Chemotherapeutic Activities of New η6-p-Cymene Ruthenium(II) and Osmium(II) Complexes with Chelating SS and Tridentate SNS Ligands
Abstract
1. Introduction
2. Results and Discussion
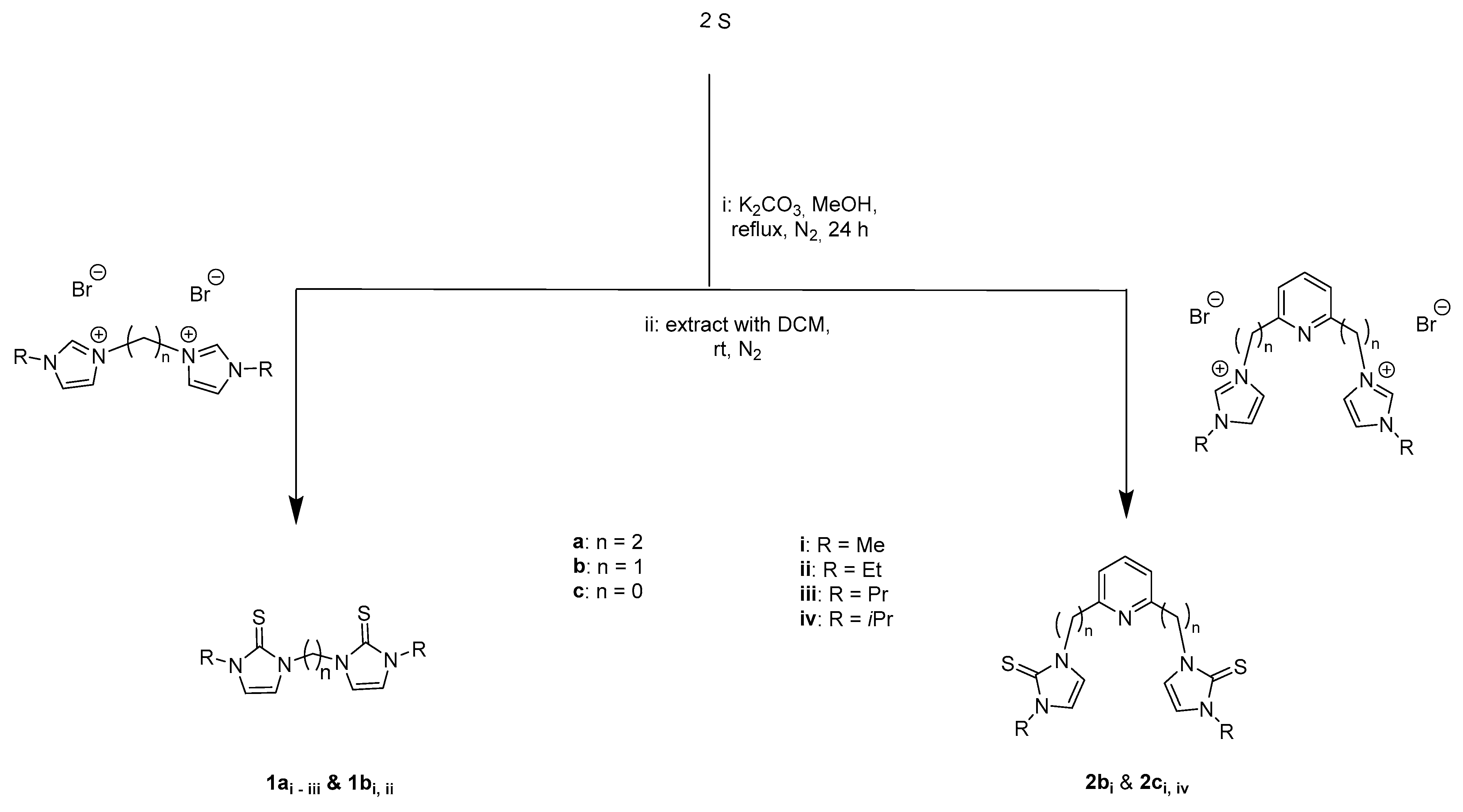
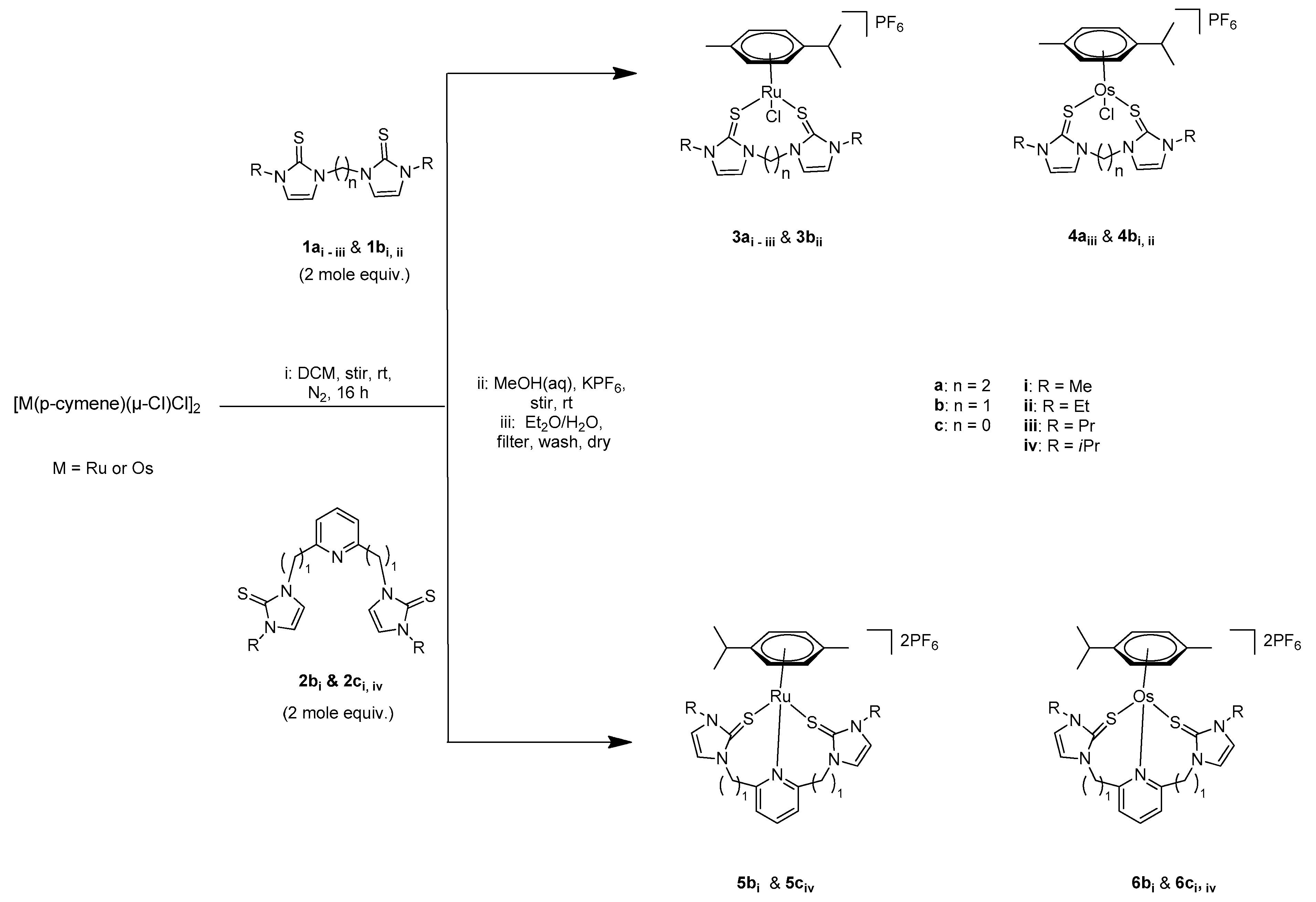
2.1. Synthesis and Characterization
2.2. Chemotherapeutic Activities of the Arene-Ru(II)/Os(II) Complexes
3. Material and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Imidazole-2-thione Ligands
- 3,3′-(ethane-1,2-diyl)bis(1-methyl-1H-imidazole-2(3H)-thione) (1ai). Colorless solid. Yield: 1.9 g (75%. m.p.: 195–197 °C). 1H NMR (400 MHz, CDCl3): δ 3.59 (s, 2NCH3, 6 H), 4.46 (s, NCH2-CH2N, 4 H), 6.58 (d, J = 8.2 Hz, imidazole, 4 H). 13C NMR (100 MHz, CDCl3): δ 35.2 (NCH3), 45.6 (NCH2-CH2N), 117.9 (imidazole), 162.4 (C=S) ppm. FTIR (solid state): υ(C=C, C=N) 1639, 1561 cm−1; υ(C-N, C=S) 1190 cm−1; υs/as(C=S, C=S) 670, 510 cm−1. ESI-HRMS (CH3CN): m/z found for [M + H]+: 255.0738; calculated: 255.0733.
- 3,3′-(ethane-1,2-diyl)bis(1-ethyl-1H-imidazole-2(3H)-thione) (1aii). Pale yellow solid. Yield: (1.84 g, 65%. m.p.: 131–133 °C). 1H NMR (400 MHz, CDCl3): δ 1.34 (t, J = 7.3 Hz, 2NCH2CH3, 6H), 4.06 (m, 2NCH2CH3, 4H), 4,48 (s, NCH2-CH2N, 4H), 6.58 (d, J = 14.0 Hz, imidazole, 4H). 13C NMR (100 MHz, CDCl3): δ 14.3 (NCH2CH3), 42.9 (NCH2CH3), 45.4 (NCH2-CH2N), 115.9, 118.1 (imidazole), 161.6 (C=S). FTIR (solid state): υ(=C-H) 3154, 3117, 3084 cm−1; υ(CH3) 2981, 2940 cm−1; υ(C=C, C=N) 1643, 1563 cm−1; υ(C-N, C=S) 1177 cm−1; υs/as(C=S, C=S) 670, 514 cm−1. ESI-HRMS (CH3CN): m/z found for [M + H]+: 283.1049; calculated: 283.1051. ESI-HRMS (CH3CN): m/z found for [M + H]+: 283.1049; calculated: 283.1046.
- 3,3′-(ethane-1,2-diyl)bis(1-propyl-1H-imidazole-2(3H)-thione) (1aiii). Orange solid. Yield: (1.86 g, 60%. m.p.: 91–92 °C). 1H NMR (400 MHz, CDCl3): δ 0.92 (t J = 7.4 Hz, 2NCH2CH2CH3, 6H), 1.77 (m, 2NCH2CH2CH3, 4H), 3.96 (t, J = 7.3 Hz, 2NCH2CH2CH3, 4H), 4.49 (s, NCH2-CH2N, 4H), 6.54 (d, J = 11.2 Hz, imidazole, 4H). 13C NMR (100 MHz, CDCl3): δ 11.1 (NCH2CH2CH3), 22.3 (NCH2CH2CH3), 45.3 (NCH2CH2CH3), 49.5 (NCH2-CH2N) 116.7, 117.9 (imidazole), 161.8 (C=S). FTIR (solid state): υ(=C-H) 3163, 3127, 3097 cm−1; υ(CH3) 2959, 2933 cm−1; υ(C=C, C=N) 1641, 1564, 1526 cm−1, υ(C-N, C=S) 1179 cm−1; υs/as(C=S, C=S) 665, 532 cm−1. ESI-HRMS (CH3CN): m/z found for [M + H]+: 311.1364; calculated: 311.1364.
- 3,3′-(methane-1,1-diyl)bis(1-methyl-1H-imidazole-2(3H)-thione) (1bi). Pale yellow solid. Yield: 2.02 g, 84%. m.p.: 194–197 °C). 1H NMR (400 MHz, CDCl3): δ 3.56 (s, 2NCH3, 6H), 6.30 (s, NCH2N, 2H), 6.59 (d, J = 2.1 Hz, imidazole, 2H), 7.59 (d, J = 2.1, imidazole, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ 35.3 (NCH3), 56.2 (NCH2N), 117.8, 118.7 (imidazole), 163.9 (C=S). FTIR (solid state): υ(C=C, C=N) 1654, 1569 cm−1; υ(C-N, C=S) 1162 cm−1; υs/as(C=S, C=S) 968, 671, 521 cm−1. ESI-HRMS (CH3CN): m/z found for [M + H]+: 241.0592; calculated: 241.0537.
- 3,3′-(methane-1,1-diyl)bis(1-ethyl-1H-imidazole-2(3H)-thione) (1bii). Colorless solid. Yield: (1.88 g 70%. m.p.: 175–177 °C). 1H NMR (400 MHz, CDCl3): δ 1.35 (t, J = 7.3 Hz, 2NCH2CH3, 6H), 4.05 (m, 2NCH2CH3, 4H), 6.33 (s, NCH2N, 2H), 6.62 (d, J = 2.3 Hz, imidazole, 2H), 7.64 (d, J = 2.3 Hz, imidazole, 2H). 13C NMR (100 MHz, CDCl3): δ 14.1 (NCH2CH3), 42.9 (NCH2CH3), 55.8 (NCH2N), 116.0, 116.9 (imidazole), 162.9 (C=S). FTIR (solid state): υ(=C-H) 3109, 3080 cm−1; υ(CH3) 2989, 2945 cm−1; υ(C=C, C=N) 1681, 1566 cm−1; υ(C-N, C=S) 1070 cm−1; υs/as(C=S, C=S) 715, 515 cm−1. ESI-HRMS (CH3CN): m/z found for [M + H]+: 269.0904; calculated: 269.0850.
- 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-methyl-1H-imidazole-2(3H)-thione) (2bi). Pale yellow solid. Yield: (2.32 g, 70%. m.p.: 190–193 °C). 1H NMR (400 MHz, CDCl3): δ 3.59 (s, 2CH3, 6H), 5.33 (s, NCH2py, 4 H), 6.67 (d, J = 1.7 Hz, imidazole, 2H), 6.81 (d, J = 1.4 Hz, imidazole, 2H), 7.2 (d, J = 7.8 Hz, py, 2H), 7.59 (t, J = 7.7 Hz, py 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 35.4 (CH3), 52.6 (NCH2py), 117.3, 118.0 (imidazole), 122.1, 138.3, 155.3 (py) 163.1 (C=S). FTIR (solid state): υ(C=C, C=N) 1666, 1570 cm−1; υ(C-N, C=S) 1140, 772 cm−1; υs/as(C=S, C=S) 723, 527 cm−1. ESI-HRMS (CH3CN): m/z found for [M + H]+: 332.1013; calculated: 332.0959.
- 3,3′-(pyridine-2,6-diyl)bis(1-methyl-1H-imidazole-2(3H)-thione) (2ci). Colorless solid. Yield: (1.91 g 63%. m.p.: 270–273 °C). 1H NMR (400 MHz, CDCl3): δ 3.66 (s, 2CH3, 6H), 6.79 (s, imidazole, 2H), 7.48 (s, imidazole, 2H), 8.02 (t, J = 8.1 Hz, pyridine, 1H), 8.90 (d, J = 8.0 Hz, pyridine, 2H). 13C NMR (100 MHz, CDCl3): δ 35.3 (CH3), 115.9 (imidazole), 116.7 (imidazole), 118.7 (pyridine), 140.0 (pyridine), 148.6 (pyridine), 162.9 (C=S) ppm. FTIR (solid state): υ(C=C, C=N) 1688, 1577 cm−1; υ(C-N, C=S) 1084 cm−1, υs/as(C=S, C=S) 791, 546 cm−1. ESI-HRMS (CH3CN): m/z found for [M + H]+: 304.0686; calculated: 304.0691.
- 3,3′-(pyridine-2,6-diyl)bis(1-isopropyl-1H-imidazole-2(3H)-thione) (2civ). Colorless solid. Yield: (2.69 g 75%. m.p.: 189–190 °C). 1H NMR (400 MHz, CDCl3): δ 1.39 (d, J = 6.7 Hz, 2(CH3)2CH), 12H), 5.26 (m, 2(CH3)2CH, 2H), 6.86 (s, imidazole, 2H), 7.50 (s, imidazole, 2H), 8.02 (t, J = 7.9 Hz, pyridine, 1H), 8.86 (d, J = 8.0 Hz, pyridine, 2H). 13C NMR (100 MHz, CDCl3): δ 21.8 ((CH3)2CH), 48.7 ((CH3)2CH), 113.8 (pyridine), 116.8 (imidazole), 117.6 (imidazole), 139.7 (pyridine), 148.6 (pyridine), 161.6 (C=S) ppm. FTIR (solid state): υ(C=C, C=N) 1669, 1599 cm−1; υ(C-N, C=S) 1571, 1127 cm−1, υs(C=S)+υas(C=S) 783, 666, 527 cm−1. ESI-HRMS (CH3CN): m/z found for [M + H]+: 360.1313; calculated: 360.1317.
3.3. General Procedure for the Synthesis of the Chelating Bidentate (SS) Alkylimidazole-2-thione-Ru(II)/Os(II) Complexes and the Tridentate (SNS) Pyridine-2,6-diylimidazole-2-thione-Ru(II)/Os(II) Complexes
- [(η6-cymene)Ru(L)Cl]PF6 (3ai: L = 1ai). Dark brown solid. Yield: (49.01 mg, 73%. m.p.: 187–188 °C). 1H NMR (400 MHz, CDCl3): δ 1.32 (d, J = 6.9 Hz, (CH3)2CHC6H4(CH3)-p), 6H), 2.23 (s, (CH3)2CHC6H4(CH3)-p), 3H), 2.88 (m, (CH3)2CHC6H4(CH3)-p), 1H), 3.59 (s, 2NCH3, 6H), 4.35, 5.23 (m, NCH2-CH2N, 4H), 5.70, 5.83 (d, J = 6.1 Hz, (CH3)2CHC6H4(CH3)-p), 4H), 7.20, 7.25 (d, J = 2.0 Hz, imidazole, 4H). 13C NMR (100 MHz, CDCl3): δ 19.0 (CH3)2CHC6H4(CH3)-p), 22.8 (CH3)2CHC6H4(CH3)-p), 31.7 (CH3)2CHC6H4(CH3)-p), 37.1 (NCH3), 48.4 (NCH2-CH2), 86.9, 88.7, 105.0, 107.6 (CH3)2CHC6H4(CH3)-p), 123.7, 123.8 (imidazole), 154.0 (C=S). FTIR (solid state): υ(C=C, C=N) 1567 cm−1; υ(C-N, C=S) 1200 cm−1; υ(P-F) 824 cm−1; υs/as(C=S, C=S) 739, 685, 555 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF6]+: 525.0483; calculated: 525.0377.
- [(η6-cymene)Ru(L)Cl]PF6 (3aii: L = 1aii). Dark brown solid. Yield: (46.01 mg, 66%. m.p.: 146–147 °C). 1H NMR (400 MHz, CD3CN): δ 1.33 (m, 2NCH2CH3, CH3)2CHC6H4(CH3)-p, 12H), 2.16 (s, CH3)2CHC6H4(CH3)-p, 3H), 2.88 (m, CH3)2CHC6H4(CH3)-p, 1H), 3.87–4.38 (m, 2NCH2CH3, NCH2-CH2N, 8H), 5.32 (d, J = 5.7 Hz, CH3)2CHC6H4(CH3)-p, 1H), 5.49 (d, J = 5.7 Hz, CH3)2CHC6H4(CH3)-p, 1H), 5.69 (d, J = 6.0, CH3)2CHC6H4(CH3)-p, 1H), 5.82 (d, J = 6.0 Hz, CH3)2CHC6H4(CH3)-p, 1H), 7.05 (d, J = 21.6 Hz, imidazole, 2H), 7.25 (d, J = 9.4 Hz, imidazole, 2H). 13C NMR (100 MHz, CD3CN): δ 14.8 (NCH2CH3), 18.9 (CH3)2CHC6H4(CH3)-p), 22.9 (CH3)2CHC6H4(CH3)-p), 31.6 (CH3)2CHC6H4(CH3)-p), 45.2 (NCH2CH3), 47.7 (NCH2-CH2N), 84.5, 85.3, 101.2, 104.0 (CH3)2CHC6H4(CH3)-p), 120.7, 122.3 (imidazole), 155.2 (C=S) ppm. FTIR (solid state): υ(C=C, C=N) 1565 cm−1; υ(C-N, C=S) 1150 cm−1; υ(P-F) 830 cm−1, υs/as(C=S, C=S) 736, 679, 556 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF6]+: 553.0801; calculated: 553.0800.
- [(η6-cymene)Ru(L)Cl]PF6 (3aiii: L = 1aiii). Dark brown solid. Yield: (51 mg, 70%. m.p.: 111–113 °C). 1H NMR (400 MHz, CDCl3): δ 0.91 (t, J = 7.4 Hz, 2NCH2CH2CH3, 6H), 1.32 (d, J = 6.9 Hz, (CH3)2CHC6H4(CH3)-p), 6H), 1.76 (m, 2NCH2CH2CH3, 4H), 2.30 (CH3)2CHC6H4(CH3)-p), 3H), 2.82 (m, (CH3)2CHC6H4(CH3)-p), 1H), 3.78 (m, NCH2CH2CH3, 2H), 4.05 (m, NCH2CH2CH3, 2H), 4.47 (m, NCH2-CH2N, 2H), 5.33 (m, NCH2-CH2N, 2H), 2H), 5.87 (d, J = 5.8 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.02 (d, J = 5.8 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 7.23 (d, J = 2.1 Hz, imidazole, 2H), 7.33 (d, J = 2.1 Hz, imidazole, 2H). 13C NMR (100 MHz, CDCl3): δ 11.4 (NCH2CH2CH3), 18.8 (CH3)2CHC6H4(CH3)-p), 23.1 (NCH2CH2CH3), 24.0 (CH3)2CHC6H4(CH3)-p), 31.6 (CH3)2CHC6H4(CH3)-p), 49.0 (NCH2CH2CH3), 51.5 (NCH2-CH2N), 79.3, 81.5, 97.8, 100.4 (CH3)2CHC6H4(CH3)-p), 122.8, 124.6 (imidazole), 152.3 (C=S). FTIR (solid state): υ(C=C, C=N) 1564 cm−1; υ(C-N, C=S) 1115 cm−1, 685; υ(P-F) 823 cm−1; υs/as(C=S, C=S) 738, 555 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF6]+: 581.1107; calculated: 581.1003.
- [(η6-cymene)Ru(L)Cl]PF6 (3bii: L = 1bii). Red brown solid. Yield: (35 mg, 51%. m.p.: 199–201 °C). 1H NMR (400 MHz CD3CN): δ 1.29 (d, J = 6.9 Hz, (CH3)2CHC6H4(CH3)-p), 6H), 1.39 (t, J = 7.8 Hz, 2NCH2CH3, 6H), 2.13 (s, (CH3)2CHC6H4(CH3)-p), 3H), 2.90 (m, (CH3)2CHC6H4(CH3)-p), 1H), 4.08 (m, 2NCH2CH3, 4H), 5.33 (d, J = 5.8 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 5.51 (d, J = 5.8 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.16 (dd, J = 264.3 Hz, NCH2N, 2H), 7.16 (d, J = 2.0 Hz, imidazole, 2H), 7.43 (d, J = 2.0 Hz, imidazole, 2H). 13C NMR (100 MHz, CD3CN): δ 15.1 (NCH2CH3), 19.0 (CH3)2CHC6H4(CH3)-p), 22.9 (CH3)2CHC6H4(CH3)-p), 31.2 (CH3)2CHC6H4(CH3)-p), 45.5 (NCH2CH3), 58.4 (NCH2N), 84.7, 85.0, 101.3, 104.2 (CH3)2CHC6H4(CH3)-p), 120.9, 121.7 (imidazole), 158.3 (C=S). FTIR (solid state): υ(C=C, C=N) 1571 cm−1; υ(C-N, C=S) 1059 cm−1; υ(P-F) 826 cm−1; υs/as(C=S, C=S) 743, 554 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF6]+: 539.0646; calculated: 539.0644.
- [(η6-cymene)Ru(L)]PF6 (5civ: L = 2civ). Dark yellow solid. Yield: (58 mg, 65%. m.p.: 217–219 °C). 1H NMR (400 MHz, CDCl3): δ (d, J = 6.9 Hz, (CH3)2CHC6H4(CH3)-p), 6H), 1.50–1.74 (m, (CH3)2CHC6H4(CH3)-p), 2NCH(CH3)2, 15H), 2.78 (m, (CH3)2CHC6H4(CH3)-p), 1H), 5.22 (2NCH(CH3)2, 2H), 5.32 (d, J = 6.1 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 5.62 (d, J = 6.0 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 7.55 (d, J = 2.5 Hz, pyridine, 2H), 7.68 (m, imidazole, 4H), 8.35 (t, J = 8.1 Hz, pyridine) ppm. 13C NMR (100 MHz, CDCl3): δ 19.1 (CH3)2CHC6H4(CH3)-p), 22.2 (CH3)2CHC6H4(CH3)-p), 22.97 (NCH(CH3)2), 32.1 (CH3)2CHC6H4(CH3)-p), 53.4 (NCH(CH3)2, 88.5, 89.8, 106.4, 106.6 (CH3)2CHC6H4(CH3)-p), 121.0 (pyridine), 122.0, 123.9 (imidazole), 146.2, 149.5 (pyridine), 158.3 (C=S) ppm. FTIR (solid state): υ(C=C, C=N) 1609, 1580 cm−1; υ(C-N, C=S) 1155, 680 cm−1; υ(P-F) 830 cm−1, υs/as(C=S, C=S) 739, 555 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF6]+: 740.1014; calculated: 740.1019; ESI-HRMS (CH3CN): m/z found for [M + Li]+: 892.0860; calculated: 892.0821.
- [(η6-cymene)Ru(L)]PF6 (5bi: L = 2bi). Dark brown solid. Yield: (51 mg, 60%. m.p.: 196–198 °C). 1H NMR (400 MHz, CDCl3): δ 1.27 (d, J = 6.9 Hz, (CH3)2CHC6H4(CH3)-p), 6H), 1.69(s, (CH3)2CHC6H4(CH3)-p), 3H), 2.90 (m, (CH3)2CHC6H4(CH3)-p), 1H), 3.57 (s, 2NCH3, 6H), 5.25 (d, J = 14.9 Hz, NCH2, 2H), 5.79 (d, J = 6.0 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.06 (d, J = 6.0 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.28 (d, J = 14.8 Hz, NCH2, 2H), 6.96 (d, J = 1.9 Hz, imidazole, 2H), 7.04 (d, J = 1.9 Hz, imidazole, 2H), 7.91 (d, J = 7.6 Hz, pyridine, 2H), 8.11 (t, J = 7.7 Hz, pyridine, 1H). 13C NMR (100 MHz, CDCl3): δ 18.3 (CH3)2CHC6H4(CH3)-p), 22.5, (CH3)2CHC6H4(CH3)-p), 31.2 (CH3)2CHC6H4(CH3)-p), 36.7 (NCH3), 57.5 (NCH3), 89.5, 105.1, 108.1 (CH3)2CHC6H4(CH3)-p), 119.6, 123.9 (imidazole), 129.9, 143.3, 153.0 (pyridine), 161.5 (C=S). FTIR (solid state): υ(C=C, C=N) 1569 cm−1; υ(C-N, C=S) 1145, 699 cm−1; υ(P-F) 826 cm−1, υs/as(C=S, C=S) 739, 555 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF6−2CH3]2+: 684.0386; calculated: 684.0393, for [M−2PF6−2CH3]2+: 538.0687 calculated: 539.0751.
- [(η6-cymene)Os(L)Cl]PF6 (4aiii: L = 1aiii). Yellow solid. Yield: (55 mg, 68%. m.p.: 125–127 °C). 1H NMR (400 MHz, CDCl3): δ 0.91 (t, J = 7.4 Hz, 2NCH2CH2CH3, 6H), 1.32 (d, J = 6.9 Hz, (CH3)2CHC6H4(CH3)-p), 6H), 1.76 (m, 2NCH2CH2CH3, 4H), 2.30 (CH3)2CHC6H4(CH3)-p), 3H), 2.82 (m, (CH3)2CHC6H4(CH3)-p), 1H), 3.78 (m, NCH2CH2CH3, 2H), 4.05 (m, NCH2CH2CH3, 2H), 4.47 (m, NCH2-CH2N, 2H), 5.33 (m, NCH2-CH2N, 2H), 2H), 5.87 (d, J = 5.8 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.02 (d, J = 5.8 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 7.23 (d, J = 2.1 Hz, imidazole, 2H), 7.33 (d, J = 2.1 Hz, imidazole, 2H). 13C NMR (100 MHz, CDCl3): δ 11.4 (NCH2CH2CH3), 18.8 (CH3)2CHC6H4(CH3)-p), 23.1 (NCH2CH2CH3), 24.0 (CH3)2CHC6H4(CH3)-p), 31.6 (CH3)2CHC6H4(CH3)-p), 49.0 (NCH2CH2CH3), 51.5 (NCH2-CH2N), 79.3, 81.5, 97.8, 100.4 (CH3)2CHC6H4(CH3)-p), 122.8, 124.6 (imidazole), 152.3 (C=S). FTIR (solid state): υ(C=C, C=N) 1566 cm−1; υ(C-N, C=S) 1113, 691 cm−1; υ(P-F) 827 cm−1; υs/as(C=S, C=S) 739, 556 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF6−Cl]+: 635.1862; calculated: 635.4136.
- [(η6-cymene)Os(L)Cl]PF6 (4bi: L = 1bi). Yellow solid. Yield: (47 mg, 63%. m.p.: 158–160 °C). 1H NMR (400 MHz CDCl3): δ 1.29 (d, J = 6.9 Hz, (CH3)2CHC6H4(CH3)-p), 6H), 2.29 (s, (CH3)2CHC6H4(CH3)-p), 3H), 2.87 (m, (CH3)2CHC6H4(CH3)-p), 1H), 3.73 (s, 2NCH3, 6H), 5.84 (d, J = 5.7 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.01 (d, J = 5.7 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.01 (d, J = 5.7 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.15 (d, J = 14.1 Hz, NCH2N, 1H), 6.57 (d, J = 14.0 Hz, NCH2N, 1H), 7.34 (d, J = 1.8 Hz, imidazole, 2H), 7.63 (d, J = 1.8 Hz, imidazole, 2H). 13C NMR (100 MHz, CDCl3): δ 18.8 (CH3)2CHC6H4(CH3)-p), 23.1 (CH3)2CHC6H4(CH3)-p), 31.6 (CH3)2CHC6H4(CH3)-p), 37.6 (NCH3), 59.7 (NCH2N), 78.9, 80.7, 97.8, 101.0 (CH3)2CHC6H4(CH3)-p), 122.2, 125.1 (imidazole), 156.5 (C=S) ppm. FTIR (solid state): υ(C=C, C=N) 1572 cm−1; υ(C-N, C=S) 1098 cm−1; υ(P-F) 820 cm−1; υs/as(C=S, C=S) 751, 555 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF6]+: 601.0878; calculated: 601.0902.
- [(η6-cymene)Os(L)Cl]PF6 (4bii: L = 1bii). Yellow solid. Yield: (43 mg, 56%. m.p.: 161–163 °C). 1H NMR (400 MHz, CDCl3): δ 1.28–1.41 (m, 2NCH2CH3, (CH3)2CHC6H4(CH3)-p), 12H), 2.27 (s (CH3)2CHC6H4(CH3)-p), 3H), 2.84 (m, (CH3)2CHC6H4(CH3)-p), 1H), 4.00–4.26 (m. 2NCH2CH3, 4H), 5.84–5.88 (m, (CH3)2CHC6H4(CH3)-p), 2H), 6.02 (d, J = 5.7 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.16 (d, J = 14.0 Hz, NCH2N, 1H), 6.58 (d, J = 14.1 Hz, NCH2N, 1H), 7.18 (d, J = 1.9 Hz, imidazole, 1H), 7.41 (d, J = 1.9 Hz, imidazole, 1H), 7.50 (d, J = 2.0 Hz, imidazole, 1H), 7.68 (d, J = 1.9 Hz, imidazole, 1H). 13C NMR (100 MHz, CDCl3): δ 15.6 (NCH2CH3), 18.8 (CH3)2CHC6H4(CH3)-p), 23.0 (CH3)2CHC6H4(CH3)-p), 31.5 (CH3)2CHC6H4(CH3)-p), 46.2 (NCH2CH3), 59.6 (NCH2N), 79.3, 81.0, 97.8, 100.8 (CH3)2CHC6H4(CH3)-p), 122.8, 123.5 (imidazole), 155.5 (C=S). FTIR (solid state): υ(C=C, C=N) 1572 cm−1; υ(C-N, C=S) 1090 cm−1; υ(P-F) 823 cm−1; υs/as(C=S, C=S) 769, 555 cm−1. ESI-HRMS (CH3CN): m/z found for [(M−PF6−Cl)]2+: 593.1472; calculated: 594.1527.
- [(η6-cymene)Os(L)]PF6 (6ci: L = 2ci). Yellow solid. Yield: (62 mg, 68%. m.p.: 259–260 °C). 1H NMR (400 MHz, CDCl3): δ 1.20 (d, J = 6.9 Hz, (CH3)2CHC6H4(CH3)-p), 6H), 1.83 (CH3)2CHC6H4(CH3)-p), 3H), 2.69 (m, (CH3)2CHC6H4(CH3)-p), 1H), 3.88 (s, 2NCH3, 6H), 5.76 (d, J = 5.8 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.00 (d, J = 5.8 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 7.39 (d, J = 2.4 Hz, imidazole, 2H), 7.60 (d, J = 2.4 Hz, imidazole, 2H), 7.66 (d, J = 7.9 Hz, pyridine, 2H), 8.25 (t, J = 8.2 Hz, pyridine, 1H). 13C NMR (100 MHz, CDCl3): δ 19.2 (CH3)2CHC6H4(CH3)-p), 23.0 (CH3)2CHC6H4(CH3)-p), 32.1 (CH3)2CHC6H4(CH3)-p), 37.2 (NCH3), 80.6, 81.9, 98.8, 99.4 (CH3)2CHC6H4(CH3)-p), 120.9, 123.5 (imidazole), 125.8, 146.6, 149.3 (pyridine), 159.2 (C=S). FTIR (solid state): υ(C=C, C=N) 1607 cm−1; υ(C-N, C=S) 1158, 1096 cm−1; υ(P-F) 822 cm−1; υs/as(C=S, C=S) 722, 554 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF6]+: 772.0970; calculated: 774.0964.
- [(η6-cymene)Os(L)]PF6 (6civ: L = 2civ). Yellow solid. Yield: (60 mg, 62%. m.p.: 209–210 °C). 1H NMR (400 MHz, CDCl3): δ 1.20 (d, J = 6.9 Hz, (CH3)2CHC6H4(CH3)-p), 6H), 1.48 (d, J = 6.7 Hz, NCH(CH3)2, 6H), 1.69 (d, J = 6.7 Hz, NCH(CH3)2, 6H), 1.83 (s, (CH3)2CHC6H4(CH3)-p), 3H), 2.69 (m, (CH3)2CHC6H4(CH3)-p), 1H), 5.11 (m, 2NCH(CH3)2, 2H), 5.70(d, J = 5.7 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 5.92 (d, J = 5.7 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 7.52 (d, J = 2.4 Hz, pyridine, 2H), 7.66 (m, imidazole, 4H), 8.27 (t, J = 8.2 Hz, pyridine). 13C NMR (100 MHz, CDCl3): δ 19.3 (CH3)2CHC6H4(CH3)-p), 22.2 (CH3)2CHC6H4(CH3)-p), 22.9 (NCH(CH3)2, 32.0 (CH3)2CHC6H4(CH3)-p), 53.4 (NCH(CH3)2, 80.8, 82.2, 98.5, 99.5 (CH3)2CHC6H4(CH3)-p), 121.2 (pyridine), 122.2, 123.9 (imidazole), 146.5, 149.2 (pyridine), 157.9 (C=S). FTIR (solid state): υ(C=C, C=N) 1609, 1580 cm−1; υ(C-N, C=S) 1153, 734 cm−1; υ(P-F) 827 cm−1, υs/as(C=S, C=S) 680, 556 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF6]+: 830.1597; calculated: 830.1590.
- [(η6-cymene)Os(L)]PF6 (6bi: L = 2bi). Yellow solid. Yield: (57 mg, 60%. m.p.: 275–277 °C). 1H NMR (400 MHz, CDCl3): δ 1.27 (d, J = 6.8 Hz, (CH3)2CHC6H4(CH3)-p), 1.78 (s, (CH3)2CHC6H4(CH3)-p), 3H), 2.83 (m, (CH3)2CHC6H4(CH3)-p), 1H), 3.59 (s, 2NCH3, 6H), 5.22 (d, J = 14.9 Hz, NCH2, 2H), 6.07 (d, J = 5.6 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.19 (d, J = 14.9 Hz, NCH2, 2H), 6.37 (d, J = 5.6 Hz, (CH3)2CHC6H4(CH3)-p), 2H), 6.99 (d, J = 1.7 Hz, imidazole, 2H), 7.07 (d, J = 1.8 Hz, imidazole, 2H), 7.88 (d, J = 7.7 Hz, pyridine, 2H), 8.02 (t, J = 7.7 Hz, pyridine, 1H). 13C NMR (100 MHz, CDCl3): δ = 18.1 (CH3)2CHC6H4(CH3)-p), 22.6 (CH3)2CHC6H4(CH3)-p), 31.1 (CH3)2CHC6H4(CH3)-p), 36.8 (NCH3), 58.3 (NCH2), 81.8, 97.5, 101.8, 120.0 (CH3)2CHC6H4(CH3)-p), 123.6, 129.8 (imidazole), 139.5, 143.5, 152.3 pyridine, 161.6 (C=S). FTIR (solid state): υ(C=C, C=N) 1606 cm−1; υ(C-N, C=S) 1162 cm−1; υ(P-F) 823 cm−1, υs/as(C=S, C=S) 722, 555 cm−1. ESI-HRMS (CH3CN): m/z found for [M−PF−2CH3]2+: 774.1000; calculated: 774.0964.
3.4. X-ray Crystallography
3.5. In Vitro Anti-Cancer Activity
3.5.1. Chemicals
3.5.2. MTT Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, I.; Lone, M.N.; Aboul-Enein, H.Y. Imidazoles as Potential Anticancer Agents. Medchemcomm 2017, 8, 1742–1773. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gust, R. Update on Metal N-Heterocyclic Carbene Complexes as Potential Anti-Tumor Metallodrugs. Coord. Chem. Rev. 2016, 329, 191–213. [Google Scholar] [CrossRef]
- Tong, K.K.H.; Hanif, M.; Lovett, J.H.; Hummitzsch, K.; Harris, H.H.; Söhnel, T.; Jamieson, S.M.F.; Hartinger, C.G. Thiourea-Derived Chelating Ligands and Their Organometallic Compounds: Investigations into Their Anticancer Activity. Molecules 2020, 25, 3661. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.G.; Huang, Y.B.; Lin, Y.J.; Wang, G.L.; Jin, G.X. Nickel Complexes and Cobalt Coordination Polymers with Organochalcogen (S, Se) Ligands Bearing an N-Methylimidazole Moiety: Syntheses, Structures, and Properties. Eur. J. Inorg. Chem. 2008, 2008, 4063–4073. [Google Scholar] [CrossRef]
- Williams, D.J.; Jones, R.L.; Menaldino, D.S. Main Group Metal Halide Complexes with Sterically Hindered Thioureas XI. Complexes of Antimony(II1) and Bismuth(II1) Chlorides with a New Bidentate Thiourea-1,l’-Methylenebis(3-Methyl-2H-Imidazole-2-Thione). Inorg. Chim. Acta 1989, 165, 173–178. [Google Scholar] [CrossRef]
- Jia, W.G.; Huang, Y.B.; Jin, G.X. Synthesis, Characterization of Novel Half-Sandwich Iridium and Rhodium Complexes Containing Pyridine-Based Organochalcogen Ligands. J. Organomet. Chem. 2009, 694, 4008–4013. [Google Scholar] [CrossRef]
- Slivarichova, M.; Correa da Costa, R.; Nunn, J.; Ahmad, R.; Haddow, M.F.; Sparkes, H.A.; Gray, T.; Owen, G.R. Two Synthetic Routes to Bis(1-Methyl-Imidazole-2-Thione)Methane and Bis(1-Benzyl-Imidazole-2-Thione)Methane Complexes Including Sulfur Atom Insertion into Copper−NHC Bonds. J. Organomet. Chem. 2017, 847, 224–233. [Google Scholar] [CrossRef]
- Roy, G.; Jayaram, P.N.; Mugesh, G. Inhibition of Lactoperoxidase-Catalyzed Oxidation by Imidazole-Based Thiones and Selones: A Mechanistic Study. Chem. Asian J. 2013, 8, 1910–1921. [Google Scholar] [CrossRef]
- Savjani, J.; Gajjar, A. ChemInform Abstract: Pharmaceutical Importance and Synthetic Strategies for Imidazolidine-2-Thione and Imidazole… Cite This Paper. Pak. J. Biol. Sci. 2011, 14, 1076–1089. [Google Scholar] [CrossRef][Green Version]
- Abu Almaaty, A.H.; Toson, E.E.M.; El-Sayed, E.S.H.; Tantawy, M.A.M.; Fayad, E.; Abu Ali, O.A.; Zaki, I. 5-Aryl-1-Arylideneamino-1h-Imidazole-2(3h)-Thiones: Synthesis and in Vitro Anticancer Evaluation. Molecules 2021, 26, 1706. [Google Scholar] [CrossRef] [PubMed]
- Kimani, M.M.; Brumaghim, J.L.; VanDerveer, D. Probing the Antioxidant Action of Selenium and Sulfur Using Cu(I)-Chalcogenone Tris(Pyrazolyl)Methane and -Borate Complexes. Inorg. Chem. 2010, 49, 9200–9211. [Google Scholar] [CrossRef]
- Rong, Y.; Al-Harbi, A.; Kriegel, B.; Parkin, G. Structural Characterization of 2-Imidazolones: Comparison with Their Heavier Chalcogen Counterparts. Inorg. Chem. 2013, 52, 7172–7182. [Google Scholar] [CrossRef]
- Feng, C.; Wang, L.; Yan, Y.; Liu, J.; Li, S. Synthesis and Antitumor Evaluation of Some 1,3,4-Oxadiazole-2(3H)-Thione and 1,2,4-Triazole-5(1H)-Thione Derivatives. Med. Chem. Res. 2012, 21, 315–320. [Google Scholar] [CrossRef]
- Jomaa, M.Y.; Altaf, M.; Ahmad, S.; Alhoshani, A.; Baig, N.; Kawde, A.-N.; Bhatia, G.; Singh, J.; Isab, A.A. Synthesis, Characterization and Anticancer Evaluation of Transplatin Derivatives with Heterocyclic Thiones. Polyhedron 2018, 141, 360–368. [Google Scholar] [CrossRef]
- Jomaa, M.Y.; Altaf, M.; Ahmad, S.; Bhatia, G.; Singh, J.; Altuwaijri, S.; Isab, A.A. Synthesis, Spectroscopic Characterization and in Vitro Anticancer Activity of New Platinum(II) Complexes with Some Thione Ligands in the Presence of Triethylphosphine. BioMetals 2017, 30, 787–795. [Google Scholar] [CrossRef]
- de Moura, T.R.; Cavalcanti, S.L.; de Godoy, P.R.D.V.; Sakamoto-Hojo, E.T.; Rocha, F.V.; de Almeida, E.T.; Deflon, V.M.; Mauro, A.E.; Netto, A.V.G. Synthesis, Characterization and Antitumor Activity of Palladium(II) Complexes of Imidazolidine-2-Thione. Transit. Metal. Chem. 2017, 42, 565–574. [Google Scholar] [CrossRef]
- Beheshti, A.; Nozarian, K.; Mousavifard, E.S.; Abrahams, C.T.; Mayer, P.; Gajda, R.; Woźniak, K.; Motamedi, H. Design and Construction of the Imidazole-2-Thione-Based Copper(I) Complexes by Varying the Co-Anion and Synthesis Conditions and Verifying Their Antimicrobial Activity. J. Solid. State Chem. 2021, 294, 121874. [Google Scholar] [CrossRef]
- Namiecińska, E.; Sadowska, B.; Wiȩckowska-Szakiel, M.; Dołȩga, A.; Pasternak, B.; Grazul, M.; Budzisz, E. Anticancer and Antimicrobial Properties of Novel H6-: P -Cymene Ruthenium(Ii) Complexes Containing a N,S-Type Ligand, Their Structural and Theoretical Characterization. RSC Adv. 2019, 9, 38629–38645. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Pandey, D.S.; Xu, Q.; Braunstein, P. Recent Advances in Supramolecular and Biological Aspects of Arene Ruthenium(II) Complexes. Coord. Chem. Rev. 2014, 270–271, 31–56. [Google Scholar] [CrossRef]
- Fricker, S.P. Metal Based Drugs: From Serendipity to Design. Dalton Trans. 2007, 4903–4917. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.F.A.; Sadler, P.J. Medicinal Organometallic Chemistry: Designing Metal Arene Complexes as Anticancer Agents. Chem. Asian J. 2008, 3, 1890–1899. [Google Scholar] [CrossRef]
- Süss-Fink, G. Arene Ruthenium Complexes as Anticancer Agents. Dalton Trans. 2010, 39, 1673–1688. [Google Scholar] [CrossRef]
- Rocha, F.V.; Barra, C.V.; Netto, A.V.G.; Mauro, A.E.; Carlos, I.Z.; Frem, R.C.G.; Ananias, S.R.; Quilles, M.B.; Stevanato, A.; da Rocha, M.C. 3,5-Dimethyl-1-Thiocarbamoylpyrazole and Its Pd(II) Complexes: Synthesis, Spectral Studies and Antitumor Activity. Eur. J. Med. Chem. 2010, 45, 1698–1702. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure–Activity Relationships for Ruthenium and Osmium Anticancer Agents—Towards Clinical Development. Chem. Soc. Rev. 2018, 47, 909–928. [Google Scholar] [CrossRef]
- Peacock, A.F.A.; Melchart, M.; Deeth, R.J.; Habtemariam, A.; Parsons, S.; Sadler, P.J. Osmium(II) and Ruthenium(II) Arene Maltolato Complexes: Rapid Hydrolysis and Nucleobase Binding. Chem.—A Eur. J. 2007, 13, 2601–2613. [Google Scholar] [CrossRef]
- Hanif, M.; Babak, M.V.; Hartinger, C.G. Development of Anticancer Agents: Wizardry with Osmium. Drug Discov. Today 2014, 19, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Maksimoska, J.; Williams, D.S.; Atilla-Gokcumen, G.E.; Smalley, K.S.M.; Carroll, P.J.; Webster, R.D.; Filippakopoulos, P.; Knapp, S.; Herlyn, M.; Meggers, E. Similar Biological Activities of Two Isostructural Ruthenium and Osmium Complexes. Chemistry 2008, 14, 4816–4822. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, S.A.; Patil, R.; Keri, R.S.; Budagumpi, S.; Balakrishna, G.R.; Tacke, M. N-Heterocyclic Carbene Metal Complexes as Bio-Organometallic Antimicrobial and Anticancer Drugs. Future Med. Chem. 2015, 7, 1305–1333. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Friedrich, H.B.; Omondi, B.; Singh, M.; Naicker, K.; Chenia, H.Y. Synthesis, Characterization, and Cytotoxic and Antimicrobial Activities of Ruthenium(II) Arene Complexes with N,N-Bidentate Ligands. J. Coord. Chem. 2016, 69, 3531–3544. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Friedrich, H.B.; Omondi, B.; Naicker, K.; Singh, M.; Chenia, H.Y. Synthesis, Characterization, Antiproliferative, and Antimicrobial Activity of Osmium(II) Half-Sandwich Complexes. J. Coord. Chem. 2018, 71, 342–354. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Omondi, B.; Lazarus, G.; Singh, M.; Shaikh, N.; Chenia, H.Y.; Friedrich, H.B. Influence of Halogen Substitution in the Ligand Sphere on the Antitumor and Antibacterial Activity of Half-Sandwich Ruthenium(II) Complexes [RuX(H6-Arene)(C5H4N-2-CH=N-Ar)]+. Z. Anorg. Allg. Chem. 2017, 643, 699–711. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Lo, W.; Lynn, M.A.; O’Loughlin, B.E.; Dimarzio, A.P.; Martinez, A.M.; Lampe, L.; Foley, K.M.; Keilich, L.C.; Lisi, G.P.; et al. Syntheses, Characterization, Density Functional Theory Calculations, and Activity of Tridentate SNS Zinc Pincer Complexes. Inorganica Chim. Acta 2011, 376, 515–524. [Google Scholar] [CrossRef][Green Version]
- Tao, X.L.; Lei, M.; Wang, Y.G. Unexpected Microwave Reaction of 1,3-Disubstituted Imidazolium Salts: A Novel Synthesis of 1,3-Disubstituted Imidazole-2-Thiones. Synth. Commun. 2007, 37, 399–408. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, D.; Yu, K.; Xu, J. 1,1′-(1,2-Ethanediyl)Bis(2,3-Dihydro-3-Methyl-1H-Imidazole-2-Thione). Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, o356–o357. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Lo, W.; Lynn, M.A.; Jain, S.; Keilich, L.C.; Kloczko, N.F.; O’Loughlin, B.E.; Dimarzio, A.P.; Foley, K.M.; Lisi, G.P.; et al. Syntheses, Characterization, Density Functional Theory Calculations, and Activity of Tridentate SNS Zinc Pincer Complexes Based on Bis-Imidazole or Bis-Triazole Precursors. Inorganica Chim. Acta 2012, 387, 25–36. [Google Scholar] [CrossRef]
- Silva, R.M.; Smith, M.D.; Gardinier, J.R. Unexpected New Chemistry of the Bis(Thioimidazolyl)Methanes. J. Org. Chem. 2005, 70, 8755–8763. [Google Scholar] [CrossRef]
- Jia, W.G.; Du, T.T.; Gao, L.L.; Du, J. Synthesis, Characterization, and Catalytic Activity of Half-Sandwich Ruthenium Complexes with Pyridine/Phenylene Bridged NHC = E (NHC = N-Heterocyclic Carbene, E = S, Se) Ligands. Appl. Organomet. Chem. 2020, 34, e5651. [Google Scholar] [CrossRef]
- Ibrahim, H.; Bala, M.D. Air Stable Pincer (CNC) N-Heterocyclic Carbene-Cobalt Complexes and Their Application as Catalysts for C-N Coupling Reactions. J. Organomet. Chem. 2015, 794, 301–310. [Google Scholar] [CrossRef]
- Sharma, A.K.; Joshi, H.; Sharma, K.N.; Gupta, P.L.; Singh, A.K. 2-Propanol vs Glycerol as Hydrogen Source in Catalytic Activation of Transfer Hydrogenation with (H6-Benzene)Ruthenium(II) Complexes of Unsymmetrical Bidentate Chalcogen Ligands. Organometallics 2014, 33, 3629–3639. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Venkataraghavan, R. The C=S Stretching Frequency and the “−N−C=S Bands” in the Infrared. Spectrochim. Acta A 1989, 45, 299–305. [Google Scholar] [CrossRef]
- Singh, B.; Thakur, K.P. Thioamide Bands and Nature of Bonding-II. J. Inorg. Nucl. Chem. 1974, 36, 1735–1737. [Google Scholar] [CrossRef]
- Atkinson, E.R.; Gardiner, D.J.; Jackson, A.R.W.; Raper, E.S. Tris-(1-Methylimidazoline-2(3H)-Thione)Copper(I) Nitrate: Preparation, Thermal Analysis and Crystal Structure. Inorganica Chim. Acta 1985, 98, 35–41. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.K. Transfer Hydrogenation of Ketones and Catalytic Oxidation of Alcohols with Half-Sandwich Complexes of Ruthenium(II) Designed Using Benzene and Tridentate (S, N, E) Type Ligands (E = S, Se, Te). Organometallics 2010, 29, 6433–6442. [Google Scholar] [CrossRef]
- Prakash, O.; Sharma, K.N.; Joshi, H.; Gupta, P.L.; Singh, A.K. Half Sandwich Complexes of Chalcogenated Pyridine Based Bi-(N, S/Se) and Terdentate (N, S/Se, N) Ligands with (H6-Benzene)Ruthenium(Ii): Synthesis, Structure and Catalysis of Transfer Hydrogenation of Ketones and Oxidation of Alcohols. Dalton Trans. 2013, 42, 8736–8747. [Google Scholar] [CrossRef] [PubMed]
- Sollert, C.; Orthaber, A.; Pilarski, L.T. Crystal Structure of Acetonitrile[H6-1-Methyl-4-(1-Methylethyl)Benzene][1-(Pyrimidin-2-Yl)-3H-Indol-1-Ium-2-Yl-Κ2 N,C]Ruthenium(II) Bis(Hexafluoridoantimonate). Acta Crystallogr. E Crystallogr. Commun. 2015, 71, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Foreman, M.R.S.J.; Ma, C.; Hill, A.F.; Otten, N.E.; Sharma, M.; Tshabang, N.; Ward, J.S. Dihydrobis(Methimazolyl)Borato Complexes of Ruthenium and Osmium. Dalton Trans. 2017, 46, 14957–14972. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T.; et al. Colorectal Cancer Screening for Average-risk Adults: 2018 Guideline Update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Wahid, M.; Mandal, R.K.; Dar, S.A.; Jawed, A.; Lohani, M.; Areeshi, M.Y.; Akhter, N.; Haque, S. Therapeutic Potential and Critical Analysis of Trastuzumab and Bevacizumab in Combination with Different Chemotherapeutic Agents against Metastatic Breast/Colorectal Cancer Affecting Various Endpoints. Crit. Rev. Oncol. Hematol. 2016, 104, 124–130. [Google Scholar] [CrossRef]
- Vásquez, D.; Rodríguez, J.A.; Theoduloz, C.; Calderon, P.B.; Valderrama, J.A. Studies on Quinones. Part 46. Synthesis and in Vitro Antitumor Evaluation of Aminopyrimidoisoquinolinequinones. Eur. J. Med. Chem. 2010, 45, 5234–5242. [Google Scholar] [CrossRef]
- Waseem, T.; Ullah, N.; Rajput, T.A. Review of Synthetic Accessibility And Pharmacological Activity of 1,2,4-Triazole and 2-Methylbenzimidazole Derivatives. Pharm. Chem. J. 2022, 56, 943–947. [Google Scholar] [CrossRef]
- Ayaz, F.; Yetkin, D.; Yüzer, A.; Demircioğlu, K.; Ince, M. Non-Canonical Anti-Cancer, Anti-Metastatic, Anti-Angiogenic and Immunomodulatory PDT Potentials of Water Soluble Phthalocyanine Derivatives with Imidazole Groups and Their Intracellular Mechanism of Action. Photodiagnosis Photodyn. Ther. 2022, 39, 103035. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, C.; Selvan, S.T.; Saminathan, M.; Safin, D.A. Crystal Structure, Quantum Computational, Molecular Docking and in Vitro Anti-Proliferative Investigations of 1H-imidazole-2-thione Analogues Derivative. J. Mol. Struct. 2022, 1250, 131833. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Chapter 4—Purification of Organic Chemicals. In Purification of Laboratory Chemicals (Sixth Edition); Armarego, W.L.F., Chai, C.L.L., Eds.; Butterworth-Heinemann: Oxford, UK, 2009; pp. 88–444. ISBN 978-1-85617-567-8. [Google Scholar]
- Bennet, M.A.; Huang, T.-N.; Matheson, T.W.; Smith, A.K.; Itel, S.; Nickerson, W. Hexmethylbenzene)Ruthenium Complexes. Inorg. Synth. 2007, 17, 74–78. [Google Scholar]
- Jensen, S.B.; Rodgers, S.J.; Spicer, M.D. Facile Preparation of N6-p-Cymene Ruthenium Diphosphine Complexes. Crystal Structure of [(N6-p-Cymene)Ru(Dppf)Cl]PF6. J. Organomet. Chem. 1998, 556, 151–158. [Google Scholar] [CrossRef]
- Castarlenas, R.; Esteruelas, M.A.; Oñate, E. N-Heterocyclic Carbene-Osmium Complexes for Olefin Metathesis Reactions. Organometallics 2005, 24, 4343–4346. [Google Scholar] [CrossRef]
- Werner, H.; Zenkert, K. Aromaten(Phosphan)Metall-Komplexe: XIII. Osmium(II)- Und Osmium(O)-Komplexe Mit p-Cymen Als Aromatischem Liganden. J. Organomet. Chem. 1988, 345, 151–166. [Google Scholar] [CrossRef]
- Cao, C.; Zhuang, Y.; Zhao, J.; Liu, H.; Geng, P.; Pang, G.; Shi, Y. Green Synthesis of Alkane Bridged Bisimidazolium Salts under Solvent-Free Conditions. Synth. Commun. 2012, 42, 380–387. [Google Scholar] [CrossRef]
- Lee, K.-M.; Chen, J.C.C.; Lin, I.J.B. Helical Mono and Dinuclear Mercury(II) N-Heterocyclic Carbene Complexes. J. Organomet. Chem. 2001, 617, 364–375. [Google Scholar] [CrossRef]
- Ibrahim, H.; Bala, M.D. Improved Methods for the Synthesis and Isolation of Imidazolium Based Ionic Salts. Tetrahedron Lett. 2014, 55, 6351–6353. [Google Scholar] [CrossRef]
- Peris, E.; Loch, J.A.; Mata, J.; Crabtree, R.H. A Pd Complex of a Tridentate Pincer CNC Bis-Carbene Ligand as a Robust Homogenous Heck Catalyst. Chem. Commun. 2001, 201–202. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Daniels, A.; Singh, S.; Singh, M. Histidine-Tagged Folate-Targeted Gold Nanoparticles for Enhanced Transgene Expression in Breast Cancer Cells in Vitro. Pharmaceutics 2022, 14, 53. [Google Scholar] [CrossRef]
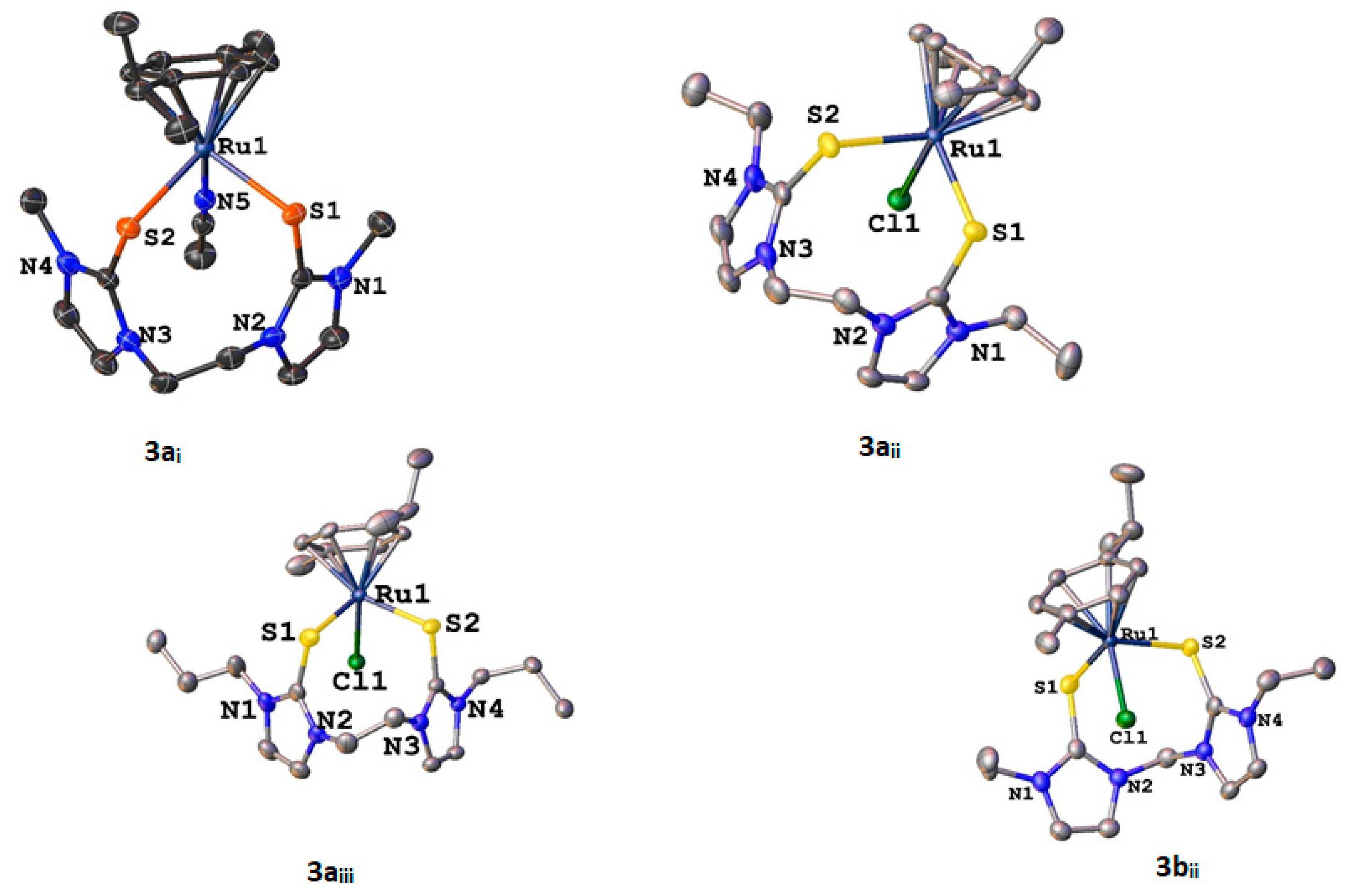
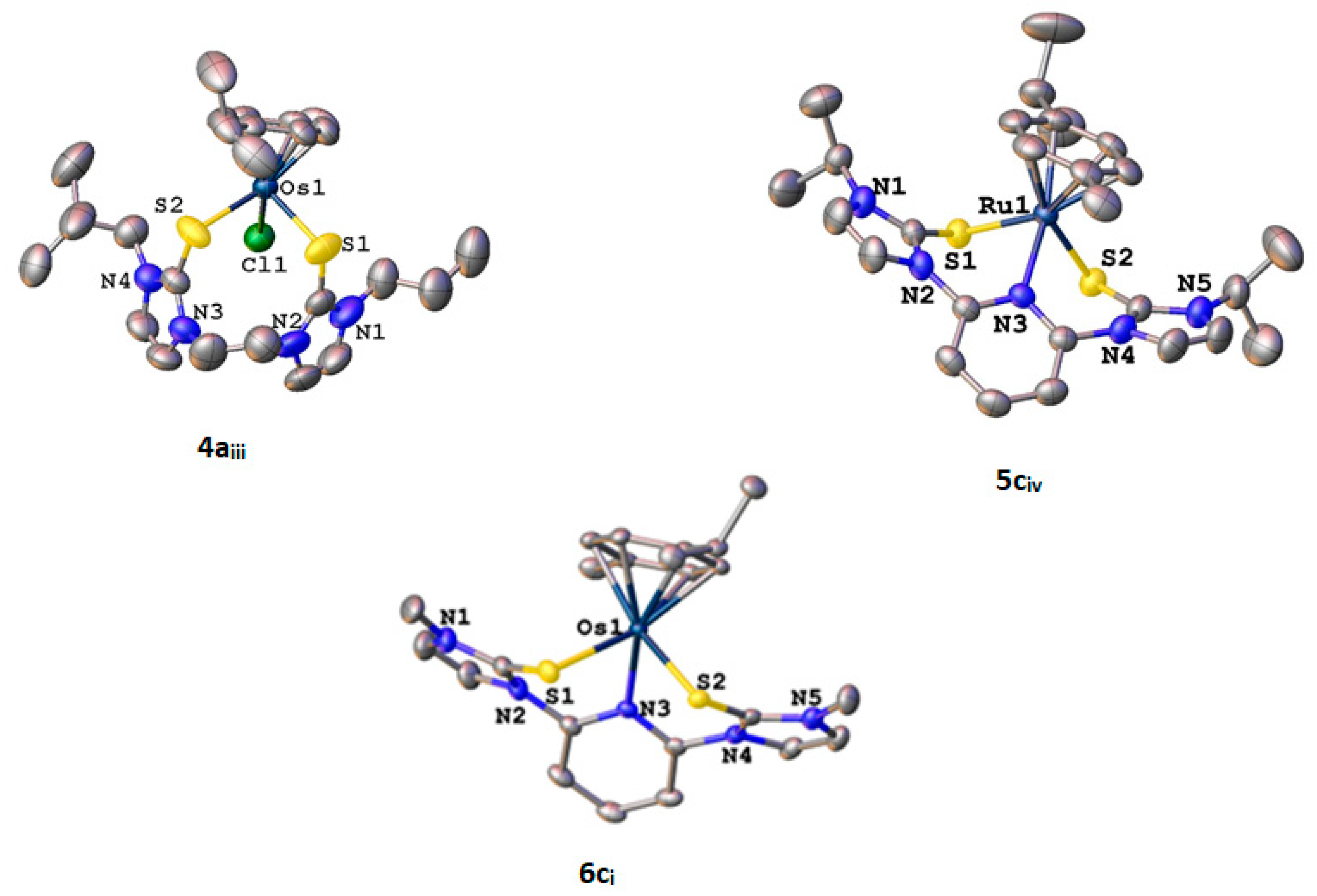
| Compound | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | 3ai | 3aii | 3aiii | 3bii | 4aiii | 5civ | 6ci |
| Formula | C22H31F6N5PRuS2 | C22H32ClF6N4PRuS2 | C24H36ClF6N4PRuS2 | C21H30ClF6N4PRuS2 | C24H36ClF6N4OsPS2 | C27H35F12N5P2RuS2 | C23H27F12N5OsP2S2 |
| Formula weight | 675.68 | 698.12 | 726.18 | 684.10 | 815.31 | 884.73 | 917.75 |
| Crystal system | triclinic | Monoclinic | monoclinic | Monoclinic | monoclinic | monoclinic | Triclinic |
| Space group | P-1 | P21/n | P21/c | P21/n | P21/n | C2/c | P-1 |
| a/Å | 9.2668(3) | 13.9150(2) | 20.0391(3) | 11.7443(2) | 14.3155(13) | 27.0151(7) | 11.4823(7) |
| b/Å | 11.4774(3) | 14.4134(2) | 14.6704(2) | 17.4566(4) | 14.9434(15) | 11.2276(3) | 11.9080(6) |
| c/Å | 16.7090(5) | 14.0586(2) | 20.3946(3) | 14.1523(3) | 14.5774(14) | 26.3787(7) | 13.5933(8) |
| α/° | 70.0180(10) | 90 | 90 | 90 | 90 | 90 | 73.725(2) |
| β/° | 88.453(2) | 92.5410(10) | 91.8760(10) | 111.8930(10) | 90.364(5) | 113.2150(10) | 65.784(2) |
| γ/° | 69.1310(10) | 90 | 90 | 90 | 90 | 90 | 64.656(2) |
| Volume/Å3 | 1551.49(8) | 2816.85(7) | 5992.42(15) | 2692.19(10) | 3118.4(5) | 7353.2(3) | 1519.53(15) |
| Z | 2 | 4 | 8 | 4 | 4 | 8 | 2 |
| ρcalc g/cm3 | 1.446 | 1.646 | 1.610 | 1.688 | 1.737 | 1.598 | 2.006 |
| Temperature/K | 149.9 | 149.99 | 149.99 | 149.98 | 296.15 | 296 | 150.0 |
| μ/mm−1 | 0.747 | 0.916 | 0.865 | 0.957 | 4.417 | 0.715 | 4.538 |
| λ/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| F(000) | 686.0 | 1416.0 | 2960.0 | 1384.0 | 1608.0 | 3568.0 | 892.0 |
| Cryst size, mm3 | 0.31 × 0.25 × 0.14 | 0.35 × 0.27 × 0.14 | 0.28 × 0.24 × 0.13 | 0.25 × 0.17 × 0.13 | 0.18 × 0.12 × 0.08 | 0.26 × 0.21 × 0.14 | 0.18 × 0.16 × 0.11 |
| θmin, θmax,◦ | 3.972 to 55.754 | 6.354 to 56.844 | 2.034 to 56.666 | 5.604 to 56.682 | 4.82 to 56.546 | 3.982 to 56.618 | 4.368 to 57 |
| No. of reflns. Collected | 24,335 | 28,323 | 97,276 | 44,694 | 46,353 | 40,270 | 30,272 |
| No of indep. reflns. | 7328 | 6969 | 14,899 | 6643 | 7666 | 9032 | 7443 |
| Completeness to theta | 27.877(99.1%) | 28.422(98.4%) | 28.333(99.6%) | 28.341(98.9%) | 28.273(99.1%) | 28.309(98.7%) | 28.500(96.6%) |
| Absorbed correction | MULTI-SCAN | MULTI-SCAN | MULTI-SCAN | MULTI-SCAN | MULTI-SCAN | MULTI-SCAN | MULTI-SCAN |
| Goodness-of-fit on F2 | 1.025 | 1.060 | 1.034 | 1.097 | 1.041 | 1.048 | 1.038 |
| Final R indices | 0.0255, 0.0624 | 0.0209, 0.0488 | 0.0331, 0.0758 | 0.0388, 0.1145 | 0.0342, 0.0770 | 0.0587, 0.1727 | 0.0180, 0.0430 |
| R indices (all data) | 0.0284, 0.0645 | 0.0245, 0.0508 | 0.0445, 0.0834 | 0.0440, 0.1192 | 0.0565, 0.0871 | 0.0700, 0.1839 | 0.0199, 0.0439 |
| Largest diff. peak/hole/eÅ−3 | 0.69/−0.38 | 0.62/−0.32 | 1.08/−0.72 | 1.31/−1.22 | 1.04/−0.67 | 1.48/−0.78 | 0.90/−0.47 |
| Compounds | |||||||
|---|---|---|---|---|---|---|---|
| Bond Distances (Å) | |||||||
| Bond/Angle | 3ai (Ru) | 3aii (Ru) | 3aiii (Ru) | 3bii (Ru) | 4aiii (Os) | 5civ (Ru) | 6ci (Os) |
| M-S | Ru(1)-S(1) 2.4182(5) | Ru(1)-S(1) 2.4405(4) | Ru(1)-S(1) 2.4426(6) | Ru(1)-S(1) 2.4479(7) | Os(1)-S(1) 2.4478(13) | Ru(1)-S(1) 2.3857(10) | Os(1)-S(1) 2.4113(6) |
| Ru(1)-S(2) 2.4656(5) | Ru(1)-S(2) 2.4726(4) | Ru(1)-S(2) 2.4698(6) | Ru(1)-S(2) 2.4320(6) | Os(1)-S(2) 2.4488(13) | Ru(1)-S(2) 2.4071(10) | Os(1)-S(2) 2.3905(5) | |
| M-Cl | Ru(1)-Cl(1) 2.4072(4) | Ru(1)-Cl(1) 2.4030(6) | Ru(1)-Cl(1) 2.4018(7) | Os(1)-Cl(1) 2.4027(12) | |||
| M-N | Ru(1)-N(5) 2.0710(16) | Ru(1)-N(3) 2.168(3) | Os(1)-N(3) 2.1733(18) | ||||
| C-S | S(1)-C(2) 1.7222(19) | S(1)-C(5) 1.7196(15) | S(1)-C(4) 1.720(2) | S(1)-C(5) 1.718(3) | S(1)-C(16) 1.715(6) | S(1)-C(16) 1.706(4) | S(1)-C(4) 1.706(2) |
| S(2)-C(7) 1.7170(19) | S(2)-C(10) 1.7186(17) | S(2)-C(9) 1.719(2) | S(2)-C(7) 1.716(3) | S(2)-C(22) 1.715(5) | S(2)-C(22) 1.701(4) | S(2)-C(10) 1.699(2) | |
| Bond angles (°) | |||||||
| S-M-S | S(1)-Ru(1)-S(2) 88.255(17) | S(1)-Ru(1)-S(2) 92.835(14) | S(1)-Ru(1)-S(2) 91.02(2) | S(1)-Ru(1)-S(2) 87.33(2) | S(1)-Os(1)-S(2) 90.94(6) | S(1)-Ru(1)-S(2) 84.70(4) | S(2)-Os(1)-S(1) 86.07(2) |
| S-M-Cl | C1(1)-Ru(1)-S(1) 89.034(13) | Cl(1)-Ru(1)-S(1) 89.98(2) | Cl(1)-Ru(1)-S(1) 91.42(2) | Cl(1)-Os(1)-S(1) 89.61(5) | |||
| Cl(1)-Ru(1)-S(2) 90.985(13) | Cl(1)-Ru(1)-S(2) 91.16(2) | Cl(1)-Ru(1)-S(2) 90.43(2) | Cl(1)-Os(1)-S(2) 90.18(4) | ||||
| S-M-N | N(5)-Ru(1)-S(1) 90.65(4) | N(3)-Ru(1)-S(1) 84.62(9) | N(3)-Os(1)-S(1) 84.41(5) | ||||
| N(5)-Ru(1)-S(2) 90.74(4) | N(3)-Ru(1)-S(2) 84.23(9) | N(3)-Os(1)-S(2) 84.67(5) | |||||
| S/No. | Compound | IC50/μM a | |
|---|---|---|---|
| HEK293 | HeLa | ||
| 1 | 3ai | 180 ± 23 | 219 ± 55 |
| 2 | 3aii | 155 ± 13 | 132 ± 41 |
| 3 | 3aiii | 159 ± 33 | 344 ± 26 |
| 4 | 3bii | 29 ± 7 | 191 ± 25 |
| 5 | 4aiii | 173 ± 23 | 170 ± 16 |
| 6 | 4bi | 47 ± 18 | 469 ± 21 |
| 7 | 4bii | 211 ± 11 | 315 ± 62 |
| 8 | 5bi | 155 ± 5 | 86 ± 9 |
| 9 | 5civ | 169 ± 7 | 147 ± 21 |
| 10 | 6bi | 18 ± 7 | 151 ± 12 |
| 11 | 6ci | 121 ± 8 | 36 ± 10 |
| 12 | 6civ | 121 ± 4 | 40 ± 4 |
| 13 | 5-Fluorouracil | 180 ± 20 | 87 ± 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ywaya, D.O.; Ibrahim, H.; Friedrich, H.B.; Bala, M.D.; Soobramoney, L.; Daniels, A.; Singh, M. Chemotherapeutic Activities of New η6-p-Cymene Ruthenium(II) and Osmium(II) Complexes with Chelating SS and Tridentate SNS Ligands. Molecules 2024, 29, 944. https://doi.org/10.3390/molecules29050944
Ywaya DO, Ibrahim H, Friedrich HB, Bala MD, Soobramoney L, Daniels A, Singh M. Chemotherapeutic Activities of New η6-p-Cymene Ruthenium(II) and Osmium(II) Complexes with Chelating SS and Tridentate SNS Ligands. Molecules. 2024; 29(5):944. https://doi.org/10.3390/molecules29050944
Chicago/Turabian StyleYwaya, David O., Halliru Ibrahim, Holger B. Friedrich, Muhammad D. Bala, Lynette Soobramoney, Aliscia Daniels, and Moganavelli Singh. 2024. "Chemotherapeutic Activities of New η6-p-Cymene Ruthenium(II) and Osmium(II) Complexes with Chelating SS and Tridentate SNS Ligands" Molecules 29, no. 5: 944. https://doi.org/10.3390/molecules29050944
APA StyleYwaya, D. O., Ibrahim, H., Friedrich, H. B., Bala, M. D., Soobramoney, L., Daniels, A., & Singh, M. (2024). Chemotherapeutic Activities of New η6-p-Cymene Ruthenium(II) and Osmium(II) Complexes with Chelating SS and Tridentate SNS Ligands. Molecules, 29(5), 944. https://doi.org/10.3390/molecules29050944






