Conjugated Polymer-Based Hydrogel Film for a Fast and Sensitive Detection of Fe(Ⅲ) in Vegetables
Abstract
1. Introduction
2. Results and Discussion
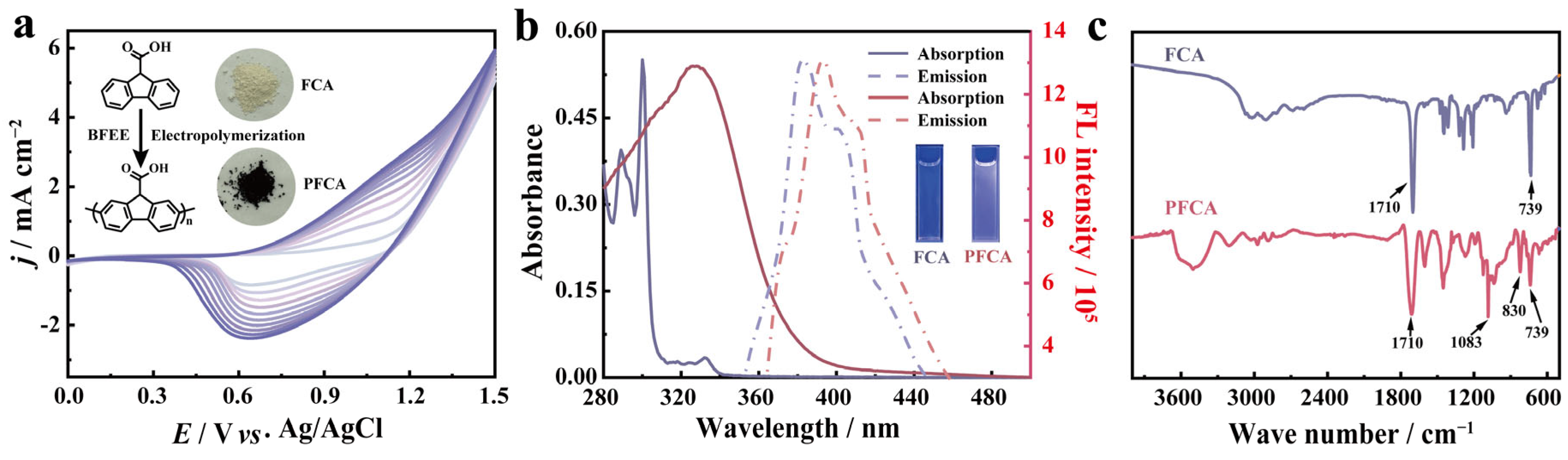
2.1. Electrosynthesis and Characterization of PFCA
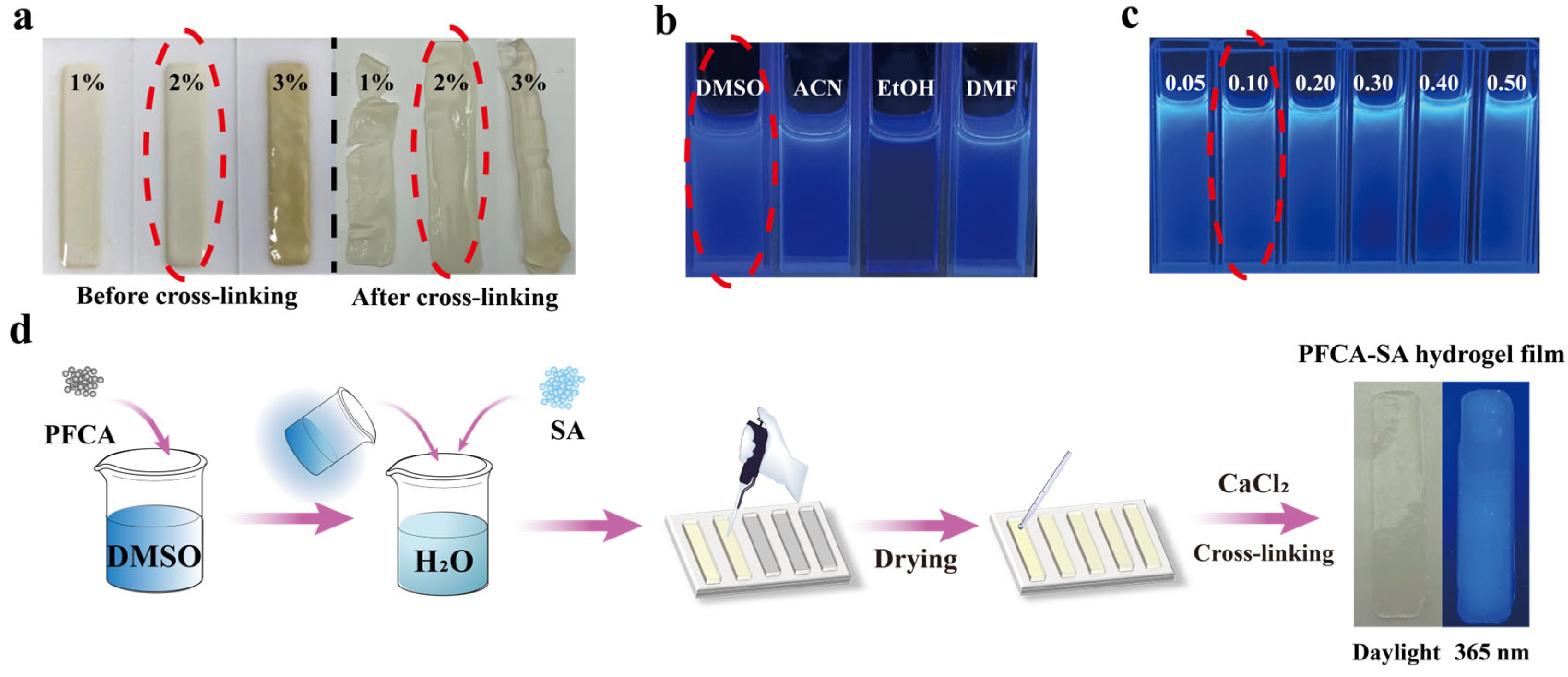
2.2. Preparation and Characterization of PFCA-SA Hydrogel Films
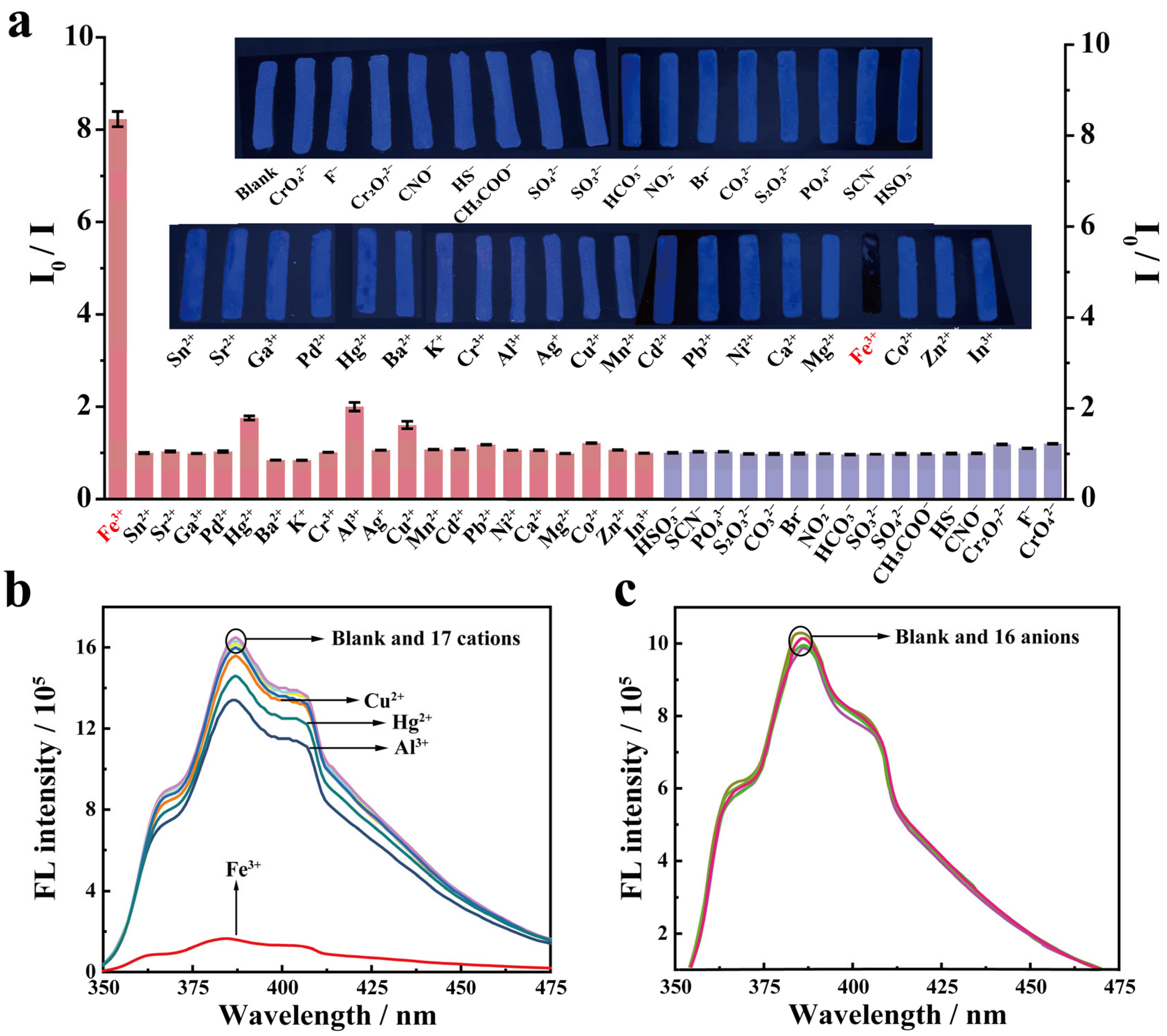
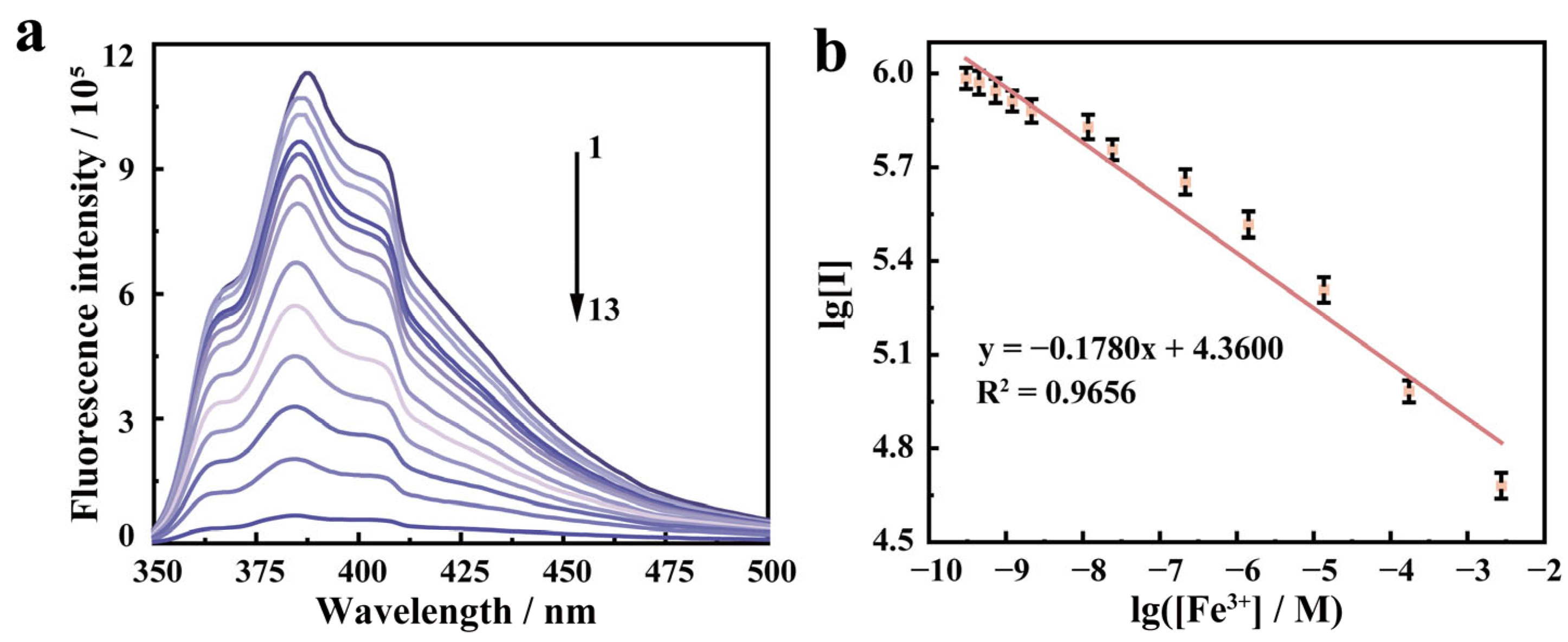
2.3. Detection of Fe3+ Using PFCA-SA Hydrogel Films
2.4. Application of the PFCA-SA Hydrogel Film
3. Experimental Section
3.1. Chemicals and Materials
3.2. Instrumentation
3.3. Methods
3.3.1. Preparation of PFCA
3.3.2. Preparation of the PFCA-SA Hydrogel Film
3.3.3. Preparation of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanikenne, M.; Esteves, S.M.; Fanara, S.; Rouached, H. Coordinated homeostasis of essential mineral nutrients: A focus on iron. J. Exp. Bot. 2021, 72, 2136–2153. [Google Scholar] [CrossRef]
- Watari, T.; Nansai, K.; Nakajima, K. Major metals demand, supply, and environmental impacts to 2100: A critical review. Resour. Conserv. Recycl. 2021, 164, 105107. [Google Scholar] [CrossRef]
- Coates, T.D.; Cazzola, M. Introduction to a review series on iron metabolism and its disorders. Blood 2019, 133, 1–2. [Google Scholar] [CrossRef] [PubMed]
- van Swelm, R.P.L.; Wetzels, J.F.M.; Swinkels, D.W. The multifaceted role of iron in renal health and disease. Nat. Rev. Nephrol. 2020, 16, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.X.; Ardehali, H.; Min, J.X.; Wang, F.D. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Yuan, L.; Li, W.; Li, J.Y. Ferroptosis in Parkinson’s disease: Glia-neuron crosstalk. Trends Mol. Med. 2022, 28, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Li, H.T.; Huang, H.A.; Cao, X.H.; Chen, X.D.; Cao, D.P. Porous organic polymers as a platform for sensing applications. Chem. Soc. Rev. 2022, 51, 2031–2080. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Ma, J.; Sun, D.W. Aggregates-based fluorescence sensing technology for food hazard detection: Principles, improvement strategies, and applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2977–3010. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.M.; Yan, B. Facile fabrication of luminescent Eu(III) functionalized HOF hydrogel film with multifunctionailities: Quinolones fluorescent Sensor and Anticounterfeiting Platform. Adv. Funct. Mater. 2021, 31, 2103321. [Google Scholar] [CrossRef]
- Huang, R.R.; Li, M.Q.; Lin, D.H.; Shao, Y.T.; Shang, C.D.; Liu, Q.Q.; Liu, G.J.; Li, N.; Miao, R.; Peng, H.N.; et al. A fluorescent film sensor for high-performance detection of Listeria monocytogenes via vapor sampling. Aggregate 2023, 4, e203. [Google Scholar] [CrossRef]
- Wang, X.B.; Yu, C.; Guo, H.; Cheng, Y.Q.; Li, Y.W.; Zheng, D.Y.; Feng, S.S.; Lin, Y.X. Robust fluorescent detection of iodine vapor by a film sensor based on a polymer of intrinsic microporosity. Chem. Eng. J. 2022, 438, 135641. [Google Scholar] [CrossRef]
- Cao, X.H.; Gao, A.P.; Hou, J.T.; Yi, T. Fluorescent supramolecular self-assembly gels and their application as sensors: A review. Coord. Chem. Rev. 2021, 434, 213792. [Google Scholar] [CrossRef]
- Ding, W.C.; Xu, J.K.; Wen, Y.P.; Zhang, H.; Zhang, J. Facile fabrication of fluorescent poly(5-cyanoindole) thin film sensor via electropolymerization for detection of Fe3+ in aqueous solution. J. Photochem. Photobiol. A Chem. 2016, 314, 22–28. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Sun, X.K.; Guan, S.W.; Zhang, G.; Baumgarten, M.; Müllen, K. A dendrimer-based highly sensitive and selective fluorescence quenching sensor for Fe3+ both in solution and as film. Biosens. Bioelectron. 2016, 85, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Kamaci, M.; Kaya, I. A highly selective, sensitive and stable fluorescent chemosensor based on Schiff base and poly(azomethine-urethane) for Fe3+ ions. J. Ind. Eng. Chem. 2017, 46, 234–243. [Google Scholar] [CrossRef]
- Namgung, H.; Kim, J.; Gwon, Y.; Lee, T.S. Synthesis of poly(p-phenylene) containing a rhodamine 6G derivative for the detection of Fe(III) in organic and aqueous media. RSC Adv. 2017, 7, 39852–39858. [Google Scholar] [CrossRef]
- Kamaci, M.; Kaya, I. Polymeric fluorescent film sensor based on poly(azomethine-urethane): Ion sensing and surface properties. React. Funct. Polym. 2019, 136, 1–8. [Google Scholar] [CrossRef]
- Xu, H.B.; Zhou, S.H.; Fang, W.B.; Fan, Y.Z. Synthesis of N-doped graphene quantum dots from bulk N-doped carbon nanofiber film for fluorescence detection of Fe3+ and ascorbic acid. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 218–226. [Google Scholar] [CrossRef]
- Xiao, L.Q.; Shi, J.; Nan, B.F.; Chen, W.L.; Zhang, Q.; Zhang, E.D.; Lu, M.G. Highly sensitive detection of Fe3+ ions using waterborne polyurethane-carbon dots self-healable Fluorescence Film. Macromol. Mater. Eng. 2020, 305, 12. [Google Scholar] [CrossRef]
- Jia, P.; Wang, Z.H.; Zhang, Y.F.; Zhang, D.; Gao, W.C.; Su, Y.; Li, Y.B.; Yang, C.L. Selective sensing of Fe3+ ions in aqueous solution by a biodegradable platform based lanthanide metal organic framework. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 1900810. [Google Scholar] [CrossRef]
- Li, D.M.; Zhang, S.Y.; Wan, J.Y.; Zhang, W.Q.; Yan, Y.L.; Tang, X.H.; Zheng, S.R.; Cai, S.L.; Zhang, W.G. A new hydrazone-linked covalent organic framework for Fe(III) detection by fluorescence and QCM technologies. CrystEngComm 2021, 23, 3594–3601. [Google Scholar] [CrossRef]
- Lv, G.S.; Dai, X.J.; Lu, G.M.; Ye, L.; Wang, G.; Zhou, L. Facile fabrication of portable electrospun poly(vinyl alcohol)/sulfur quantum dots film sensor for sensitive and selective detection of Fe3+. Opt. Mater. 2023, 135, 113227. [Google Scholar] [CrossRef]
- Mondal, S.; Sarma, D. A coordination driven ‘heat-set’ Zr-gel: Efficient fluorophore probe for selective detection of Fe3+ and nitrofuran-based antibiotics and smart approach for UV protection. Soft Matter 2023, 19, 4926–4938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, G.; Xu, J.; Wen, Y.; Lu, B.; Zhang, J.; Ding, W. Novel highly selective fluorescent sensor based on electrosynthesized poly(9-fluorenecarboxylic acid) for efficient and practical detection of iron (III) and its agricultural application. Sens. Actuators B Chem. 2016, 230, 123–129. [Google Scholar] [CrossRef]

| Sensor | Substrate | Response Time | Working Concentration Range | LOD | Interference Exclusion | Applications |
|---|---|---|---|---|---|---|
| P5CI [14] | ND | 5 min | 0.2 μM–0.5 mM | 0.897 ppb | Metal ions, anions, natural amino acids, organic acids, and carbohydrates | ND |
| PYTPAG2 [15] | ND | ND | 0–40 μM | 0.5 μM | Metal ions | Natural water |
| P-PAH [16] | ND | ND | 1–8.0 mM | 74.5 μM | Metal ions and anions | Sea, tap, and drinking water |
| R6G–PPP [17] | Cellulosic paper | 1 min | 6.3 μM–0.1 mM | 1 μM | Metal ions | ND |
| PAZU [18] | ND | ND | 1–8 mM | 86.15 μM; 28.90 μM | Metal ions | Drinking, sea, and tap water |
| N-GQDs [19] | ND | ND | 0.5 μM–50 μM | ND | Metal ions | Tap water |
| WBPU-N-C-dots [20] | ND | ND | 0–0.2 mM | 2.19 μM | Metal ions | ND |
| Eu0.24Tb0.76-BHM-COOH [21] | Polylactic acid | 5 min | ND | 4.47 μM | Metal ions | ND |
| Tfpa–Mth COF [22] | Quartz crystalmicrobalance chip | ND | 0–0.1 mM | 64 nM | Metal ions and anion | ND |
| SQDs [23] | Poly(vinyl alcohol) | ND | 0–0.53 mM | 0.69 μM | Metal ions | Tap water and river water |
| H3TATAB [24] | Poly(methyl methacrylate) | ND | ND | 68 ppb | Metal ions | ND |
| PFCA [In this work] | SA | Immediately | 0.303 nM–6.01 mM | 0.10 nM | Metal ions and anions | Vegetables |
| Sample | Fe3+ Spiked (M) | Fe3+ Found ( ± SD) (M) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Wild rice stem | - | - | - | - |
| 4.90 × 10−6 | (4.93 ± 0.01) × 10−6 | 100.61 | 0.20 | |
| 4.95 × 10−5 | (4.97 ± 0.02) × 10−5 | 100.40 | 0.40 | |
| 4.70 × 10−4 | (4.71 ± 0.04) × 10−4 | 100.21 | 0.85 | |
| Water spinach | - | - | - | - |
| 4.90 × 10−6 | (4.93 ± 0.02) × 10−6 | 100.61 | 0.41 | |
| 4.95 × 10−5 | (4.97 ± 0.03) × 10−5 | 100.40 | 0.60 | |
| 4.70 × 10−4 | (4.65 ± 0.03) × 10−4 | 98.94 | 0.65 | |
| Cabbage | - | - | - | - |
| 4.90 × 10−6 | (4.94 ± 0.07) × 10−6 | 100.82 | 1.42 | |
| 4.95 × 10−5 | (4.92 ± 0.02) × 10−5 | 99.39 | 0.41 | |
| 4.70 × 10−4 | (4.67 ± 0.09) × 10−4 | 99.36 | 1.93 | |
| Celery | - | - | - | - |
| 4.90 × 10−6 | (4.92 ± 0.02) × 10−6 | 100.41 | 0.41 | |
| 4.95 × 10−5 | (4.90 ± 0.06) × 10−5 | 98.98 | 1.22 | |
| 4.70 × 10−4 | (4.66 ± 0.01) × 10−4 | 99.15 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Sheng, L.; Zhang, G.; Ji, M.; Li, Y. Conjugated Polymer-Based Hydrogel Film for a Fast and Sensitive Detection of Fe(Ⅲ) in Vegetables. Molecules 2024, 29, 925. https://doi.org/10.3390/molecules29050925
Ding X, Sheng L, Zhang G, Ji M, Li Y. Conjugated Polymer-Based Hydrogel Film for a Fast and Sensitive Detection of Fe(Ⅲ) in Vegetables. Molecules. 2024; 29(5):925. https://doi.org/10.3390/molecules29050925
Chicago/Turabian StyleDing, Xingli, Li Sheng, Ge Zhang, Min Ji, and Yu Li. 2024. "Conjugated Polymer-Based Hydrogel Film for a Fast and Sensitive Detection of Fe(Ⅲ) in Vegetables" Molecules 29, no. 5: 925. https://doi.org/10.3390/molecules29050925
APA StyleDing, X., Sheng, L., Zhang, G., Ji, M., & Li, Y. (2024). Conjugated Polymer-Based Hydrogel Film for a Fast and Sensitive Detection of Fe(Ⅲ) in Vegetables. Molecules, 29(5), 925. https://doi.org/10.3390/molecules29050925








