Water-Soluble Trityl Radicals for Fluorescence Imaging
Abstract
1. Introduction
2. Results and Discussion
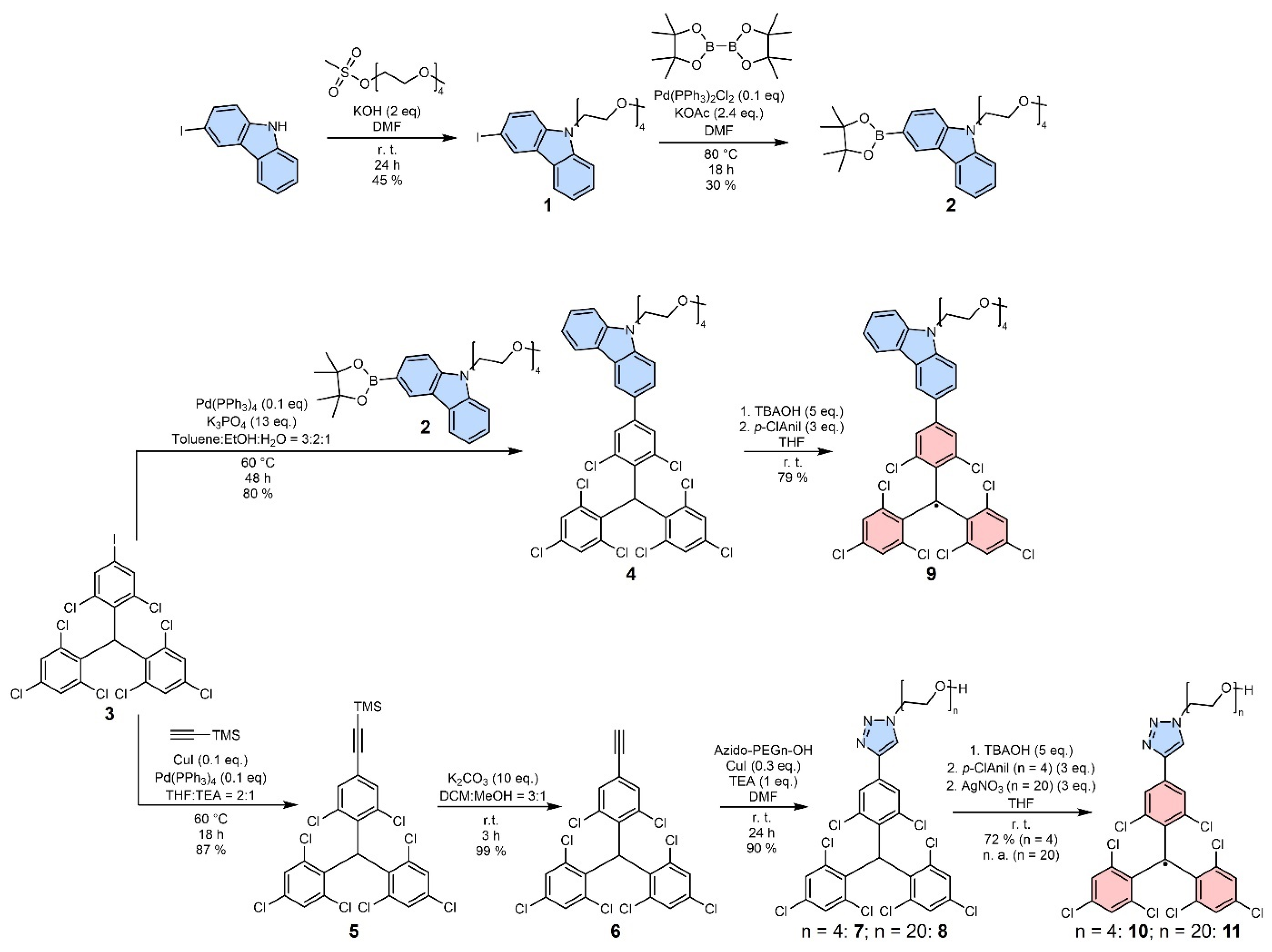
2.1. Synthesis
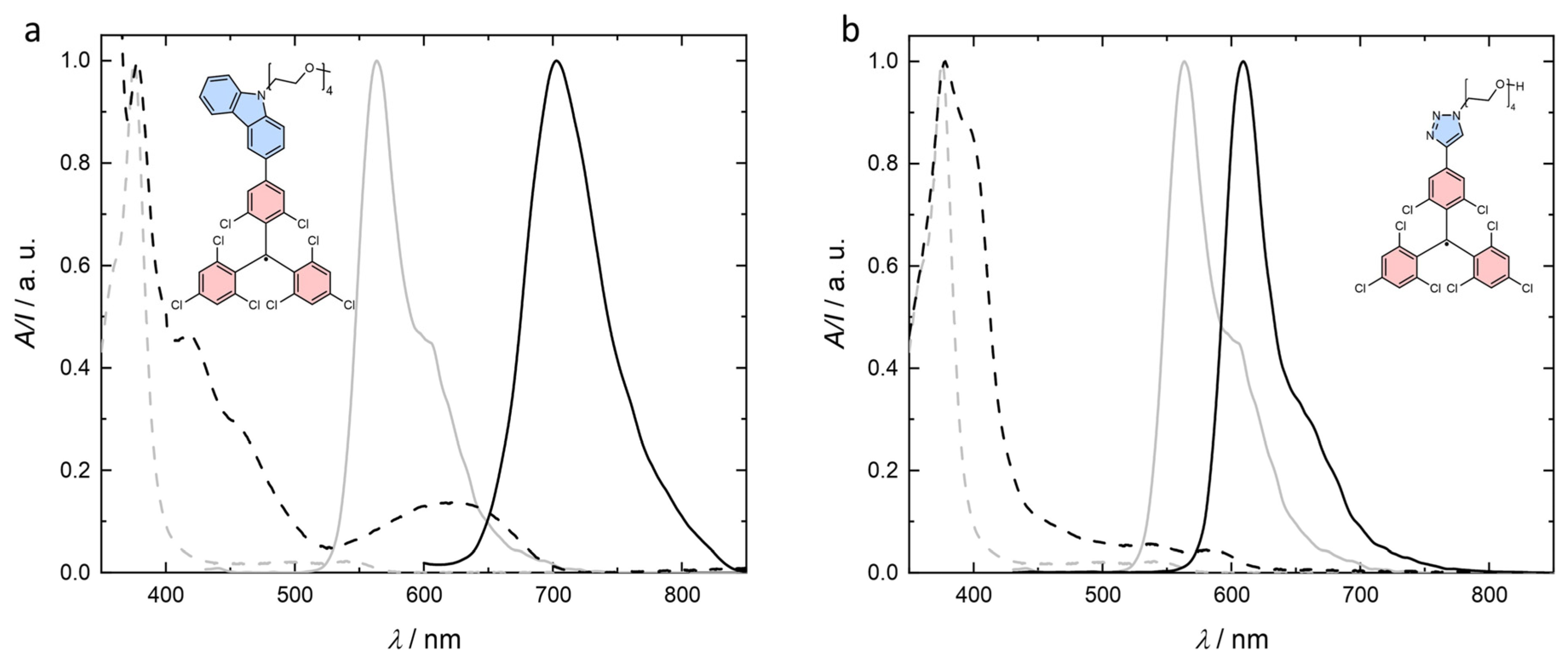
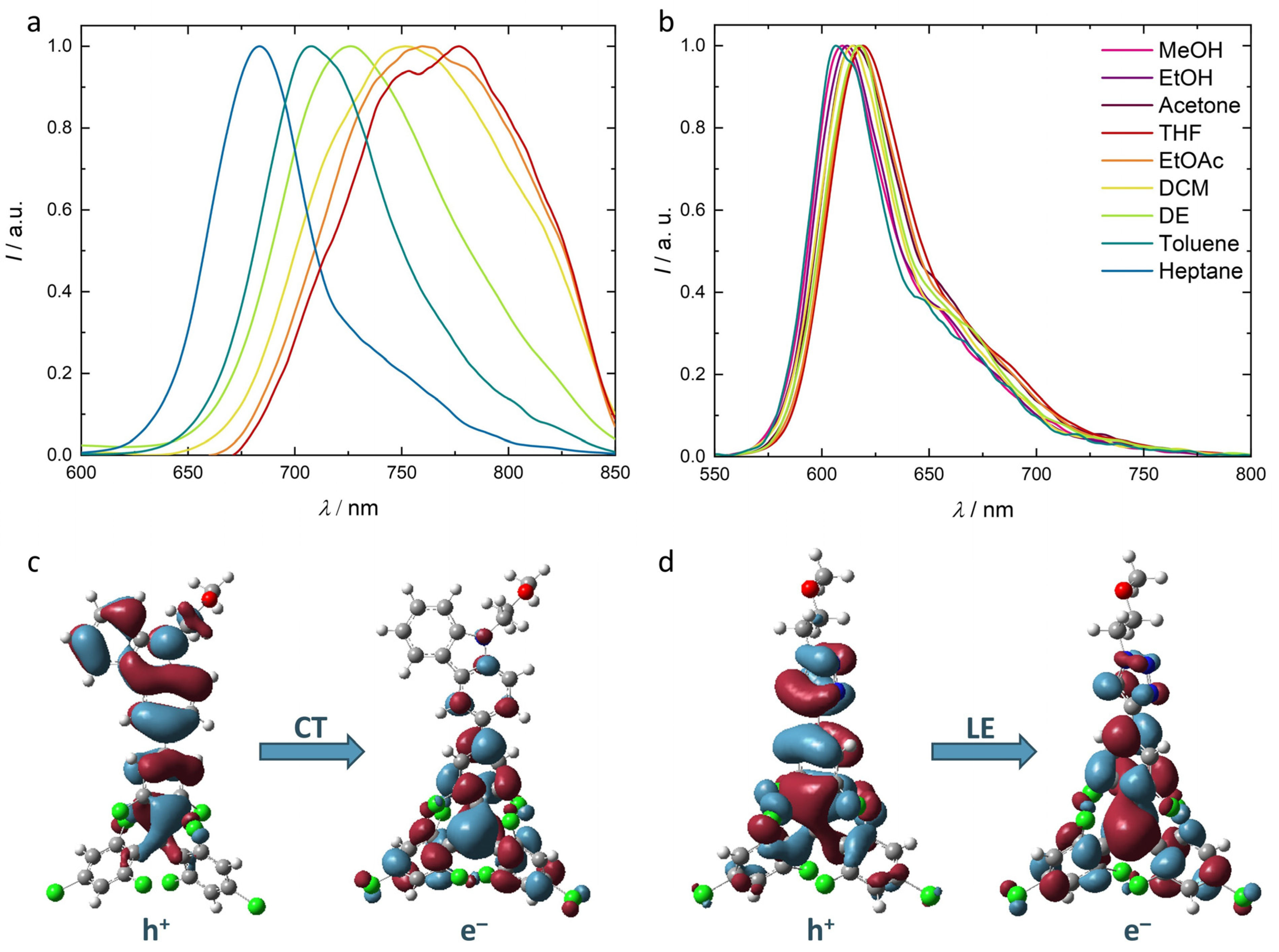
2.2. Optical Characterization of Radicals Carrying OEG Chains in Organic Solvents
2.3. Quantum Chemical Calculations
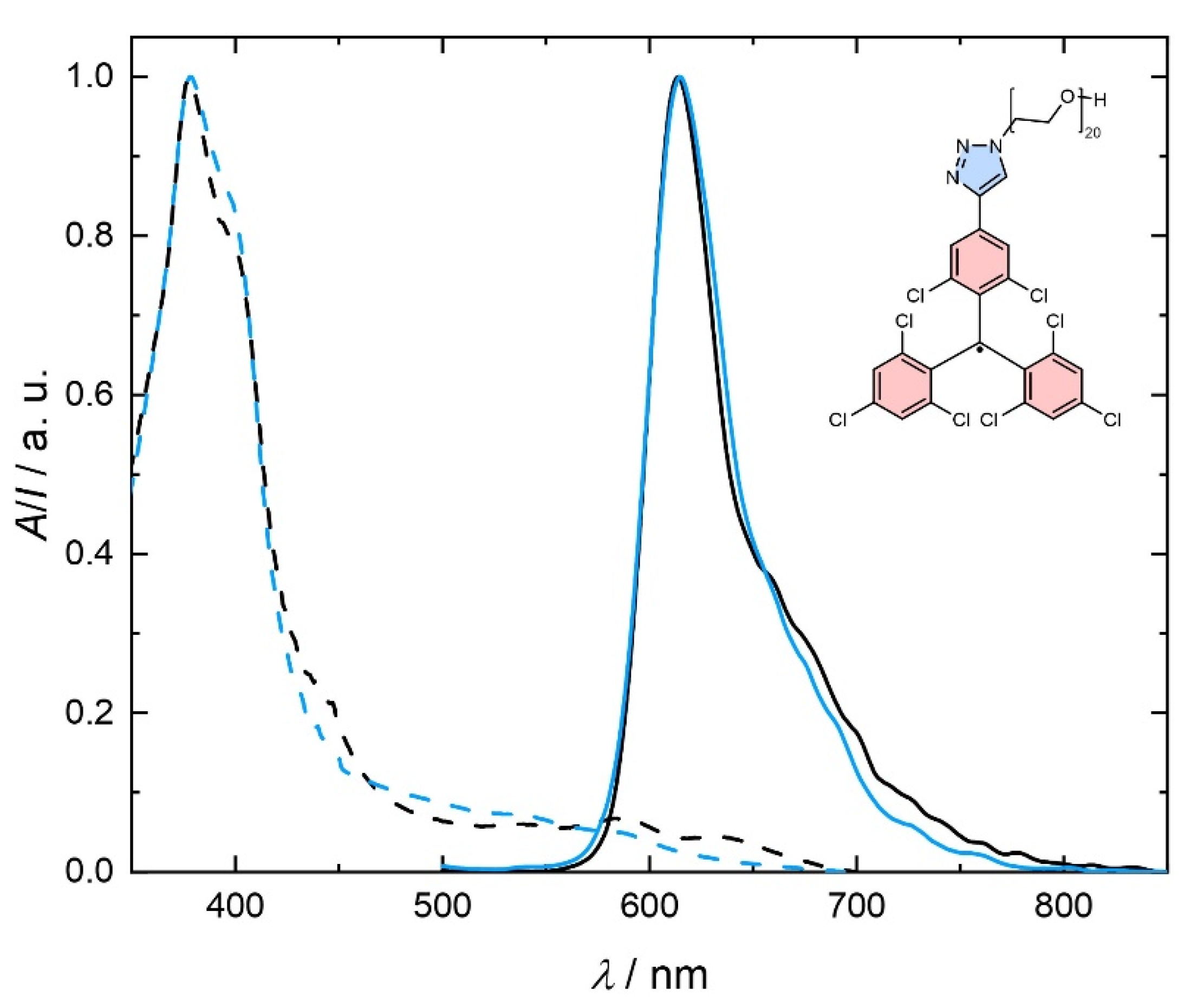
2.4. Water-Soluble TTM
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdurahman, A.; Hele, T.J.H.; Gu, Q.; Zhang, J.; Peng, Q.; Zhang, M.; Friend, R.H.; Li, F.; Evans, E.W. Understanding the luminescent nature of organic radicals for efficient doublet emitters and pure-red light-emitting diodes. Nat. Mater. 2020, 19, 1224–1229. [Google Scholar] [CrossRef]
- Ding, J.; Dong, S.; Zhang, M.; Li, F.J. Efficient pure near-infrared organic light-emitting diodes based on tris (2, 4, 6-trichlorophenyl) methyl radical derivatives. Mater. Chem. C Mater. 2022, 10, 14116–14121. [Google Scholar] [CrossRef]
- Gamero, V.; Velasco, D.; Latorre, S.; López-Calahorra, F.; Brillas, E.; Juliá, L. [4-(N-carbazolyl)-2,6-dichlorophenyl]bis(2,4,6-trichlorophenyl)methyl radical an efficient red light-emitting paramagnetic molecule. Tetrahedron Lett. 2006, 47, 2305–2309. [Google Scholar] [CrossRef]
- Ai, X.; Evans, E.W.; Dong, S.; Gillett, A.J.; Guo, H.; Chen, Y.; Hele, T.J.H.; Friend, R.H.; Li, F. Efficient radical-based light-emitting diodes with doublet emission. Nature 2018, 563, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Obolda, A.; Zhang, M.; Li, F. Organic Light-Emitting Diodes Using a Neutral π Radical as Emitter: The Emission from a Doublet. Angew. Chem. Int. Ed. 2015, 54, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Obolda, A.; Ai, X.; Zhang, M.; Li, F. Up to 100% Formation Ratio of Doublet Exciton in Deep-Red Organic Light-Emitting Diodes Based on Neutral π-Radical. ACS Appl. Mater. Interfaces 2016, 8, 35472–35478. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Wang, P.; Chzhan, M.; Zweier, J.L. High resolution electron paramagnetic resonance imaging of biological samples with a single line paramagnetic label. Magn. Reason. Med. 1997, 37, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Rudolf, T.; Blinder, R.; Suryadevara, N.; Dalmeida, A.; Welscher, P.J.; Lamla, M.; Arnold, M.; Herr, U.; Jelezko, F.; et al. Red-Fluorescing Paramagnetic Conjugated Polymer Nanoparticles—Triphenyl Methyl Radicals as Monomers in C–C Cross-Coupling Dispersion Polymerization. Macromolecules 2023, 56, 2104–2112. [Google Scholar] [CrossRef]
- Mesa, J.A.; Velázquez-Palenzuela, A.; Brillas, E.; Torres, J.L.; Juliá, L. Synthesis of a new stable and water-soluble tris (4-hydroxysulfonyltetrachlorophenyl) methyl radical with selective oxidative capacity. Tetrahedron 2011, 67, 3119–3123. [Google Scholar] [CrossRef]
- Mesa, J.A.; Velázquez-Palenzuela, A.; Brillas, E.; Coll, J.; Torres, J.L.; Juliá, L.J. Preparation and Characterization of Persistent Maltose-Conjugated Triphenylmethyl Radicals. Org. Chem. 2012, 77, 1081–1086. [Google Scholar] [CrossRef]
- Bai, X.; Tan, W.; Abdurahman, A.; Li, X.; Li, F. Stable red nanoparticles loaded neutral luminescent radicals for fluorescence imaging. Dye. Pigment. 2022, 202, 110260. [Google Scholar] [CrossRef]
- Blasi, D.; Gonzalez-Pato, N.; Rodriguez, X.R.; Diez-Zabala, I.; Srinivasan, S.Y.; Camarero, N.; Esquivias, O.; Roldán, M.; Guasch, J.; Laromaine, A.; et al. Ratiometric Nanothermometer Based on a Radical Excimer for In Vivo Sensing. Small 2023, 19, e2207806. [Google Scholar] [CrossRef]
- Gonzalez-Pato, N.; Blasi, D.; Nikolaidou, D.M.; Bertocchi, F.; Cerdá, J.; Terenziani, F.; Ventosa, N.; Aragó, J.; Lapini, A.; Veciana, J.; et al. Nanothermometer Based on Polychlorinated Trityl Radicals Showing Two-Photon Excitation and Emission in the Biological Transparency Window: Temperature Monitoring of Biological Tissues. Small Methods, 2023; Early View. [Google Scholar] [CrossRef]
- Chen, L.; Arnold, M.; Kittel, Y.; Blinder, R.; Jelezko, F.; Kuehne, A.J.C. 2,7-Substituted N-Carbazole Donors on Tris(2,4,6-trichlorophenyl)methyl Radicals with High Quantum Yield. Adv. Opt. Mater. 2022, 10, 2102101. [Google Scholar] [CrossRef]
- Nakamura, K.; Matsuda, K.; Rui, X.; Furukori, M.; Miyata, S.; Hosokai, T.; Anraku, K.; Nakao, K.; Albrecht, K. Effects of halogen atom substitution on luminescent radicals: A case study on tris(2,4,6-trichlorophenyl)methyl radical-carbazole dyads. Faraday Discuss 2023. [Google Scholar] [CrossRef]
- Arnold, M.E.; Kuehne, A.J.C. (2,6-Dichloro-4-iodophenyl)bis(2,4,6-trichlorophenyl)methane as a precursor in efficient cross-coupling reactions for donor and acceptor functionalized triphenylmethyl radicals. Dye. Pigment. 2022, 208, 110863. [Google Scholar] [CrossRef]
- Armet, O.; Veciana, J.; Rovira, C.; Riera, J.; Castañer, J.; Molins, E.; Rius, J.; Miravitlles, C.; Olivella, S.; Brichfeus, J.J. Inert carbon free radicals. 8. Polychlorotriphenylmethyl radicals: Synthesis, structure, and spin-density distribution. Phys. Chem. 1987, 91, 5608–5616. [Google Scholar] [CrossRef]
- Gross, M.; Zhang, F.; Arnold, M.E.; Ravat, P.; Kuehne, A.J.C. Aza[7]helicene Functionalized Triphenylmethyl Radicals with Circularly Polarized Doublet Emission. Adv. Opt. Mater. 2023, 12, 2301707. [Google Scholar] [CrossRef]
- Fajarí, L.; Papoular, R.; Reig, M.; Brillas, E.; Jorda, J.L.; Vallcorba, O.; Rius, J.; Velasco, D.; Juliá, L.J. Charge transfer States in stable neutral and oxidized radical adducts from carbazole derivatives. Org. Chem. 2014, 79, 1771–1777. [Google Scholar]
- Dong, S.; Xu, W.; Guo, H.; Yan, W.; Zhang, M.; Li, F. Effects of substituents on luminescent efficiency of stable triaryl methyl radicals. Phys. Chem. Chem. Phys. 2018, 20, 18657–18662. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Cho, E.; Wan, K.; Wu, C.; Gao, Y.; Coropceanu, V.; Brédas, J.L.; Li, F. Achieving Nearly 100% Photoluminescence Quantum Efficiency in Organic Radical Emitters by Fine-Tuning the Effective Donor-Acceptor Distance. Adv. Funct. Mater. 2024; Early View. [Google Scholar] [CrossRef]
- He, C.; Li, Z.; Lei, Y.; Zou, W.; Suo, B.J. Unraveling the Emission Mechanism of Radical-Based Organic Light-Emitting Diodes. Phys. Chem. Lett. 2019, 10, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kusamoto, T.; Nishihara, H. Highly photostable luminescent open-shell (3,5-dihalo-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radicals: Significant effects of halogen atoms on their photophysical and photochemical properties. RSC Adv. 2015, 5, 64802–64805. [Google Scholar] [CrossRef]
- Abroshan, H.; Coropceanu, V.; Brédas, J.L. Radiative and Nonradiative Recombinations in Organic Radical Emitters: The Effect of Guest–Host Interactions. Adv. Funct. Mater. 2020, 30, 2002916. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Z.W.; Huang, M.H.; Peng, Y. Polymerizable ionic liquids and polymeric ionic liquids: Facile synthesis of ionic liquids containing ethylene oxide repeating unit via methanesulfonate and their electrochemical properties. RSC Adv. 2017, 7, 5394–5401. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V.J. Toward reliable density functional methods without adjustable parameters: The PBE0 model. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. GaussView; Semichem Inc.: Shawnee Mission, KS, USA, 2019. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnold, M.E.; Schoeneburg, L.; Lamla, M.; Kuehne, A.J.C. Water-Soluble Trityl Radicals for Fluorescence Imaging. Molecules 2024, 29, 995. https://doi.org/10.3390/molecules29050995
Arnold ME, Schoeneburg L, Lamla M, Kuehne AJC. Water-Soluble Trityl Radicals for Fluorescence Imaging. Molecules. 2024; 29(5):995. https://doi.org/10.3390/molecules29050995
Chicago/Turabian StyleArnold, Mona E., Larissa Schoeneburg, Markus Lamla, and Alexander J. C. Kuehne. 2024. "Water-Soluble Trityl Radicals for Fluorescence Imaging" Molecules 29, no. 5: 995. https://doi.org/10.3390/molecules29050995
APA StyleArnold, M. E., Schoeneburg, L., Lamla, M., & Kuehne, A. J. C. (2024). Water-Soluble Trityl Radicals for Fluorescence Imaging. Molecules, 29(5), 995. https://doi.org/10.3390/molecules29050995





