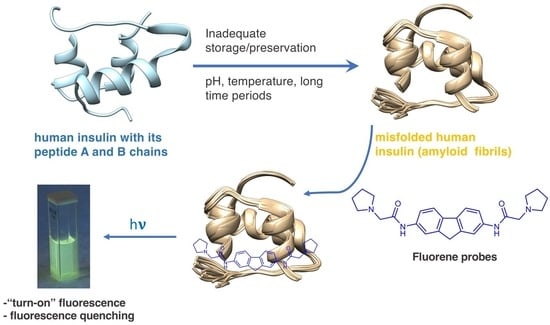
Fluorimetric Detection of Insulin Misfolding by Probes Derived from Functionalized Fluorene Frameworks
Abstract
1. Introduction
2. Results and Discussion
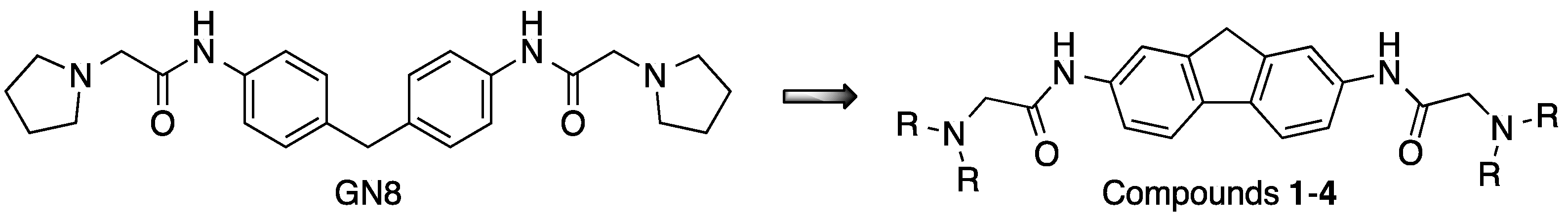
2.1. Synthesis
2.2. Spectrophotometric and Spectrofluorimetric Study of Fluorene Derivatives
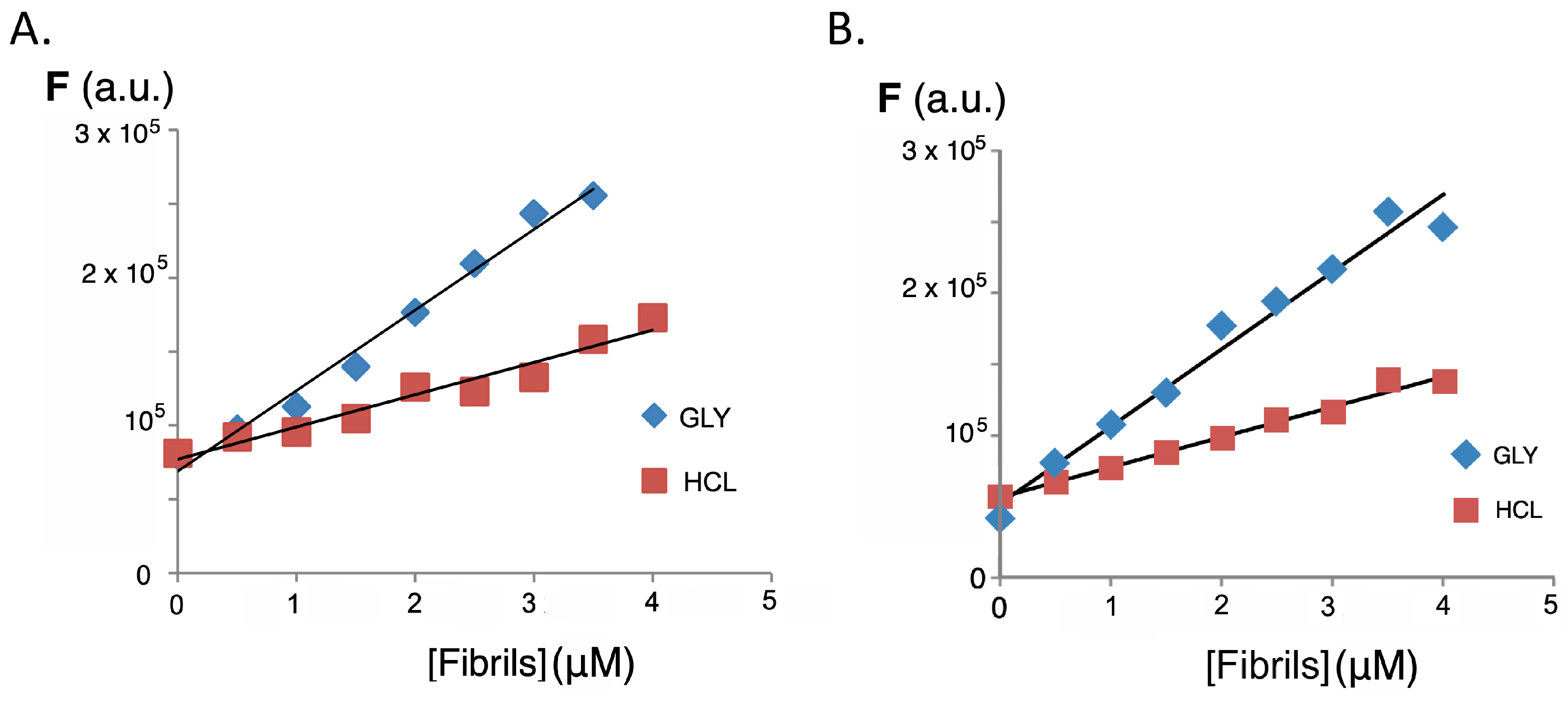
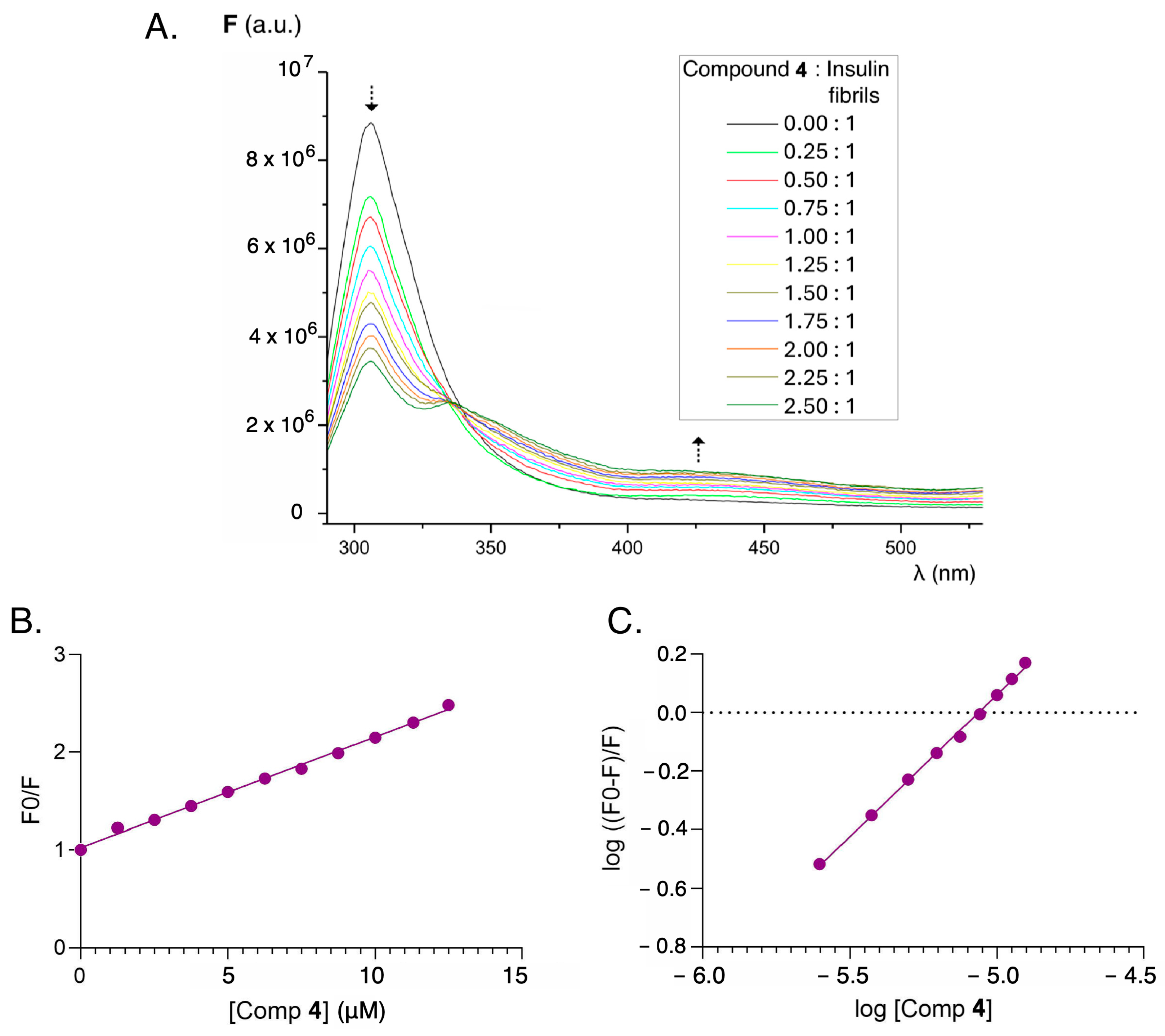
2.3. Interaction with Insulin and Insulin Fibrils
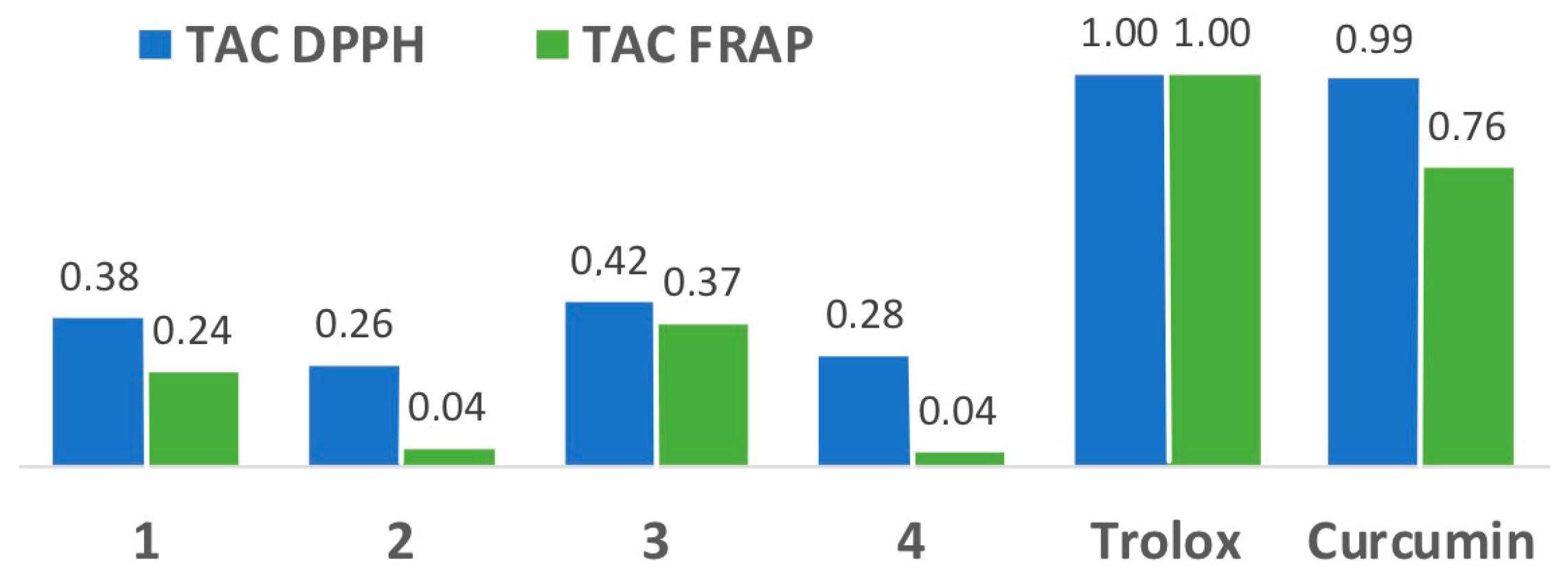
2.4. Antioxidant Capacity
3. Materials and Methods
3.1. General Experimental Information
3.2. Synthesis of Fluorene Derivatives. General Procedure
3.2.1. N,N′-(9H-Fluorene-2,7-diyl)bis(2-(4-methylpiperazin-1-yl)acetamide) (1)
3.2.2. N,N′-(9H-Fluorene-2,7-diyl)bis(2-(4-phenylpiperazin-1-yl)acetamide) (2)
3.2.3. N,N′-(9H-Fluorene-2,7-diyl)bis(dimethylamino)acetamide (3)
3.2.4. N,N′-(9H-Fluorene-2,7-diyl)bis(2-(pyrrolidin-1-yl)acetamide) (4)
3.3. Synthesis of GN8
3.4. Preparation of Amyloid β Fibrils from Human Insulin
3.5. Spectrophotometric and Spectrofluorimetric Study of Functionalized Fluorene Derivatives
3.6. Fluorescence Studies of Fluorene Sensors Interaction with Native Insulin Protein
3.7. Fluorene Sensors’ Interaction with Fibrils of β-amyloid Human Insulin Protein
3.8. Fluorescence Quenching for Evidencing Sensor–Protein Interaction
3.9. Determination of the Antioxidant Capacity of Fluorene Derivatives
3.9.1. DPPH Method: 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Activity
3.9.2. FRAP Method: Ferric Ion Reducing Antioxidant Power
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kell, D.D.; Pretorius, E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: Lessons from and for blood clotting. Prog. Biophys. Mol. Biol. 2017, 123, 16–41. [Google Scholar] [CrossRef] [PubMed]
- DeToma, A.S.; Salamekh, S.; Ramamoorthy, A.; Lim, M.H. Misfolded proteins in Alzheimer’s disease and type II diabetes. Chem. Soc. Rev. 2012, 41, 608–621. [Google Scholar] [CrossRef]
- Mukherjee, A.; Morales-Scheihing, D.; Butler, P.C.; Soto, C. Type 2 diabetes as a protein misfolding disease. Trends Mol. Med. 2015, 21, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Mankad, A.K.; Sesay, I.; Shah, K.B. Light-chain cardiac amyloidosis. Curr. Probl. Cancer 2017, 41, 144–156. [Google Scholar] [CrossRef]
- Iliyasu, M.O.; Musa, S.A.; Oladele, S.B.; Iliya, A.I. Amyloid-beta aggregation implicates multiple pathways in Alzheimer’s disease: Understanding the mechanisms. Front. Neurosci. 2023, 17, 1081938. [Google Scholar] [CrossRef]
- Hernández, F.; Ferrer, I.; Pérez, M.; Zabala, J.C.; del Río, J.A.; Ávila, J. Tau aggregation. Neuroscience 2023, 518, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Forloni, G. Alpha synuclein: Neurodegeneration and inflammation. Int. J. Mol. Sci. 2023, 24, 5914. [Google Scholar] [CrossRef]
- Eftekharzadeh, B.; Hyman, B.T.; Wegmann, S. Structural studies on the mechanism of protein aggregation in age-related neurodegenerative diseases. Mech. Ageing Dev. 2016, 156, 1–13. [Google Scholar] [CrossRef]
- Bertrand, E.; Demongin, C.; Dobra, I.; Rengifo-González, J.C.; Singatulina, A.S.; Sukhanova, M.V.; Lavrik, O.I.; Pastré, D.; Hamon, L. FUS fibrillation occurs through a nucleation-based process below the critical concentration required for liquid–liquid phase separation. Sci. Rep. 2023, 13, 7772. [Google Scholar] [CrossRef]
- Babazadeh, A.; Rayner, S.L.; Lee, A.; Chung, R.S. TDP-43 as a therapeutic target in neurodegenerative diseases: Focusing on motor neuron disease and frontotemporal dementia. Ageing Res. Rev. 2023, 92, 102085. [Google Scholar] [CrossRef]
- Vaquer-Alicea, J.; Diamond, M.I. Propagation of protein aggregation in neurodegenerative diseases. Annu. Rev. Biochem. 2019, 88, 785–810. [Google Scholar] [CrossRef]
- Collinge, J. Mammalian prions and their wider relevance in neurodegenerative diseases. Nature 2016, 539, 217–226. [Google Scholar] [CrossRef]
- Ascari, L.M.; Rocha, S.C.; Gonçalves, P.B.; Vieira, T.C.; Cordeiro, Y. Challenges and advances in antemortem diagnosis of human transmissible spongiform encephalopathies. Front. Bioeng. Biotechnol. 2020, 8, 585896. [Google Scholar] [CrossRef]
- Heumüller, S.E.; Hornberger, A.C.; Hebestreit, A.S.; Hossinger, A.; Vorberg, I.M. Propagation and dissemination strategies of transmissible spongiform encephalopathy agents in mammalian cells. Int. J. Mol. Sci. 2022, 23, 2909. [Google Scholar] [CrossRef] [PubMed]
- Kheirbakhsh, R.; Chinisaz, M.; Amanpour, S.; Amini, S.; Khodayari, S.; Khodayari, H.; Dilmaghanian, A.; Haddadi, M.; Ebrahim-Habibi, A. Turmeric effect on subcutaneous insulin-induced amyloid mass: An in vivo study. Drug Chem. Toxicol. 2017, 40, 1–6. [Google Scholar] [CrossRef]
- Lewis, B.E.; Mulka, A.; Mao, L.; Sharafieh, R.; Qiao, Y.; Kesserwan, S.; Wu, R.; Kreutzer, D.; Klueh, U. Insulin derived fibrils induce cytotoxicity in vitro and trigger inflammation in murine models. J. Diabetes Sci. Technol. 2023, 17, 163–171. [Google Scholar] [CrossRef]
- Delbeck, S.; Heise, H.M. Quality assurance of commercial insulin formulations: Novel assay using infrared spectroscopy. J. Diabetes Sci. Technol. 2021, 15, 865–873. [Google Scholar] [CrossRef]
- D’Souza, A.; Theis, J.D.; Vrana, J.A.; Dogan, A. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid 2014, 21, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Shikama, Y.; Kitazawa, J.; Yagihashi, N.; Uehara, O.; Murata, Y.; Yajima, N.; Wada, R.; Yagihashi, S. Localized amyloidosis at the site of repeated insulin injection in a diabetic patient. Intern. Med. 2010, 49, 397–401. [Google Scholar] [CrossRef]
- Nagase, T.; Iwaya, K.; Iwaki, Y.; Kotake, F.; Uchida, R.; Oh-i, T.; Sekine, H.; Miwa, K.; Murakami, S.; Odaka, T.; et al. Insulin-derived amyloidosis and poor glycemic control: A case series. Am. J. Med. 2014, 127, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Hrudka, J.; Sticová, E.; Krbcová, M.; Schwarzmannová, K. Localized insulin-derived amyloidosis in diabetes mellitus Type 1 patient: A case report. Diagnostics 2023, 13, 2415. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, C.F.; Norrman, M.; Wahlund, P.O.; Benie, A.J.; Petersen, B.O.; Jessen, C.M.; Pedersen, T.Å.; Vestergaard, K.; Steensgaard, D.B.; Pedersen, J.S.; et al. Structure, aggregation, and activity of a covalent insulin dimer formed during storage of neutral formulation of human insulin. J. Pharm. Sci. 2016, 105, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.; Braune, K.; Carter, A.; Zayani, A.; Krämer, L.A. Insulin storage: A critical reappraisal. J. Diabetes Sci. Technol. 2021, 15, 147–159. [Google Scholar] [CrossRef]
- Nilsson, M.R. Insulin amyloid at injection sites of patients with diabetes. Amyloid 2016, 23, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Ghasemi, Y.; Uversky, V.N.; Yousefi, R. Chemical modifications of insulin: Finding a compromise between stability and pharmaceutical performance. Int. J. Pharm. 2018, 547, 450–468. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liang, Y.; Wang, Y.; Zhang, J.; Wang, C.; Sun, Y.; Feng, X.; Zhao, G. Orthogonal conformation regulation enables highly efficient phosphorescence emission in BODIPY-fluorene derivatives without heavy atoms. Dye. Pigm. 2023, 208, 110791. [Google Scholar] [CrossRef]
- Neelambra, A.U.; Govind, C.; Devassia, T.T.; Somashekharappa, G.M.; Karunakaran, V. Direct evidence of solvent polarity governing the intramolecular charge and energy transfer: Ultrafast relaxation dynamics of push–pull fluorene derivatives. Phys. Chem. Chem. Phys. 2019, 21, 11087–11102. [Google Scholar] [CrossRef]
- Waseem, M.T.; Junaid, H.M.; Gul, H.; Khan, Z.A.; Yu, C.; Shahzad, S.A. Fluorene based fluorescent and colorimetric sensors for ultrasensitive detection of nitroaromatics in aqueous medium. J. Photochem. Photobiol. A 2022, 425, 113660. [Google Scholar]
- Batool, R.; Riaz, N.; Junaid, H.M.; Waseem, M.T.; Khan, Z.A.; Nawazish, S.; Farooq, U.; Yu, C.; Shahzad, S.A. Fluorene-based fluorometric and colorimetric conjugated polymers for sensitive detection of 2,4,6-trinitrophenol explosive in aqueous medium. ACS Omega 2021, 7, 1057–1070. [Google Scholar] [CrossRef]
- Rostamnezhad, F.; Fatemi, M.H. Comprehensive investigation of binding of some polycyclic aromatic hydrocarbons with bovine serum albumin: Spectroscopic and molecular docking studies. Bioorg. Chem. 2022, 120, 105656. [Google Scholar] [CrossRef]
- Capodilupo, A.L.; Manni, F.; Corrente, G.A.; Accorsi, G.; Fabiano, E.; Cardone, A.; Giannuzzi, R.; Beneduci, A.; Gigli, G. Arylamino-fluorene derivatives: Optically induced electron transfer investigation, redox-controlled modulation of absorption and fluorescence. Dye. Pigment. 2020, 177, 108325. [Google Scholar] [CrossRef]
- Shaya, J.; Corridon, P.R.; Al-Omari, B.; Aoudi, A.; Shunnar, A.; Mohideen, M.I.H.; Qurashi, A.; Michel, B.Y.; Burger, A. Design, photophysical properties, and applications of fluorene-based fluorophores in two-photon fluorescence bioimaging: A review. J. Photochem. Photobiol. C 2022, 52, 100529. [Google Scholar] [CrossRef]
- Shvadchak, V.V.; Kucherak, O.; Afitska, K.; Dziuba, D.; Yushchenko, D.A. Environmentally sensitive probes for monitoring protein-membrane interactions at nanomolar concentrations. Biochim. Biophys. Acta Biomembr. 2017, 1859, 852–859. [Google Scholar] [CrossRef]
- Antimonova, O.I.; Grudinina, N.A.; Egorov, V.V.; Ilyin, V.V.; Zabrodskaya, Y.A.; Ramsay, E.S.; Shabalin, K.A.; Protasov, A.V.; Yakimov, A.P.; Polukeev, V.A.; et al. Time machine: Can a dye from 1928 be re-purposed for modern, fluorescence-based detection of amyloid-like fibrils? Dye. Pigment. 2020, 172, 107863. [Google Scholar] [CrossRef]
- Mei, L.-J.; Fan, C.; Xu, C.-R.; Yu, Q.; Li, C.; Wang, Y.-L.; Zhu, M.Q. Cationic molecular probes based on aggregation-induced emission for fluorescent sensing and super-resolution imaging of insulin fibrosis. Chem. Eng. J. 2023, 451, 139027. [Google Scholar] [CrossRef]
- Hussain, S.; Chen, X.; Wang, C.; Hao, Y.; Tian, X.; He, Y.; Li, J.; Shahid, M.; Iyer, P.K.; Gao, R. Aggregation and binding-directed FRET modulation of conjugated polymer materials for selective and point-of-care monitoring of serum albumins. Anal. Chem. 2022, 94, 10685–10694. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Ly, S.; Hilt, S.; Petrlova, J.; Maezawa, I.; Kálai, T.; Hideg, K.; Jin, L.-W.; Lawrence, T.A.; Voss, J.C. Protective spin-labeled fluorenes maintain amyloid beta peptide in small oligomers and limit transitions in secondary structure. Biochim. Biophys. Acta 2015, 1854, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Petrlova, J.; Kálai, T.; Maezawa, I.; Altman, R.; Harishchandra, G.; Hong, H.S.; Bricarello, D.A.; Parikh, A.N.; Lorigan, G.A.; Jin, L.-W.; et al. The influence of spin-labeled fluorene compounds on the assembly and toxicity of the Aβ peptide. PLoS ONE 2012, 7, e35443. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Q.; Li, M.; Bao, B.; Tang, Y. A ratiometric fluorescent conjugated oligomer for amyloid β recognition, aggregation inhibition, and detoxification. Small 2021, 17, 2104581. [Google Scholar] [CrossRef]
- Ghosh, D.; Dutta, P.; Chakraborty, C.; Singh, P.K.; Anoop, A.; Jha, N.N.; Jacob, R.S.; Mondal, M.; Mankar, S.; Das, S.; et al. Complexation of amyloid fibrils with charged conjugated polymers. Langmuir 2014, 30, 3775–3786. [Google Scholar] [CrossRef]
- Pasieka, A.; Panek, D.; Zaręba, P.; Sługocka, E.; Gucwa, N.; Espargaró, A.; Latacz, G.; Khan, N.; Bucki, A.; Sabaté, R.; et al. Novel drug-like fluorenyl derivatives as selective butyrylcholinesterase and β-amyloid inhibitors for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2023, 88, 117333. [Google Scholar] [CrossRef] [PubMed]
- Kálai, T.; Petrlova, J.; Balog, M.; Aung, H.H.; Voss, J.C.; Hideg, K. Synthesis and study of 2-amino-7-bromofluorenes modified with nitroxides and their precursors as dual anti-amyloid and antioxidant active compounds. Eur. J. Med. Chem. 2011, 46, 1348–1355. [Google Scholar] [CrossRef]
- Hilt, S.; Altman, R.; Kálai, T.; Maezawa, I.; Gong, Q.; Wachsmann-Hogiu, S.; Jin, L.-W.; Voss, J.C. A bifunctional anti-amyloid blocks oxidative stress and the accumulation of intraneuronal amyloid-beta. Molecules 2018, 23, 2010. [Google Scholar] [CrossRef] [PubMed]
- Bouziane, H.; Chouarfia, A. Sequence- and structure-based prediction of amyloidogenic regions in proteins. Soft Comput. 2020, 24, 3285–3308. [Google Scholar] [CrossRef]
- Kuwata, K.; Nishida, N.; Matsumoto, T.; Kamatari, Y.O.; Hosokawa-Muto, J.; Kodama, K.; Nakamura, H.K.; Kimura, K.; Kawasaki, M.; Takakura, Y.; et al. Hot spots in prion protein for pathogenic conversión. Proc. Natl. Acad. Sci. USA 2007, 104, 11921–11926. [Google Scholar] [CrossRef]
- Kimura, T.; Hosokawa-Muto, J.; Kamatari, Y.O.; Kuwata, K. Synthesis of GN8 derivatives and evaluation of their antiprion activity in TSE-infected cells. Bioorg. Med. Chem. Lett. 2011, 21, 1502–1507. [Google Scholar] [CrossRef]
- Staderini, M.; Vanni, S.; Colini Baldeschi, A.; Giachin, G.; Zattoni, M.; Celauro, L.; Ferracin, C.; Bistaffa, E.; Moda, F.; Pérez, D.I.; et al. Bifunctional carbazole derivatives for simultaneous therapy and fluorescence imaging in prion disease murine cell models. Eur. J. Med. Chem. 2023, 245, 114923. [Google Scholar] [CrossRef]
- Berlman, I.B. Handbook of Fluorescence Spectra of Aromatic Molecules, 2nd ed.; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Guilbault, G.G. (Ed.) Practical Fluorescence; Marcel Dekker Inc.: New York, NY, USA, 1990. [Google Scholar]
- Dong, X.; Wan, W.; Zeng, L.; Jin, W.; Huang, Y.; Shen, D.; Bai, Y.; Zhao, Q.; Zang, L.; Liu, Y.; et al. Regulation of fluorescence solvatochromism to resolve cellular polarity upon protein aggregation. Anal. Chem. 2021, 93, 16447–16455. [Google Scholar] [CrossRef] [PubMed]
- Amdursky, N.; Erez, Y.; Huppert, D. Molecular rotors: What lies behind the high sensitivity of the thioflavin-T fluorescent marker. Acc. Chem. Res. 2012, 45, 1548–1557. [Google Scholar] [CrossRef]
- Morais, G.R.; Miranda, H.V.; Santos, I.C.; Santos, I.; Outeiro, T.F.; Paulo, A. Synthesis and in vitro evaluation of fluorinated styryl benzazoles as amyloid-probes. Bioorg. Med. Chem. 2011, 19, 7698–7710. [Google Scholar] [CrossRef]
- Biancalana, M.; Makabe, K.; Koide, A.; Koide, S. Molecular mechanism of thioflavin-T binding to the surface of β-rich peptide self-assemblies. J. Mol. Biol. 2009, 385, 1052–1063. [Google Scholar] [CrossRef]
- Freire, S.; de Araujo, M.H.; Al-Soufi, W.; Novo, M. Photophysical study of thioflavin T as fluorescence marker of amyloid fibrils. Dye. Pigment. 2014, 110, 97–105. [Google Scholar] [CrossRef]
- Zhao, H.; Ge, M.; Zhang, Z.; Wang, W.; Wu, G. Spectroscopic studies on the interaction between riboflavin and albumins. Spectrochim. Acta A Mol. 2006, 65, 811–817. [Google Scholar] [CrossRef]
- Sen, P.; Fatima, S.; Ahmad, B.; Khan, R.H. Interactions of thioflavin T with serum albumins: Spectroscopic analyses. Spectrochim. Acta A Mol. 2009, 74, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Morgenstern, J.L.; Lamb, J.R.; Lockhart, A. Characterisation of the binding of amyloid imaging tracers to rodent Aβ fibrils and rodent-human Aβ co-polymers. Biochem. Biophys. Res. Commun. 2006, 347, 669–677. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Ishak, S.; Yeap, G.Y.; Sasidharan, S.; Sangeetha, T.; Kaneko, K.; Ito, M.M. Experimental and computational studies of fluorene derivatives containing two identical quinoline and pyridine moieties. J. Mol. Struct. 2022, 1253, 132174. [Google Scholar] [CrossRef]
- Wigenius, J.; Andersson, M.R.; Esbjörner, E.K.; Westerlund, F. Interactions between a luminescent conjugated polyelectrolyte and amyloid fibrils investigated with flow linear dichroism spectroscopy. Biophys. Res. Commun. 2011, 408, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Craparo, E.F.; Podestà, A.; Bulone, D.; Carrotta, R.; Martorana, V.; Tiana, G.; San Biagio, P.L.J. Kinetics of different processes in human insulin amyloid formation. J. Mol. Biol. 2007, 366, 258–274. [Google Scholar]
- Nilsson, K.; Herland, A.; Hammarström, P.; Inganäs, O. Conjugated polyelectrolytes: Conformation-sensitive optical probes for detection of amyloid fibril formation. Biochemistry 2005, 44, 3718–3724. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, K.; Narayanaswamy, N.; Murugan, N.A.; Kuang, G.; Ågren, H.; Govindaraju, T. A high affinity red fluorescence and colorimetric probe for amyloid aggregates. Sci. Rep. 2016, 6, 23668. [Google Scholar] [CrossRef]
- Li, Q.; Min, J.; Ahn, Y.-H.; Namm, J.; Kim, E.M.; Lui, R.; Kim, H.Y.; Ji, Y.; Wu, H.; Wisniewski, T.; et al. Styryl-based compounds as potential in vivo imaging agents for β-amyloid plaques. ChemBioChem 2007, 8, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Chapter 2—Instrumentation for fluorescence spectroscopy. In Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; p. 54. [Google Scholar]
- Mussardo, P.; Corda, E.; González-Ruiz, V.; Rajesh, J.; Girotti, S.; Martín, M.A.; Olives, A.I. Study of non-covalent interactions of luotonin A derivatives and the DNA minor groove as a first step in the study of their analytical potential as DNA probes. Anal. Bioanal. Chem. 2011, 400, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Nuengchamnong, N.; De Jong, C.F.; Bruyneel, B.; Niessen, W.M.A.; Irth, H.; Ingkaninan, K. HPLC coupled on-line to ESI-MS and a DPPH-based assay for the rapid identification of anti-oxidants in Butea superba. Phytochem. Anal. 2005, 16, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Xing, H.; Lu, X.; Zhao, L.; Qu, K.; Bi, K. Evaluation of antioxidant activity of ten compounds in different tea samples by means of an on-line HPLC–DPPH assay. Food Res. Int. 2013, 53, 847–856. [Google Scholar] [CrossRef]
- Cores, Á.; Abril, S.; Michalska, P.; Duarte, P.; Olives, A.I.; Martín, M.A.; Villacampa, M.; León, R.; Menéndez, J.C. Bisavenathramide analogues as NRF2 inductors and neuroprotectors in in vitro models of oxidative stress and hyperphosphorylation. Antioxidants 2021, 10, 941. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A. Approach to optimization of FRAP methodology for studies based on selected monoterpenes. Molecules 2020, 25, 5267. [Google Scholar] [CrossRef]





| Compound 1 | Kd 2 |
|---|---|
| Thioflavin T | 16.46 |
| 1 | 5.59 |
| 2 | 5.60 |
| 4 | 10.12 |
| Compound | KSV | K |
|---|---|---|
| Thioflavin T | 2.11 × 104 | 1.26 × 103 |
| GN8 | 1.63 × 104 | 1.01 × 104 |
| 4 | 1.09 × 105 | 1.46 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarabia-Vallejo, Á.; Molina, A.; Martínez-Orts, M.; D’Onofrio, A.; Staderini, M.; Bolognesi, M.L.; Martín, M.A.; Olives, A.I.; Menéndez, J.C. Fluorimetric Detection of Insulin Misfolding by Probes Derived from Functionalized Fluorene Frameworks. Molecules 2024, 29, 1196. https://doi.org/10.3390/molecules29061196
Sarabia-Vallejo Á, Molina A, Martínez-Orts M, D’Onofrio A, Staderini M, Bolognesi ML, Martín MA, Olives AI, Menéndez JC. Fluorimetric Detection of Insulin Misfolding by Probes Derived from Functionalized Fluorene Frameworks. Molecules. 2024; 29(6):1196. https://doi.org/10.3390/molecules29061196
Chicago/Turabian StyleSarabia-Vallejo, Álvaro, Ana Molina, Mónica Martínez-Orts, Alice D’Onofrio, Matteo Staderini, Maria Laura Bolognesi, M. Antonia Martín, Ana I. Olives, and J. Carlos Menéndez. 2024. "Fluorimetric Detection of Insulin Misfolding by Probes Derived from Functionalized Fluorene Frameworks" Molecules 29, no. 6: 1196. https://doi.org/10.3390/molecules29061196
APA StyleSarabia-Vallejo, Á., Molina, A., Martínez-Orts, M., D’Onofrio, A., Staderini, M., Bolognesi, M. L., Martín, M. A., Olives, A. I., & Menéndez, J. C. (2024). Fluorimetric Detection of Insulin Misfolding by Probes Derived from Functionalized Fluorene Frameworks. Molecules, 29(6), 1196. https://doi.org/10.3390/molecules29061196