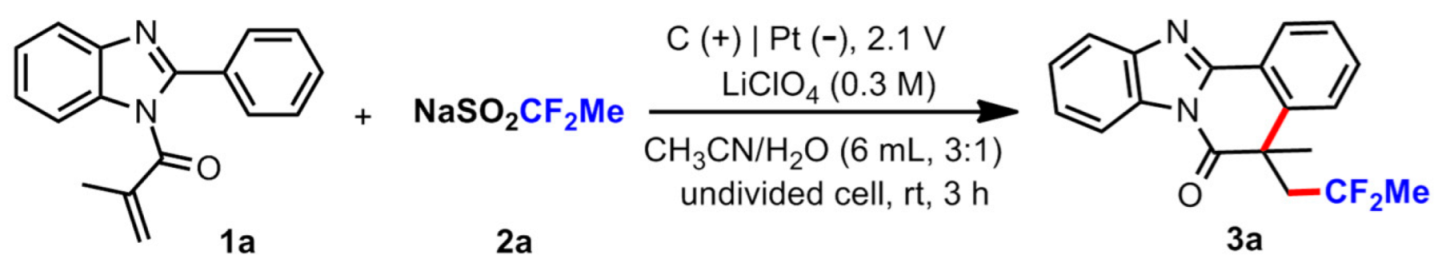
3.2. General Procedure for the Reaction
A 20 mL test tube with a stir bar was charged with, 2-arylbenzimidazoles/2-arylindoles (1 equiv., 0.2 mmol), MeCF
2SO
2Na, or sodium cyclopropyldifluoromethylsulfinate (3 equiv., 0.6 mmol), LiClO
4 (0.3 M), MeCN (4.5 mL), H
2O (1.5 mL). The tube was equipped with a carbon plate (10 mm × 10 mm × 3 mm) as the anode and a platinum plate (10 mm × 10 mm × 0.2 mm) as the cathode. The reaction mixture was electrolyzed in an undivided cell at room temperature under a constant voltage of 2.1 V for 3h. Upon completion, the mixture was extracted with EtOAc (10 mL × 3). The combined organic phases were dried over Na
2SO
4 and condensed under vacuum. The residue was purified by silica gel column chromatography to afford the final products (
1H NMR,
19F NMR, and
13C NMR of compounds (
3a–
v and 5a–
i) are shown in
Supplementary Materials).
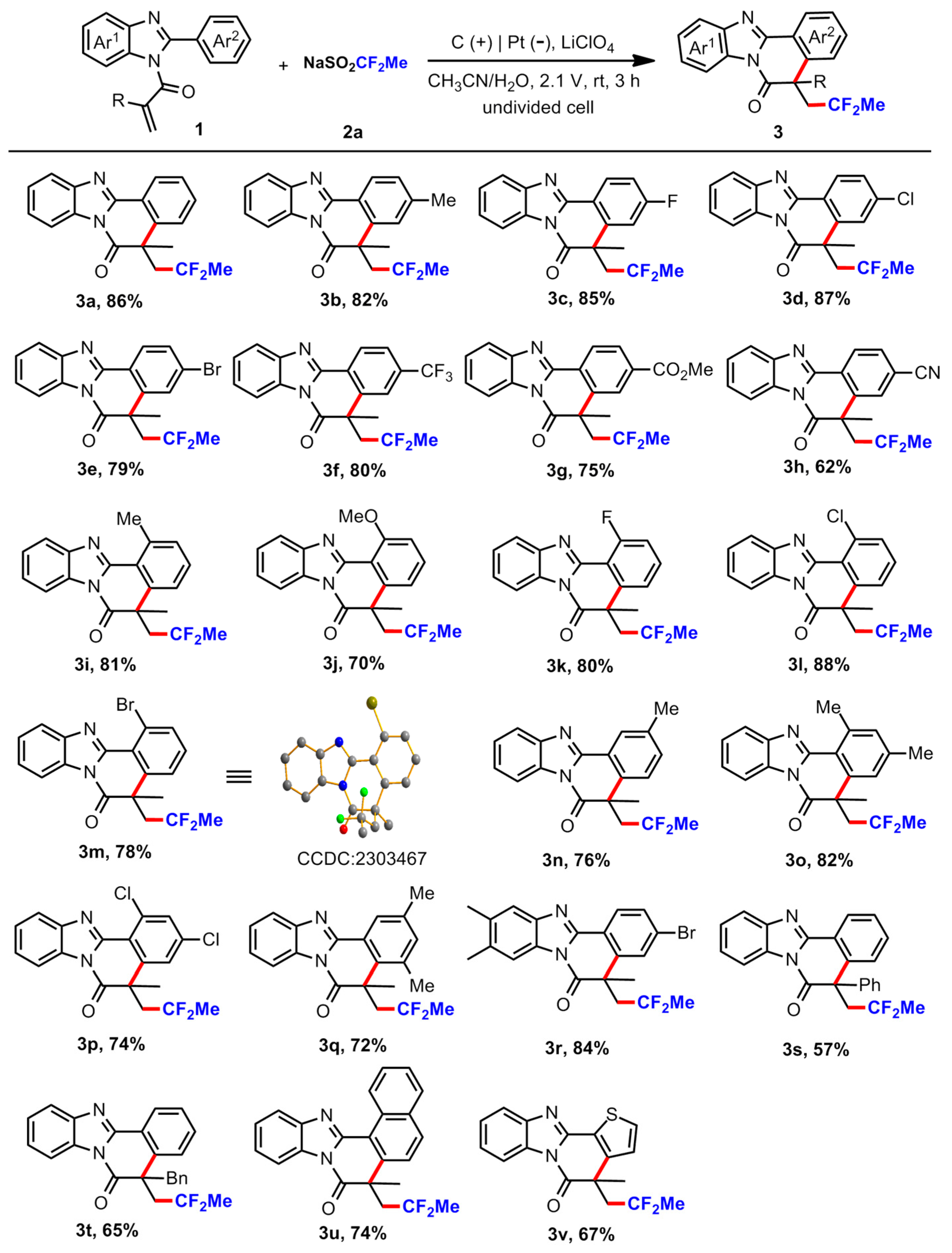
5-(2,2-Difluoropropyl)-5-methylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (
3a) [
39]. A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 153–154 °C, 56.1 mg, 86 % yield.
1H NMR (400 MHz, CDCl
3):
δ 8.52–8.49 (m, 1H), 8.37–8.35 (m, 1H), 7.84–7.82 (m, 1H), 7.59–7.55 (m, 1H), 7.50 (t,
J = 7.2 Hz, 2H), 7.47–7.40 (m, 2H), 3.30–3.18 (m, 1H), 2.79–2.67 (m, 1H), 1.72 (s, 3H), 1.34 (t,
J = 18.8 Hz, 3H).
13C{
1H} NMR (100 MHz, CDCl
3):
δ 172.2, 149.6, 144.0, 140.0, 131.5, 131.3, 127.9, 126.8, 126.0, 125.9, 125.6, 122.6 (t,
J = 240.5 Hz), 122.3, 119.8, 115.7, 48.2 (t,
J = 23.9 Hz), 45.5, 31.1, 24.7 (t,
J = 27.3 Hz).
19F NMR (471 MHz, CDCl
3):
δ −86.07–−86.30 (m, 2F). HRMS (ESI-TOF)
m/
z: Calcd for C
19H
16F
2N
2O (M + H)
+ 327.1303; found 327.1306. IR (KBr) ν 3402, 3057, 2931, 1943, 1840, 1700, 1619, 1547, 985, 803, 644, 549 cm
−1.
5-(2,2-Difluoropropyl)-3,5-dimethylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3b). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 132–133 °C, 55.7 mg, 82 % yield. 1H NMR (500 MHz, CDCl3): δ 8.37 (d, J = 8.0 Hz, 1H), 8.35–8.33 (m, 1H), 7.80–7.79 (m, 1H), 7.44–7.38 (m, 2H), 7.30 (d, J = 8.5 Hz, 1H), 7.26 (s, 1H), 3.21 (q, J = 15.0 Hz, 1H), 2.70 (q, J = 15.5 Hz, 1H), 2.45 (s, 3H), 1.70 (s, 3H), 1.32 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 172.3, 149.8, 144.1, 141.8, 140.1, 131.4, 129.0, 127.1, 126.0, 125.8, 125.3, 122.5 (t, J = 240.6 Hz), 119.7, 119.6, 115.6, 48.2 (t, J = 24.1Hz), 45.5, 31.0, 24.7(t, J = 27.4 Hz), 21.8. 19F NMR (471 MHz, CDCl3): δ −86.01–−86.20 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C20H18F2N2O (M + H)+ 341.1460; found 341.1461. IR (KBr) ν 3415, 3068, 2964, 2361, 1909, 1715, 1613, 1557, 1238, 915, 801, 615 cm−1.
5-(2,2-Difluoropropyl)-3-fluoro-5-methylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3c). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate =10/1), mp 134–135 °C, 58.5 mg, 85 % yield. 1H NMR (400 MHz, CDCl3): δ 8.50 (dd, J = 8.4, 6.0 Hz, 1H), 8.34 (d, J = 7.6 Hz, 1H), 7.80 (d, J = 7.6 Hz, 1H), 7.46–7.39 (m, 2H), 7.22–7.15 (m, 2H), 3.29–3.17 (m, 1H), 2.71–2.59 (m, 1H), 1.70 (s, 3H), 1.40 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.6, 164.6 (d, J = 252.5 Hz), 148.8, 144.0, 142.9 (d, J = 7.9 Hz), 131.4, 128.5 (d, J = 9.1Hz), 125.7 (d, J = 37.5 Hz), 122.4 (t, J = 240.6 Hz), 119.7, 118.89, 118.87, 115.9 (d, J = 22.4 Hz), 115.6, 113.7 (d, J = 23.2 Hz), 48.3 (t, J = 23.7 Hz), 45.7, 30.9, 24.7 (t, J = 27.3Hz). 19F NMR (471 MHz, CDCl3): δ −85.51–−86.22 (m, 1F), −87.32–−88.02 (m, 1F), −106.94–-106.99 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15F3N2O (M + H)+ 345.1209; found 345.1211. IR (KBr) ν 3380, 3111, 3068, 2988, 2920, 2877, 2620, 2370, 1956, 1913, 1802, 1715, 1625, 1566, 1008, 986, 786, 655, 578 cm−1.
3-Chloro-5-(2,2-difluoropropyl)-5-methylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3d). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 174–175 °C, 62.6 mg, 87 % yield. 1H NMR (400 MHz, CDCl3): δ 8.43 (d, J = 8.4 Hz, 1H), 8.34 (d, J = 6.8 Hz, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.47–7.42 (m, 4H), 3.28–3.16 (m, 1H), 2.73–2.61 (m, 1H), 1.71 (s, 3H), 1.40 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.5, 148.7, 143.9, 141.8, 137.5, 131.4, 128.5, 127.4, 127.0, 126.0, 125.8, 122.5 (t, J = 239.0 Hz), 120.9, 119.8, 115.6, 48.2 (t, J = 23.6 Hz), 45.5 (d, J = 3.3Hz), 30.9, 24.8 (t, J = 27.2 Hz). 19F NMR (471 MHz, CDCl3): δ −85.56–−86.27 (m, 1F), −87.33–−88.03 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15ClF2N2O (M + H)+ 361.0914; found 361.0915. IR (KBr) ν 3355, 3005, 2923, 2851, 2369, 1916, 1720, 1577, 1450, 1356, 1076, 902, 835, 765, 619 cm−1.
3-Bromo-5-(2,2-difluoropropyl)-5-methylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3e). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 207–208 °C, 64.0 mg, 79 % yield. 1H NMR (500 MHz, CDCl3): δ 8.37–8.33 (m, 2H), 7.82–7.80 (m, 1H), 7.63–7.61 (m, 2H), 7.46–7.41 (m, 2H), 3.27–3.17 (m, 1H), 2.72–2.63 (m, 1H), 1.71 (s, 3H), 1.41 (t, J = 18.5 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.4, 148.7, 143.9, 141.9, 131.4, 131.3, 129.9, 127.5, 126.0, 125.8, 122.5 (t, J = 238.5 Hz), 121.4, 119.9, 115.6, 48.2 (t, J = 23.7 Hz), 45.5 (d, J = 3.3Hz), 30.8, 24.8 (t, J = 27.2 Hz). 19F NMR (471 MHz, CDCl3): δ −85.57–−86.28 (m, 1F), −87.26–−87.96 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15BrF2N2O (M + H)+ 405.0408; found 405.0409. IR (KBr) ν 3420, 3074, 3004, 2946, 2713, 2359, 1910, 1793, 1725, 1543, 1420, 1292, 1072, 983, 905, 832, 716, 654 cm−1.
5-(2,2-Difluoropropyl)-5-methyl-3-(trifluoromethyl)benzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3f). A light-yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 151–152 °C, 63.0 mg, 80 % yield. 1H NMR (500 MHz, CDCl3): δ 8.63 (d, J = 8.0 Hz, 1H), 8.38–8.36 (m, 1H), 7.86–7.85 (m, 1H), 7.75–7.72 (m, 2H), 7.49–7.45 (m, 2H), 3.33–3.24 (m, 1H), 2.80–2.71 (m, 1H), 1.75 (s, 3H), 1.43 (t, J = 18.5 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.4, 148.1, 143.9, 140.7, 132.8 (q, J = 32.8 Hz), 131.5, 126.7, 126.24, 126.20, 125.6, 124.7 (q, J = 3.6 Hz), 124.0–123.9 (m), 122.5 (t, J = 240.1Hz), 120.2, 115.8, 48.1 (t, J = 23.5 Hz), 45.7 (d, J = 3.3Hz), 30.8, 24.8 (t, J = 27.2 Hz). 19F NMR (471 MHz, CDCl3): δ −62.91 (s, 3F), −85.44–−86.13 (m, 1F), −87.85–−88.51 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C20H15F5N2O (M + H)+ 395.1177; found 395.1179. IR (KBr) ν 3418, 3065, 2975, 2946, 2874, 2357, 1927, 1802, 1723, 1613, 1555, 1456, 1076, 991, 905, 839, 694, 546 cm−1.
Methyl 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo [4,5]imidazo [2,1-a]isoquinoline-3-carboxylate (3g). A light-yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1), 57.6 mg, 75 % yield. 1H NMR (400 MHz, CDCl3): δ 8.57 (d, J = 8.0 Hz, 1H), 8.38–8.36 (m, 1H), 8.18 (s, 1H), 8.13 (dd, J = 8.4, 1.6 Hz, 1H), 7.86–7.84 (m, 1H), 7.49–7.44 (m, 2H), 3.98 (s, 3H), 3.31–3.19 (m, 1H), 2.87–2.75 (m, 1H), 1.76 (s, 3H), 1.40 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.7, 166.1, 148.6, 144.1, 140.3, 132.4, 131.5, 128.7, 128.3, 126.18, 126.16, 126.14, 126.12, 122.6 (t, J = 238.6 Hz), 120.1, 115.8, 52.5, 48.3 (t, J = 23.5 Hz), 45.7 (d, J = 3.4 Hz), 30.8, 24.8 (t, J = 27.2 Hz). 19F NMR (471 MHz, CDCl3): δ −85.83–−86.54 (m, 1F), −87.26–−87.96 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H18F2N2O3 (M + H)+ 385.1358; found 385.1360. IR (thin film) ν 3417, 3080, 2980, 2848, 2354, 1922, 1843, 1717, 1614, 1550, 1475, 1359, 982, 903, 758, 565 cm−1.
5-(2,2-Difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo [4,5]imidazo [2,1-a]isoquinoline-3-carbonitrile (3h). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1), mp 243–244 °C, 43.5 mg, 62% yield. 1H NMR (500 MHz, CDCl3): δ 8.59 (d, J = 8.0 Hz, 1H), 8.36–8.34 (m, 1H), 7.86–7.84 (m, 1H), 7.78 (s, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.48–7.46 (m, 2H), 3.31–3.21 (m, 1H), 2.76–2.66 (m, 1H), 1.73 (s, 3H), 1.46 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 170.9, 147.6, 143.9, 141.0, 131.4, 131.04, 131.03, 130.9, 126.7, 126.5, 126.3, 122.5 (t, J = 238.6 Hz), 120.3, 118.0, 115.8, 114.5, 48.1 (t, J = 23.3 Hz), 45.5 (d, J = 3.1 Hz), 30.6, 29.6, 24.8 (t, J = 27.1Hz). 19F NMR (471 MHz, CDCl3): δ −85.22–−85.93 (m, 1F), −88.52–−89.22 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C20H15F2N3O (M + H)+ 352.1256; found 352.1260. IR (KBr) ν 3414, 3088, 2991, 2943, 2733, 2351, 2224, 1942, 1816, 1723, 1615, 1573, 1552, 1479, 1449, 1243, 1126, 1081, 911, 846, 741, 662 cm−1.
5-(2,2-Difluoropropyl)-1,5-dimethylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3i). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 149–150 °C, 55.1 mg, 81% yield. 1H NMR (400 MHz, CDCl3): δ 8.38 (d, J = 8.0 Hz, 1H), 8.36–7.34 (m, 1H), 7.81–7.79 (m, 1H), 7.45–7.38 (m, 2H), 7.31 (d, J = 8.0 Hz, 1H), 7.27 (s, 1H), 3.22 (q, J = 15.2 Hz, 1H), 2.72 (q, J = 15.2 Hz, 1H), 2.47 (s, 3H), 1.71 (s, 3H), 1.34 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.3, 149.8, 144.0, 141.8, 140.0, 131.4, 129.1, 127.2, 125.9, 125.8, 125.3, 122.6 (t, J = 240.6 Hz), 119.7, 119.6, 115.6, 48.2 (t, J = 23.9 Hz), 45.5, 31.1, 24.7 (t, J = 27.3 Hz), 21.9. 19F NMR (471 MHz, CDCl3): δ −85.99–−86.18 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C20H18F2N2O (M + H)+ 341.1460; found 341.1462. IR (KBr) ν 3410, 3065, 2991, 2963, 2854, 2720, 2361, 1909, 1843, 1796, 1716, 1619, 1559, 1242, 985, 915, 802, 659 cm−1.
5-(2,2-Difluoropropyl)-1-methoxy-5-methylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3j). A brown solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 165–166 °C, 49.8 mg, 70% yield. 1H NMR (500 MHz, CDCl3): δ 8.39–8.37 (m, 1H), 7.91–7.89 (m, 1H), 7.51 (t, J = 8.0 Hz, 1H), 7.43–7.39 (m, 2H), 7.12 (d, J = 8.0 Hz, 1H), 7.06 (d, J = 8.5 Hz, 1H), 4.14 (s, 3H), 3.23 (q, J = 15.0 Hz, 1H), 2.71 (q, J = 15.5 Hz, 1H), 1.72 (s, 3H), 1.32 (t, J = 18.5 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.1, 158.7, 147.7, 144.3, 142.6, 131.7, 130.3, 125.6, 125.5, 122.5 (t, J = 240.6 Hz), 120.5, 119.1, 115.5, 111.8, 110.4, 56.6, 48.6 (t, J = 23.9 Hz), 45.4, 31.5, 24.7 (t, J = 27.4 Hz). 19F NMR (471 MHz, CDCl3): δ −85.88–−86.06 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C20H18F2N2O2 (M + H)+ 357.1409; found 357.1410. IR (KBr) ν 3395, 3198, 3063, 3007, 2923, 2848, 2684, 2544, 2369, 2226, 1936, 1792, 1703, 1612, 1540, 1480, 1242, 1098, 1055, 935, 861, 803, 594 cm−1.
5-(2,2-Difluoropropyl)-1-fluoro-5-methylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3k). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 153–154 °C, 55.0 mg, 80% yield. 1H NMR (500 MHz, CDCl3): δ 8.38–8.37 (m, 1H), 7.94–7.92 (m, 1H), 7.51 (td, J = 8.0, 5.0 Hz, 1H), 7.47–7.43 (m, 2H), 7.31 (d, J = 8.0 Hz, 1H), 7.24–7.21 (m, 1H), 3.29–3.19 (m, 1H), 2.76–2.67 (m, 1H), 1.72 (s, 3H), 1.39 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.6, 160.4 (d, J = 262.1Hz), 145.8 (d, J = 8.4 Hz), 144.2, 142.5, 131.8 (d, J = 9.6 Hz), 130.4, 126.0 (d, J = 14.0 Hz), 122.8, 122.5 (t, J = 240.6 Hz), 120.5, 115.8, 115.6, 115.5, 111.8 (d, J = 9.9 Hz), 48.5 (t, J = 23.7 Hz), 45.4, 31.3, 24.8 (t, J = 27.3 Hz). 19F NMR (471 MHz, CDCl3): δ −85.44–−86.11 (m, 1F), −87.00–−87.69 (m, 1F), −107.12–−107.16 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15F3N2O (M + H)+ 345.1209; found 345.1211. IR (KBr) ν 3358, 3115, 3065, 2918, 2848, 2681, 2360, 1943, 1800, 1705, 1625, 1580, 1525,1452, 1345, 1240, 932, 896, 799, 765, 602 cm−1.
1-Chloro-5-(2,2-difluoropropyl)-5-methylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3l). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 210–211 °C, 63.4 mg, 88% yield. 1H NMR (400 MHz, CDCl3): δ 8.39 (dd, J = 6.0, 3.2 Hz, 1H), 7.93 (dd, J = 6.0, 3.2 Hz, 1H), 7.58–7.56 (m, 1H), 7.47–7.42 (m, 4H), 3.30–3.19 (m, 1H), 2.77–2.65 (m, 1H), 1.72 (s, 3H), 1.38 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.4, 147.0, 143.9, 142.9, 133.5, 131.5, 130.6, 130.4, 126.3, 125.9, 125.7, 122.5 (t, J = 238.8 Hz), 120.7, 120.5, 115.7, 48.49 (t, J = 23.7 Hz), 45.76 (d, J = 3.5 Hz), 31.43 (s), 24.80 (t, J = 27.3Hz). 19F NMR (471 MHz, CDCl3): δ −85.21–−85.92 (m, 1F), −86.99–−87.70 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15ClF2N2O (M + H)+ 361.0914; found 361.0916. IR (KBr) ν 3410, 3064, 3003, 2944, 2848, 2363, 2112, 1956, 1802, 1726, 1610, 1573, 1532,1456, 1239, 1093, 1045, 951, 902, 755, 575 cm−1.
1-Bromo-5-(2,2-difluoropropyl)-5-methylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3m). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 196–197 °C, 63.2 mg, 78% yield. 1H NMR (500 MHz, CDCl3): δ 8.38 (dd, J = 6.0, 3.0 Hz, 1H), 7.93 (dd, J = 6.0, 3.0 Hz, 1H), 7.82 (d, J = 8.0 Hz, 1H), 7.49–7.44 (m, 3H), 7.33 (t, J = 8.0 Hz, 1H), 3.29–3.20 (m, 1H), 2.76–2.67 (m, 1H), 1.72 (s, 3H), 1.38 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.3, 147.1, 143.5, 143.1, 135.3, 130.8, 130.6, 126.4, 126.3, 125.9, 122.5 (t, J = 240.7 Hz), 121.8, 121.3, 120.8, 115.7, 48.4 (t, J = 23.5 Hz), 45.9 (d, J = 3.2 Hz), 31.5, 24.8 (t, J = 27.3Hz). 19F NMR (471 MHz, CDCl3): δ −85.15–−85.86 (m, 1F), −86.99–−87.69 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15BrF2N2O (M + H)+ 405.0408; found 405.0412. IR (KBr) ν 3415, 3090, 3063, 3000, 2924, 2848, 2680, 2363, 1957, 1803, 1726, 1610, 1565, 1535, 1450, 1236, 1092, 1039, 951, 898, 755, 569 cm−1.
5-(2,2-Difluoropropyl)-2,5-dimethylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3n). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 116–117 °C, 51.7 mg, 76% yield. 1H NMR (500 MHz, CDCl3): δ 8.37–8.35 (m, 1H), 8.32 (s, 1H), 7.83–7.81 (m, 1H), 7.45–7.40 (m, 2H), 7.37 (s, 2H), 3.22 (q, J = 15.5 Hz, 1H), 2.70 (q, J = 15.5 Hz, 1H), 2.46 (s, 3H), 1.69 (s, 3H), 1.33 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.4, 149.8, 143.9, 137.9, 137.2, 132.4, 131.5, 126.7, 126.1, 125.8, 125.5, 122.6 (t, J = 240.5 Hz), 122.0, 119.7, 115.6, 48.2 (t, J = 24.0 Hz), 45.3 (d, J = 2.2 Hz), 31.1, 24.7 (t, J = 27.3Hz), 20.9. 19F NMR (471 MHz, CDCl3): δ −86.02–−86.20 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C20H18F2N2O (M + H)+ 341.1460; found 341.1462. IR (KBr) ν 3417, 3115, 3073, 2923, 2850, 2671, 2369, 1943, 1716, 1613, 1547, 1495, 1335, 1238, 1172, 1012, 878, 826, 763, 555 cm−1.
5-(2,2-Difluoropropyl)-1,3,5-trimethylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3o). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 150–151 °C, 58.0 mg, 82% yield. 1H NMR (500 MHz, CDCl3): δ 8.39–8.38 (m, 1H), 7.83–7.81 (m, 1H), 7.44–7.39 (m, 2H), 7.15 (s, 2H), 3.27–3.18 (m, 1H), 3.02 (s, 3H), 2.76–2.67 (m, 1H), 2.42 (s, 3H), 1.71 (s, 3H), 1.32 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.5, 150.0, 144.2, 141.1, 140.2, 139.7, 132.3, 130.6, 125.5, 125.4, 125.3, 122.7 (t, J = 240.6 Hz), 119.9, 118.4, 115.6, 48.5 (t, J = 24.1Hz), 45.4, 31.7, 24.7 (t, J = 27.3Hz), 24.6, 21.6. 19F NMR (471 MHz, CDCl3): δ −85.66–−85.96 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C21H20F2N2O (M + H)+ 355.1616; found 355.1619. IR (KBr) ν 3394, 3115, 3061, 3001, 2924, 2853, 2746, 2359, 1953, 1914, 1805, 1716, 1615, 1533, 1462, 1229, 1081, 905, 803, 615, 544 cm−1.
1,3-Dichloro-5-(2,2-difluoropropyl)-5-methylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3p). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 216–217 °C, 58.5 mg, 74% yield. 1H NMR (500 MHz, CDCl3): δ 8.38–8.36 (m, 1H), 7.92–7.91 (m, 1H), 7.58 (d, J = 2.0 Hz, 1H), 7.48–7.44 (m, 2H), 7.40 (d, J = 1.5 Hz, 1H), 3.29–3.20 (m, 1H), 2.72–2.63 (m, 1H), 1.73 (s, 3H), 1.45 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 170.7, 146.3, 144.1, 143.8, 136.2, 134.4, 131.3, 130.5, 126.5, 126.1, 122.4 (t, J = 240.8 Hz), 120.8, 119.2, 115.6, 48.4 (t, J = 23.4 Hz), 45.8 (d, J = 3.0 Hz), 31.3, 24.9 (t, J = 27.1 Hz). 19F NMR (471 MHz, CDCl3): δ −85.15–−85.86 (m, 1F), −87.94–−88.64 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H14Cl2F2N2O (M + H)+ 395.0524; found 395.0527. IR (KBr) ν 3418, 3144, 3070, 3007, 2947, 2781, 2369, 1954, 1919, 1803, 1725, 1612, 1577, 1446, 1230, 1083, 1033, 983, 911, 802, 755, 618, 555 cm−1.
5-(2,2-Difluoropropyl)-2,4,5-trimethylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3q). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 170–171 °C, 51.0 mg, 72% yield. 1H NMR (400 MHz, CDCl3): δ 8.35–8.33 (m, 2 H), 7.46–7.38 (m, 1H), 7.42 (pd, J = 7.2, 1.6 Hz, 2H), 7.18 (s, 1H), 3.34–3.11 (m, 2H), 2.62 (s, 3H), 2.41 (s, 3H), 1.80 (s, 3H), 1.38 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 173.5, 150.4, 144.1, 137.7, 137.5, 136.4, 134.6, 131.5, 125.9, 125.2, 122.9 (t, J = 240.3Hz), 122.9, 119.6, 115.7, 46.6, 44.9 (t, J = 23.5 Hz), 27.2, 24.5 (t, J = 27.5 Hz), 22.8, 20.6. 19F NMR (471 MHz, CDCl3): δ −88.43–−88.61 (m, 1F), −88.66–−88.85 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H20F2N2O (M + H)+ 355.1616; found 355.1619. IR (KBr) ν 3385, 3060, 3010, 2917, 2851, 2357, 1994, 1909, 1795, 1706, 1612, 1542, 1455, 1228, 1152, 1026, 946, 888, 799, 765, 656, 561 cm−1.
3-Bromo-5-(2,2-difluoropropyl)-5,9,10-trimethylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3r). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 226–227 °C, 72.7 mg, 84% yield. 1H NMR (500 MHz, CDCl3): δ 8.31 (d, J = 9.0 Hz, 1H), 8.12 (s, 1H), 7.60–7.58 (m, 2H), 7.56 (s, 1H), 3.26–3.16 (m, 1H), 2.70–2.61 (m, 1H), 2.41 (s, 3H), 2.39 (s, 3H), 1.70 (s, 3H), 1.39 (t, J = 18.5 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.3, 148.0, 142.4, 141.7, 135.2, 135.0, 131.2, 129.9, 129.7, 127.2, 125.3, 122.4 (t, J = 240.7 Hz), 121.7, 120.0, 115.9, 48.2 (t, J = 23.8 Hz), 45.4 (d, J = 3.3Hz), 30.8, 24.7 (t, J = 27.2 Hz), 20.5, 20.4. 19F NMR (471 MHz, CDCl3): δ −85.57–−86.28 (m, 1F), −87.17–−87.87 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H19BrF2N2O (M + H)+ 433.0721; found 433.0723. IR (KBr) ν 3402, 3090, 2978, 2737, 2586, 2361, 2193, 1933, 1720, 1596, 1539, 1458, 1230, 1082, 948, 859, 698, 654, 562 cm−1.
5-(2,2-Difluoropropyl)-5-phenylbenzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3s). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 44.2 mg, 57% yield. 1H NMR (400 MHz, CDCl3): δ 8.58 (dd, J = 8.0, 1.2 Hz, 1H), 8.26 (d, J = 7.6 Hz, 1H), 7.84 (d, J = 7.6 Hz, 1H), 7.56–7.37 (m, 4H), 7.32–7.24 (m, 3H), 7.22–7.17 (m, 3H), 3.98–3.07 (m, 1H), 3.18–3.07 (m, 1H), 1.47 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 170.3, 149.7, 144.1, 142.3, 139.8, 131.6, 131.2, 129.3, 129.1, 128.2, 128.1, 126.8, 125.93, 125.87, 125.7, 123.6, 122.8 (t, J = 239.8 Hz), 119.9, 115.7, 53.4, 46.0 (t, J = 23.5 Hz), 25.3 (t, J = 27.5 Hz). 19F NMR (471 MHz, CDCl3): δ −84.84–−85.02 (m, 1F), −85.04–−85.22 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C24H18F2N2O (M + H)+ 389.1460; found 389.1463. IR (thin film) ν 3427, 3064, 2921, 2847, 2720, 2350, 1943, 1829, 1717, 1612, 1552, 1445, 1366, 1108, 1043, 942, 848, 701, 624 cm−1.
5-Benzyl-5-(2,2-difluoropropyl)benzo [4,5]imidazo [2,1-a]isoquinolin-6(5H)-one (3t). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 160–161 °C, 52.3 mg, 65% yield. 1H NMR (500 MHz, CDCl3): δ 8.36–8.34 (m, 1H), 8.29 (d, J = 8.0 Hz, 1H), 7.68–7.66 (m, 1H), 7.63–7.62 (m, 2H), 7.49–7.46 (m, 1H), 7.41–7.36 (m, 2H), 6.87 (t, J = 7.5 Hz, 1H), 6.77 (t, J = 7.5 Hz, 2H), 6.49 (d, J = 7.5 Hz, 2H), 3.53 (d, J = 12.5 Hz, 1H), 3.49–3.41 (m, 1H), 3.17 (d, J = 12.5 Hz, 1H), 2.99–2.90 (m, 1H), 1.43 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.2, 149.2, 143.6, 137.4, 133.2, 130.9, 130.8, 129.1, 128.0, 127.8, 127.3, 125.72, 125.69, 125.4, 124.1, 122.6 (t, J = 240.8 Hz), 119.6, 115.4, 51.9, 50.7, 46.5 (t, J = 23.8 Hz), 25.1 (t, J = 27.3Hz). 19F NMR (471 MHz, CDCl3): δ −83.32–−84.00 (m, 1F), −84.83–−85.51 (m, 1F). HRMS (ESI-TOF) m/z: C25H20F2N2O (M + H)+ 403.1616; found 403.1617. IR (KBr) ν 3398, 3033, 3001, 2960, 2934, 2851, 2678, 2366, 2054, 1792, 1712, 1606, 1585, 1555, 1495, 1448, 1263, 1242, 1175, 1113, 1045, 946, 833, 694, 536 cm−1.
7-(2,2-Difluoropropyl)-7-methylbenzo[h]benzo [4,5]imidazo [2,1-a]isoquinolin-8(7H)-one (3u). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 195–196 °C, 55.6 mg, 74% yield. 1H NMR (500 MHz, CDCl3): δ 10.56 (d, J = 8.5 Hz, 1H), 8.47–8.45 (m, 1H), 8.01 (d, J = 8.5 Hz, 1H), 7.96–7.94 (m, 1H), 7.91 (d, J = 8.0 Hz, 1H), 7.84– 7.81 (m, 1H), 7.64 (t, J = 7.5 Hz, 1H), 7.56 (d, J = 8.5 Hz, 1H), 7.50–7.46 (m, 2H), 3.37–3.27 (m, 1H), 2.90–2.81 (m, 1H), 1.78 (s, 3H), 1.33 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.3, 149.7, 144.0, 140.6, 132.7, 132.0, 130.4, 130.3, 128.7, 128.4, 128.2, 126.9, 125.9, 125.8, 122.5 (d, J = 240.5 Hz), 123.7, 120.1, 117.6, 115.7, 47.9 (t, J = 24.1Hz), 45.9, 31.0, 24.6 (t, J = 27.3Hz). 19F NMR (471 MHz, CDCl3): δ −86.07–−86.30 (m, 1F), −86.32–−86.46 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C23H18F2N2O (M + H)+ 377.1460; found 377.1462. IR (KBr) ν 3405, 3104, 3058, 3003, 2940, 2726, 2356, 1940, 1789, 1715, 1619, 1572, 1526, 1453, 1305, 1266, 1182, 1078, 975, 939, 905, 823, 749, 606, 586 cm−1.
4-(2,2-Difluoropropyl)-4-methylbenzo [4,5]imidazo [1,2-a]thieno [2,3-c]pyridin-5(4H)-one (3v). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), mp 187–188 °C, 44.5 mg, 67% yield. 1H NMR (400 MHz, CDCl3): δ 8.33–8.30 (m, 1H), 7.78–7.75 (m, 1H), 7.59 (d, J = 4.8 Hz, 1H), 7.44–7.38 (m, 2H), 7.11 (d, J = 5.2Hz, 1H), 3.24–3.12 (m, 1H), 2.66–2.54 (m, 1H), 1.67 (s, 3H), 1.38 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 172.6, 146.4, 145.9, 143.9, 130.9, 130.4, 125.9, 125.8, 125.5, 123.6, 122.4 (t, J = 240.5 Hz), 119.7, 115.2, 48.2 (t, J = 24.3Hz), 45.6–45.5 (m), 29.9, 24.5 (t, J = 27.3Hz). 19F NMR (471 MHz, CDCl3): δ −86.86–−87.18 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C17H14F2N2OS (M + H)+333.0868; found 333.0870. IR (KBr) ν 3358, 3117, 3065, 2921, 2851, 2681, 2357, 1942, 1866, 1706, 1619, 1579, 1525, 1455, 1425, 1348, 1248, 1168, 1098, 935, 895, 831, 802, 768, 674, 599 cm−1.
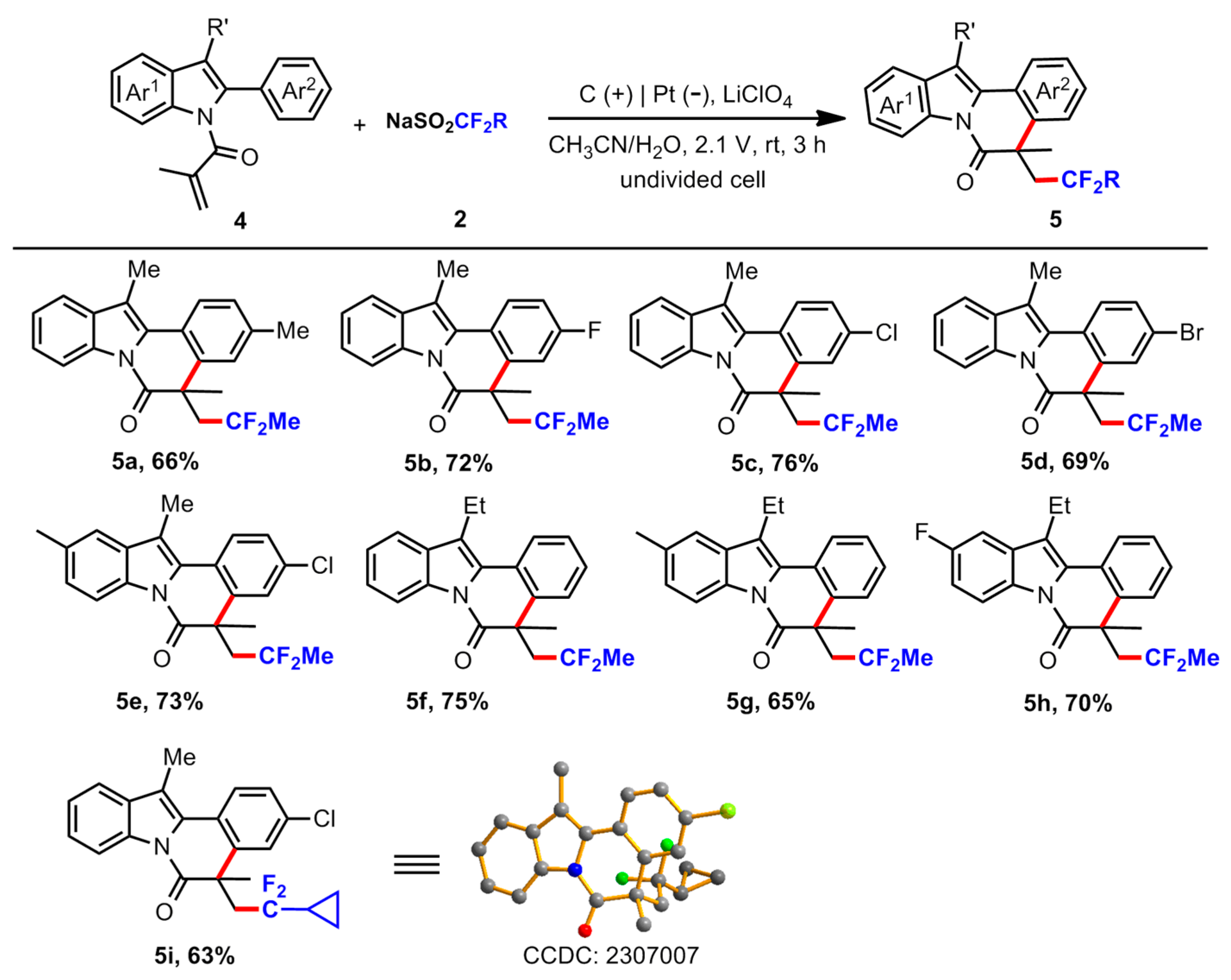
5-(2,2-Difluoropropyl)-3,5,12-trimethylindolo [2,1-a]isoquinolin-6(5H)-one (5a). A white gummy after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 46.6 mg, 66% yield. 1H NMR (400 MHz, CDCl3): δ 8.62 (d, J = 7.2Hz, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.60–7.58 (m, 1H), 7.41–7.34 (m, 3H), 7.23 (d, J = 8.4 Hz, 1H), 3.28–3.16 (m, 1H), 2.71–2.60 (m, 1H), 2.65 (s, 3H), 2.44 (s, 3H), 1.69 (s, 3H), 1.31 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.0, 137.3, 137.0, 134.0 (d, J = 30.9 Hz), 132.6, 129.6 (d, J = 24.5 Hz), 128.3, 127.7, 125.4, 124.9, 124.2, 123.2, 122.9 (t, J = 240.2Hz), 118.2, 116.7, 113.6, 48.1 (t, J = 24.2Hz), 45.0–44.8 (m), 31.4, 24.5 (t, J = 27.4 Hz), 21.5, 11.5. 19F NMR (471 MHz, CDCl3): δ −84.11–−84.82 (m, 1F), −85.19–-85.89 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C22H21F2NO (M + H)+ 354.1664; found 354.1666. IR (thin film) ν 3358, 3184, 3071, 3001, 2923, 2850, 2567, 2363, 1919, 1716, 1627, 1570, 1489, 1392, 1320, 1158, 1091, 1012, 839, 766, 672 cm−1.
5-(2,2-Difluoropropyl)-3-fluoro-5,12-dimethylindolo [2,1-a]isoquinolin-6(5H)-one (5b). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 51.4 mg, 72 % yield. 1H NMR (500 MHz, CDCl3): δ 8.61 (d, J = 8.0 Hz, 1H), 8.03 (dd, J = 8.8, 6.0 Hz, 1H), 7.59 (d, J = 7.0 Hz, 1H), 7.42–7.35 (m, 2H), 7.17–7.11 (m, 2H), 3.28–3.18 (m, 1H), 2.63 (s, 3H), 2.62–2.55 (m, 1H), 1.69 (s, 3H), 1.38 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.2, 161.8 (d, J = 248.4 Hz), 139.8 (d, J = 6.9 Hz), 134.2, 132.4, 128.9, 126.9 (d, J = 8.3Hz), 125.7, 124.3, 122.7 (t, J = 240.6 Hz), 122.4 (d, J = 2.5 Hz), 118.3, 116.7, 114.9 (d, J = 21.8 Hz), 114.1, 113.9, 48.1 (t, J = 24.1Hz), 45.1, 31.2, 24.6 (t, J = 27.4 Hz), 11.4. 19F NMR (471 MHz, CDCl3): δ −84.17–−84.88 (m, 1F), −86.70–-87.41 (m, 1F), −112.80–−112.85 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H18F3NO (M + H)+ 358.1413; found 358.1415. IR (thin film) ν 3355, 3043, 3014, 2973, 2867, 2747, 2591, 2470, 2360, 1940, 1903, 1787, 1702, 1569, 1502, 1016, 975, 819, 755, 625, 589, 531 cm−1.
3-Chloro-5-(2,2-difluoropropyl)-5,10,12-trimethylindolo [2,1-a]isoquinolin-6(5H)-one (5c). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 56.8 mg, 76 % yield. 1H NMR (400 MHz, CDCl3): δ 8.62–8.59 (m, 1H), 7.97 (d, J = 8.4 Hz, 1H), 7.61–7.58 (m, 1H), 7.43 (d, J = 1.6 Hz, 1H), 7.41–7.35 (m, 3H), 3.28–3.16 (m, 1H), 2.63 (s, 3H), 2.67–2.55 (m, 1H), 1.69 (s, 3H), 1.38 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.2, 138.9, 134.2, 133.1, 132.3, 128.6, 127.6, 127.4, 126.2, 125.9, 124.5, 124.4, 122.8 (t, J = 240.4 Hz), 118.4, 116.7, 114.9, 48.0 (t, J = 23.9 Hz), 44.9, 31.2, 24.7 (t, J = 27.3Hz), 11.5. 19F NMR (471 MHz, CDCl3): δ −84.35–−85.06 (m, 1F), −86.79–-87.49 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H18ClF2NO (M + H)+ 374.1118; found 374.1120. IR (thin film) ν 3343, 3195, 3124, 3054, 2983, 2921, 2873, 2727, 2360, 2226, 1939, 1793, 1686, 1602, 1560, 1458, 1285, 1233, 1082, 1013, 978, 902, 811, 711, 619, 546 cm−1.
3-Bromo-5-(2,2-difluoropropyl)-5,12-dimethylindolo [2,1-a]isoquinolin-6(5H)-one (5d). A yellowish liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 57.7 mg, 69% yield. 1H NMR (500 MHz, CDCl3): δ 8.60 (d, J = 7.5 Hz, 1H), 7.90 (d, J = 8.5 Hz, 1H), 7.60–7.58 (m, 2H), 7.52 (dd, J = 8.5, 2.0 Hz, 1H), 7.43–7.35 (m, 2H), 3.27–3.17 (m, 1H), 2.63 (s, 3H), 2.65–2.56 (m, 1H), 1.68 (s, 3H), 1.38 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.1, 139.2, 134.3, 132.3, 130.5, 130.3, 128.7, 126.4, 126.0, 124.9, 124.4, 122.7 (t, J = 240.5 Hz), 121.2, 118.5, 116.8, 115.1, 48.1 (t, J = 23.9 Hz), 44.9, 31.2, 24.7 (t, J = 27.3Hz), 11.5. 19F NMR (471 MHz, CDCl3): δ −84.40–-85.07 (m, 1F), −86.79–-87.47 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H18BrF2NO (M + H)+ 420.0594; found 420.0598. IR (thin film) ν 3340, 3188, 3124, 3054, 2868, 2727, 2357, 1940, 1902, 1870, 1789, 1693, 1603, 1553, 1289, 1238, 1079, 1015, 973, 903, 803, 761, 701, 618 cm−1.
3-Chloro-5-(2,2-difluoropropyl)-5,10,12-trimethylindolo [2,1-a]isoquinolin-6(5H)-one (5e). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 56.6 mg, 73% yield. 1H NMR (500 MHz, CDCl3): δ 8.34 (d, J = 8.5 Hz, 1H), 7.84 (d, J = 8.5 Hz, 1H), 7.30 (s, 1H), 7.25 (d, J = 9.0 Hz, 1H), 7.15 (s, 1H), 7.11 (d, J = 8.0 Hz, 1H), 3.14–3.04 (m, 1H), 2.50 (s, 3H), 2.52–2.43 (m, 1H), 2.38 (s, 3H), 1.56 (s, 3H), 1.25 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 170.9, 139.0, 134.1, 133.0, 132.5, 132.5, 128.8, 127.6, 127.4, 127.2, 126.1, 124.6, 122.7 (t, J = 240.4 Hz), 118.5, 116.4, 114.7, 48.1 (t, J = 24.0 Hz), 44.9, 31.2, 24.6 (t, J = 27.4 Hz), 21.6, 11.5. 19F NMR (471 MHz, CDCl3): δ −84.19–-84.90 (m, 1F), −86.72–−87.42 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C22H20ClF2NO (M + H)+ 388.1274; found 388.1275. IR (thin film) ν 3357, 3094, 2980, 2938, 2861, 2733, 2359, 1877, 1692, 1592, 1556, 1492, 1462, 1296, 1242, 1172, 1073, 939, 901, 801, 714, 664, 619, 591 cm−1.
5-(2,2-Difluoropropyl)-12-ethyl-5-methylindolo [2,1-a]isoquinolin-6(5H)-one (5f). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 52.9 mg, 75% yield. 1H NMR (500 MHz, CDCl3): δ 8.64 (d, J = 7.5 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.61 (d, J = 7.0 Hz, 1H), 7.47 (d, J = 7.5 Hz, 1H), 7.44–7.35 (m, 4 H), 3.26–3.13 (m, 3H), 2.70–2.61 (m, 1H), 1.70 (s, 3H), 1.42 (t, J = 7.5 Hz, 3H), 1.31 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 172.0, 137.1, 134.5, 131.8, 128.8, 127.5, 127.4, 127.3, 125.7, 124.7, 124.3, 122.9 (t, J = 240.2Hz), 121.1, 118.2, 116.9, 48.1 (t, J = 24.3Hz), 44.9, 31.3, 24.5 (t, J = 27.4 Hz), 18.6, 13.3. 19F NMR (471 MHz, CDCl3): δ −84.21–−84.92 (m, 1F), −85.41–−86.12 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C22H21F2NO (M + H)+ 354.1664; found 354.1667. IR (thin film) ν 3364, 3118, 3068, 2973, 2931, 2878, 2681, 2367, 1845, 1720, 1700, 1677, 1606, 1559, 1456, 1395, 1335, 1262, 1128, 1085, 903, 805, 736, 702, 552 cm−1.
5-(2,2-Difluoropropyl)-12-ethyl-5,10-dimethylindolo [2,1-a]isoquinolin-6(5H)-one (5g). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), mp 151–152 °C, 47.7 mg, 65% yield. 1H NMR (500 MHz, CDCl3): δ 8.50 (d, J = 8.5 Hz, 1H), 7.98 (d, J = 8.0 Hz, 1H), 7.46 (d, J = 7.5 Hz, 1H), 7.45–7.35 (m, 3H), 7.22 (d, J = 8.0 Hz, 1H), 3.25–3.16 (m, 1H), 3.13 (q, J =7.5 Hz, 2H), 2.69–2.60 (m, 1H), 2.51 (s, 3H), 1.69 (s, 3H), 1.41 (t, J = 7.5 Hz, 3H), 1.30 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.7, 137.0, 133.9, 132.6, 131.9, 128.9, 127.4, 127.3, 127.0, 125.8, 124.6, 122.9 (t, J = 240.2Hz), 121.0, 118.2, 116.6, 48.1 (t, J = 24.4 Hz), 44.8, 31.2, 24.4 (t, J = 27.4 Hz), 21.6, 18.6, 13.3. 19F NMR (471 MHz, CDCl3): δ −84.10–−84.77 (m, 1F), −85.39–−86.06 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C23H23F2NO (M + H)+ 368.1820; found 368.1824. IR (KBr) ν 3358, 3121, 3068, 2968, 2936, 2871, 2733, 2369, 2249, 1917, 1770, 1693, 1592, 1560, 1493, 1466, 1309, 1238, 1122, 1079, 943, 896, 816, 734, 561 cm−1.
5-(2,2-Difluoropropyl)-12-ethyl-10-fluoro-5-methylindolo [2,1-a]isoquinolin-6(5H)-one (5h). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 51.9 mg, 70% yield. 1H NMR (500 MHz, CDCl3): δ 8.57 (dd, J = 9.0, 5.0 Hz, 1H), 7.98 (d, J = 7.5 Hz, 1H), 7.47 (d, J = 7.5 Hz, 1H), 7.44–7.37 (m, 2H), 7.23 (dd, J = 9.0, 2.5 Hz, 1H), 7.09 (td, J = 9.0, 2.5 Hz, 1H), 3.24–3.15 (m, 1H), 3.12–3.07 (m, 2H), 2.69–2.60 (m, 1H), 1.69 (s, 3H), 1.40 (t, J = 7.5 Hz, 3H), 1.31 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.8, 160.4 (d, J = 241.1Hz), 137.3, 133.2 (d, J = 9.4 Hz), 130.7, 130.4, 127.65 (d, J = 41.6 Hz), 127.3, 125.4, 124.8, 122.8 (d, J = 240.4 Hz), 120.6 (d, J = 4.1Hz), 118.0 (d, J = 9.0 Hz), 113.1 (d, J = 24.6 Hz), 104.0 (d, J = 24.0 Hz), 48.3 (t, J = 24.2Hz), 44.8, 31.2, 24.6 (t, J = 27.4 Hz), 18.7, 13.2. 19F NMR (471 MHz, CDCl3): δ −84.78–−85.42 (m, 1F), −85.86–−86.52 (m, 1F), −118.11–−118.16 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C22H20F3NO (M + H)+ 372.1570; found 372.1575. IR (thin film) ν 3360, 3124, 3075, 3038, 2960, 2928, 2877, 2850, 2764, 2367, 2249, 2172, 2039, 1967, 1940, 1889, 1826, 1692, 1616, 1562, 1399, 1340, 1173, 976, 902, 846, 734, 691,652, 595 cm−1.
3-Chloro-5-(2-cyclopropyl-2,2-difluoroethyl)-5,12-dimethylindolo [2,1-a]isoquinolin-6(5H)-one (5i). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), mp 155–156 °C, 50.4 mg, 63% yield. 1H NMR (500 MHz, CDCl3): δ 8.61 (d, J = 7.5 Hz, 1H), 7.97 (d, J = 8.5 Hz, 1H), 7.59 (d, J = 8.0 Hz, 1H), 7.44 (s, 1H), 7.42–7.36 (m, 3H), 3.40–3.30 (m, 1H), 2.76–2.67 (m, 1H), 2.63 (s, 3H), 1.69 (s, 3H), 1.02–0.95 (m, 1H), 0.44–0.23 (m, 4 H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.2, 139.2, 134.3, 133.1, 132.3, 128.8, 127.5, 126.1, 125.9, 124.4, 124.3, 122.4 (t, J = 242.3Hz), 118.4, 116.8, 114.7, 47.7 (t, J = 25.9 Hz), 45.0, 31.7, 16.6 (t, J = 28.9 Hz), 11.5, 1.3–1.2 (m). 19F NMR (471 MHz, CDCl3): δ −94.49–−95.10 (m, 1F), −99.95–−100.56 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C23H20ClF2NO (M + H)+ 400.1274; found 400.1279. IR (KBr) ν 3341, 3190, 3110, 3053, 3021, 2985, 2948, 2911, 2860, 2724, 2630, 2350, 2080, 1939, 1869, 1789, 1679, 1609, 1563, 1459, 1430, 1280, 1245, 1135, 1059, 1016, 925, 895, 813, 714, 639, 539 cm−1.