Antibacterial Textile Coating Armoured with Aggregation-Induced Emission Photosensitisers to Prevent Healthcare-Associated Infections
Abstract
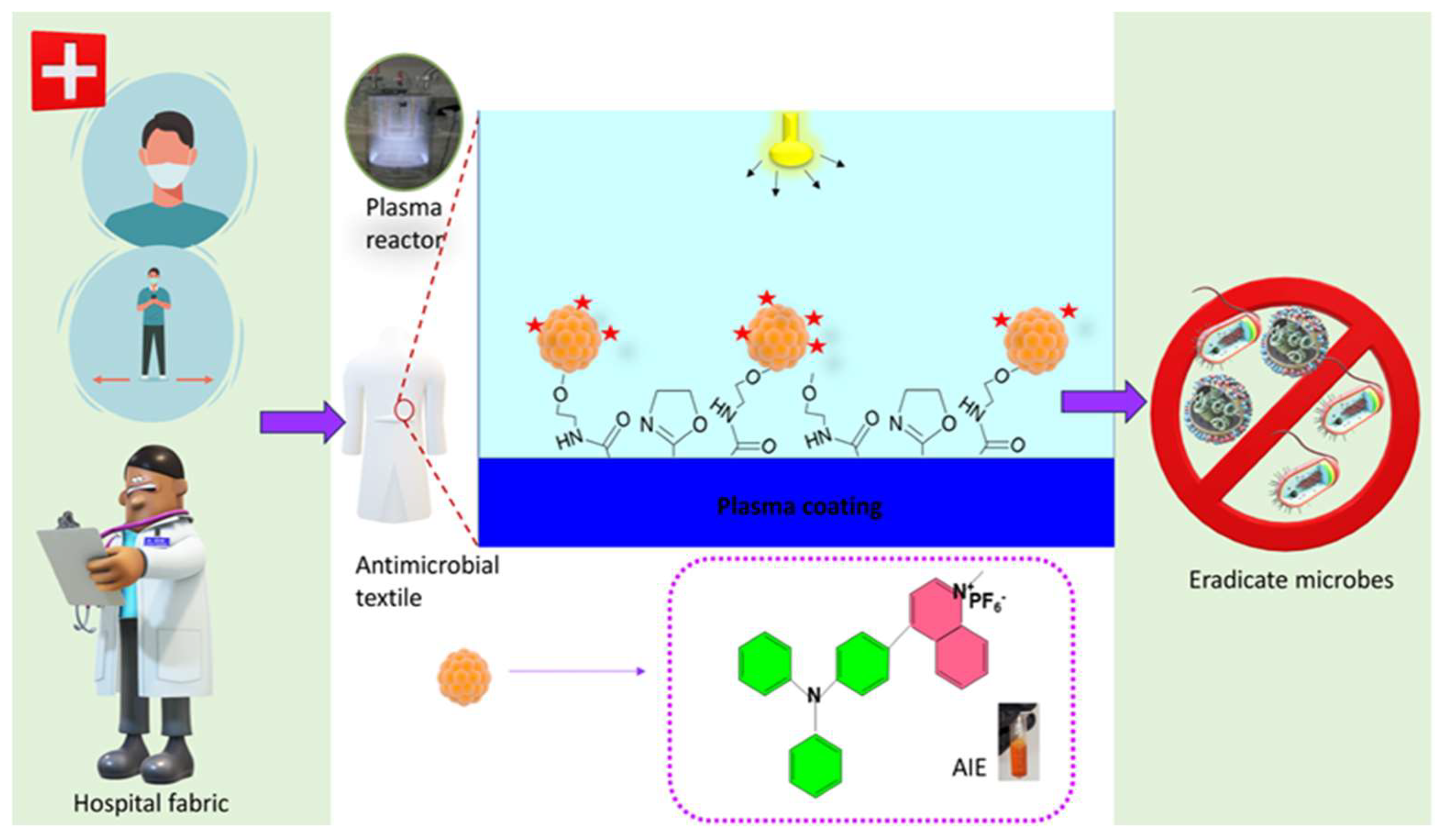
1. Introduction
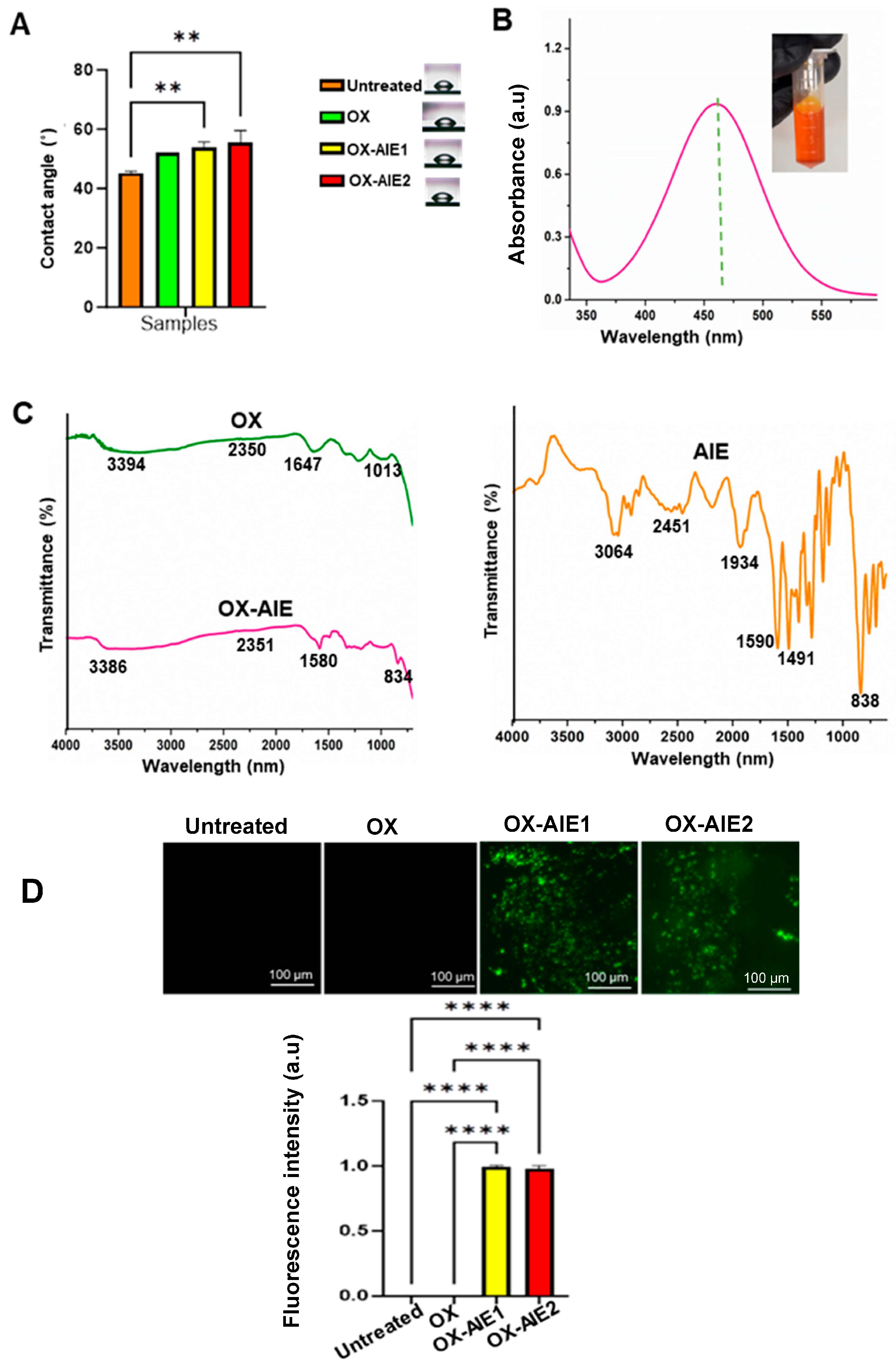
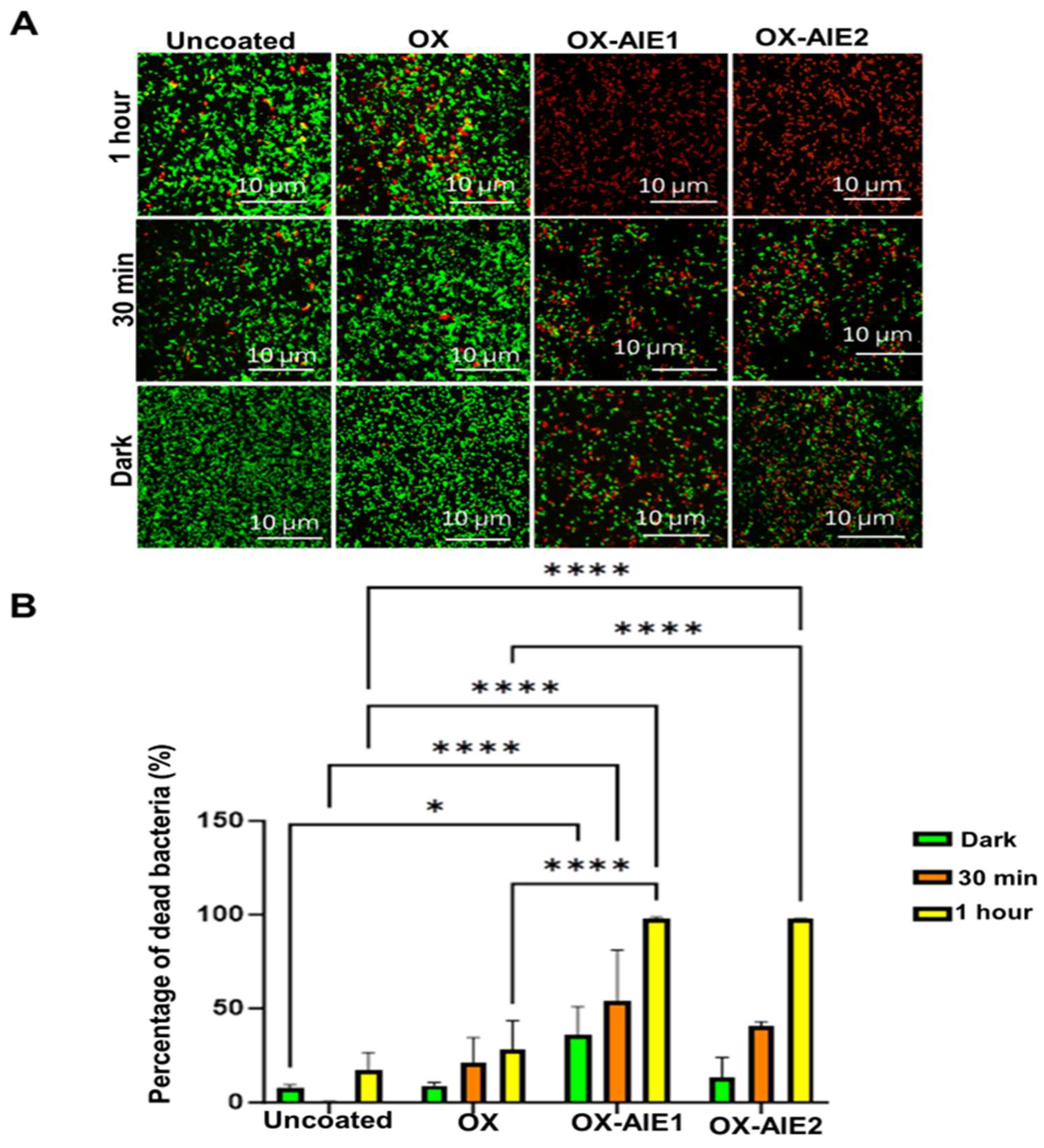
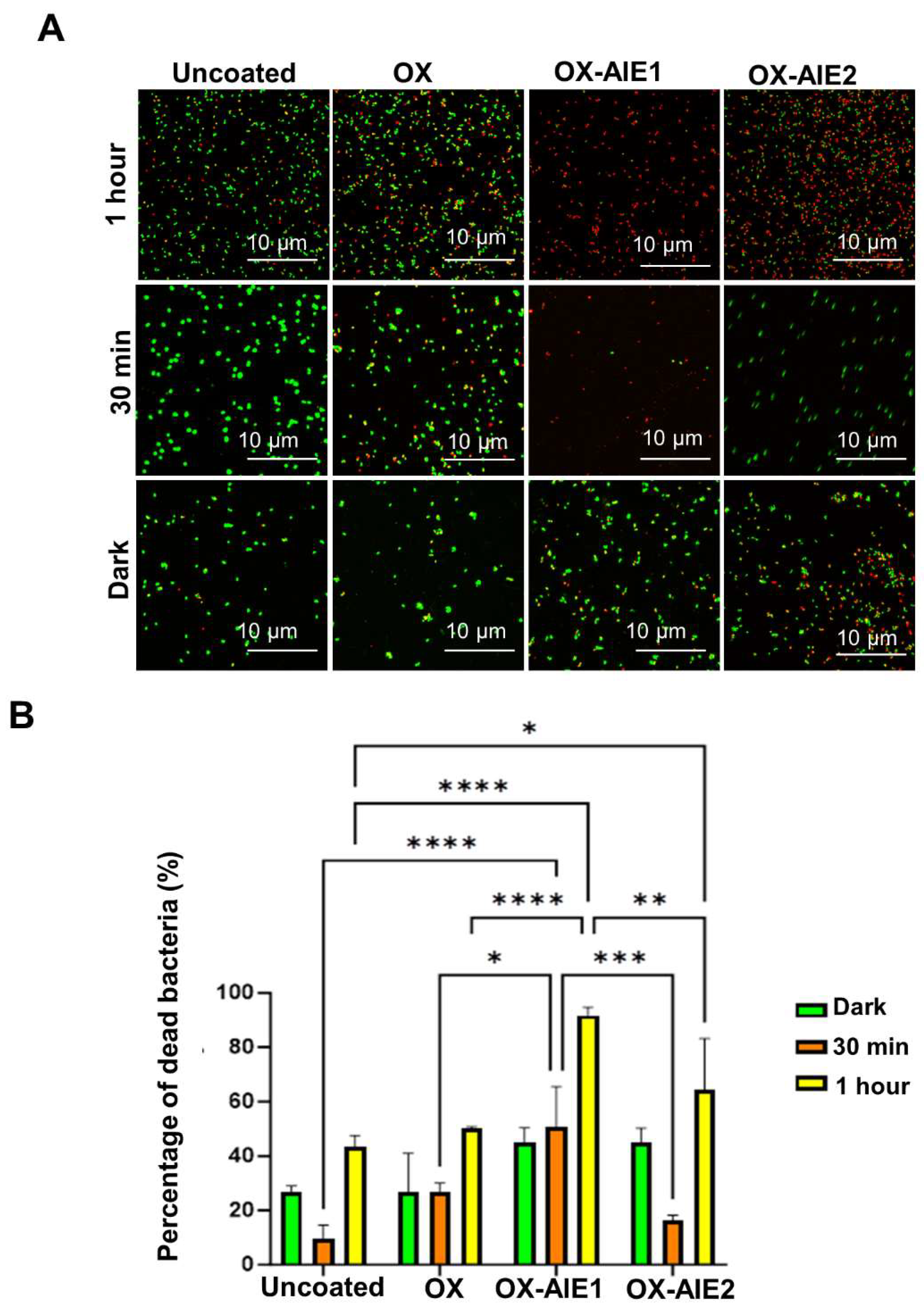
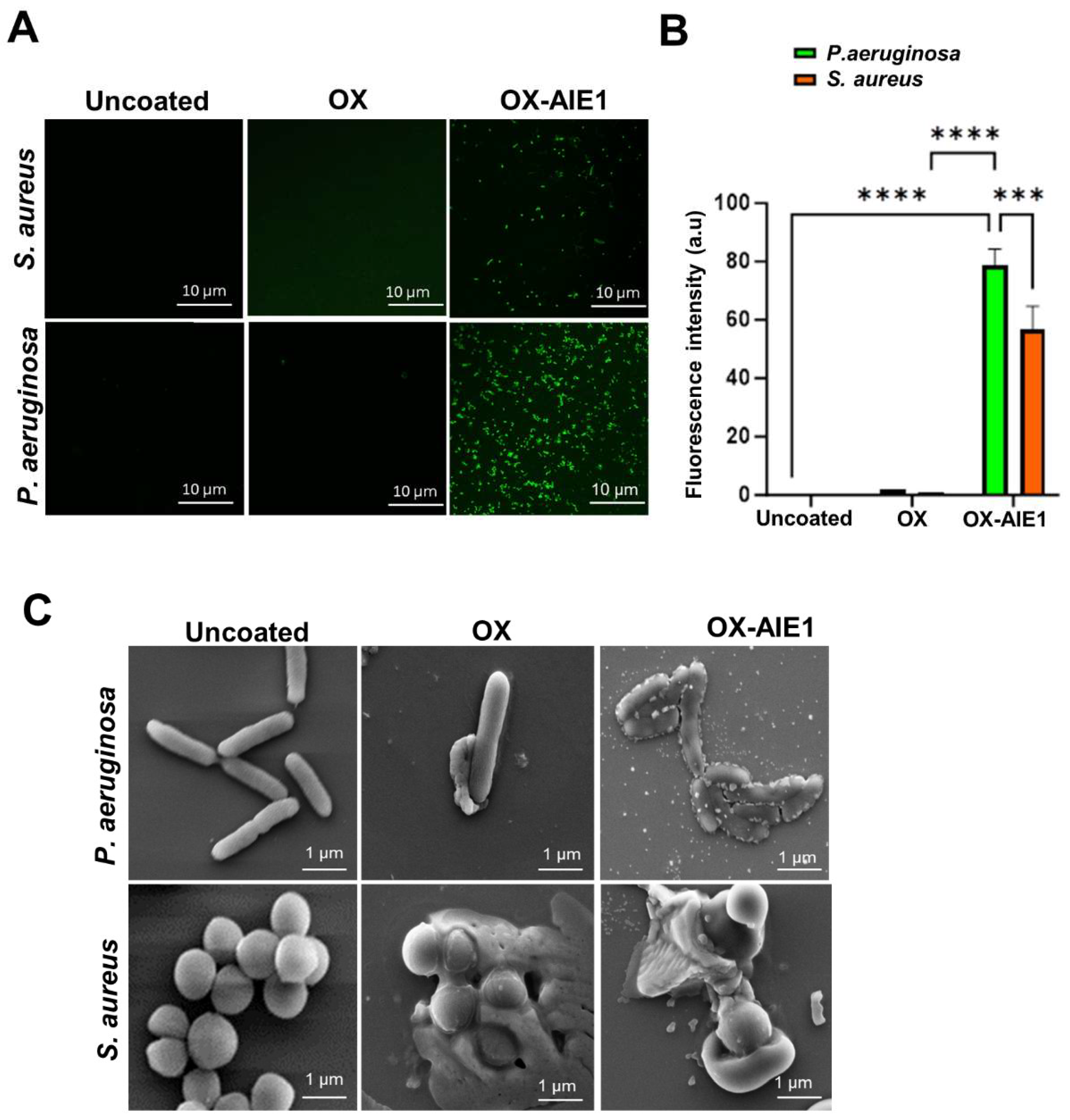
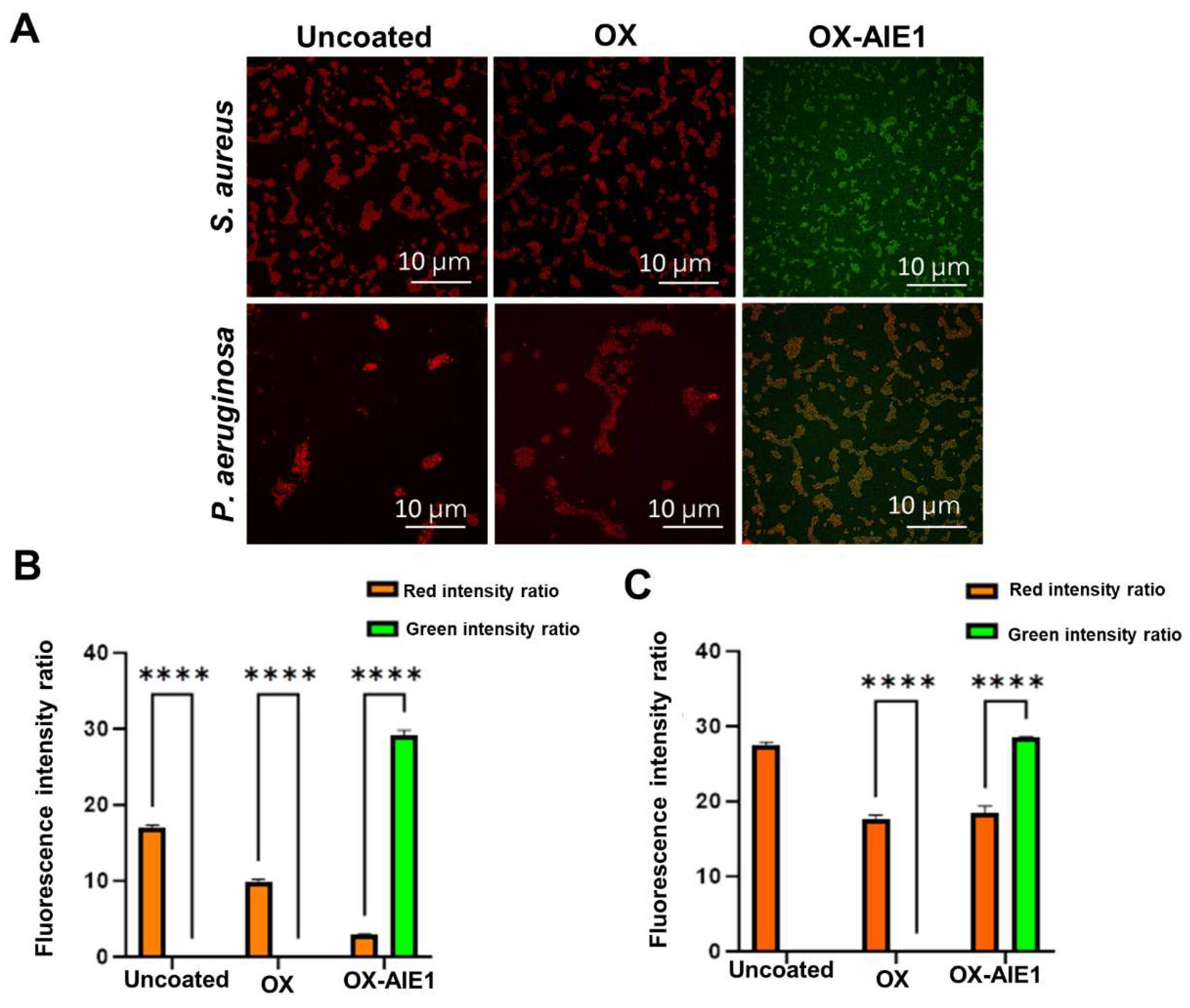
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Plasma-Functionalised Surfaces
3.2.2. Surface Immobilisation Using AIE PS
3.2.3. Ellipsometry
3.2.4. Water Contact Angle
3.2.5. UV Visible Spectroscopy
3.2.6. Fourier Transform Infrared Microscopy (FTIR)
3.2.7. Fluorescent Microscope
3.2.8. Scanning Electron Microscopy (SEM)
3.2.9. Live/Dead Antibacterial Assay
3.2.10. Colony Enumeration
3.2.11. Reactive Oxygen Species (ROS) Assay
3.2.12. Membrane Potential
3.2.13. Membrane Integrity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Hegde, V.; Zoller, S.D.; Park, H.Y.; Hart, C.M.; Kondo, T.; Hamad, C.D.; Hu, Y.; Loftin, A.H.; Johansen, D.O.; et al. Point-of-care antimicrobial coating protects orthopaedic implants from bacterial challenge. Nat. Commun. 2021, 12, 5473. [Google Scholar] [CrossRef] [PubMed]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Bartolo, M.; Zucchella, C.; Aabid, H.; Valoriani, B.; Copetti, M.; Fontana, A.; Intiso, D.; Mancuso, M. Impact of healthcare-associated infections on functional outcome of severe acquired brain injury during inpatient rehabilitation. Sci. Rep. 2022, 12, 5245. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Turk, S. Hospital textiles, are they a possible vehicle for healthcare-associated infections? Int. J. Environ. Res. Public Health 2012, 9, 3330–3343. [Google Scholar] [CrossRef]
- Mitchell, A.; Spencer, M.; Edmiston, C., Jr. Role of healthcare apparel and other healthcare textiles in the transmission of pathogens: A review of the literature. J. Hosp. Infect. 2015, 90, 285–292. [Google Scholar] [CrossRef]
- Qian, J.; Dong, Q.; Chun, K.; Zhu, D.; Zhang, X.; Mao, Y.; Culver, J.N.; Tai, S.; German, J.R.; Dean, D.P.; et al. Highly stable, antiviral, antibacterial cotton textiles via molecular engineering. Nat. Nanotechnol. 2023, 18, 168–176. [Google Scholar] [CrossRef]
- Capatina, D.; Lupoi, T.; Feier, B.; Olah, D.; Cristea, C.; Oprean, R. Highly Sensitive Detection of PQS Quorum Sensing in Pseudomonas aeruginosa Using Screen-Printed Electrodes Modified with Nanomaterials. Biosensors 2022, 12, 638. [Google Scholar] [CrossRef]
- Berlanda, J.; Kiesslich, T.; Engelhardt, V.; Krammer, B.; Plaetzer, K. Comparative in vitro study on the characteristics of different photosensitizers employed in PDT. J. Photochem. Photobiol. B 2010, 100, 173–180. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, Z.; Zabihi, F.; Chen, Z.; Zhu, M. Progress and Perspective of Antiviral Protective Material. Adv. Fiber Mater. 2020, 2, 123–139. [Google Scholar] [CrossRef]
- Peng, J.; Liu, P.; Peng, W.; Sun, J.; Dong, X.; Ma, Z.; Gan, D.; Liu, P.; Shen, J. Poly(hexamethylene biguanide) (PHMB) as high-efficiency antibacterial coating for titanium substrates. J. Hazard. Mater. 2021, 411, 125110. [Google Scholar] [CrossRef] [PubMed]
- Gutman, O.; Natan, M.; Banin, E.; Margel, S. Characterization and antibacterial properties of N-halamine-derivatized cross-linked polymethacrylamide nanoparticles. Biomaterials 2014, 35, 5079–5087. [Google Scholar] [CrossRef] [PubMed]
- Ninan, N.; Joseph, B.; Visalakshan, R.M.; Bright, R.; Denoual, C.; Zilm, P.; Dalvi, Y.B.; Priya, P.V.; Mathew, A.; Grohens, Y.; et al. Plasma assisted design of biocompatible 3D printed PCL/silver nanoparticle scaffolds: In vitro and in vivo analyses. Mater. Adv. 2021, 2, 6620–6630. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ikram, S. A comprehensive review on bio-mimicked multimolecular frameworks and supramolecules as scaffolds for enzyme immobilization. Biotechnol. Bioeng. 2023, 120, 352–398. [Google Scholar] [CrossRef] [PubMed]
- González García, L.E.; Ninan, N.; Simon, J.; Madathiparambil Visalakshan, R.; Bright, R.; Wahono, S.K.; Ostrikov, K.; Mailänder, V.; Landfester, K.; Goswami, N.; et al. Ultra-small gold nanoclusters assembled on plasma polymer-modified zeolites: A multifunctional nanohybrid with anti-haemorrhagic and anti-inflammatory properties. Nanoscale 2021, 13, 19936–19945. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, Z.; Zhao, Z.; Tang, B.Z. TypeIAIE photosensitizers: Mechanism and application. View 2022, 3, 20200121. [Google Scholar] [CrossRef]
- Chua, M.H.; Chin, K.L.O.; Loh, X.J.; Zhu, Q.; Xu, J. Aggregation-Induced Emission-Active Nanostructures: Beyond Biomedical Applications. ACS Nano 2023, 17, 1845–1878. [Google Scholar] [CrossRef]
- Tavakoli, J.; Gascooke, J.; Xie, N.; Tang, B.Z.; Tang, Y. Enlightening Freeze–Thaw Process of Physically Cross-Linked Poly(vinyl alcohol) Hydrogels by Aggregation-Induced Emission Fluorogens. ACS Appl. Polym. Mater. 2019, 1, 1390–1398. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, P.; Wang, Z.; Li, H.; Yu, L.; Sun, D.; Chen, M.; Bi, Y.; Xin, X.; Hao, J. Metal–Organic Gels from Silver Nanoclusters with Aggregation-Induced Emission and Fluorescence-to-Phosphorescence Switching. Angew. Chem. Int. Ed. 2020, 59, 9922–9927. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, L.; Xian, L.; Wang, Z.; Jin, J.; Zheng, C.; Chen, R. AIE-active organic resonance molecules for highly sensitive dynamic explosive detection. J. Mater. Chem. C 2022, 10, 8241–8245. [Google Scholar] [CrossRef]
- He, Z.; Jiang, R.; Long, W.; Huang, H.; Liu, M.; Feng, Y.; Zhou, N.; Ouyang, H.; Zhang, X.; Wei, Y. Red aggregation-induced emission luminogen and Gd3+ codoped mesoporous silica nanoparticles as dual-mode probes for fluorescent and magnetic resonance imaging. J. Colloid Interface Sci. 2020, 567, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ma, Y.; Yang, T.; Yang, X.; Tao, R.; Jin, Y.; Zhang, W.; Qiu, L. Rehealable and Recyclable Aggregation-Induced Emission (AIE)-Active Luminescent Vitrimers. ACS Mater. Lett. 2023, 5, 2348–2354. [Google Scholar] [CrossRef]
- Wang, J.-L.; Xia, F.-W.; Wang, Y.; Shi, H.-Z.; Wang, L.-J.; Zhao, Y.; Song, J.-X.; Wu, M.-Y.; Feng, S. Molecular Charge and Antibacterial Performance Relationships of Aggregation-Induced Emission Photosensitizers. ACS Appl. Mater. Interfaces 2023, 15, 17433–17443. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Y.; Chen, Y.; Chen, J.; Feng, G.; Tang, B.Z. Cationic AIE-active photosensitizers for highly efficient photodynamic eradication of drug-resistant bacteria. Mater. Chem. Front. 2023, 7, 96–105. [Google Scholar] [CrossRef]
- Gao, F.; Shao, T.; Yu, Y.; Xiong, Y.; Yang, L. Surface-bound reactive oxygen species generating nanozymes for selective antibacterial action. Nat. Commun. 2021, 12, 745. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Varshney, S.; Gupta, D.; Sharma, S. Textiles as fomites in the healthcare system. Appl. Microbiol. Biotechnol. 2023, 107, 3887–3897. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, J.M.; Asally, M. The Microbiologist’s Guide to Membrane Potential Dynamics. Trends Microbiol. 2020, 28, 304–314. [Google Scholar] [CrossRef]
- Pham, T.; Nguyen, T.T.; Nguyen, N.H.; Hayles, A.; Li, W.; Pham, D.Q.; Nguyen, C.K.; Nguyen, T.; Vongsvivut, J.; Ninan, N.; et al. Transforming Spirulina maxima Biomass into Ultrathin Bioactive Coatings Using an Atmospheric Plasma Jet: A New Approach to Healing of Infected Wounds. Small 2023, 2305469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahu, R.; Ninan, N.; Nguyen, N.H.; Wang, J.; Vasilev, K.; Truong, V.K.; Tang, Y. Antibacterial Textile Coating Armoured with Aggregation-Induced Emission Photosensitisers to Prevent Healthcare-Associated Infections. Molecules 2024, 29, 1209. https://doi.org/10.3390/molecules29061209
Sahu R, Ninan N, Nguyen NH, Wang J, Vasilev K, Truong VK, Tang Y. Antibacterial Textile Coating Armoured with Aggregation-Induced Emission Photosensitisers to Prevent Healthcare-Associated Infections. Molecules. 2024; 29(6):1209. https://doi.org/10.3390/molecules29061209
Chicago/Turabian StyleSahu, Resmarani, Neethu Ninan, Ngoc Huu Nguyen, Jianzhong Wang, Krasimir Vasilev, Vi Khanh Truong, and Youhong Tang. 2024. "Antibacterial Textile Coating Armoured with Aggregation-Induced Emission Photosensitisers to Prevent Healthcare-Associated Infections" Molecules 29, no. 6: 1209. https://doi.org/10.3390/molecules29061209
APA StyleSahu, R., Ninan, N., Nguyen, N. H., Wang, J., Vasilev, K., Truong, V. K., & Tang, Y. (2024). Antibacterial Textile Coating Armoured with Aggregation-Induced Emission Photosensitisers to Prevent Healthcare-Associated Infections. Molecules, 29(6), 1209. https://doi.org/10.3390/molecules29061209








