Effect of Diacetylcurcumin Manganese Complex on Rotenone-Induced Oxidative Stress, Mitochondria Dysfunction, and Inflammation in the SH-SY5Y Parkinson’s Disease Cell Model
Abstract
1. Introduction
2. Results
2.1. In Vitro Antioxidant Assays of DiAc-Cp and DiAc-Cp-Mn
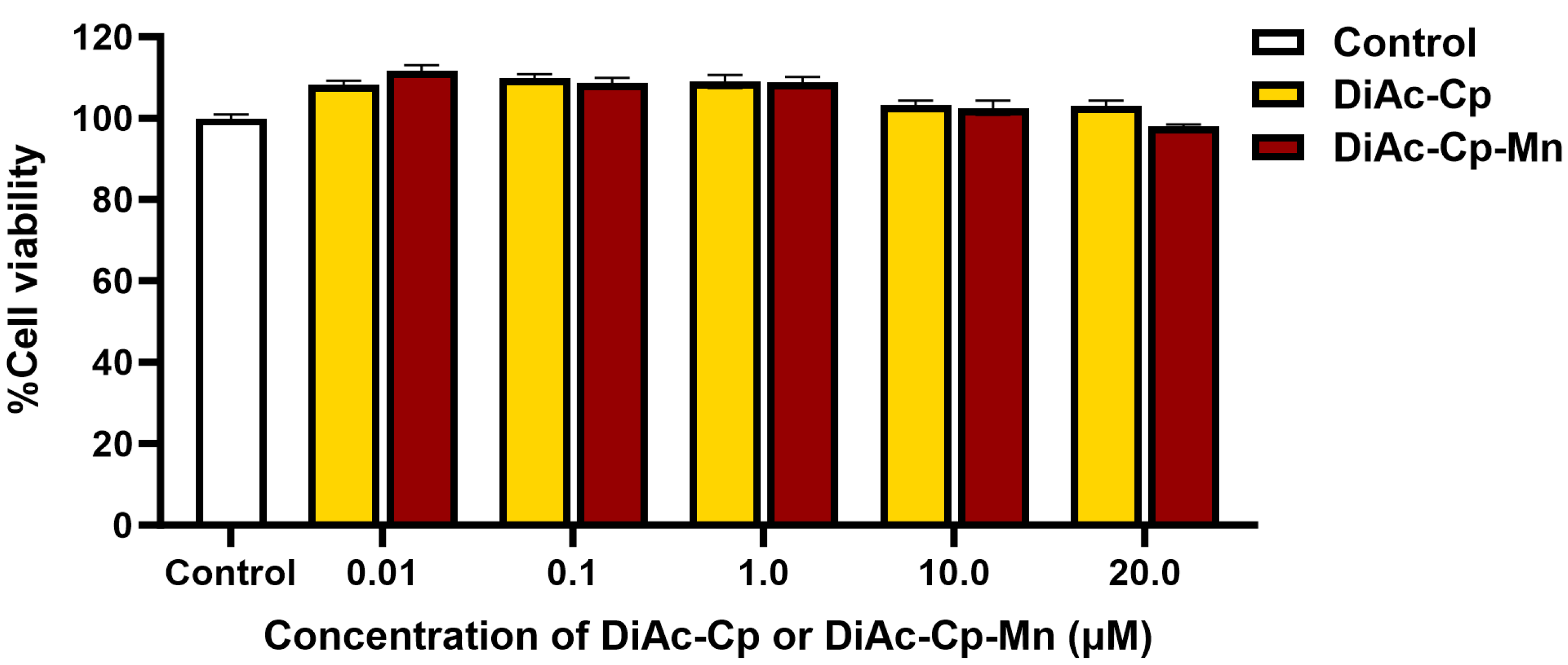
2.2. Effect of DiAc-Cp and DiAc-Cp-Mn on the Viability of SH-SY5Y Cells
2.3. Effect of Rotenone on the Viability of SH-SY5Y Cells
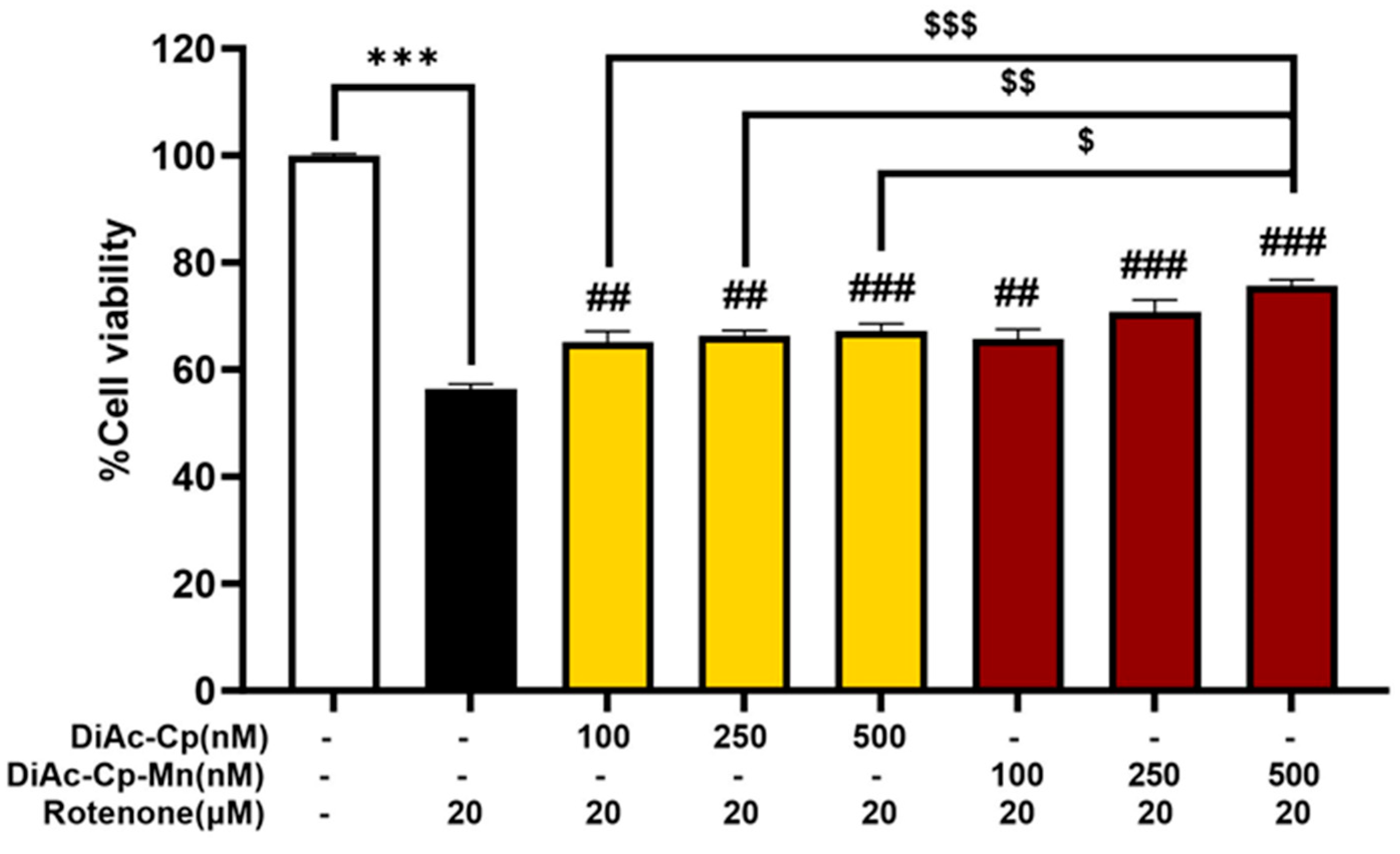
2.4. Effect of DiAc-Cp and DiAc-Cp-Mn on Rotenone-Induced Neurotoxicity in SH-SY5Y Cells
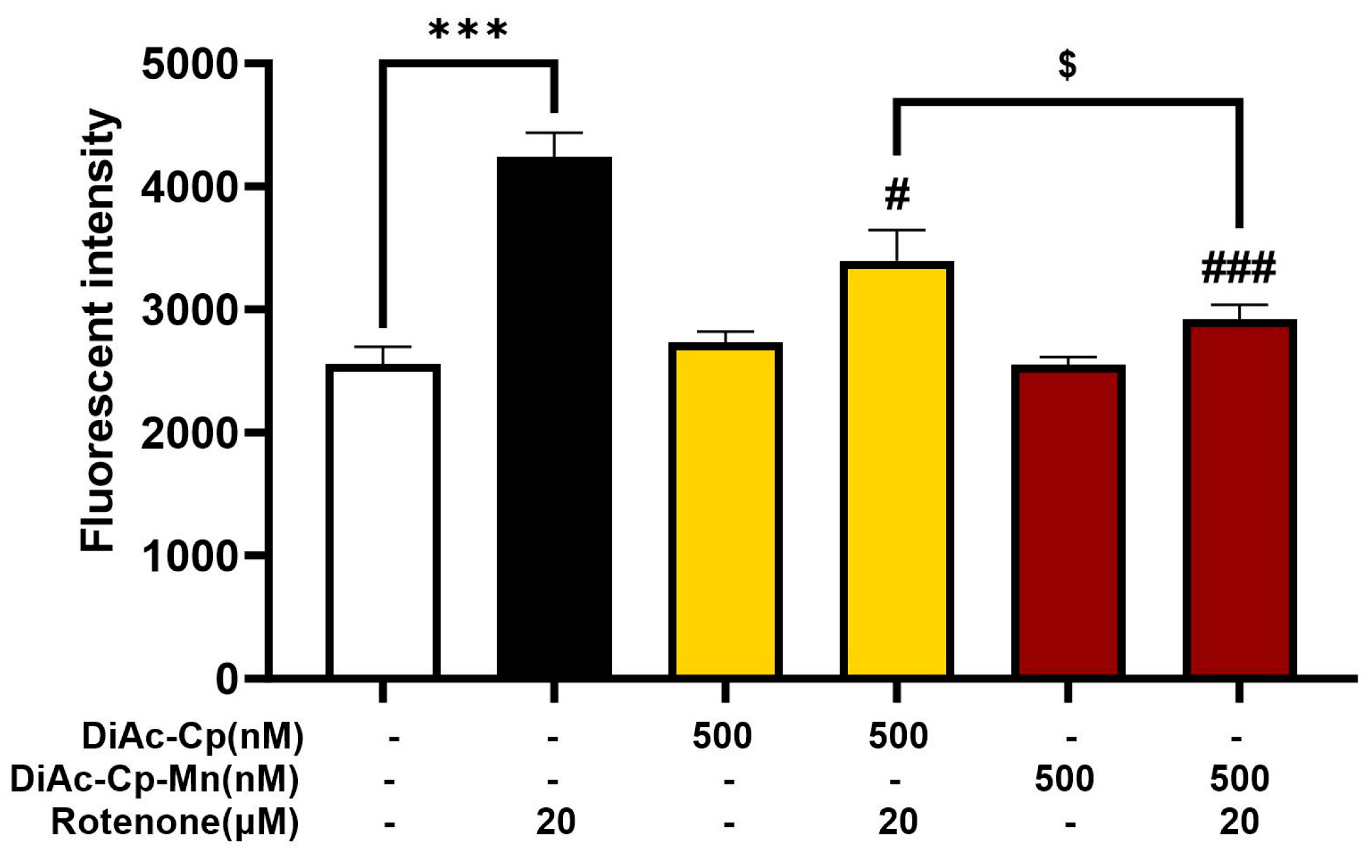
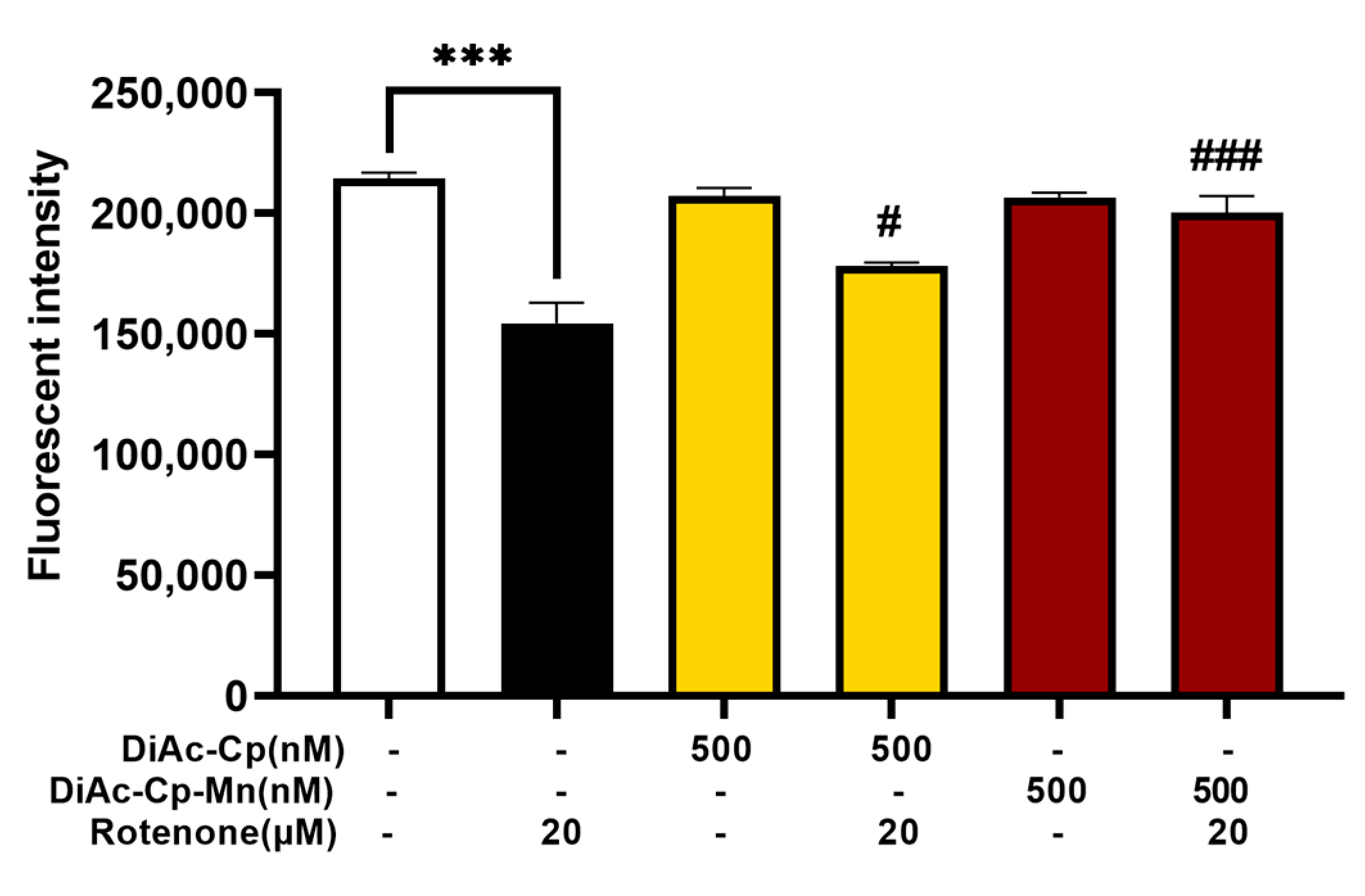
2.5. DiAc-Cp and DiAc-Cp-Mn Suppress Rotenone-Induced Intracellular ROS Overproduction in SH-SY5Y Cells
2.6. DiAc-Cp and DiAc-Cp-Mn Restored Mitochondrial Membrane Potential in Rotenone-Induced Mitochondrial Dysfunction in SH-SY5Y Cells
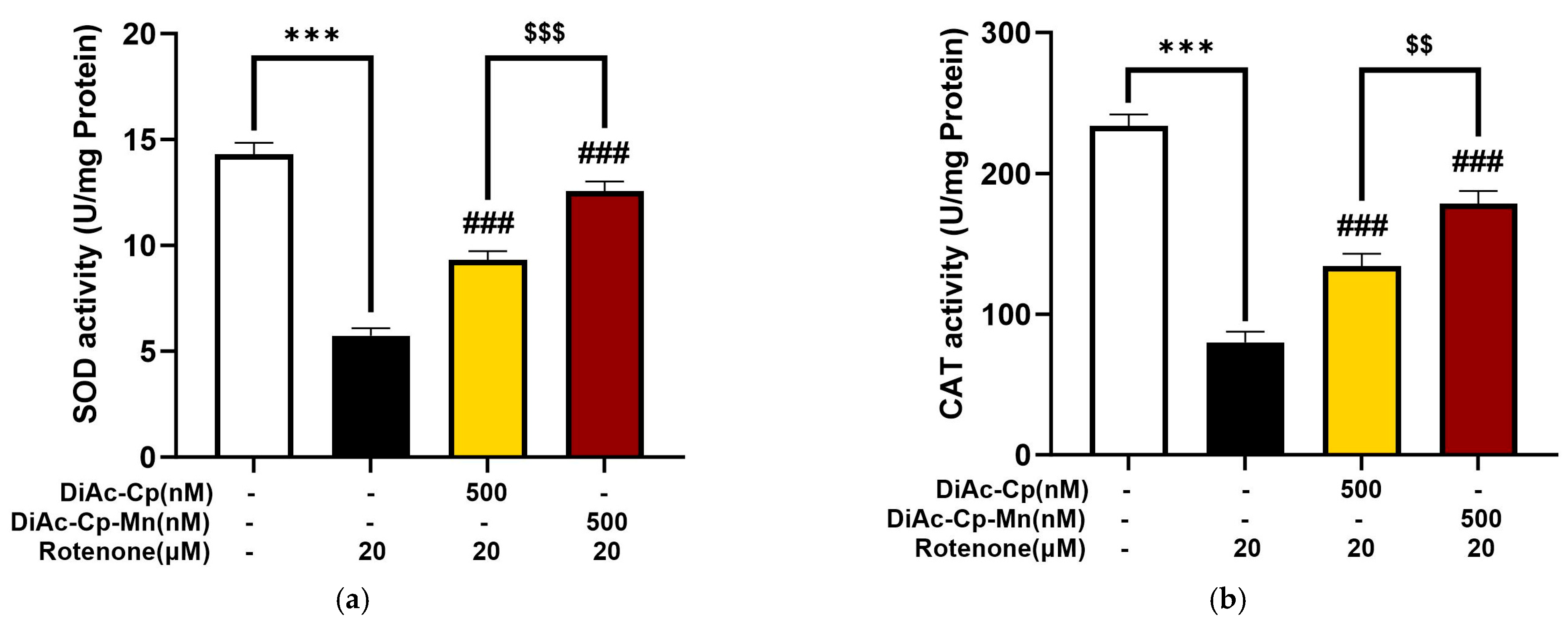
2.7. DiAc-Cp and DiAc-Cp-Mn Increased Antioxidant Enzyme Activity during Rotenone-Induced Oxidative Stress in SH-SY5Y Cells
2.8. Effect of DiAc-Cp and DiAc-Cp-Mn on Gene Expression in Rotenone-Induced SH-SY5Y Cells
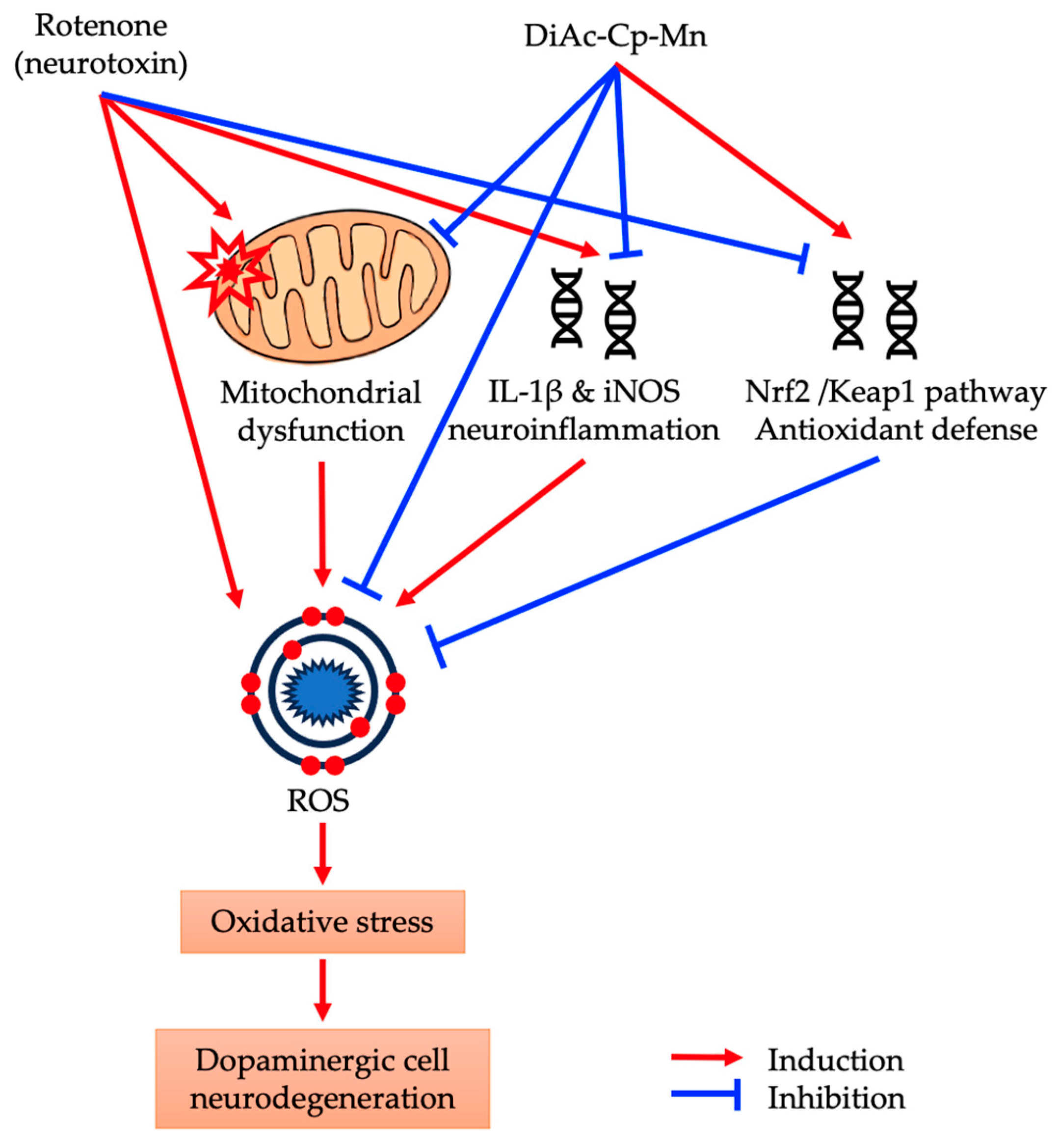
3. Discussion
4. Materials and Methods
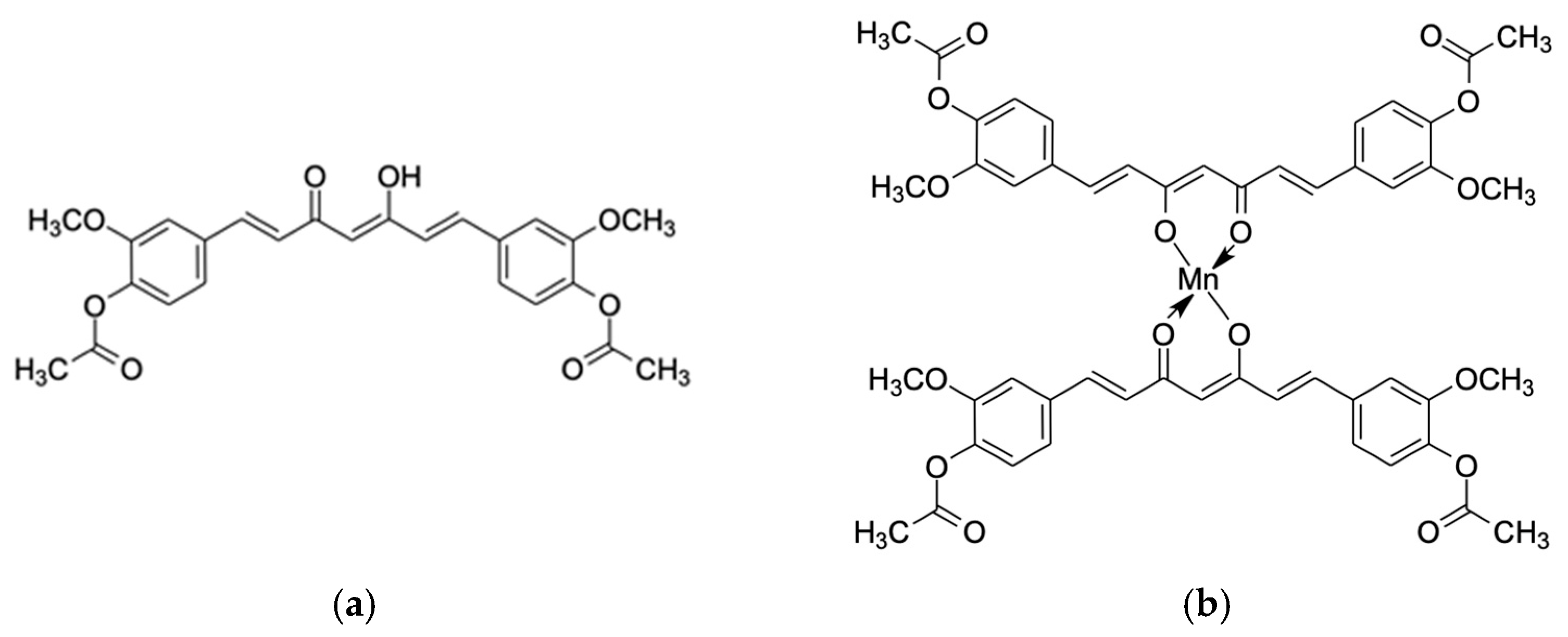
4.1. Synthesis of Diacetylcurcumin Manganese Complex (DiAc-Cp-Mn)
4.2. Superoxide Radical Scavenging Activity Assay
4.3. Hydroxyl Radical Scavenging Activity Assay
4.4. Hydrogen Peroxide Scavenging Activity Assay
4.5. Neuronal Cell Culture
4.6. Cell Viability Assay
4.6.1. Effect of DiAc-Cp and DiAc-Cp-Mn on Neuronal Cell Viability
4.6.2. Rotenone-Induced Cytotoxicity in SH-SY5Y Cells
4.6.3. DiAc-Cp and DiAc-Cp-Mn Treatment against Rotenone-Induced Neurotoxicity
4.7. Measurement of Intracellular Reactive Oxygen Species
4.8. Measurement of Mitochondrial Membrane Potential
4.9. Measurement of Antioxidant Enzyme Activities
4.10. Quantitative Real-Time Polymerase Chain Reaction (q-PCR)
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Desai, S.R.; Doke, R.R.; Pansare, P.A.; Sainani, S.R.; Bhalchim, V.M. Natural Products: An Emerging Tool in Parkinson’s Disease Therapeutics. IP Indian J. Neurosci. 2019, 5, 95–105. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, C.-H. Effect of Reactive Oxygen Species on the Endoplasmic Reticulum and Mitochondria during Intracellular Pathogen Infection of Mammalian Cells. Antioxidants 2021, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J.; Brand, M.D. Reactive Oxygen Species Production by Mitochondria. In Mitochondrial DNA; Stuart, J.A., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 554, pp. 165–181. ISBN 978-1-934115-60-2. [Google Scholar]
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health Benefits of Cyanidin-3-Glucoside as a Potent Modulator of Nrf2-Mediated Oxidative Stress. Inflammopharmacology 2021, 29, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Vijayanathan, Y.; Lim, S.M.; Tan, M.P.; Lim, F.T.; Majeed, A.B.A.; Ramasamy, K. Adult Endogenous Dopaminergic Neuroregeneration Against Parkinson’s Disease: Ideal Animal Models? Neurotox. Res. 2021, 39, 504–532. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Martínez, C.; Guzmán-Gutiérrez, S.L.; Carrillo-López, M.I.; Deveze-Álvarez, M.A.; Trujillo-Valdivia, A.; Meza-Morales, W.; Enríquez, R.G. Diacetylcurcumin: Its Potential Antiarthritic Effect on a Freund’s Complete Adjuvant-Induced Murine Model. Molecules 2019, 24, 2643. [Google Scholar] [CrossRef] [PubMed]
- Vajragupta, O.; Boonchoong, P.; Sumanont, Y.; Watanabe, H.; Wongkrajang, Y.; Kammasud, N. Manganese-Based Complexes of Radical Scavengers as Neuroprotective Agents. Bioorg. Med. Chem. 2003, 11, 2329–2337. [Google Scholar] [CrossRef]
- Sumanont, Y.; Murakami, Y.; Tohda, M.; Vajragupta, O.; Watanabe, H.; Matsumoto, K. Prevention of Kainic Acid-Induced Changes in Nitric Oxide Level and Neuronal Cell Damage in the Rat Hippocampus by Manganese Complexes of Curcumin and Diacetylcurcumin. Life Sci. 2006, 78, 1884–1891. [Google Scholar] [CrossRef]
- Sumanont, Y.; Murakami, Y.; Tohda, M.; Vajragupta, O.; Watanabe, H.; Matsumoto, K. Effects of Manganese Complexes of Curcumin and Diacetylcurcumin on Kainic Acid-Induced Neurotoxic Responses in the Rat Hippocampus. Biol. Pharm. Bull. 2007, 30, 1732–1739. [Google Scholar] [CrossRef]
- Vajragupta, O. Manganese Complexes of Curcumin and Its Derivatives: Evaluation for the Radical Scavenging Ability and Neuroprotective Activity. Free Radic. Biol. Med. 2003, 35, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Henrich, M.T.; Oertel, W.H.; Surmeier, D.J.; Geibl, F.F. Mitochondrial Dysfunction in Parkinson’s Disease—A Key Disease Hallmark with Therapeutic Potential. Mol. Neurodegener. 2023, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Cooper, J.M.; Dexter, D.; Jenner, P.; Clark, J.B.; Marsden, C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. Lancet 1989, 333, 1269. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.D.; Parks, J.K. Mitochondrial ND5 Mutations in Idiopathic Parkinson’s Disease. Biochem. Biophys. Res. Commun. 2005, 326, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.D.; Parks, J.K.; Swerdlow, R.H. Complex I Deficiency in Parkinson’s Disease Frontal Cortex. Brain Res. 2008, 1189, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez De La Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Borgstahl, G. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef]
- Sumanont, Y.; Murakami, Y.; Tohda, M.; Vajragupta, O.; Matsumoto, K.; Watanabe, H. Evaluation of the Nitric Oxide Radical Scavenging Activity of Manganese Complexes of Curcumin and Its Derivative. Biol. Pharm. Bull. 2004, 27, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Vajragupta, O.; Boonchoong, P.; Berliner, L.J. Manganese complexes of curcumin analogues: Evaluation of hydroxyl radical scavenging ability, superoxide dismutase activity and stability towards hydrolysis. Free Radic Res. 2004, 38, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-F.; Guo, F.; Cao, Y.-Z.; Shi, W.; Xia, Q. Neuroprotection by Manganese Superoxide Dismutase (MnSOD) Mimics: Antioxidant Effect and Oxidative Stress Regulation in Acute Experimental Stroke. CNS Neurosci. Ther. 2012, 18, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Meza-Morales, W.; Estévez-Carmona, M.M.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Cassani, J.; Ramírez-Apan, M.T.; Escobedo-Martínez, C.; Soriano-García, M.; Reynolds, W.F.; Enríquez, R.G. Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-Level Cytotoxicity In Vitro with Minimal Acute Toxicity In Vivo. Molecules 2019, 24, 1598. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Chen, Z.; Yao, R.; Xu, Z.; Xu, C.; He, F.; Pei, H.; Hao, C. SIRT1-Dependent Mitochondrial Biogenesis Supports Therapeutic Effects of Vidarabine against Rotenone-Induced Neural Cell Injury. Heliyon 2023, 9, e21695. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Lin, X.; Su, A.; Zhang, Y.; Xing, Z.; Mi, L.; Wei, T.; Li, Z.; Wu, W. Mitochondrial Dysfunction-Targeting Therapeutics of Natural Products in Parkinson’s Disease. Front. Pharmacol. 2023, 14, 1117337. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-A.; Ellis, A.C. Dietary Antioxidants and Parkinson’s Disease. Antioxidants 2020, 9, 570. [Google Scholar] [CrossRef]
- Zeng, X.-S.; Geng, W.-S.; Jia, J.-J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 175909141877743. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Yang, D.; Smith, W.W. Curcumin Protects against Rotenone-Induced Neurotoxicity in Cell and Drosophila Models of Parkinson’s Disease. Adv. Park. Dis. 2013, 2, 18–27. [Google Scholar] [CrossRef]
- Zhang, N.; Dou, D.; Ran, X.; Kang, T. Neuroprotective Effect of Arctigenin against Neuroinflammation and Oxidative Stress Induced by Rotenone. RSC Adv. 2018, 8, 2280–2292. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Zhao, S.; Ghosh, A.; Lo, C.-S.; Chenier, I.; Scholey, J.W.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.-L.; Chan, J.S.D. Nrf2 Deficiency Upregulates Intrarenal Angiotensin-Converting Enzyme-2 and Angiotensin 1–7 Receptor Expression and Attenuates Hypertension and Nephropathy in Diabetic Mice. Endocrinology 2018, 159, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Dasgupta, P.; Sinha Roy, D.; Palchoudhuri, S.; Chatterjee, I.; Ali, S.; Ghosh Dastidar, S. A Sensitive In Vitro Spectrophotometric Hydrogen Peroxide Scavenging Assay Using 1,10-Phenanthroline. Free Radic. Antioxid. 2016, 6, 124–132. [Google Scholar] [CrossRef]
- Fakhri, S.; Pesce, M.; Patruno, A.; Moradi, S.Z.; Iranpanah, A.; Farzaei, M.H.; Sobarzo-Sánchez, E. Attenuation of Nrf2/Keap1/ARE in Alzheimer’s Disease by Plant Secondary Metabolites: A Mechanistic Review. Molecules 2020, 25, 4926. [Google Scholar] [CrossRef] [PubMed]
- Celic, T.; Španjol, J.; Bobinac, M.; Tovmasyan, A.; Vukelic, I.; Reboucas, J.S.; Batinic-Haberle, I.; Bobinac, D. Mn Porphyrin-Based SOD Mimic, MnTnHex-2-PyP 5+, and Non-SOD Mimic, MnTBAP 3−, Suppressed Rat Spinal Cord Ischemia/Reperfusion Injury via NF-κB Pathways. Free Radic. Res. 2014, 48, 1426–1442. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, A.P. In Vitro Antioxidant and Free Radical Scavenging Activity of Nardostachys Jatamansi DC. J. Acupunct. Meridian Stud. 2012, 5, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Nartey, D.; Gyesi, J.N.; Borquaye, L.S. Chemical Composition and Biological Activities of the Essential Oils of Chrysophyllum albidum G. Don (African Star Apple). Biochem. Res. Int. 2021, 2021, 9911713. [Google Scholar] [CrossRef] [PubMed]
- Boonyarat, C.; Boonput, P.; Tongloh, N.; Kaewamatawong, R.; Chaiwiwatrakul, S.; Yenjai, C.; Waiwut, P. Nordentatin Inhibits Neuroblastoma Cell Proliferation and Migration through Regulation of GSK-3 Pathway. Curr. Issues Mol. Biol. 2022, 44, 1062–1074. [Google Scholar] [CrossRef]
- Ramkumar, M.; Rajasankar, S.; Gobi, V.V.; Dhanalakshmi, C.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M.M.; Kalandar, A.; Chidambaram, R. Neuroprotective Effect of Demethoxycurcumin, a Natural Derivative of Curcumin on Rotenone Induced Neurotoxicity in SH-SY 5Y Neuroblastoma Cells. BMC Complement. Altern. Med. 2017, 17, 217. [Google Scholar] [CrossRef]
- Feng, C.; Luo, T.; Zhang, S.; Liu, K.; Zhang, Y.; Luo, Y.; Ge, P. Lycopene Protects Human SH-SY5Y Neuroblastoma Cells against Hydrogen Peroxide-Induced Death via Inhibition of Oxidative Stress and Mitochondria-Associated Apoptotic Pathways. Mol. Med. Rep. 2016, 13, 4205–4214. [Google Scholar] [CrossRef]
- Chalermwongkul, C.; Khamphukdee, C.; Maneenet, J.; Daodee, S.; Monthakantirat, O.; Boonyarat, C.; Chotritthirong, Y.; Awale, S.; Kijjoa, A.; Chulikhit, Y. Antidepressant-like Effect of Oroxylum Indicum Seed Extract in Mice Model of Unpredictable Chronic Mild Stress. Nutrients 2023, 15, 4742. [Google Scholar] [CrossRef]
- Ganapasam, S.; Pandurangan, A.; Kumar, S.; Dharmalingam, P. Luteolin, a Bioflavonoid Inhibits Azoxymethane-Induced Colon Carcinogenesis: Involvement of iNOS and COX-2. Pharmacogn. Mag. 2014, 10, 306. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Piao, Y.; Nagaoka, K.; Watanabe, G.; Taya, K.; Li, C. Protective Effects of Nuclear Factor Erythroid 2-Related Factor 2 on Whole Body Heat Stress-Induced Oxidative Damage in the Mouse Testis. Reprod. Biol. Endocrinol. 2013, 11, 23. [Google Scholar] [CrossRef]


| Scavenging Activity Assay (IC50) | |||
|---|---|---|---|
| Sample | Superoxide Radical | Hydrogen Peroxide | Hydroxyl Radical |
| DiAc-Cp (µM) | 211.10 ± 4.27 | 218.02 ± 3.00 | 46.94 ± 1.65 |
| DiAc-Cp-Mn (µM) | 5.69 ± 0.05 | 85.57 ± 0.32 | 15.14 ± 0.19 |
| SOD (unit/mL) | 5.90 ± 0.20 | - | - |
| Trolox (µM) | - | 1118.18 ± 15.14 | 99.78 ± 14.26 |
| Treatment | Concentration (µM) | Duration (h) |
|---|---|---|
| DiAc-Cp/Rotenone | 0.1, 0.25, and 0.5/20.0 | 4/24 |
| DiAc-Cp-Mn/Rotenone | 0.1, 0.25, and 0.5/20.0 | 4/24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirunkaset, E.; Boonyarat, C.; Maneenet, J.; Khamphukdee, C.; Daodee, S.; Monthakantirat, O.; Awale, S.; Kijjoa, A.; Chulikhit, Y. Effect of Diacetylcurcumin Manganese Complex on Rotenone-Induced Oxidative Stress, Mitochondria Dysfunction, and Inflammation in the SH-SY5Y Parkinson’s Disease Cell Model. Molecules 2024, 29, 957. https://doi.org/10.3390/molecules29050957
Pirunkaset E, Boonyarat C, Maneenet J, Khamphukdee C, Daodee S, Monthakantirat O, Awale S, Kijjoa A, Chulikhit Y. Effect of Diacetylcurcumin Manganese Complex on Rotenone-Induced Oxidative Stress, Mitochondria Dysfunction, and Inflammation in the SH-SY5Y Parkinson’s Disease Cell Model. Molecules. 2024; 29(5):957. https://doi.org/10.3390/molecules29050957
Chicago/Turabian StylePirunkaset, Ekanong, Chantana Boonyarat, Juthamart Maneenet, Charinya Khamphukdee, Supawadee Daodee, Orawan Monthakantirat, Suresh Awale, Anake Kijjoa, and Yaowared Chulikhit. 2024. "Effect of Diacetylcurcumin Manganese Complex on Rotenone-Induced Oxidative Stress, Mitochondria Dysfunction, and Inflammation in the SH-SY5Y Parkinson’s Disease Cell Model" Molecules 29, no. 5: 957. https://doi.org/10.3390/molecules29050957
APA StylePirunkaset, E., Boonyarat, C., Maneenet, J., Khamphukdee, C., Daodee, S., Monthakantirat, O., Awale, S., Kijjoa, A., & Chulikhit, Y. (2024). Effect of Diacetylcurcumin Manganese Complex on Rotenone-Induced Oxidative Stress, Mitochondria Dysfunction, and Inflammation in the SH-SY5Y Parkinson’s Disease Cell Model. Molecules, 29(5), 957. https://doi.org/10.3390/molecules29050957










