Enhanced Adsorption of Trace Ethylene on Ag/NZ5 Modified with Ammonia: Hierarchical Structure and Metal Dispersion Effects
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure and Surface Morphology of Ag/NZ5(X)
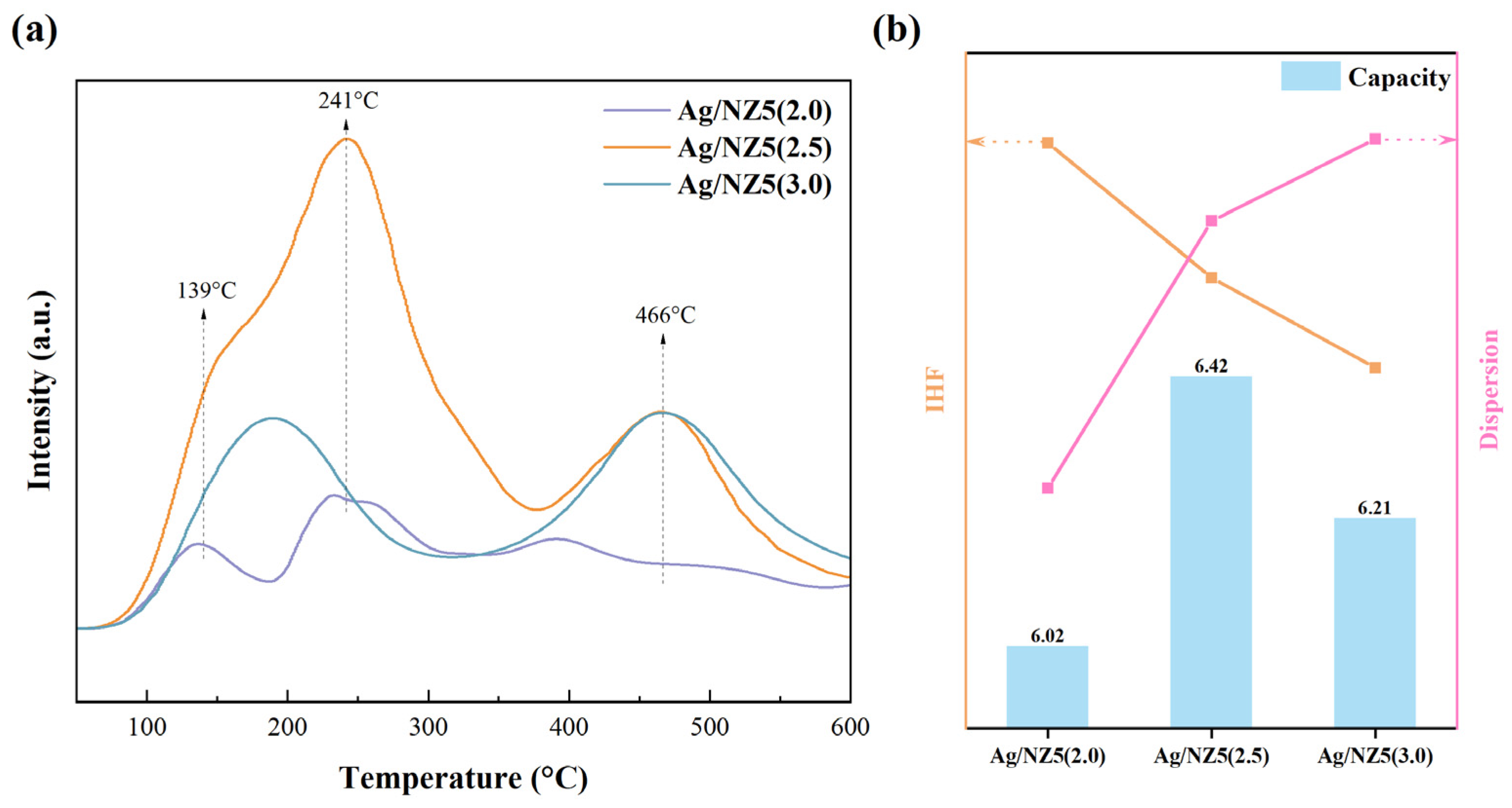
2.2. Defectiveness and Metal Dispersion of Ag/NZ5(X)
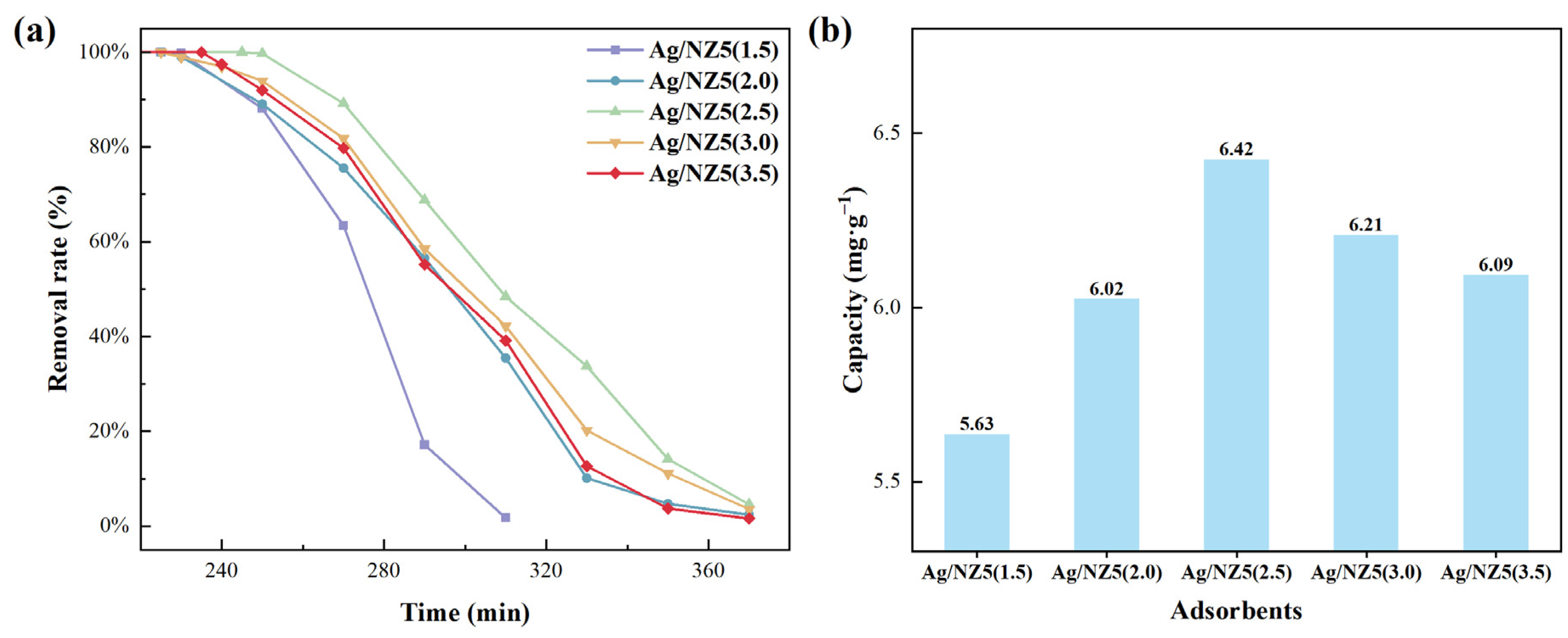
2.3. Ethylene Adsorption Performance of Ag/NZ5(X)
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of the Adsorbents
3.3. Ethylene Adsorption Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene Scavengers for the Preservation of Fruits and Vegetables: A Review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Sun, D.-W.; Pu, H.; Wei, Q. Recent Advances in Detecting and Regulating Ethylene Concentrations for Shelf-Life Extension and Maturity Control of Fruit: A Review. Trends Food Sci. Technol. 2019, 91, 66–82. [Google Scholar] [CrossRef]
- Keller, N.; Ducamp, M.-N.; Robert, D.; Keller, V. Ethylene Removal and Fresh Product Storage: A Challenge at the Frontiers of Chemistry. Toward an Approach by Photocatalytic Oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef]
- Sadeghi, K.; Lee, Y.; Seo, J. Ethylene Scavenging Systems in Packaging of Fresh Produce: A Review. Food Rev. Int. 2021, 37, 155–176. [Google Scholar] [CrossRef]
- Awalgaonkar, G.; Beaudry, R.; Almenar, E. Ethylene-removing Packaging: Basis for Development and Latest Advances. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3980–4007. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A Concise Guide to Active Agents for Active Food Packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Tzeng, J.; Weng, C.; Huang, J.; Shiesh, C.; Lin, Y.; Lin, Y. Application of Palladium-modified Zeolite for Prolonging Post-harvest Shelf Life of Banana. J. Sci. Food Agric. 2019, 99, 3467–3474. [Google Scholar] [CrossRef]
- Sultana, A.; Kathuria, A.; Gaikwad, K.K. Metal–Organic Frameworks for Active Food Packaging. A Review. Environ. Chem. Lett. 2022, 20, 1479–1495. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Y.; Wang, Y.; Liu, P.; Li, L.; Li, J. Ammonia Modification on UTSA-280 for C2H4/C2H6 Separation. Acta Chim. Sinica 2020, 78, 534. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Artés-Hernández, F.; Ávalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Ventura-Sobrevilla, J.M.; Martínez-Hernández, G.B. Current Scenario of Adsorbent Materials Used in Ethylene Scavenging Systems to Extend Fruit and Vegetable Postharvest Life. Food Bioprocess Technol. 2018, 11, 511–525. [Google Scholar] [CrossRef]
- Qi, Y.; Li, C.; Li, H.; Yang, H.; Guan, J. Elimination or Removal of Ethylene for Fruit and Vegetable Storage via Low-Temperature Catalytic Oxidation. J. Agric. Food Chem. 2021, 69, 10419–10439. [Google Scholar] [CrossRef]
- Limlamthong, M.; Yip, A.C.K. Recent Advances in Zeolite-Encapsulated Metal Catalysts: A Suitable Catalyst Design for Catalytic Biomass Conversion. Bioresour. Technol. 2020, 297, 122488. [Google Scholar] [CrossRef]
- Bereciartua, P.J.; Cantín, Á.; Corma, A.; Jordá, J.L.; Palomino, M.; Rey, F.; Valencia, S.; Corcoran, E.W.; Kortunov, P.; Ravikovitch, P.I.; et al. Control of Zeolite Framework Flexibility and Pore Topology for Separation of Ethane and Ethylene. Science 2017, 358, 1068–1071. [Google Scholar] [CrossRef]
- Aguado, S.; Bergeret, G.; Daniel, C.; Farrusseng, D. Absolute Molecular Sieve Separation of Ethylene/Ethane Mixtures with Silver Zeolite A. J. Am. Chem. Soc. 2012, 134, 14635–14637. [Google Scholar] [CrossRef]
- Monzón, J.D.; Pereyra, A.M.; Gonzalez, M.R.; Legnoverde, M.S.; Moreno, M.S.; Gargiulo, N.; Peluso, A.; Aprea, P.; Caputo, D.; Basaldella, E.I. Ethylene Adsorption onto Thermally Treated AgA-Zeolite. Appl. Surf. Sci. 2021, 542, 148748. [Google Scholar] [CrossRef]
- Min, J.G.; Kemp, K.C.; Hong, S.B. Silver ZK-5 Zeolites for Selective Ethylene/Ethane Separation. Sep. Purif. Technol. 2020, 250, 117146. [Google Scholar] [CrossRef]
- Li, C.; Yang, H.; Qi, Y.; Li, H. Synergistic Effect of Metal Oxidation States and Surface Acidity Enhanced the Trace Ethylene Adsorption of Ag/ZSM-5. New J. Chem. 2022, 46, 9048–9056. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.; Corma, A. Applications of Zeolites to C1 Chemistry: Recent Advances, Challenges, and Opportunities. Adv. Mater. 2020, 32, 2002927. [Google Scholar] [CrossRef]
- Abreu, N.J.; Valdés, H.; Zaror, C.A.; Azzolina-Jury, F.; Meléndrez, M.F. Ethylene Adsorption onto Natural and Transition Metal Modified Chilean Zeolite: An Operando DRIFTS Approach. Microporous Mesoporous Mater. 2019, 274, 138–148. [Google Scholar] [CrossRef]
- Peng, P.; Gao, X.-H.; Yan, Z.-F.; Mintova, S. Diffusion and Catalyst Efficiency in Hierarchical Zeolite Catalysts. Natl. Sci. Rev. 2020, 7, 1726–1742. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Z.; Chen, Y.; Ma, X. Syngas Methanation over Ni/SiO2 Catalyst Prepared by Ammonia-Assisted Impregnation. Int. J. Hydrogen Energy 2017, 42, 27073–27083. [Google Scholar] [CrossRef]
- Van Aelst, J.; Verboekend, D.; Philippaerts, A.; Nuttens, N.; Kurttepeli, M.; Gobechiya, E.; Haouas, M.; Sree, S.P.; Denayer, J.F.M.; Martens, J.A.; et al. Catalyst Design by NH4OH Treatment of USY Zeolite. Adv. Funct. Mater. 2015, 25, 7130–7144. [Google Scholar] [CrossRef]
- Janiszewska, E.; Kowalska-Kuś, J.; Góra-Marek, K.; Szymocha, A.; Nowińska, K.; Kowalak, S. Modification of Silicalite-1 with Ammonium Compounds Aimed at Preparation of Acidic Catalyst for Acetalization of Glycerol with Acetone. Appl. Catal. A 2019, 581, 1–10. [Google Scholar] [CrossRef]
- Guo, H.; Warnicke, P.; Griffa, M.; Müller, U.; Chen, Z.; Schaeublin, R.; Zhang, Z.; Luković, M. Hierarchical Porous Wood Cellulose Scaffold with Atomically Dispersed Pt Catalysts for Low-Temperature Ethylene Decomposition. ACS Nano 2019, 13, 14337–14347. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.C.; Bach, T.; Ziese, U.; Paulaime-van Donk, A.M.; de Jong, K.P.; Moulijn, J.A.; Pérez-Ramírez, J. Creation of Hollow Zeolite Architectures by Controlled Desilication of Al-Zoned ZSM-5 Crystals. J. Am. Chem. Soc. 2005, 127, 10792–10793. [Google Scholar] [CrossRef]
- Verboekend, D.; Mitchell, S.; Milina, M.; Groen, J.C.; Pérez-Ramírez, J. Full Compositional Flexibility in the Preparation of Mesoporous MFI Zeolites by Desilication. J. Phys. Chem. C 2011, 115, 14193–14203. [Google Scholar] [CrossRef]
- Verboekend, D.; Pérez-Ramírez, J. Design of Hierarchical Zeolite Catalysts by Desilication. Catal. Sci. Technol. 2011, 1, 879. [Google Scholar] [CrossRef]
- Chen, L.-H.; Sun, M.-H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.-L. Hierarchically Structured Zeolites: From Design to Application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef]
- Shen, Y.; Qin, Z.; Asahina, S.; Asano, N.; Zhang, G.; Qian, S.; Ma, Y.; Yan, Z.; Liu, X.; Mintova, S. The Inner Heterogeneity of ZSM-5 Zeolite Crystals. J. Mater. Chem. A 2021, 9, 4203–4212. [Google Scholar] [CrossRef]
- Qin, Z.; Pinard, L.; Benghalem, M.A.; Daou, T.J.; Melinte, G.; Ersen, O.; Asahina, S.; Gilson, J.-P.; Valtchev, V. Preparation of Single-Crystal “House-of-Cards”-like ZSM-5 and Their Performance in Ethanol-to-Hydrocarbon Conversion. Chem. Mater. 2019, 31, 4639–4648. [Google Scholar] [CrossRef]
- Santos, J.L.; Mäki-Arvela, P.; Monzón, A.; Murzin, D.Y.; Centeno, M.Á. Metal Catalysts Supported on Biochars: Part I Synthesis and Characterization. Appl. Catal. B 2020, 268, 118423. [Google Scholar] [CrossRef]
- Cisneros, L.; Gao, F.; Corma, A. Silver Nanocluster in Zeolites. ADSORPTION of ETHYLENE Traces for Fruit Preservation. Microporous Mesoporous Mater. 2019, 283, 25–30. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect Engineering in Photocatalytic Materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, Y.; Zhai, Y.; Ma, T.; Xu, C.; Zuo, S.; Zheng, L.; Zhang, J.; Ping, L. Creation of CuOx/ZSM-5 Zeolite Complex: Healing Defect Sites and Boosting Acidic Stability and Catalytic Activity. Catal. Sci. Technol. 2020, 10, 4981–4989. [Google Scholar] [CrossRef]
- Qin, Z.; Shen, W.; Zhou, S.; Shen, Y.; Li, C.; Zeng, P.; Shen, B. Defect-Assisted Mesopore Formation during Y Zeolite Dealumination: The Types of Defect Matter. Microporous Mesoporous Mater. 2020, 303, 110248. [Google Scholar] [CrossRef]
- Erigoni, A.; Newland, S.H.; Paul, G.; Marchese, L.; Raja, R.; Gianotti, E. Creating Accessible Active Sites in Hierarchical MFI Zeolites for Low-Temperature Acid Catalysis. ChemCatChem 2016, 8, 3161–3169. [Google Scholar] [CrossRef]
- Medeiros-Costa, I.C.; Dib, E.; Nesterenko, N.; Dath, J.-P.; Gilson, J.-P.; Mintova, S. Silanol Defect Engineering and Healing in Zeolites: Opportunities to Fine-Tune Their Properties and Performances. Chem. Soc. Rev. 2021, 50, 11156–11179. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Hafiz, L.; Shen, Y.; Daele, S.V.; Boullay, P.; Ruaux, V.; Mintova, S.; Gilson, J.-P.; Valtchev, V. Defect-Engineered Zeolite Porosity and Accessibility. J. Mater. Chem. A 2020, 8, 3621–3631. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Marchese, L.; Petrini, G.; Leofanti, G.; Padovan, M. Silicalite Characterization. 2. IR Spectroscopy of the Interaction of Carbon Monoxide with Internal and External Hydroxyl Groups. J. Phys. Chem. 1992, 96, 4991–4997. [Google Scholar] [CrossRef]
- Groen, J.C.; Jansen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Optimal Aluminum-Assisted Mesoporosity Development in MFI Zeolites by Desilication. J. Phys. Chem. B 2004, 108, 13062–13065. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.; Jun, Y.; Choi, M. Cooperative Effects of Zeolite Mesoporosity and Defect Sites on the Amount and Location of Coke Formation and Its Consequence in Deactivation. J. Catal. 2017, 347, 222–230. [Google Scholar] [CrossRef]
- Fodor, D.; Beloqui Redondo, A.; Krumeich, F.; van Bokhoven, J.A. Role of Defects in Pore Formation in MFI Zeolites. J. Phys. Chem. C 2015, 119, 5447–5453. [Google Scholar] [CrossRef]
- Samantaray, M.K.; D’Elia, V.; Pump, E.; Falivene, L.; Harb, M.; Ould Chikh, S.; Cavallo, L.; Basset, J.-M. The Comparison between Single Atom Catalysis and Surface Organometallic Catalysis. Chem. Rev. 2020, 120, 734–813. [Google Scholar] [CrossRef]
- Limlamthong, M.; Jia, X.; Jang, E.; Jeong, Y.; Baik, H.; Cowan, M.G.; Choi, J.; Yip, A.C.K. An Anti-Humidity Palladium-Containing MFI Composite as a Robust Ethylene Scavenger. Microporous Mesoporous Mater. 2022, 341, 112090. [Google Scholar] [CrossRef]
- Yang, H.; Ma, C.; Li, Y.; Wang, J.; Zhang, X.; Wang, G.; Qiao, N.; Sun, Y.; Cheng, J.; Hao, Z. Synthesis, Characterization and Evaluations of the Ag/ZSM-5 for Ethylene Oxidation at Room Temperature: Investigating the Effect of Water and Deactivation. Chem. Eng. J. 2018, 347, 808–818. [Google Scholar] [CrossRef]
- Wang, C.; Leng, S.; Guo, H.; Yu, J.; Li, W.; Cao, L.; Huang, J. Quantitative Arrangement of Si/Al Ratio of Natural Zeolite Using Acid Treatment. Appl. Surf. Sci. 2019, 498, 143874. [Google Scholar] [CrossRef]
- Wang, C.; Guo, H.; Leng, S.; Yu, J.; Feng, K.; Cao, L.; Huang, J. Regulation of Hydrophilicity/Hydrophobicity of Aluminosilicate Zeolites: A Review. Crit. Rev. Solid State Mater. Sci. 2021, 46, 330–348. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Rungtaweevoranit, B.; Parlinska-Wojtan, M.; Pei, X.; Yaghi, O.M.; Behm, R.J. Highly Active and Stable Single-Atom Cu Catalysts Supported by a Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 5201–5210. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, Y.; Oku, Y.; Hidaka, Y.; Kanaya, N.; Nakajima, Y.; Fukuroi, J.; Yoshida, K.; Sasaki, Y.; Sekine, Y.; Matsukata, M. Physicochemical Characterization of Highly Dispersed Platinum and Chromium on Zeolite Beta. J. Phys. Chem. C 2014, 118, 10746–10753. [Google Scholar] [CrossRef]
- Yang, H.; Ma, C.; Zhang, X.; Li, Y.; Cheng, J.; Hao, Z. Understanding the Active Sites of Ag/Zeolites and Deactivation Mechanism of Ethylene Catalytic Oxidation at Room Temperature. ACS Catal. 2018, 8, 1248–1258. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Guo, X.; Zhang, A.; Fei, X. Removal of C2H4 from a CO2 Stream by Using AgNO3-Modified Y-Zeolites. Ind. Eng. Chem. Res. 2006, 45, 6236–6242. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. High Adsorption of Ethylene by Alkali-Treated Halloysite Nanotubes for Food-Packaging Applications. Environ. Chem. Lett. 2018, 16, 1055–1062. [Google Scholar] [CrossRef]
- Chung, K.; Park, D.; Kim, K.-M.; Lee, C.-H. Adsorption Equilibria and Kinetics of Ethane and Ethylene on Zeolite 13X Pellets. Microporous Mesoporous Mater. 2022, 343, 112199. [Google Scholar] [CrossRef]
- Patdhanagul, N.; Srithanratana, T.; Rangsriwatananon, K.; Hengrasmee, S. Ethylene Adsorption on Cationic Surfactant Modified Zeolite NaY. Microporous Mesoporous Mater. 2010, 131, 97–102. [Google Scholar] [CrossRef]
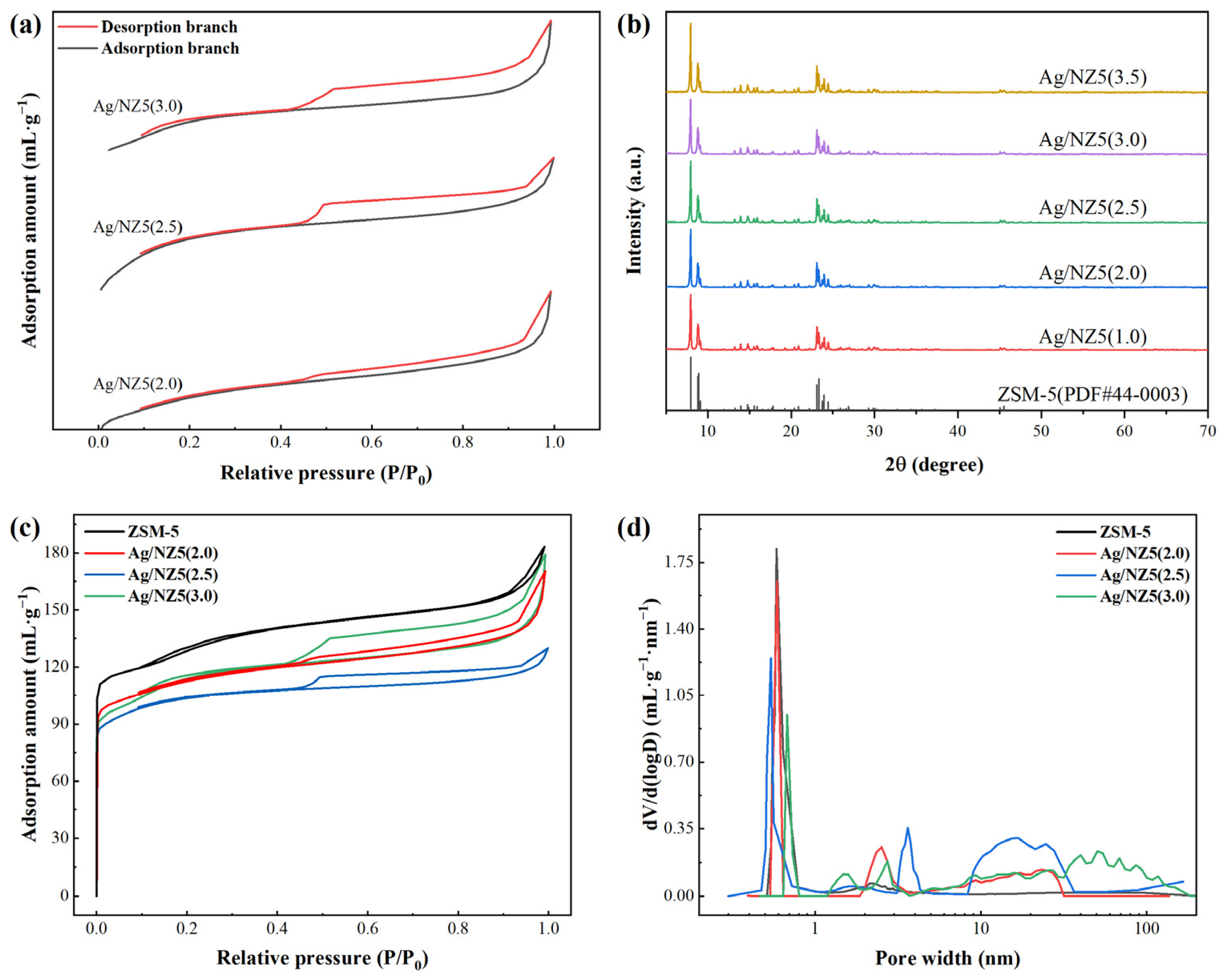

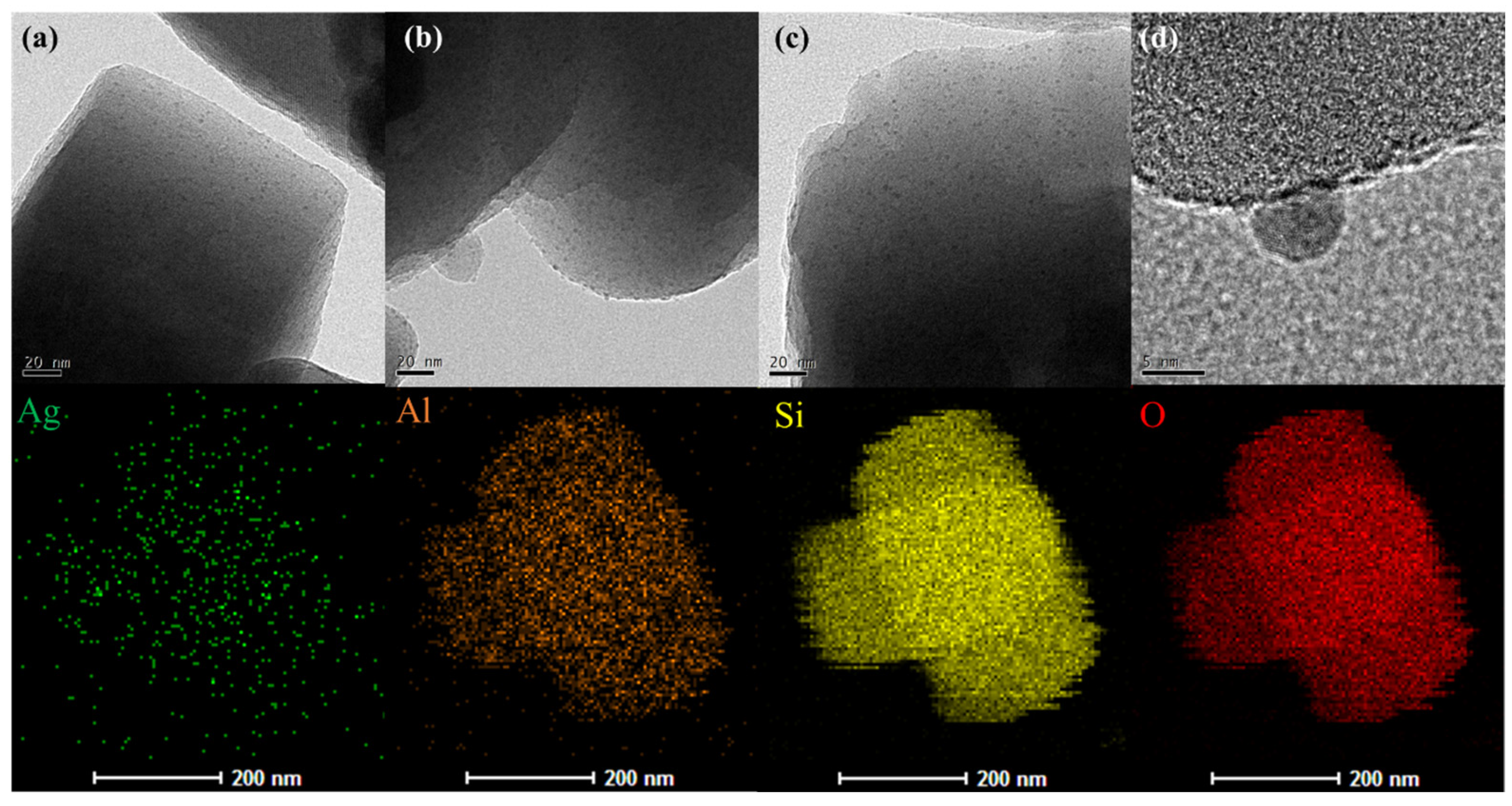
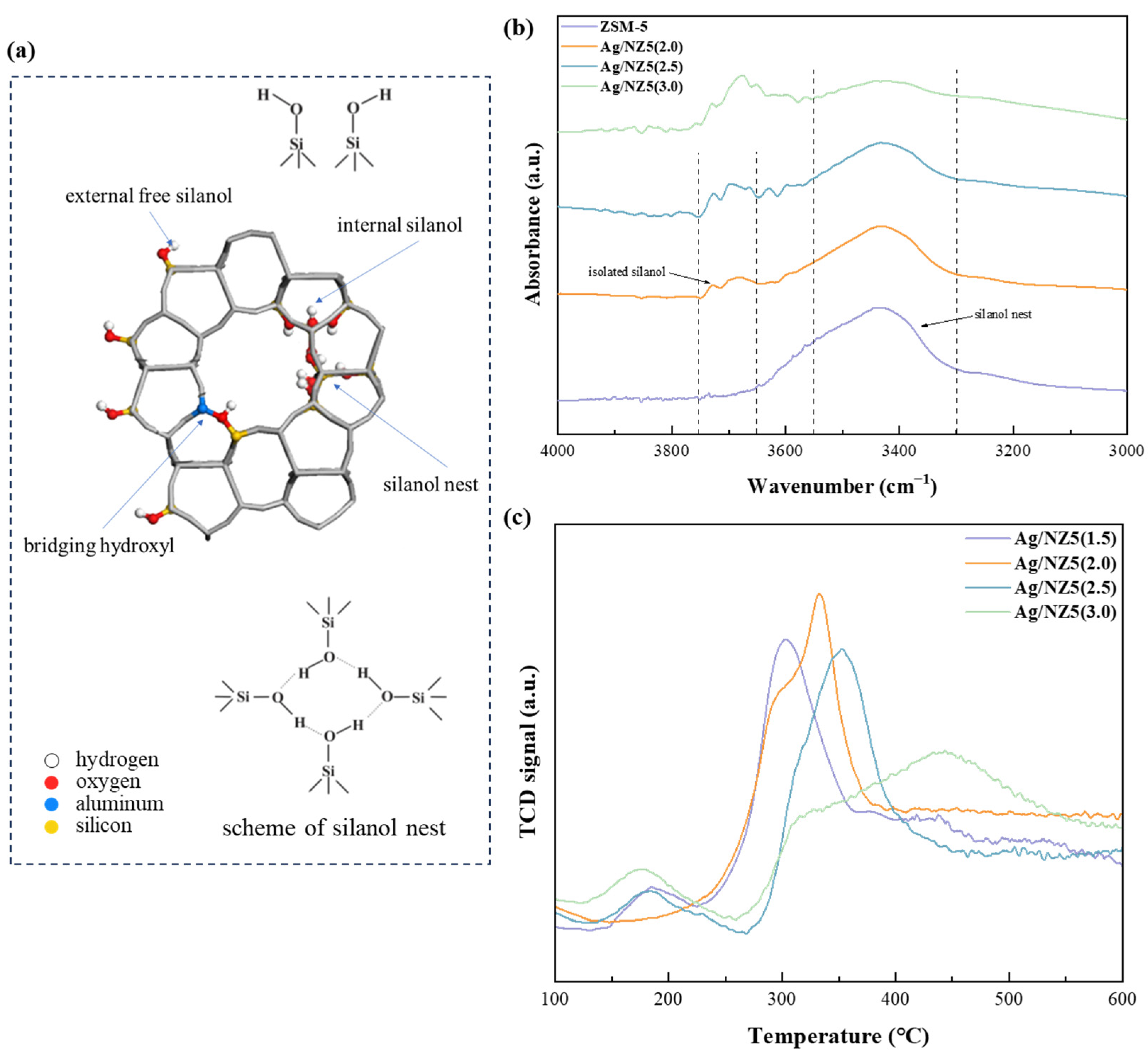
| Adsorbent | Stotal a | Smeso b | Vmicro b | Vtotal c | IHF d |
|---|---|---|---|---|---|
| (m2·g−1) | (m2·g−1) | (cm3·g−1) | (cm3·g−1) | ||
| Ag/NZ5(1.5) | 351 | 35 | 0.199 | 0.237 | 0.59 |
| Ag/NZ5(2.0) | 364 | 44 | 0.184 | 0.231 | 0.69 |
| Ag/NZ5(2.5) | 355 | 47 | 0.165 | 0.226 | 0.66 |
| Ag/NZ5(3.0) | 356 | 50 | 0.149 | 0.212 | 0.63 |
| Ag/NZ5(3.5) | 353 | 59 | 0.115 | 0.205 | 0.58 |
| parent zeolite | 377 | 19 | 0.233 | 0.253 | - |
| Adsorbent | Silver Loading a (wt.%) | Dispersion Rate b (%) | Si/Al Ratio a |
|---|---|---|---|
| Ag/NZ5(1.5) | 0.528 | 10.93 | 95 |
| Ag/NZ5(2.0) | 0.512 | 12.45 | 89 |
| Ag/NZ5(2.5) | 0.499 | 20.77 | 86 |
| Ag/NZ5(3.0) | 0.495 | 23.32 | 92 |
| Adsorbent | qm,p (mg·g−1) | qm,c (mg·g−1) | bp (kPa−1) | bc (kPa−1) | R2 |
|---|---|---|---|---|---|
| Ag/NZ5(2.0) | 2.23 | 0.038 | 5.8 × 10−3 | 3.2 × 1016 | 0.99 |
| Ag/NZ5(2.5) | 3.78 | 0.059 | 4.8 × 10−3 | 1.9 × 1021 | 0.99 |
| Ag/NZ5(3.0) | 4.13 | 0.053 | 3.9 × 10−3 | 1.4 × 1018 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Yang, H.; Li, C.; Li, H. Enhanced Adsorption of Trace Ethylene on Ag/NZ5 Modified with Ammonia: Hierarchical Structure and Metal Dispersion Effects. Molecules 2024, 29, 981. https://doi.org/10.3390/molecules29050981
Qi Y, Yang H, Li C, Li H. Enhanced Adsorption of Trace Ethylene on Ag/NZ5 Modified with Ammonia: Hierarchical Structure and Metal Dispersion Effects. Molecules. 2024; 29(5):981. https://doi.org/10.3390/molecules29050981
Chicago/Turabian StyleQi, Ying, Huaming Yang, Chunli Li, and Hao Li. 2024. "Enhanced Adsorption of Trace Ethylene on Ag/NZ5 Modified with Ammonia: Hierarchical Structure and Metal Dispersion Effects" Molecules 29, no. 5: 981. https://doi.org/10.3390/molecules29050981
APA StyleQi, Y., Yang, H., Li, C., & Li, H. (2024). Enhanced Adsorption of Trace Ethylene on Ag/NZ5 Modified with Ammonia: Hierarchical Structure and Metal Dispersion Effects. Molecules, 29(5), 981. https://doi.org/10.3390/molecules29050981







