Concentrations, Sources and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Chinese Herbal Medicines
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Extraction Conditions
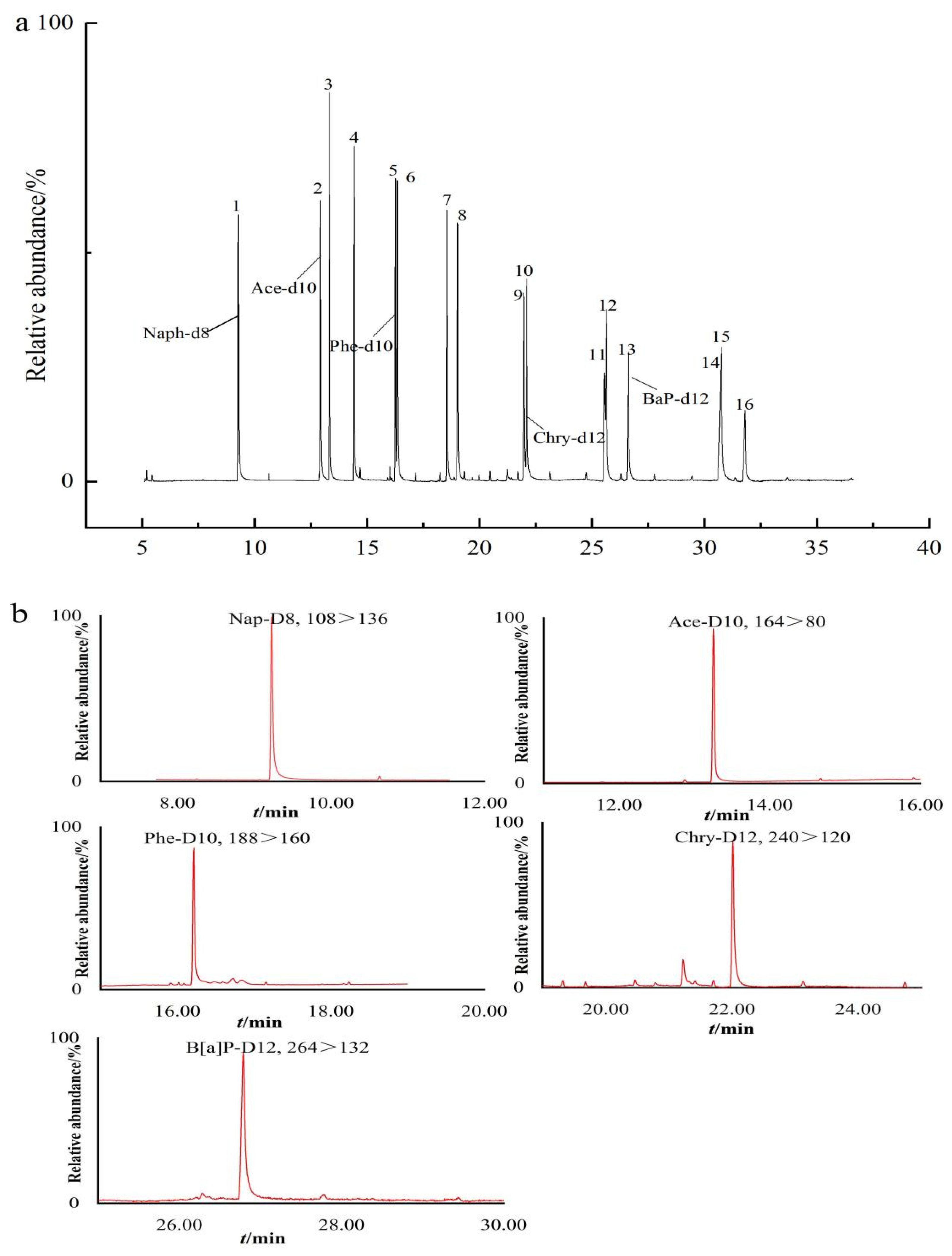
2.2. Performance of the Method
2.3. Application to Real Samples
2.4. Distributional Characteristics of PAHs
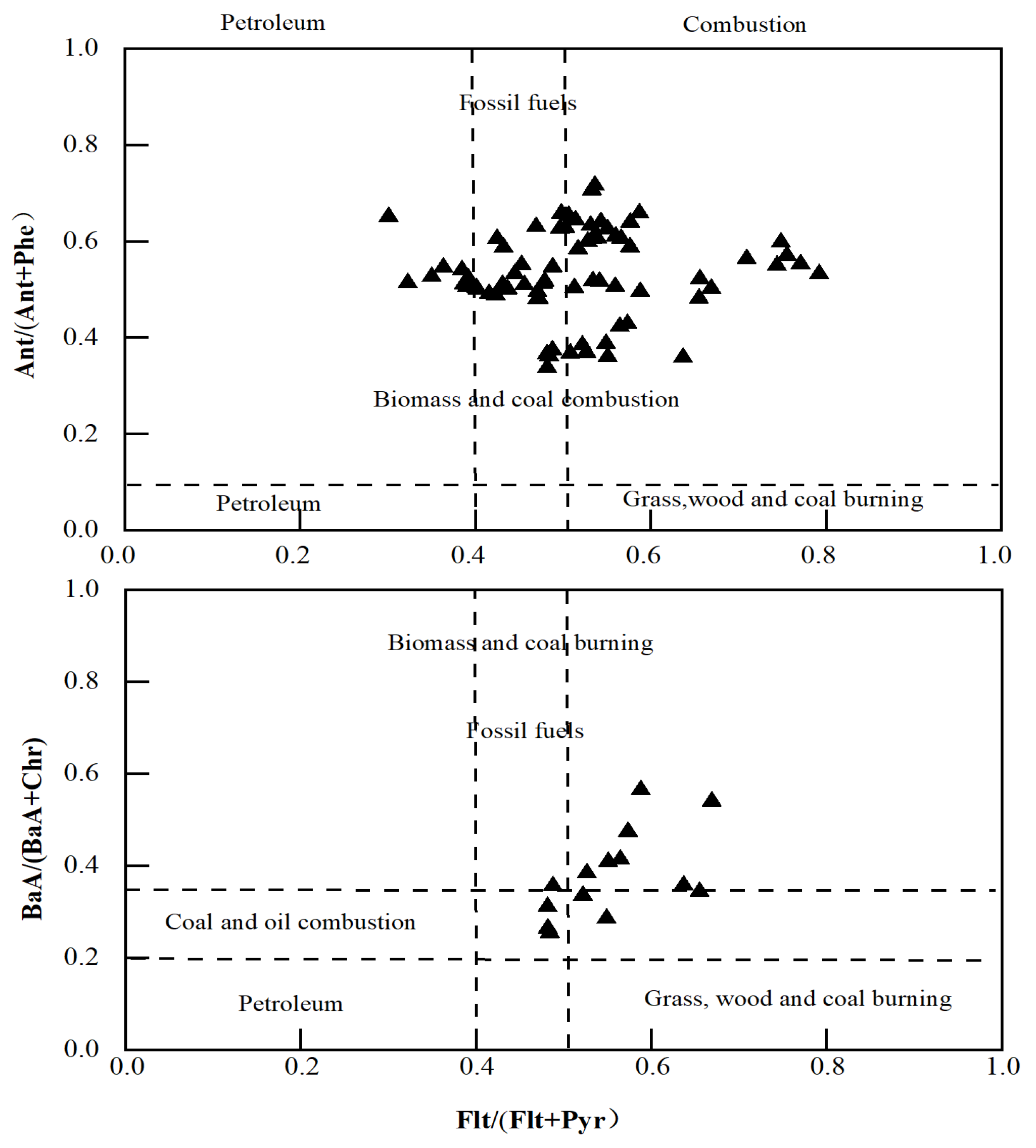
2.5. PAH Source Analysis
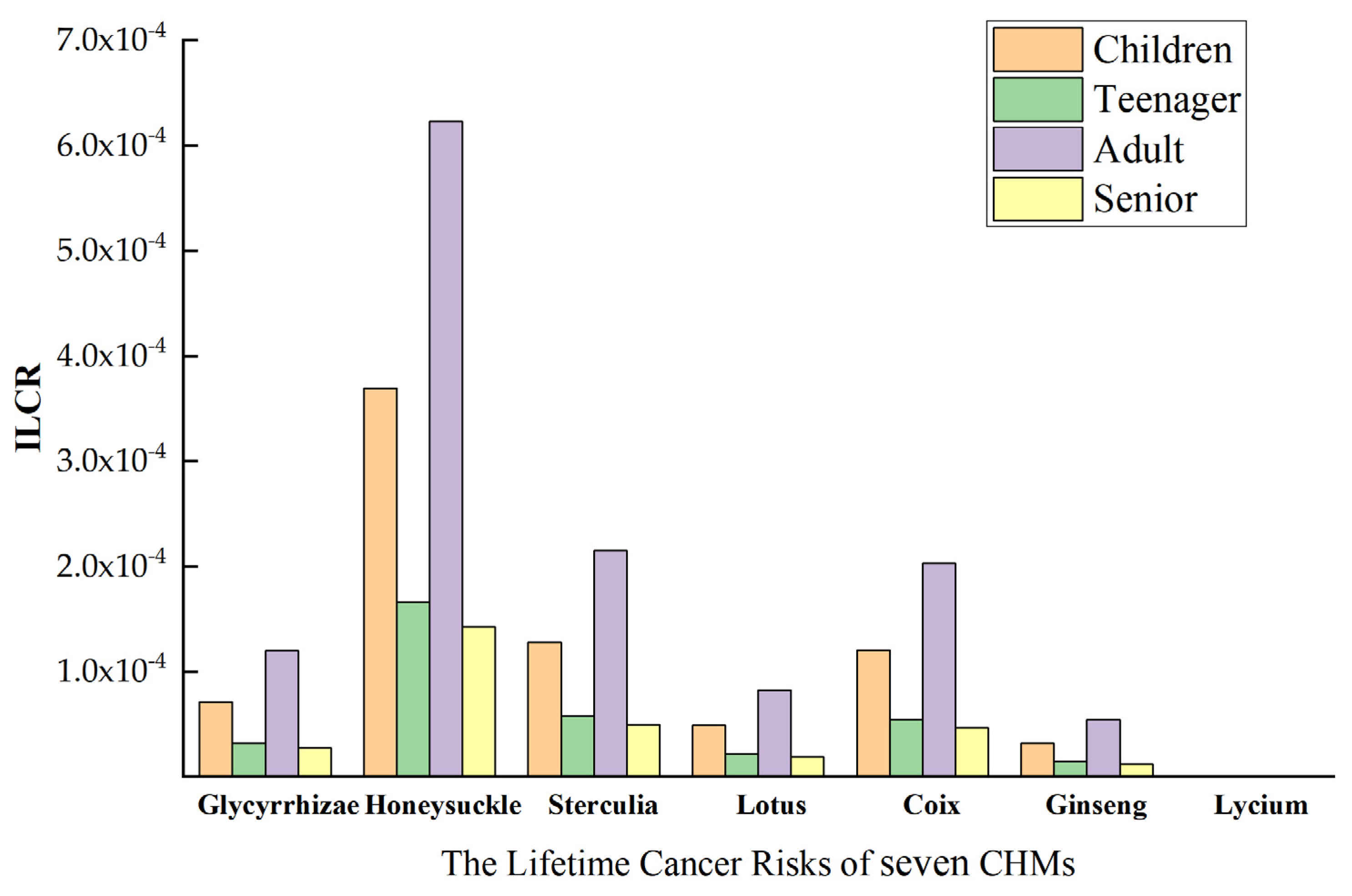
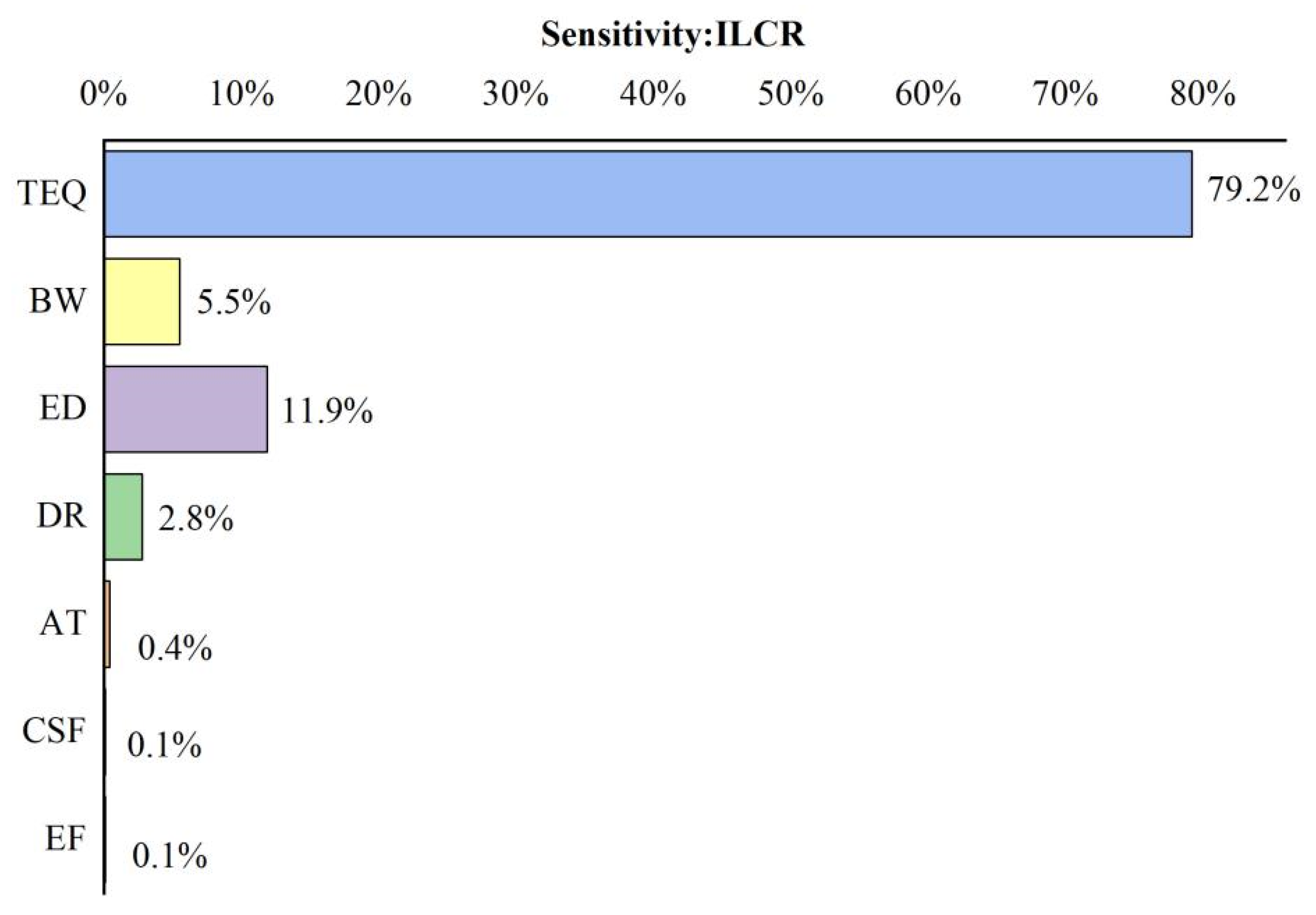
2.6. Health Risk Assessment
3. Experimental Methods
3.1. Instrumentation
3.2. Chemicals and Solutions
3.3. Sample Pretreatment
3.3.1. Extraction
3.3.2. Purify
4. Health Risk Assessment
4.1. Toxic Equivalent Content of PAHs
4.2. Carcinogenic Risk Assessment
4.3. Probabilistic Assessment and Sensitivity Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishizaki, A.; Sito, K.; Kataoka, H. Analysis of contaminant polycyclic aromatic hydrocarbons in tea products and crude drugs. Anal. Methods Adv. Methods Appl. 2011, 3, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-J.; Lee, S.-H.; Kim, Y.-Y.; Shin, H.-S. Polycyclic aromatic hydrocarbon risk assessment and analytical methods using QuEchERS pretreatment for the evaluation of herbal medicine ingredients in Korea. Foods 2021, 10, 2200. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; David, B.; Tu, P.; Barbin, Y. Recent analytical approaches in quality control of traditional Chinese medicines—A review. Anal. Chim. Acta 2010, 657, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Akerele, O. WHO Guidelines for the Assessment of Herbal Medicine. Fitoterapia 1992, 63, 99–104. [Google Scholar] [CrossRef]
- Yu, L.; Cui, Z.; Cao, Y.; Zhang, J.; Sun, H. Investigation of 15 polycyclic aromatic hydrocarbons in selected medicinal herbs used as health food additives by ultra-performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1783–1788. [Google Scholar] [CrossRef]
- Harris, E.S.J.; Cao, S.; Littlefield, B.A.; Craycroft, J.A.; Scholten, R.; Kaptchuk, T.; Fu, Y.; Wang, W.; Liu, Y.; Chen, H. Heavy metal and pesticide content in commonly prescribed individual raw Chinese Herbal Medicines. Sci. Total Environ. 2011, 409, 4297–4305. [Google Scholar] [CrossRef]
- Ling, Y.C.; Teng, H.C.; Cartwright, C. Supercritical fluid extraction and clean-up of organochlorine pesticides in Chinese herbal medicine. J. Chromatogr. A 1999, 835, 145–157. [Google Scholar] [CrossRef]
- Tong, H.; Tong, Y.; Xue, J.; Liu, D.; Wu, X. Multi-residual Pesticide Monitoring in Commercial Chinese Herbal Medicines by Gas Chromatography–Triple Quadrupole Tandem Mass Spectrometry. Food Anal. Methods 2014, 7, 135–145. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Wei, W.; Xu, R. Simultaneous determination of heavy metal pollution in commercial traditional Chinese medicines in China. J. Nat. Med. 2013, 67, 887–893. [Google Scholar] [CrossRef]
- Ting, A.; Chow, Y.; Tan, W. Microbial and heavy metal contamination in commonly consumed traditional Chinese herbal medicines. J. Tradit. Chin. Med. 2013, 33, 119–124. [Google Scholar] [CrossRef]
- Yu, L.; Cao, Y.; Zhang, J.; Cui, Z.; Sun, H. Isotope dilution-GC-MS/MS analysis of 16 polycyclic aromatic hydrocarbons in selected medicinal herbs used as health food additives. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Yue, D.; Wan, C.; Ye, Y.; Wang, X. Characterization and loading estimation of polycyclic aromatic hydrocarbons in road runoff from urban regions of Beijing, China. Environ. Toxicol. Chem. 2008, 27, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Keyte, I.J.; Harrison, R.M.; Lammel, G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons—A review. Chem. Soc. Rev. 2013, 42, 9333–9391. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
- Chen, B.H.; Chen, Y.C. Formation of polycyclic aromatic hydrocarbons in the smoke from heated model lipids and food lipids. J. Agric. Food Chem. 2001, 49, 5238–5243. [Google Scholar] [CrossRef]
- Crone, T.J.; Tolstoy, M. Magnitude of the 2010 Gulf of Mexico oil leak. Science 2010, 330, 634. [Google Scholar] [CrossRef]
- Xia, Z.; Duan, X.; Tao, S.; Qiu, W.; Liu, D.; Wang, Y.; Wei, S.; Wang, B.; Jiang, Q.; Lu, B. Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Environ. Pollut. 2013, 173, 150–156. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Patra, J.-K.; Shin, H.-S. Evaluation of analytical method and risk assessment of polycyclic aromatic hydrocarbons for fishery products in Korea. Food Control 2022, 131, 108421. [Google Scholar] [CrossRef]
- Boll, E.S.; Christensen, J.H.; Holm, P.E. Quantification and source identification of polycyclic aromatic hydrocarbons in sediment, soil, and water spinach from Hanoi, Vietnam. J. Environ. Monit. 2008, 10, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Rey-Salgueiro, L.; Martínez-Carballo, E.; García-Falcón, M.S.; Simal-Gándara, J. Effects of a chemical company fire on the occurrence of polycyclic aromatic hydrocarbons in plant foods. Food Chem. 2008, 108, 347–353. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- Stadler, R.H.; Lineback, D.R. Process-Induced Food Toxicants. Occurrance, Formation, Mitigation and Health Risks; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. The occurrence of 16 EPA PAHs in food—A review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef]
- Wennrich, L.; Popp, P.; Zeibig, M. Polycyclic aromatic hydrocarbon burden in fruit and vegetable species cultivated in allotments in an industrial area. Int. J. Environ. Anal. Chem. 2002, 82, 667–690. [Google Scholar] [CrossRef]
- Wei, M.-C.; Jen, J.-F. Determination of polycyclic aromatic hydrocarbons in aqueous samples by microwave assisted headspace solid-phase microextraction and gas chromatography/flame ionization detection. Talanta 2007, 72, 1269–1274. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Popoola, O.E.; Msagati, T.A. Determination and distribution of polycyclic aromatic hydrocarbons in rivers, sediments and wastewater effluents in Vhembe District, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 387. [Google Scholar] [CrossRef]
- Zhou, Q.; Lei, M.; Wu, Y.; Yuan, Y. Magnetic solid phase extraction of typical polycyclic aromatic hydrocarbons from environmental water samples with metal organic framework MIL-101 (Cr) modified zero valent iron nano-particles. J. Chromatogr. A 2017, 1487, 22–29. [Google Scholar] [CrossRef] [PubMed]
- De Smet, P.G.; Keller, K.; Hänsel, R.; Chandler, R. Adverse Effects of Herbal Drugs; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar] [CrossRef]
- Yunker, M.B.; Backus, S.M.; Graf Pannatier, E.; Jeffries, D.S.; Macdonald, R.W. Sources and Significance of Alkane and PAH Hydrocarbons in Canadian Arctic Rivers. Estuar. Coast. Shelf Sci. 2002, 55, 1–31. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition [Review]. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Torre-Roche, R.J.D.L.; Lee, W.Y.; Campos-Diaz, S.I. Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: Analysis of a potential problem in the United States/Mexico border region. J. Hazard. Mater. 2009, 163, 946–958. [Google Scholar] [CrossRef]
- Akyüz, M.; Cabuk, H. Gas–particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci. Total Environ. 2010, 408, 5550–5558. [Google Scholar] [CrossRef]
- Lv, M.; Luan, X.; Liao, C.; Wang, D.; Liu, D.; Zhang, G.; Jiang, G.; Chen, L. Human impacts on polycyclic aromatic hydrocarbon distribution in Chinese intertidal zones. Nat. Sustain. 2020, 3, 878–884. [Google Scholar] [CrossRef]
- Zhou, D.-B.; Han, F.; Ding, L.; Song, W.; Lv, Y.-N.; Hu, Y.-Y.; Liu, Y.-X.; Sheng, X.; Zheng, P. Magnetic C60 nanospheres based solid-phase extraction coupled with isotope dilution gas chromatography–mass spectrometry method for the determination of sixteen polycyclic aromatic hydrocarbons in Chinese herbal medicines. J. Chromatogr. B 2020, 1144, 122076. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, D.; Tan, L.-H.; Zhao, S.-P.; Wang, J.-W.; Yao, L.; Cao, W.-G. Polycyclic aromatic hydrocarbons in traditional Chinese medicines: An analytical method based on different medicinal parts, levels, distribution, and sources. RSC Adv. 2017, 7, 4671–4680. [Google Scholar] [CrossRef]
- Dorine, D.; Philippe, B.; Geneviève, C. Challenges in Tracing the Fate and Effects of Atmospheric Polycyclic Aromatic Hydrocarbon Deposition in Vascular Plants. Environ. Sci. Technol. 2013, 47, 3967–3981. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Y.; Ren, L.; Li, C.; Wang, G.; Huang, H. Characteristics and Ecological Risk Assessment of Heavy Metal Pollution in Vegetable Soils of Guangzhou Urban Districts. Guangdong Agric. Sci. 2019, 46, 73–78. [Google Scholar] [CrossRef]
- Cui, Z.; Ge, N.; Zhang, A.; Liu, Y.; Zhang, J.; Cao, Y. Comprehensive determination of polycyclic aromatic hydrocarbons in Chinese herbal medicines by solid phase extraction and gas chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 1989–1997. [Google Scholar] [CrossRef]
- Nisbet, I.C.; Lagoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Madill, R.E.; Brownlee, B.G.; Josephy, P.D.; Bunce, N.J. Comparison of the Ames Salmonella assay and Mutatox genotoxicity assay for assessing the mutagenicity of polycyclic aromatic compounds in porewater from Athabasca oil sands mature fine tailings. Environ. Sci. Technol. 1999, 33, 2510–2516. [Google Scholar] [CrossRef]
- Stroomberg, G.J.; Knecht, J.A.D.; Ariese, F.; Gestel, C.A.V.; Velthorst, N.H. Pyrene metabolites in the hepatopancreas and gut of the isopod Porcellio scaber, a new biomarker for polycyclic aromatic hydrocarbon exposure in terrestrial ecosystems. Environ. Toxicol. Chem. Int. J. 1999, 18, 2217–2224. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Risk Assessment Guidance for Superfund. Volume I Human Health Evaluation Manual. (Part A); Interim Final; U.S. Environmental Protection Agency: Washington, DC, USA, 2023.
- Jiang, D.; Xin, C.; Li, W.; Chen, J.; Li, F.; Chu, Z.; Xiao, P.; Shao, L. Quantitative analysis and health risk assessment of polycyclic aromatic hydrocarbons in edible vegetable oils marketed in Shandong of China. Food Chem. Toxicol. 2015, 83, 61–67. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, L.; Cai, Y.; Chen, Y. Distribution of polycyclic aromatic hydrocarbon (PAH) residues in several tissues of edible fishes from the largest freshwater lake in China, Poyang Lake, and associated human health risk assessment. Ecotoxicol. Environ. Saf. 2014, 104, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Kordybach, B.; Smreczak, B.; Klimkowicz-Pawlas, A. Concentrations, sources, and spatial distribution of individual polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in the Eastern part of the EU: Poland as a case study. Sci. Total Environ. 2009, 407, 3746–3753. [Google Scholar] [CrossRef]
- Phillips, L.; Moya, J. The evolution of EPA’s Exposure Factors Handbook and its future as an exposure assessment resource. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 13–21. [Google Scholar] [CrossRef]
- Phillips, L.J.; Moya, J. Exposure factors resources: Contrasting EPA’s Exposure Factors Handbook with international sources. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 233–243. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, Q.; Zheng, L.; Chen, X.; Li, C.; Ren, M. Distribution, source and health risk assessment based on the Monte Carlo method of heavy metals in shallow groundwater in an area affected by mining activities, China. Ecotoxicol. Environ. Saf. 2021, 224, 112679. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Zhang, D.; Wang, Y. Probabilistic human health risk assessment of heavy metal intake via vegetable consumption around Pb/Zn smelters in Southwest China. Int. J. Environ. Res. Public Health 2019, 16, 3267. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; Chang, S.X.; Collins, C.; Xu, J.; Liu, X. Status assessment and probabilistic health risk modeling of metals accumulation in agriculture soils across China: A synthesis. Environ. Int. 2019, 128, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, H.; Dong, L.; Huang, B.; Borggaard, O.K.; Hansen, H.C.B.; He, Y.; Holm, P.E. Source identification of heavy metals in peri-urban agricultural soils of southeast China: An integrated approach. Environ. Pollut. 2018, 237, 650–661. [Google Scholar] [CrossRef]


| Number | Compound | Abbreviation | CAS Number | Molecular Weight | Retention Time (min) | Ion Pair for Quantitative Analysis (m/z) | Ion Pair for Qualitative Analysis (m/z) |
|---|---|---|---|---|---|---|---|
| 1 | Naphthalene | Naph | 91-20-3 | 128.17 | 9.273 | 128 > 102 | 128 > 77 |
| 2 | Acenaphthylene | Acy | 208-96-8 | 152.20 | 12.925 | 152 > 151 | 152 > 126 |
| 3 | Acenaphthene | Ace | 83-32-9 | 154.20 | 13.326 | 153 > 152 | 153 > 127 |
| 4 | Fluorene | Flu | 86-73-7 | 166.22 | 14.417 | 166 > 165 | 166 > 163 |
| 5 | Phenanthrene | Phe | 85-01-8 | 178.23 | 16.345 | 178 > 176 | 178 > 152 |
| 6 | Anthracene | Ant | 120-12-7 | 178.22 | 16.358 | 178 > 176 | 178 > 152 |
| 7 | Fluoranthene | Flt | 206-44-0 | 202.25 | 18.549 | 202 > 200 | 202 > 150 |
| 8 | Pyrene | Pyr | 129-00-0 | 202.26 | 19.028 | 202 > 200 | 202 > 150 |
| 9 | Benz[a]anthracene | BaA | 56-55-3 | 228.30 | 22.102 | 228 > 226 | 228 > 202 |
| 10 | Chrysene | Chry | 218-01-9 | 228.29 | 21.971 | 228 > 226 | 228 > 202 |
| 11 | Benzo[b]fluorathene | BbF | 205-99-2 | 252.31 | 25.551 | 252 > 250 | 252 > 224 |
| 12 | Benzo[k]fluorathene | bkF | 207-08-9 | 252.31 | 25.640 | 252 > 250 | 252 > 224 |
| 13 | Benzo[a]pyrene | BaP | 50-32-8 | 252.31 | 26.618 | 252 > 250 | 252 > 224 |
| 14 | Indeno[1,2,3-c,d]pyrene | Ind(cd)P | 193-39-5 | 276.00 | 30.699 | 276 > 274 | 276 > 248 |
| 15 | Dibenz[a,h]anthracene | DahA | 53-70-3 | 278.35 | 30.744 | 278 > 276 | 278 > 274 |
| 16 | Benzo[g,h,i]perylene | BghiP | 191-24-2 | 276.33 | 31.798 | 276 > 274 | 276 > 272 |
| 17 | Naphthalene-d8 | Naph-d8 | 1146-65-2 | 136.22 | 9.232 | 136 > 108 | 136 > 134 |
| 18 | Acenaphthene-d10 | Ace-d10 | 15067-26-2 | 164.17 | 13.255 | 164 > 162 | 136 > 134 |
| 19 | Phenanthrene-d10 | Phe-d10 | 1517-22-2 | 188.29 | 16.213 | 188 > 160 | 164 > 160 |
| 20 | Chrysene-d12 | Chry-d12 | 1719-03-5 | 240.36 | 22.016 | 240 > 236 | 240 > 212 |
| 21 | Benzo[a]pyrene-d12 | BaP-d12 | 63466-71-7 | 266.399 | 26.788 | 264 > 260 | 264 > 232 |
| Analyte | Spiked 20 μg/kg | Spiked 100 μg/kg | Spiked 400 μg/kg | |||
|---|---|---|---|---|---|---|
| Mean Recovery (%) | RSD (%) | Mean Recovery (%) | RSD (%) | Mean Recovery (%) | RSD (%) | |
| Naph | 66.97 | 3.06 | 79.21 | 4.38 | 67.2 | 0.75 |
| Acy | 84.67 | 4.26 | 89.37 | 5.94 | 84.1 | 1.54 |
| Ace | 63.62 | 4.91 | 82.66 | 3.94 | 85.4 | 1.13 |
| Flu | 63.37 | 10.96 | 76.89 | 2.28 | 74.0 | 2.35 |
| Phe | 133.12 | 2.76 | 116.05 | 1.95 | 108.1 | 5.21 |
| Ant | 116.72 | 14.54 | 119.15 | 9.47 | 98.9 | 2.66 |
| Flt | 104.45 | 2.10 | 104.91 | 2.74 | 107.1 | 3.26 |
| Pyr | 113.30 | 3.27 | 107.19 | 3.63 | 106.4 | 2.44 |
| BaA | 89.27 | 8.44 | 121.45 | 1.04 | 108.6 | 4.82 |
| Chry | 85.50 | 13.09 | 117.37 | 2.86 | 99.1 | 2.50 |
| BbF | 119.23 | 3.89 | 121.85 | 6.37 | 117.0 | 7.98 |
| bkF | 119.15 | 3.00 | 117.51 | 4.49 | 111.5 | 2.06 |
| BaP | 112.71 | 14.33 | 117.96 | 5.34 | 113.1 | 6.39 |
| Ind(cd)P | 100.96 | 4.38 | 109.01 | 1.08 | 114.1 | 6.18 |
| DahA | 106.04 | 3.31 | 111.17 | 5.20 | 114.2 | 3.40 |
| BghiP | 107.02 | 0.69 | 114.56 | 2.60 | 116.1 | 6.00 |
| Analyte | Linearity Range (ng/mL) | LOD (μg/kg) | LOQ (μg/kg) | Regression Equation | Coefficient of Determination (R2) |
|---|---|---|---|---|---|
| Naph | 5–500 | 6.81 | 13.6 | y = 0.453x + 0.053 | 0.9990 |
| Acy | 5–500 | 5.20 | 10.4 | y = 0.626x − 0.013 | 0.9990 |
| Ace | 5–500 | 5.68 | 11.4 | y = 0.759x + 0.029 | 0.9996 |
| Flu | 5–500 | 5.14 | 10.3 | y = 0.517x + 0.037 | 0.9997 |
| Phe | 5–500 | 6.35 | 12.7 | y = 0.527x + 0.003 | 0.9997 |
| Ant | 5–500 | 4.96 | 9.9 | y = 0.571x − 0.020 | 0.9998 |
| Flt | 5–500 | 5.13 | 10.3 | y = 0.474x + 0.009 | 0.9999 |
| Pyr | 5–500 | 5.33 | 10.7 | y = 0.346x + 0.025 | 0.9998 |
| BaA | 5–500 | 5.60 | 11.2 | y = 0.207x + 0.032 | 0.9998 |
| Chry | 5–500 | 5.54 | 11.1 | y = 0.458x + 0.009 | 0.9992 |
| BbF | 5–500 | 6.99 | 14.0 | y = 0.276x + 0.005 | 0.9996 |
| bkF | 5–500 | 6.75 | 13.5 | y = 0.502x + 0.017 | 0.9997 |
| BaP | 5–500 | 8.14 | 16.3 | y = 0.338x + 0.016 | 0.9993 |
| Ind(cd)P | 5–500 | 5.72 | 11.4 | y = 0.440x − 0.018 | 0.9998 |
| DahA | 5–500 | 6.25 | 12.5 | y = 0.245x + 0.224 | 0.9997 |
| BghiP | 5–500 | 7.70 | 15.4 | y = 0.235x + 0.019 | 1.0000 |
| Analyte | Glycyrrhizae Radix et Rhizoma | RSD% | Honeysuckle | RSD% | Coix lacryma | RSD% | Ginseng Radix et Rhizoma | RSD% | Lotus Seed | RSD% | Seed of Sterculia lychnophora | RSD% | Lycium chinense | RSD% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naph | 42.313 | 0.39 | 36.019 | 0.25 | 21.666 | 0.36 | 60.153 | 0.59 | 37.674 | 0.40 | 40.844 | 0.26 | 48.876 | 0.31 |
| Acy | 13.600 | 0.73 | 13.609 | 0.32 | 22.913 | 0.37 | nd | - | 18.681 | 0.55 | 27.400 | 0.38 | 11.378 | 0.32 |
| Ace | 31.851 | 0.10 | 25.060 | 0.56 | 24.754 | 0.50 | 33.842 | 0.47 | 27.726 | 0.52 | 38.175 | 0.39 | 39.044 | 0.12 |
| Flu | 24.576 | 0.21 | 25.191 | 0.51 | nd | - | nd | - | nd | - | 12.241 | 0.37 | 18.880 | 0.40 |
| Phe | 153.753 | 0.05 | 160.906 | 0.21 | 82.388 | 0.58 | 103.442 | 0.22 | 80.887 | 0.30 | 168.640 | 0.12 | 47.045 | 0.18 |
| Ant | 154.849 | 0.07 | nd | - | 147.707 | 0.29 | 121.351 | 0.29 | 117.332 | 0.23 | 112.253 | 0.15 | nd | - |
| Flt | 97.790 | 0.26 | 52.555 | 0.17 | 27.020 | 0.83 | 18.462 | 0.76 | 21.164 | 0.64 | 41.773 | 0.25 | nd | - |
| Pyr | 99.612 | 0.23 | 42.752 | 0.22 | nd | - | 18.190 | 0.83 | 11.859 | 0.21 | 52.345 | 0.40 | 11.683 | 0.19 |
| BaA | 31.207 | 0.39 | nd | - | nd | - | nd | - | nd | - | nd | - | nd | - |
| Chry | 50.084 | 0.12 | 76.563 | 0.21 | nd | - | nd | - | nd | - | nd | - | nd | - |
| BbF | 148.469 | 0.08 | 941.504 | 0.08 | nd | - | nd | - | nd | - | 462.085 | 0.04 | nd | - |
| bkF | nd | - | nd | - | nd | - | nd | - | 22.594 | 0.48 | nd | - | nd | - |
| BaP | nd | - | 39.928 | 0.24 | nd | - | nd | - | nd | - | nd | - | nd | - |
| Ind(cd)P | nd | - | nd | - | nd | - | nd | - | nd | - | nd | - | nd | - |
| DahA | nd | - | nd | - | nd | - | nd | - | nd | - | nd | - | nd | - |
| BghiP | nd | - | nd | - | nd | - | nd | - | nd | - | nd | - | nd | - |
| ∑16PAHs | 848.102 | - | 1414.087 | - | 326.448 | - | 355.440 | - | 337.917 | - | 955.758 | - | 176.906 | - |
| CHMs | Distribution | Parameters | 10% | 50% | 90% | |
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| Glycyrrhizae Radix et Rhizoma | Lognormal | 2.00 × 10−5 | 5.66 × 10−5 | 1.29 × 10−6 | 7.55 × 10−6 | 4.59 × 10−5 |
| Honeysuckle | Lognormal | 6.04 × 10−4 | 1.48 × 10−4 | 2.96 × 10−5 | 2.00 × 10−4 | 1.37 × 10−4 |
| Coix lacryma | Lognormal | 7.04 × 10−5 | 4.47 × 10−4 | 1.84 × 10−4 | 1.68 × 10−5 | 1.48 × 10−4 |
| Ginseng Radix et Rhizoma | Lognormal | 5.64 × 10−5 | 1.81 × 10−4 | 1.66 × 10−4 | 1.43 × 10−5 | 1.20 × 10−4 |
| Lotus seed | Lognormal | 6.56 × 10−5 | 1.98 × 10−4 | 2.45 × 10−4 | 1.93 × 10−5 | 1.45 × 10−4 |
| seed of Sterculia lychnophora | Lognormal | 2.70 × 10−4 | 9.79 × 10−4 | 1.08 × 10−5 | 7.86 × 10−5 | 5.92 × 10−4 |
| Lycium chinense | Lognormal | 5.85 × 10−7 | 1.41 × 10−4 | −1.03 × 10−4 | 5.53 × 10−7 | 2.25 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, D.; Zhu, Z.; Zhao, S.; Zhang, X.; Lin, J.; Wang, J.; Zeng, Q.; Zhu, M. Concentrations, Sources and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Chinese Herbal Medicines. Molecules 2024, 29, 972. https://doi.org/10.3390/molecules29050972
Cao D, Zhu Z, Zhao S, Zhang X, Lin J, Wang J, Zeng Q, Zhu M. Concentrations, Sources and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Chinese Herbal Medicines. Molecules. 2024; 29(5):972. https://doi.org/10.3390/molecules29050972
Chicago/Turabian StyleCao, Deyan, Zhu Zhu, Siyuan Zhao, Xi Zhang, Jianzai Lin, Junji Wang, Qinghong Zeng, and Meilin Zhu. 2024. "Concentrations, Sources and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Chinese Herbal Medicines" Molecules 29, no. 5: 972. https://doi.org/10.3390/molecules29050972
APA StyleCao, D., Zhu, Z., Zhao, S., Zhang, X., Lin, J., Wang, J., Zeng, Q., & Zhu, M. (2024). Concentrations, Sources and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Chinese Herbal Medicines. Molecules, 29(5), 972. https://doi.org/10.3390/molecules29050972







