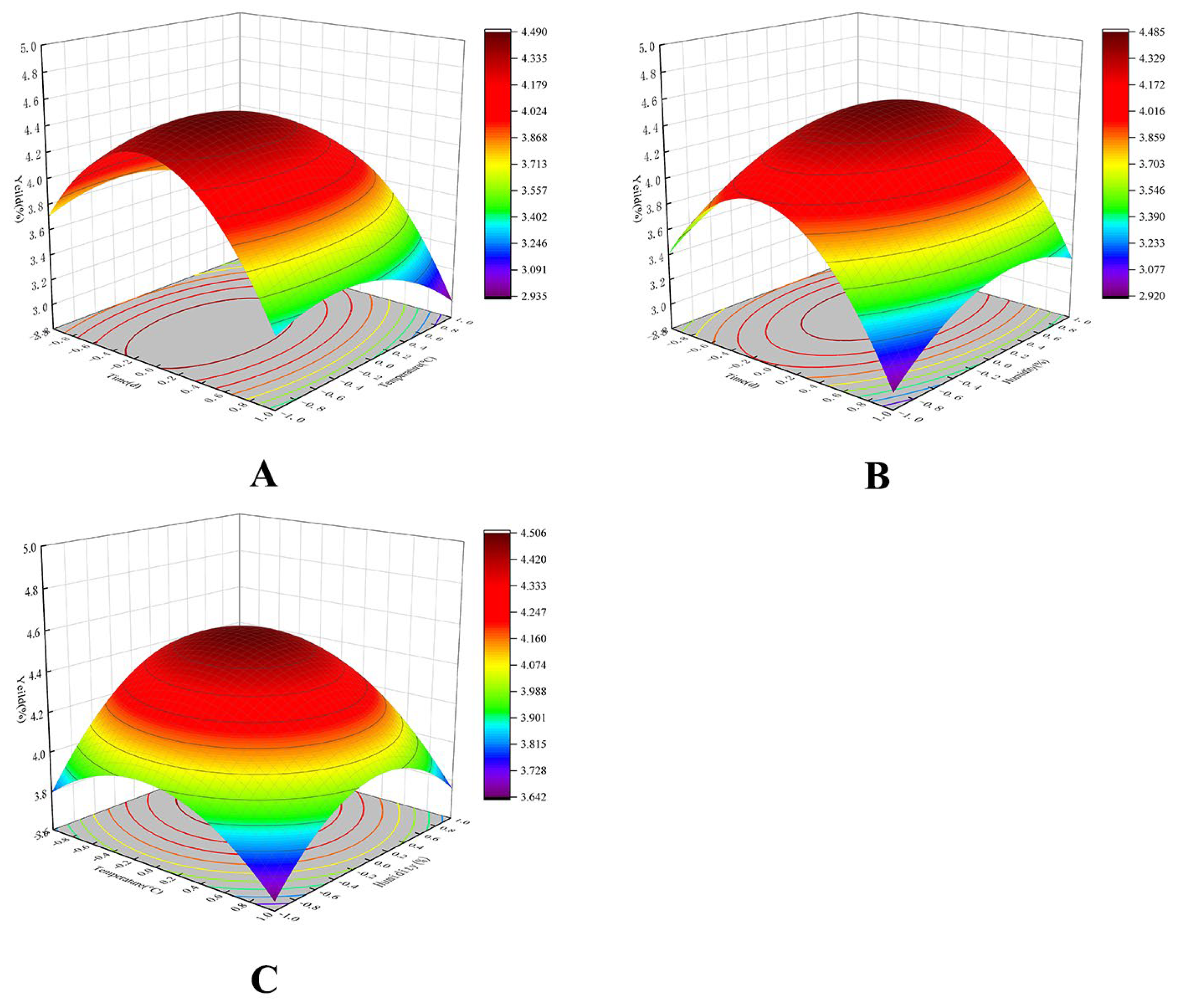
| Run | X1: Time of Fermentation (d) | X2: Temperature of Fermentation (°C) | X3: Humidity of Fermentation (%) | Y: Yield (%) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 3.73 |
| 2 | −1 | 1 | 0 | 3.396 |
| 3 | 1 | −1 | 0 | 3.345 |
| 4 | 1 | 1 | 0 | 2.908 |
| 5 | 0 | −1 | 1 | 4.301 |
| 6 | 0 | −1 | −1 | 3.737 |
| 7 | 0 | 1 | −1 | 3.633 |
| 8 | 0 | 1 | 1 | 3.809 |
| 9 | −1 | 0 | −1 | 3.431 |
| 10 | 1 | 0 | −1 | 2.958 |
| 11 | −1 | 0 | 1 | 3.624 |
| 12 | 1 | 0 | 1 | 3.257 |
| 13 | 0 | 0 | 0 | 4.434 |
| 14 | 0 | 0 | 0 | 4.415 |
| 15 | 0 | 0 | 0 | 4.522 |
| 16 | 0 | 0 | 0 | 4.456 |
| 17 | 0 | 0 | 0 | 4.414 |
| Sample Name | Time Field (min) | Weight-Average Molecular Mass/u | Content (%) | Mr/u |
|---|---|---|---|---|
| P1 | 7.358–9.439 | 2.396 × 106 ± 0.824 | 21.9 | 6.004 × 106 |
| 9.469–12.999 | 2.259 × 105 ± 0.658 | 27.6 | ||
| 13.873–14.929 | 1.210 × 104 ± 2.026 | 25.6 | ||
| 14.929–17.131 | 2.952 × 104 ± 1.985 | 20.6 | ||
| 17.131–20.118 | 9.613 × 104 ± 3.688 | 4.3 | ||
| P2 | 7.055–9.296 | 2.564 × 106 ± 0.910 | 20.6 | 7.812 × 104 |
| 9.296–13.506 | 2.019 × 105 ± 1.112 | 44.4 | ||
| 14.021–15.020 | 1.333 × 104 ± 2.741 | 17.6 | ||
| 15.020–17.080 | 2.670 × 104 ± 2.641 | 17.1 | ||
| 17.080–18.988 | 4.502 × 105 ± 3.430 | 0.4 |
| Variate Conditions | Variable Level | Control Conditions | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Time (d) | 1 | 2 | 3 | 4 | 5 | TEMP: 25 °C, HUM: 75% |
| Temperature (°C) | 10 | 15 | 20 | 25 | 30 | TIME: 3 d, HUM: 75% |
| Humidity (%) | 55 | 65 | 75 | 85 | 95 | TEMP: 25 °C, TIME: 3 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Lei, Q.; Chen, Q.; Song, D.; Zhou, M.; Wang, H.; Wang, L. Biphasic Fermentation of Trapa bispinosa Shells by Ganoderma sinense and Characterization of Its Polysaccharides and Alcoholic Extract and Analysis of Their Bioactivity. Molecules 2024, 29, 1238. https://doi.org/10.3390/molecules29061238
Sun X, Lei Q, Chen Q, Song D, Zhou M, Wang H, Wang L. Biphasic Fermentation of Trapa bispinosa Shells by Ganoderma sinense and Characterization of Its Polysaccharides and Alcoholic Extract and Analysis of Their Bioactivity. Molecules. 2024; 29(6):1238. https://doi.org/10.3390/molecules29061238
Chicago/Turabian StyleSun, Xiaoyan, Qiuqi Lei, Qinyi Chen, Dandan Song, Min Zhou, Hongxun Wang, and Limei Wang. 2024. "Biphasic Fermentation of Trapa bispinosa Shells by Ganoderma sinense and Characterization of Its Polysaccharides and Alcoholic Extract and Analysis of Their Bioactivity" Molecules 29, no. 6: 1238. https://doi.org/10.3390/molecules29061238
APA StyleSun, X., Lei, Q., Chen, Q., Song, D., Zhou, M., Wang, H., & Wang, L. (2024). Biphasic Fermentation of Trapa bispinosa Shells by Ganoderma sinense and Characterization of Its Polysaccharides and Alcoholic Extract and Analysis of Their Bioactivity. Molecules, 29(6), 1238. https://doi.org/10.3390/molecules29061238





